Identification of Alcohol Use Prior to Major Cancer Surgery: Timeline Follow Back Interview Compared to Four Other Markers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Outcomes
2.4. Collection of Data
2.5. Ethics
2.6. Statistical Analysis
3. Results
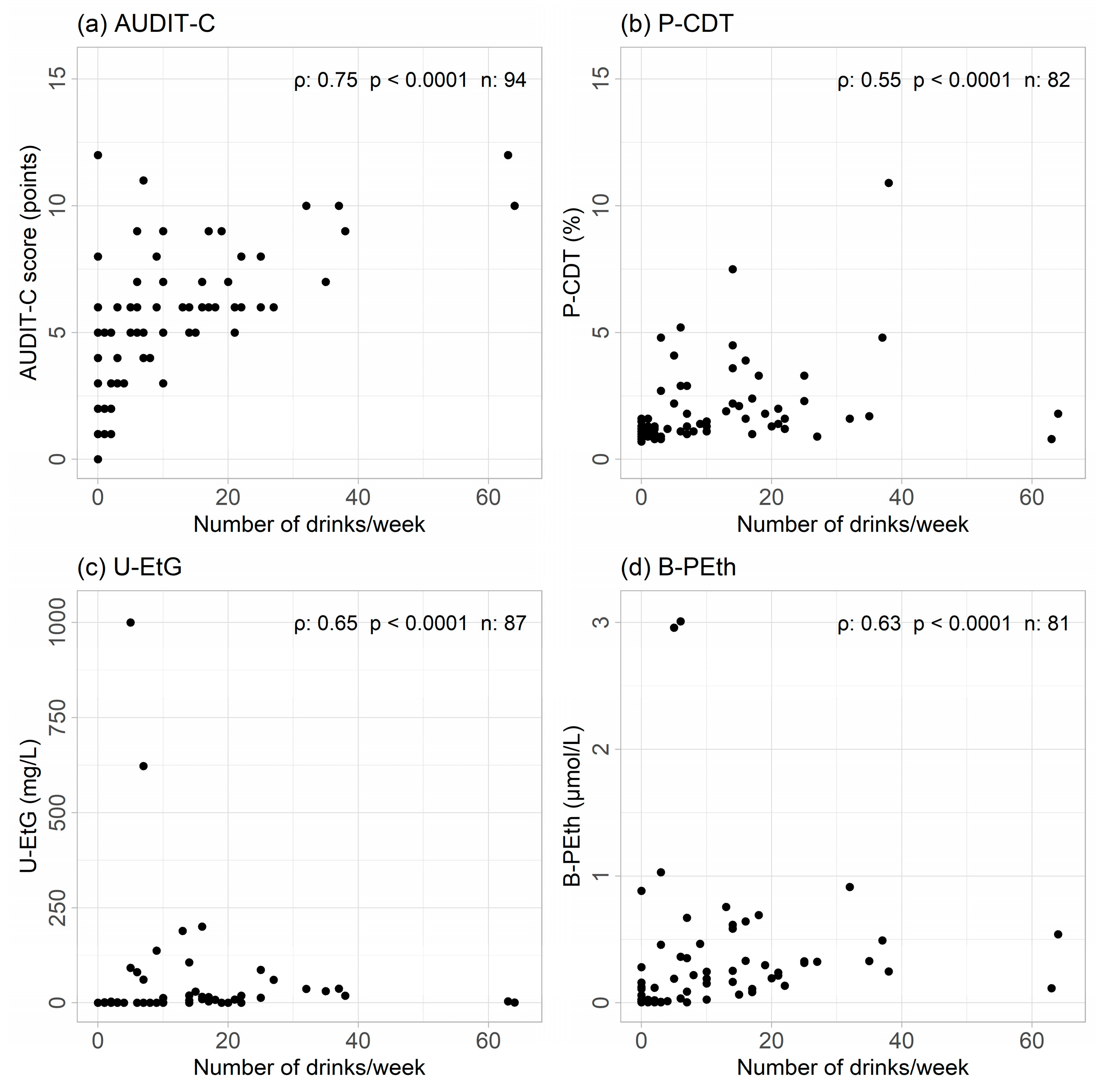
3.1. Correlations
3.2. Sensitivity, Specificity, and Predictive Values
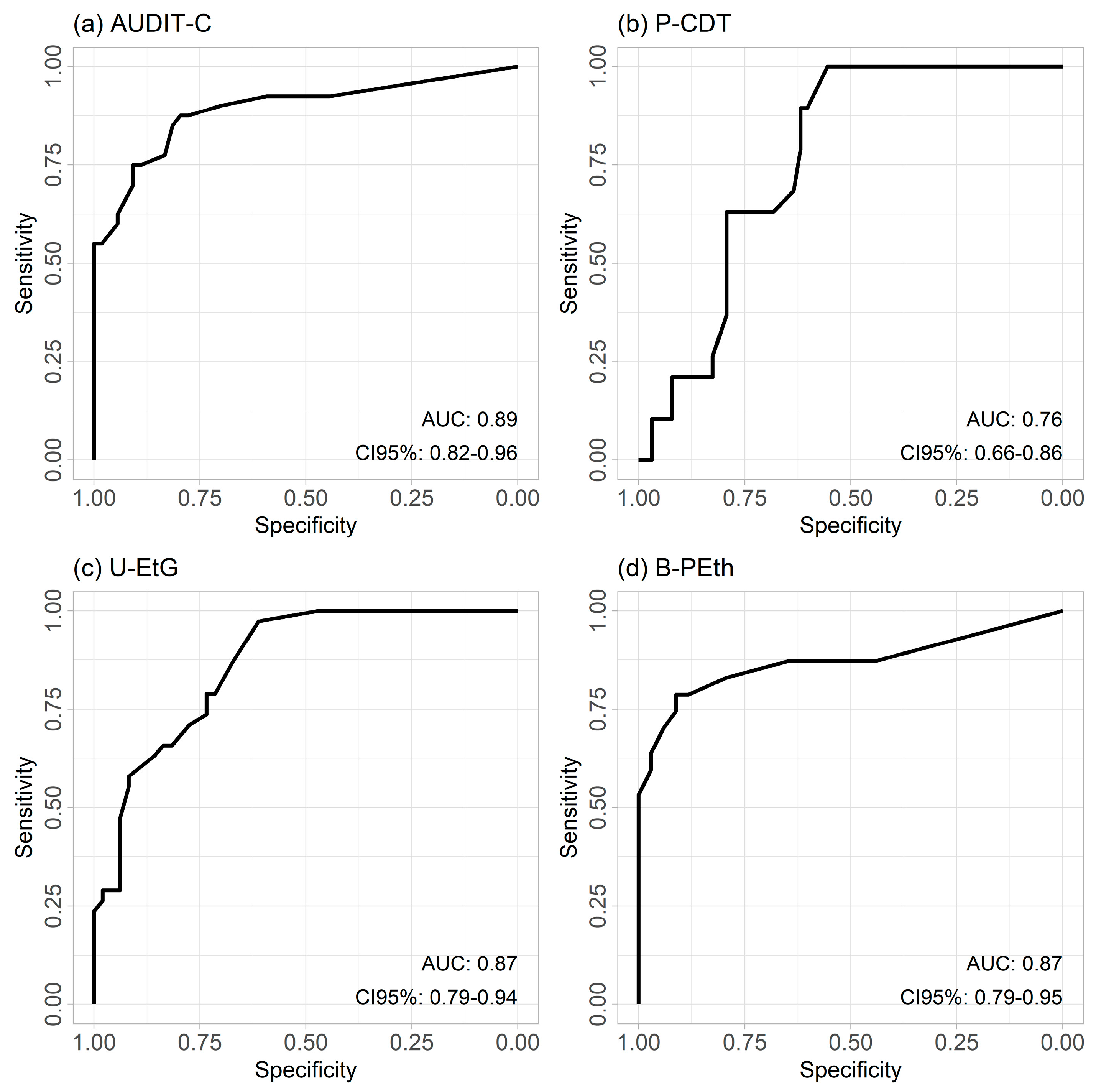
3.3. ROC Curves
4. Discussion
4.1. Bias and Limitations
4.2. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shabsigh, A.; Korets, R.; Vora, K.C.; Brooks, C.M.; Cronin, A.M.; Savage, C.; Raj, G.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur. Urol. 2009, 55, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Eliasen, M.; Grønkjær, M.; Skov-Ettrup, L.S.; Mikkelsen, S.S.; Becker, U.; Tolstrup, J.S.; Flensborg-Madsen, T. Preoperative alcohol consumption and postoperative complications: A systematic review and meta-analysis. Ann. Surg. 2013, 258, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Silveira, S.Q.; da Silva, L.M.; Gomes, R.F.; de Campos Vieira Abib, A.; Vieira, J.E.; Ho, A.M.; de Oliveira Lima, H.; Bellicieri, F.N.; Camire, D.; Nersessian, R.S.F.; et al. An evaluation of the accuracy and self-reported confidence of clinicians in using the ASA-PS Classification System. J. Clin. Anesth. 2022, 79, 110794. [Google Scholar] [CrossRef] [PubMed]
- Abouleish, A.E.; Leib, M.L.; Cohen, N.H. ASA Provides Examples to Each ASA Physical Status Class. ASA Newsl. 2015, 79, 38–49. [Google Scholar]
- Tønnesen, H.; Nielsen, P.R.; Lauritzen, J.B.; Møller, A.M. Smoking and alcohol intervention before surgery: Evidence for best practice. Br. J. Anaesth. 2009, 102, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Egholm, J.W.; Pedersen, B.; Møller, A.M.; Adami, J.; Juhl, C.B.; Tønnesen, H. Perioperative alcohol cessation intervention for postoperative complications. Cochrane Database Syst. Rev. 2018, 11, Cd008343. [Google Scholar] [CrossRef] [PubMed]
- Snowden, C.; Lynch, E.; Avery, L.; Haighton, C.; Howel, D.; Mamasoula, V.; Gilvarry, E.; McColl, E.; Prentis, J.; Gerrand, C.; et al. Preoperative behavioural intervention to reduce drinking before elective orthopaedic surgery: The PRE-OP BIRDS feasibility RCT. Health Technol. Assess. 2020, 24, 1–176. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.C.; Chapman, L.; Ren, T.Y.; Baxley, C.; Hallway, A.K.; Tang, M.J.; Waljee, J.F.; Friedmann, P.D.; Mello, M.; Borsari, B.; et al. Preoperative alcohol interventions for elective surgical patients: Results from a randomized pilot trial. Surgery 2022, 172, 1673–1681. [Google Scholar] [CrossRef]
- Sobell, L.; Sobell, M. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In Measuring Alcohol Consumption: Psychosocial and Biochemical Methods; Humana Press: Totowa, NJ, USA, 1992; pp. 41–72. [Google Scholar]
- Olsson, R.; Ljungqvist, O.; Tönnesen, H.; Gustafsson, U.O. Optimering av Riskfaktorer Inför Kirurgi Förbises. Available online: https://lakartidningen.se/opinion/debatt/2024/02/optimering-av-riskfaktorer-infor-kirurgi-forbises/ (accessed on 13 June 2024).
- Babor, T.F.; Higgins-Biddle, J.; Saunders, J.B.; Monteiro, M. AUDIT-The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Heath Care, 2nd ed.; WHO: Geneva, Switzerland, 2001; pp. 1–40. [Google Scholar]
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef]
- Helander, A.; Péter, O.; Zheng, Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol. Alcohol. 2012, 47, 552–557. [Google Scholar] [CrossRef]
- Holford, N.H. Clinical pharmacokinetics of ethanol. Clin. Pharmacokinet. 1987, 13, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Javors, M.A.; Johnson, B.A. Current status of carbohydrate deficient transferrin, total serum sialic acid, sialic acid index of apolipoprotein J and serum beta-hexosaminidase as markers for alcohol consumption. Addiction 2003, 98 (Suppl. 2), 45–50. [Google Scholar] [CrossRef]
- Jones, A.W. Brief history of the alcohol biomarkers CDT, EtG, EtS, 5-HTOL, and PEth. Drug Test. Anal. 2024, 16, 570–587. [Google Scholar] [CrossRef]
- Andresen-Streichert, H.; Müller, A.; Glahn, A.; Skopp, G.; Sterneck, M. Alcohol Biomarkers in Clinical and Forensic Contexts. Dtsch. Arztebl. Int. 2018, 115, 309–315. [Google Scholar] [CrossRef]
- Kummer, N.; Lambert, W.E.; Samyn, N.; Stove, C.P. Alternative sampling strategies for the assessment of alcohol intake of living persons. Clin. Biochem. 2016, 49, 1078–1091. [Google Scholar] [CrossRef]
- Varga, A.; Hansson, P.; Johnson, G.; Alling, C. Normalization rate and cellular localization of phosphatidylethanol in whole blood from chronic alcoholics. Clin. Chim. Acta 2000, 299, 141–150. [Google Scholar] [CrossRef]
- Schmitt, G.; Aderjan, R.; Keller, T.; Wu, M. Ethyl glucuronide: An unusual ethanol metabolite in humans. Synthesis, analytical data, and determination in serum and urine. J. Anal. Toxicol. 1995, 19, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Helander, A.; Beck, O. Ethyl sulfate: A metabolite of ethanol in humans and a potential biomarker of acute alcohol intake. J. Anal. Toxicol. 2005, 29, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, G.; Droenner, P.; Skopp, G.; Aderjan, R. Ethyl glucuronide concentration in serum of human volunteers, teetotalers, and suspected drinking drivers. J. Forensic Sci. 1997, 42, 1099–1102. [Google Scholar] [CrossRef]
- Lauridsen, S.V.; Thomsen, T.; Jensen, J.B.; Kallemose, T.; Schmidt Behrend, M.; Steffensen, K.; Poulsen, A.M.; Jacobsen, A.; Walther, L.; Isaksson, A.; et al. Effect of a Smoking and Alcohol Cessation Intervention Initiated Shortly Before Radical Cystectomy-the STOP-OP Study: A Randomised Clinical Trial. Eur. Urol. Focus 2022, 8, 1650–1658. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 13 June 2024).
- Stockwell, T.; Zhao, J.; Greenfield, T.; Li, J.; Livingston, M.; Meng, Y. Estimating under- and over-reporting of drinking in national surveys of alcohol consumption: Identification of consistent biases across four English-speaking countries. Addiction 2016, 111, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Perilli, M.; Toselli, F.; Franceschetto, L.; Cinquetti, A.; Ceretta, A.; Cecchetto, G.; Viel, G. Phosphatidylethanol (PEth) in Blood as a Marker of Unhealthy Alcohol Use: A Systematic Review with Novel Molecular Insights. Int. J. Mol. Sci. 2023, 24, 12175. [Google Scholar] [CrossRef] [PubMed]
- Dagne, K.; Myers, B.; Mihretu, A.; Teferra, S. Scoping review of assessment tools for, magnitudes of and factors associated with problem drinking in population-based studies. BMJ Open 2024, 14, e080657. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.; Lydom, L.N.; Joensen, U.N.; Egerod, I.; Pappot, H.; Lauridsen, S.V. STRONG for Surgery & Strong for Life—Against all odds: Intensive prehabilitation including smoking, nutrition, alcohol and physical activity for risk reduction in cancer surgery—A protocol for an RCT with nested interview study (STRONG-Cancer). Trials 2022, 23, 333. [Google Scholar] [CrossRef] [PubMed]
- Grottenthaler, J.M.; Konzelmann, A.; Stiegler, A.; Hinterleitner, C.; Bott, S.M.; Klag, T.; Werner, C.R.; Hinterleitner, M.; Königsrainer, A.; Batra, A.; et al. Significance and clinical impact of routinely tested urinary ethyl glucuronide after liver transplantation—Development of a risk score. Transpl. Int. 2021, 34, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Rubinsky, A.D.; Bishop, M.J.; Maynard, C.; Henderson, W.G.; Hawn, M.T.; Harris, A.H.; Beste, L.A.; Tønnesen, H.; Bradley, K.A. Postoperative risks associated with alcohol screening depend on documented drinking at the time of surgery. Drug Alcohol. Depend. 2013, 132, 521–527. [Google Scholar] [CrossRef]
- Merrill, J.E.; Fan, P.; Wray, T.B.; Miranda, R., Jr. Assessment of Alcohol Use and Consequences: Comparison of Data Collected Via Timeline Followback Interview and Daily Reports. J. Stud. Alcohol. Drugs 2020, 81, 212–219. [Google Scholar] [CrossRef]
| Biomarkers | Carbo-Deficient Transferrin (CDT) [13,15,16,17] | Phosphatidyl-Ethanol (PEth) [16,17,18,19] | Ethyl Glucuronide (EtG) [16,17,20,21,22] |
|---|---|---|---|
| Half-life | 2 weeks | 4 days | 2–3 h |
| Influenced by: | |||
| Memory | - | - | - |
| Liver disease | + | - | - |
| Dehydration | + | - | Unknown |
| Diuresis | - | - | + |
| Blood transfusion | + | + | + |
| Intake for a positive result | ~5 drinks/day for 2 weeks | ~2–3 drinks/day for a few days | Any recent intake |
| Cut off value | >2% CDT/transferrin | ≥0.050 µmol/L | >0.5 mg/L |
| Preoperative Characteristics | Values |
|---|---|
| Age (years) | 67 [43–82] |
| Men | 72 (77%) |
| Daily smokers | 72 (77%) |
| Body–Mass Index (kg/m2) | 25 [15–41] |
| Physical activity < ½ hour per day | 33 (34%) |
| Living alone | 42 (45%) |
| Education: none or only short courses | 31 (33%) |
| Alcohol characteristics | |
| TLFB (drinks the last week) | 4 [0–64] |
| ≥21 units last week | 13 (14%) |
| 14–20 units last week | 14 (15%) |
| 1–13 units last week | 40 (43%) |
| 0 units last week | 27 (29%) |
| AUDIT–C (points: 0–12) | 5 [0–12] |
| P–CDT (% of total transferrin) | 1.3 [0.7–10.9] |
| U–EtG (mg/L) | 0.3 [0.0–1000] |
| B–PEth (µmol/L) | 0.115 [0.003–3.010] |
| History of disease | |
| Tumor stage: cancer in situ | 3 (3%) |
| Stage 1 | 31 (33%) |
| Stage 2 | 34 (36%) |
| Stage 3 | 21 (22%) |
| Stage 4 | 5 (5%) |
| Preoperative neoadjuvant chemotherapy | 28 (30%) |
| Charlson Comorbidity Index ≥ 2 | 32 (34%) |
| Test | Positive Tests | Sensitivity | Specificity | Pos. Predictive Value | Neg. Predictive Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| AUDIT–C | 40/94 | 75 | (63–83) | 96 | (79–99) | 98 | (90–100) | 55 | (40–69) |
| P–CDT | 19/82 | 82 | (71–90) | 38 | (21–59) | 79 | (68–88) | 42 | (23–64) |
| U–EtG | 38/87 | 70 | (60–79) | 86 | (65–95) | 94 | (83–98) | 47 | (32–63) |
| B–PEth | 47/81 | 56 | (43–67) | 100 | (84–0) | 100 | (90–100) | 43 | (30–58) |
| Any test positive | 59/86 | 43 | (31–55) | 100 | (86–100) | 100 | (88–100) | 40 | (28–52) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicklasson, J.; Sjödell, M.; Tønnesen, H.; Lauridsen, S.V.; Rasmussen, M. Identification of Alcohol Use Prior to Major Cancer Surgery: Timeline Follow Back Interview Compared to Four Other Markers. Cancers 2024, 16, 2261. https://doi.org/10.3390/cancers16122261
Nicklasson J, Sjödell M, Tønnesen H, Lauridsen SV, Rasmussen M. Identification of Alcohol Use Prior to Major Cancer Surgery: Timeline Follow Back Interview Compared to Four Other Markers. Cancers. 2024; 16(12):2261. https://doi.org/10.3390/cancers16122261
Chicago/Turabian StyleNicklasson, Johanna, Moa Sjödell, Hanne Tønnesen, Susanne Vahr Lauridsen, and Mette Rasmussen. 2024. "Identification of Alcohol Use Prior to Major Cancer Surgery: Timeline Follow Back Interview Compared to Four Other Markers" Cancers 16, no. 12: 2261. https://doi.org/10.3390/cancers16122261
APA StyleNicklasson, J., Sjödell, M., Tønnesen, H., Lauridsen, S. V., & Rasmussen, M. (2024). Identification of Alcohol Use Prior to Major Cancer Surgery: Timeline Follow Back Interview Compared to Four Other Markers. Cancers, 16(12), 2261. https://doi.org/10.3390/cancers16122261







