Should Hypervascular Incidentalomas Detected on Per-Interventional Cone Beam Computed Tomography during Intra-Arterial Therapies for Hepatocellular Carcinoma Impact the Treatment Plan in Patients Waiting for Liver Transplantation?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Pre-Interventional Imaging
2.3. Per-Interventional Imaging
2.4. Imaging Analysis
2.5. Anatomopathological Analysis
2.6. Patient Follow-Up and Prognosis Evaluation
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Imaging Data Analysis
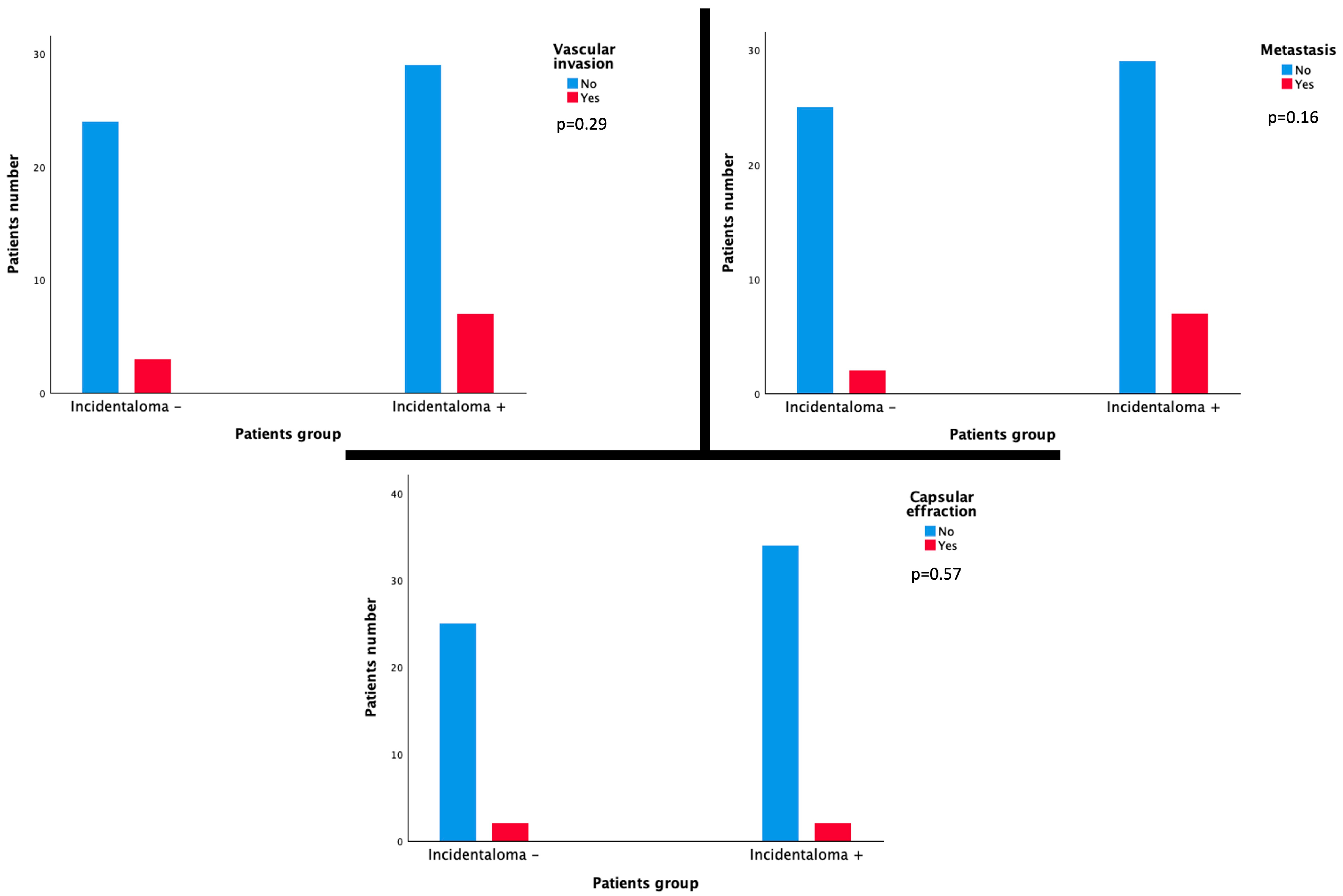
3.3. Correlation with Histopathological Features
3.4. Correlation with Patient Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Mehta, N.; Bhangui, P.; Yao, F.Y.; Mazzaferro, V.; Toso, C.; Akamatsu, N.; Durand, F.; Ijzermans, J.; Polak, W.; Zheng, S.; et al. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.; Mehta, N.; Sapisochin, G.; Roberts, J.P.; Yao, F.Y. Alpha-Fetoprotein Level > 1000 Ng/mL as an Exclusion Criterion for Liver Transplantation in Patients with Hepatocellular Carcinoma Meeting the Milan Criteria. Liver Transpl. 2014, 20, 945–951. [Google Scholar] [CrossRef]
- Filgueira, N.A. Hepatocellular Carcinoma Recurrence after Liver Transplantation: Risk Factors, Screening and Clinical Presentation. World J. Hepatol. 2019, 11, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Costentin, C.E.; Amaddeo, G.; Decaens, T.; Boudjema, K.; Bachellier, P.; Muscari, F.; Salamé, E.; Bernard, P.-H.; Francoz, C.; Dharancy, S.; et al. Prediction of Hepatocellular Carcinoma Recurrence after Liver Transplantation: Comparison of Four Explant-Based Prognostic Models. Liver Int. 2017, 37, 717–726. [Google Scholar] [CrossRef]
- Hui, T.C.H.; Chuah, T.K.; Low, H.M.; Tan, C.H. Predicting Early Recurrence of Hepatocellular Carcinoma with Texture Analysis of Preoperative MRI: A Radiomics Study. Clin. Radiol. 2018, 73, 1056.e11–1056.e16. [Google Scholar] [CrossRef]
- Sandow, T.; Pavlus, J.; Field, D.; Lacayo, E.; Cohen, E.; Lynskey, G.; Caridi, T.; Buckley, D.; Cardella, J.; Kallakury, B.; et al. Bridging Hepatocellular Carcinoma to Transplant: Transarterial Chemoembolization Response, Tumor Biology, and Recurrence after Transplantation in a 12-Year Transplant Cohort. J. Vasc. Interv. Radiol. 2019, 30, 995–1003. [Google Scholar] [CrossRef]
- Kulik, L.; Heimbach, J.K.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Wang, Z.; Murad, M.H.; Mohammed, K. Therapies for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation: A Systematic Review and Meta-Analysis. Hepatology 2018, 67, 381–400. [Google Scholar] [CrossRef]
- Sheth, R.A.; Patel, M.S.; Koottappillil, B.; Shah, J.A.; Oklu, R.; Mueller, P.; Vagefi, P.A.; Ganguli, S. Role of Locoregional Therapy and Predictors for Dropout in Patients with Hepatocellular Carcinoma Listed for Liver Transplantation. J. Vasc. Interv. Radiol. 2015, 26, 1761–1768, quiz 1768. [Google Scholar] [CrossRef]
- Yao, F.Y.; Bass, N.M.; Nikolai, B.; Merriman, R.; Davern, T.J.; Kerlan, R.; Ascher, N.L.; Roberts, J.P. A Follow-up Analysis of the Pattern and Predictors of Dropout from the Waiting List for Liver Transplantation in Patients with Hepatocellular Carcinoma: Implications for the Current Organ Allocation Policy. Liver Transpl. 2003, 9, 684–692. [Google Scholar] [CrossRef]
- Sugawara, Y.; Hibi, T. Liver Transplantation for Patients with Hepatocellular Carcinoma: Its Current Status and Advances. Biosci. Trends 2022, 16, 207–211. [Google Scholar] [CrossRef]
- Lucatelli, P.; De Rubeis, G.; Ginnani Corradini, L.; Basilico, F.; Di Martino, M.; Lai, Q.; Ginanni Corradini, S.; Cannavale, A.; Nardis, P.G.; Corona, M.; et al. Intra-Procedural Dual Phase Cone Beam Computed Tomography Has a Better Diagnostic Accuracy over Pre-Procedural MRI and MDCT in Detection and Characterization of HCC in Cirrhotic Patients Undergoing TACE Procedure. Eur. J. Radiol. 2020, 124, 108806. [Google Scholar] [CrossRef]
- Shah, S.A.; Tan, J.C.C.; McGilvray, I.D.; Cattral, M.S.; Cleary, S.P.; Levy, G.A.; Greig, P.D.; Grant, D.R. Accuracy of Staging as a Predictor for Recurrence after Liver Transplantation for Hepatocellular Carcinoma. Transplantation 2006, 81, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting Survival after Liver Transplantation in Patients with Hepatocellular Carcinoma beyond the Milan Criteria: A Retrospective, Exploratory Analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Miyayama, S.; Yamashiro, M.; Hattori, Y.; Orito, N.; Matsui, K.; Tsuji, K.; Yoshida, M.; Matsui, O. Efficacy of Cone-Beam Computed Tomography during Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma. Jpn. J. Radiol. 2011, 29, 371–377. [Google Scholar] [CrossRef]
- Loffroy, R.; Lin, M.; Rao, P.; Bhagat, N.; Noordhoek, N.; Radaelli, A.; Blijd, J.; Geschwind, J.-F. Comparing the Detectability of Hepatocellular Carcinoma by C-Arm Dual-Phase Cone-Beam Computed Tomography during Hepatic Arteriography with Conventional Contrast-Enhanced Magnetic Resonance Imaging. Cardiovasc. Interv. Radiol. 2012, 35, 97–104. [Google Scholar] [CrossRef]
- Young, K.; Fidelman, N.; Yao, F.Y.; Hills, N.K.; Kohi, M.P.; Kolli, K.P.; Taylor, A.G.; Kerlan, R.K. Implications of Discordant Findings between Hepatic Angiography and Cross-Sectional Imaging in Transplant Candidates with Hepatocellular Carcinoma. Liver Transpl. 2015, 21, 454–467. [Google Scholar] [CrossRef]
- Kim, T.K.; Lee, E.; Jang, H.-J. Imaging Findings of Mimickers of Hepatocellular Carcinoma. Clin. Mol. Hepatol. 2015, 21, 326–343. [Google Scholar] [CrossRef]
- Moreno Planas, J.M.; López Monclús, J.; Gómez Cruz, A.; Rubio González, E.; Pérez Arangüena, R.; Boullosa Graña, E.; Garcia Suárez, A.; Pérez-Picouto, J.L.; Fernández Ruiz, M.; Lucena de la Poza, J.L.; et al. Efficacy of Hepatocellular Carcinoma Locoregional Therapies on Patients Waiting for Liver Transplantation. Transplant. Proc. 2005, 37, 1484–1485. [Google Scholar] [CrossRef]
- Si, T.; Chen, Y.; Ma, D.; Gong, X.; Guan, R.; Shen, B.; Peng, C. Transarterial Chemoembolization Prior to Liver Transplantation for Patients with Hepatocellular Carcinoma: A Meta-Analysis. J. Gastroenterol. Hepatol. 2017, 32, 1286–1294. [Google Scholar] [CrossRef]
- Sun, H.-C.; Zhu, X.-D. Downstaging Conversion Therapy in Patients with Initially Unresectable Advanced Hepatocellular Carcinoma: An Overview. Front. Oncol. 2021, 11, 772195. [Google Scholar] [CrossRef]
- Shi, X.-J.; Jin, X.; Wang, M.-Q.; Wei, L.-X.; Ye, H.-Y.; Liang, Y.-R.; Luo, Y.; Dong, J.-H. Outcomes of Loco-Regional Therapy for down-Staging of Hepatocellular Carcinoma Prior to Liver Transplantation. HBPD INT 2011, 10, 143–150. [Google Scholar] [CrossRef]
- Stampfl, U.; Bermejo, J.L.; Sommer, C.M.; Hoffmann, K.; Weiss, K.H.; Schirmacher, P.; Schemmer, P.; Kauczor, H.-U.; Richter, G.M.; Radeleff, B.A.; et al. Efficacy and Nontarget Effects of Transarterial Chemoembolization in Bridging of Hepatocellular Carcinoma Patients to Liver Transplantation: A Histopathologic Study. J. Vasc. Interv. Radiol. 2014, 25, 1018–1026.e4. [Google Scholar] [CrossRef]
- Crocetti, L.; Bozzi, E.; Scalise, P.; Bargellini, I.; Lorenzoni, G.; Ghinolfi, D.; Campani, D.; Balzano, E.; De Simone, P.; Cioni, R. Locoregional Treatments for Bridging and Downstaging HCC to Liver Transplantation. Cancers 2021, 13, 5558. [Google Scholar] [CrossRef]
- Miyayama, S.; Yamashiro, M.; Hashimoto, M.; Hashimoto, N.; Ikuno, M.; Okumura, K.; Yoshida, M.; Matsui, O. Identification of Small Hepatocellular Carcinoma and Tumor-Feeding Branches with Cone-Beam CT Guidance Technology during Transcatheter Arterial Chemoembolization. J. Vasc. Interv. Radiol. 2013, 24, 501–508. [Google Scholar] [CrossRef]
- Gallix, B.; Aufort, S. Incidentalomes. Available online: www.em-premium.com/data/revues/02210363/00887-8-C2/1048/2008 (accessed on 21 June 2024).
- Choi, Y.R.; Chung, J.W.; Yu, M.H.; Lee, M.; Kim, J.H. Diagnostic Accuracy of Contrast-Enhanced Dynamic CT for Small Hypervascular Hepatocellular Carcinoma and Assessment of Dynamic Enhancement Patterns: Results of Two-Year Follow-up Using Cone-Beam CT Hepatic Arteriography. PLoS ONE 2018, 13, e0203940. [Google Scholar] [CrossRef]
- Kim, E.H.; Oh, J.S.; Chun, H.J.; Choi, B.G.; Lee, H.G. Usefulness of Fusion Images of Unenhanced and Contrast-Enhanced Arterial Phase Cone-Beam CT in the Detection of Viable Hepatocellular Carcinoma during Transarterial Chemoembolization. Diagn. Interv. Radiol. 2018, 24, 262–267. [Google Scholar] [CrossRef]
- Yao, X.; Yan, D.; Jiang, X.; Li, X.; Zeng, H.; Liu, D.; Li, H. Dual-Phase Cone-Beam CT-Based Navigation Imaging Significantly Enhances Tumor Detectability and Aids Superselective Transarterial Chemoembolization of Liver Cancer. Acad. Radiol. 2018, 25, 1031–1037. [Google Scholar] [CrossRef]
- de Baere, T.; Arai, Y.; Lencioni, R.; Geschwind, J.-F.; Rilling, W.; Salem, R.; Matsui, O.; Soulen, M.C. Treatment of Liver Tumors with Lipiodol TACE: Technical Recommendations from Experts Opinion. Cardiovasc. Interv. Radiol. 2016, 39, 334–343. [Google Scholar] [CrossRef]
- Padia, S.A.; Lewandowski, R.J.; Johnson, G.E.; Sze, D.Y.; Ward, T.J.; Gaba, R.C.; Baerlocher, M.O.; Gates, V.L.; Riaz, A.; Brown, D.B.; et al. Radioembolization of Hepatic Malignancies: Background, Quality Improvement Guidelines, and Future Directions. J. Vasc. Interv. Radiol. 2017, 28, 1–15. [Google Scholar] [CrossRef]
- Gaba, R.C.; Lokken, R.P.; Hickey, R.M.; Lipnik, A.J.; Lewandowski, R.J.; Salem, R.; Brown, D.B.; Walker, T.G.; Silberzweig, J.E.; Baerlocher, M.O.; et al. Quality Improvement Guidelines for Transarterial Chemoembolization and Embolization of Hepatic Malignancy. J. Vasc. Interv. Radiol. 2017, 28, 1210–1223.e3. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology 2012, 143, 986–994.e3. [Google Scholar] [CrossRef]
- Roux, M.; Pigneur, F.; Baranes, L.; Calderaro, J.; Chiaradia, M.; Decaens, T.; Kastahian, S.; Charles-Nelson, A.; Tselikas, L.; Costentin, C.; et al. Differentiating Focal Nodular Hyperplasia from Hepatocellular Adenoma: Is Hepatobiliary Phase MRI (HBP-MRI) Using Linear Gadolinium Chelates Always Useful? Abdom. Radiol. 2018, 43, 1670–1681. [Google Scholar] [CrossRef]
- Mulé, S.; Galletto Pregliasco, A.; Tenenhaus, A.; Kharrat, R.; Amaddeo, G.; Baranes, L.; Laurent, A.; Regnault, H.; Sommacale, D.; Djabbari, M.; et al. Multiphase Liver MRI for Identifying the Macrotrabecular-Massive Subtype of Hepatocellular Carcinoma. Radiology 2020, 295, 562–571. [Google Scholar] [CrossRef]
- Reizine, E.; Amaddeo, G.; Pigneur, F.; Baranes, L.; Legou, F.; Mulé, S.; Zegai, B.; Roche, V.; Laurent, A.; Rahmouni, A.; et al. Quantitative Correlation between Uptake of Gd-BOPTA on Hepatobiliary Phase and Tumor Molecular Features in Patients with Benign Hepatocellular Lesions. Eur. Radiol. 2018, 28, 4243–4253. [Google Scholar] [CrossRef]
- Derbel, H.; Kobeiter, H.; Pizaine, G.; Ridouani, F.; Luciani, A.; Radaelli, A.; Van der Sterren, W.; Chiaradia, M.; Tacher, V. Accuracy of a Cone-Beam CT Virtual Parenchymal Perfusion Algorithm for Liver Cancer Targeting during Intra-Arterial Therapy. J. Vasc. Interv. Radiol. 2018, 29, 254–261.e2. [Google Scholar] [CrossRef]
- Tacher, V.; Radaelli, A.; Lin, M.; Geschwind, J.-F. How I Do It: Cone-Beam CT during Transarterial Chemoembolization for Liver Cancer. Radiology 2015, 274, 320–334. [Google Scholar] [CrossRef]
- M Cunha, G.; Fowler, K.J.; Roudenko, A.; Taouli, B.; Fung, A.W.; Elsayes, K.M.; Marks, R.M.; Cruite, I.; Horvat, N.; Chernyak, V.; et al. How to Use LI-RADS to Report Liver CT and MRI Observations. Radiographics 2021, 41, 1352–1367. [Google Scholar] [CrossRef]
- Menahem, B.; Lubrano, J.; Duvoux, C.; Mulliri, A.; Alves, A.; Costentin, C.; Mallat, A.; Launoy, G.; Laurent, A. Liver Transplantation versus Liver Resection for Hepatocellular Carcinoma in Intention to Treat: An Attempt to Perform an Ideal Meta-Analysis. Liver Transpl. 2017, 23, 836–844. [Google Scholar] [CrossRef]
- Iwazawa, J.; Ohue, S.; Hashimoto, N.; Abe, H.; Hamuro, M.; Mitani, T. Detection of Hepatocellular Carcinoma: Comparison of Angiographic C-Arm CT and MDCT. AJR Am. J. Roentgenol. 2010, 195, 882–887. [Google Scholar] [CrossRef]
- Lucatelli, P.; Argirò, R.; Ginanni Corradini, S.; Saba, L.; Cirelli, C.; Fanelli, F.; Ricci, C.; Levi Sandri, G.B.; Catalano, C.; Bezzi, M. Comparison of Image Quality and Diagnostic Performance of Cone-Beam CT during Drug-Eluting Embolic Transarterial Chemoembolization and Multidetector CT in the Detection of Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2017, 28, 978–986. [Google Scholar] [CrossRef]
- Tacher, V.; Lin, M.; Bhagat, N.; Abi Jaoudeh, N.; Radaelli, A.; Noordhoek, N.; Carelsen, B.; Wood, B.J.; Geschwind, J.-F. Dual-Phase Cone-Beam Computed Tomography to See, Reach, and Treat Hepatocellular Carcinoma during Drug-Eluting Beads Transarterial Chemo-Embolization. J. Vis. Exp. 2013, 82, e50795. [Google Scholar] [CrossRef]
- Lucatelli, P.; Argirò, R.; Levi Sandri, G.B.; Munneke, G.; Catalano, C.; Bezzi, M. Single-Injection Dual-Phase Cone-Beam CT Is Better than Split-Bolus Single-Phase Cone-Beam CT for Liver Catheter-Based Procedures. J. Vasc. Interv. Radiol. 2018, 29, 748–749. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Fan, H.-K.; Ren, Z.-G.; Fan, J.; Gao, Q. Identifying Clonal Origin of Multifocal Hepatocellular Carcinoma and Its Clinical Implications. Clin. Transl. Gastroenterol. 2019, 10, e00006. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Perálvarez, M.; Luong, T.V.; Andreana, L.; Meyer, T.; Dhillon, A.P.; Burroughs, A.K. A Systematic Review of Microvascular Invasion in Hepatocellular Carcinoma: Diagnostic and Prognostic Variability. Ann. Surg. Oncol. 2013, 20, 325–339. [Google Scholar] [CrossRef]
- Zori, A.G.; Ismael, M.N.; Limaye, A.R.; Firpi, R.; Morelli, G.; Soldevila-Pico, C.; Suman, A.; Vogel, J.D.; Lazarowicz, M.; Geller, B.S.; et al. Locoregional Therapy Protocols with and Without Radioembolization for Hepatocellular Carcinoma as Bridge to Liver Transplantation. Am. J. Clin. Oncol. 2020, 43, 325. [Google Scholar] [CrossRef]
- Affonso, B.B.; Galastri, F.L.; da Motta Leal Filho, J.M.; Nasser, F.; Falsarella, P.M.; Cavalcante, R.N.; de Almeida, M.D.; Felga, G.E.G.; Valle, L.G.M.; Wolosker, N. Long-Term Outcomes of Hepatocellular Carcinoma That Underwent Chemoembolization for Bridging or Downstaging. World J. Gastroenterol. 2019, 25, 5687–5701. [Google Scholar] [CrossRef]
- Salem, R.; Gabr, A.; Riaz, A.; Mora, R.; Ali, R.; Abecassis, M.; Hickey, R.; Kulik, L.; Ganger, D.; Flamm, S.; et al. Institutional Decision to Adopt Y90 as Primary Treatment for Hepatocellular Carcinoma Informed by a 1000-Patient 15-Year Experience. Hepatology 2018, 68, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Fohlen, A.; Tasu, J.P.; Kobeiter, H.; Bartoli, J.M.; Pelage, J.P.; Guiu, B. Transarterial Chemoembolization (TACE) in the Management of Hepatocellular Carcinoma: Results of a French National Survey on Current Practices. Diagn. Interv. Imaging 2018, 99, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Sanghvi, T.; Sharma, S.; Richardson, C.; Rubin, N.; Richards, M.; D’Souza, D.; Flanagan, S.; Golzarian, J. Local Recurrence Following Complete Radiologic Response in Patients Treated with Transarterial Chemoembolization for Hepatocellular Carcinoma. Diagn. Interv. Imaging 2022, 103, 143–149. [Google Scholar] [CrossRef]
- Young, S.; Sanghvi, T.; Lake, J.J.; Rubin, N.; Golzarian, J. Predicting Post-Transarterial Chemoembolization Outcomes: A Comparison of Direct and Total Bilirubin Serums Levels. Diagn. Interv. Imaging 2020, 101, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Guiu, B.; Garin, E.; Allimant, C.; Edeline, J.; Salem, R. TARE in Hepatocellular Carcinoma: From the Right to the Left of BCLC. Cardiovasc. Interv. Radiol. 2022, 45, 1599–1607. [Google Scholar] [CrossRef] [PubMed]



| Sequence | ET (ms) | RT (ms) | Flip Angle (°) | Slice Thickness (mm) | Echo Length Train |
|---|---|---|---|---|---|
| Breath-hold fat-suppressed TSE T2-weighted sequence | 2600 | 83 | 129 | 5 | 23 |
| Breath-hold HASTE T2-weighted sequence | 1000 | 118 | 120 | 3.5 | 156 |
| Breath-hold in-phase and out-of-phase T1-weighted sequences | 131 | 2.43–3.69 | 70 | 5 | NA |
| Fat-suppressed T1-WI sequences | 117 | 2.78 | 70 | 5 | NA |
| Diffusion-weighted imaging single-shot, spin-echo-planar imaging (3 b-value 50, 400 and 800 s/mm2) | 2500 | 138 | 150 | 5 | 23 |
| Dynamic breath-hold 3D VIBE T1-weighted sequences | 3.31 | 1.25 | 15 | 3 | NA |
| Characteristics | Frequency/Mean (or Median) ± SD |
|---|---|
| Sex | |
| Male | 50 (79.4%) |
| Female | 13 (20.6%) |
| Age (YO) | 59 ± 7 (33–69) |
| Underlying hepatopathy | |
| Alcohol abuse | 42 (66.7%) |
| Chronic viral hepatitis B | 9 (14.3%) |
| Chronic viral hepatitis C | 6 (9.5%) |
| MASH | 6 (9.5%) |
| Child Pugh score | |
| A | 36 (57.2%) |
| B | 16 (25.4%) |
| C | 11 (17.4%) |
| BCLC stage (before LRT) | |
| 0 or A | 46 (73%) |
| B | 16 (25.4%) |
| C | 1 (1.6%) |
| MELD score | 11.33 ± 5.25 (6–22) |
| Pre-liver-transplantation AFP level (ng/mL) | 90 ± 170 (1–830) |
| AFP score (when available) | 1.7 ± 1.38 (0–4) |
| Group Incidentaloma+ | Group Incidentaloma− | ||||||
|---|---|---|---|---|---|---|---|
| Patients (n) | 36 (57.1%) | 27 (42.9%) | |||||
| Nodules | PII | CBCT | p-value | PII | CBCT | p-value | |
| Number (median, [Q1, Q3]) | 2 (1, 5) | 4 (3, 8) | <0.001 | 2 (1, 3) | 2 (1, 3) | 0.07 | |
| Size of the largest nodule | 23.8 ± 13 mm | 24.6 ± 13 mm | 0.22 | 29.8 ± 12 mm | 30.4 ± 12 mm | 0.08 | |
| Subgroup “Bridging Therapy” | Subgroup “Downstaging Therapy” | ||
|---|---|---|---|
| n = 49 (77.8%) | n = 14 (22.2%) | ||
| Prognostic Parameter | p-Value | Prognostic Parameter | p-Value |
| Tumor recurrence | 0.052 | Tumor recurrence | 0.85 |
| Overall survival | 0.23 | Overall survival | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derbel, H.; Galletto Pregliasco, A.; Mulé, S.; Calderaro, J.; Zaarour, Y.; Saccenti, L.; Ghosn, M.; Reizine, E.; Blain, M.; Laurent, A.; et al. Should Hypervascular Incidentalomas Detected on Per-Interventional Cone Beam Computed Tomography during Intra-Arterial Therapies for Hepatocellular Carcinoma Impact the Treatment Plan in Patients Waiting for Liver Transplantation? Cancers 2024, 16, 2333. https://doi.org/10.3390/cancers16132333
Derbel H, Galletto Pregliasco A, Mulé S, Calderaro J, Zaarour Y, Saccenti L, Ghosn M, Reizine E, Blain M, Laurent A, et al. Should Hypervascular Incidentalomas Detected on Per-Interventional Cone Beam Computed Tomography during Intra-Arterial Therapies for Hepatocellular Carcinoma Impact the Treatment Plan in Patients Waiting for Liver Transplantation? Cancers. 2024; 16(13):2333. https://doi.org/10.3390/cancers16132333
Chicago/Turabian StyleDerbel, Haytham, Athena Galletto Pregliasco, Sébastien Mulé, Julien Calderaro, Youssef Zaarour, Laetitia Saccenti, Mario Ghosn, Edouard Reizine, Maxime Blain, Alexis Laurent, and et al. 2024. "Should Hypervascular Incidentalomas Detected on Per-Interventional Cone Beam Computed Tomography during Intra-Arterial Therapies for Hepatocellular Carcinoma Impact the Treatment Plan in Patients Waiting for Liver Transplantation?" Cancers 16, no. 13: 2333. https://doi.org/10.3390/cancers16132333





