Resection Margin Status and Long-Term Outcomes after Pancreaticoduodenectomy for Ductal Adenocarcinoma: A Tertiary Referral Center Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
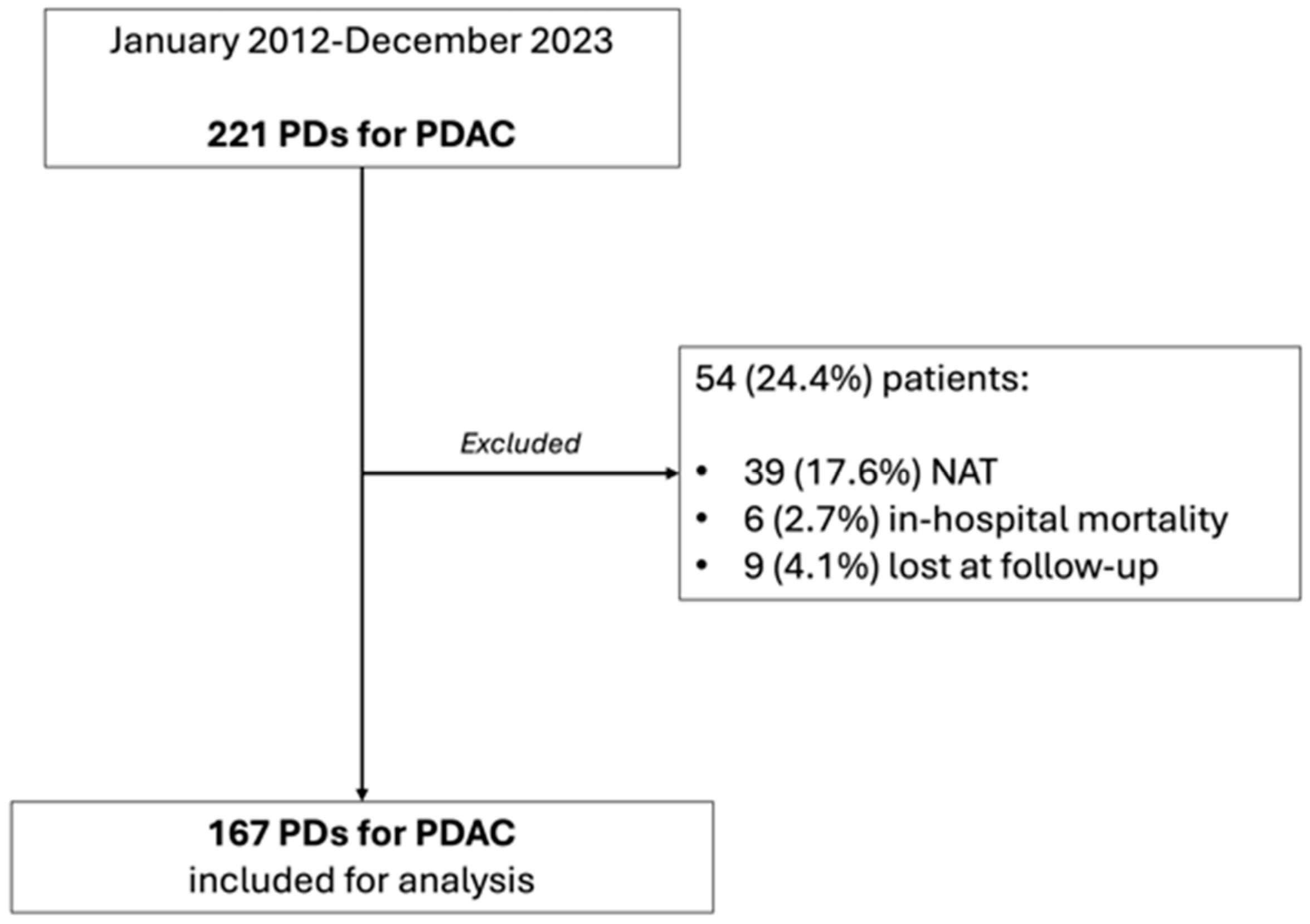
2. Materials and Methods
2.1. Surgical Technique
2.2. Post-Operative Follow-Up and Long-Term Outcomes Analysis
2.3. Pathological Assessment
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Comparison between R0 and R1 Patients (Table 1)
3.2. Impact of R Status on Long-Term Outcomes
3.3. Correlation Analysis of N and R Status with Oncological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic Cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Alexakis, N.; Halloran, C.; Raraty, M.; Ghaneh, P.; Sutton, R.; Neoptolemos, J.P. Current Standards of Surgery for Pancreatic Cancer. Br. J. Surg. 2004, 91, 1410–1427. [Google Scholar] [CrossRef]
- Ethun, C.G.; Kooby, D.A. The Importance of Surgical Margins in Pancreatic Cancer. J. Surg. Oncol. 2016, 113, 283–288. [Google Scholar] [CrossRef]
- Bilici, A. Prognostic Factors Related with Survival in Patients with Pancreatic Adenocarcinoma. World J. Gastroenterol. 2014, 20, 10802–10812. [Google Scholar] [CrossRef]
- Baldwin, S.; Kukar, M.; Gabriel, E.; Attwood, K.; Wilkinson, N.; Hochwald, S.N.; Kuvshinoff, B. Pancreatic Cancer Metastatic to a Limited Number of Lymph Nodes Has No Impact on Outcome. HPB 2016, 18, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, M.; Gravante, G.; Kosmin, M.; Riaz, A.; Al-Bahrani, A. A Systematic Review of the Prognostic Value of Lymph Node Ratio, Number of Positive Nodes and Total Nodes Examined in Pancreatic Ductal Adenocarcinoma. Ann. R. Coll. Surg. Engl. 2017, 99, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Tummers, W.S.; Groen, J.V.; Sibinga Mulder, B.G.; Farina-Sarasqueta, A.; Morreau, J.; Putter, H.; van de Velde, C.J.; Vahrmeijer, A.L.; Bonsing, B.A.; Mieog, J.S.; et al. Impact of Resection Margin Status on Recurrence and Survival in Pancreatic Cancer Surgery. Br. J. Surg. 2019, 106, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- van Roessel, S.; Kasumova, G.G.; Tabatabaie, O.; Ng, S.C.; van Rijssen, L.B.; Verheij, J.; Najarian, R.M.; van Gulik, T.M.; Besselink, M.G.; Busch, O.R.; et al. Pathological Margin Clearance and Survival After Pancreaticoduodenectomy in a US and European Pancreatic Center. Ann. Surg. Oncol. 2018, 25, 1760–1767. [Google Scholar] [CrossRef]
- Raut, C.P.; Tseng, J.F.; Sun, C.C.; Wang, H.; Wolff, R.A.; Crane, C.H.; Hwang, R.; Vauthey, J.-N.; Abdalla, E.K.; Lee, J.E.; et al. Impact of Resection Status on Pattern of Failure and Survival after Pancreaticoduodenectomy for Pancreatic Adenocarcinoma. Ann. Surg. 2007, 246, 52–60. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C.; et al. Adjuvant Chemotherapy with Fluorouracil plus Folinic Acid vs Gemcitabine Following Pancreatic Cancer Resection: A Randomized Controlled Trial. JAMA 2010, 304, 1073–1081. [Google Scholar] [CrossRef]
- Kato, K.; Yamada, S.; Sugimoto, H.; Kanazumi, N.; Nomoto, S.; Takeda, S.; Kodera, Y.; Morita, S.; Nakao, A. Prognostic Factors for Survival after Extended Pancreatectomy for Pancreatic Head Cancer: Influence of Resection Margin Status on Survival. Pancreas 2009, 38, 605–612. [Google Scholar] [CrossRef]
- Daamen, L.A.; van Goor, I.W.J.M.; Schouten, T.J.; Dorland, G.; van Roessel, S.R.; Besselink, M.G.; Bonsing, B.A.; Bosscha, K.; Brosens, L.A.A.; Busch, O.R.; et al. Microscopic Resection Margin Status in Pancreatic Ductal Adenocarcinoma—A Nationwide Analysis. Eur. J. Surg. Oncol. 2021, 47, 708–716. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Piñeros, M.; Van Eycken, E.; Weir, H.K.; Gospodarowicz, M. The TNM Classification of Malignant Tumours-towards Common Understanding and Reasonable Expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Büchler, M.; Charnley, R.M.; et al. Borderline Resectable Pancreatic Cancer: A Consensus Statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef]
- Chang, D.K.; Johns, A.L.; Merrett, N.D.; Gill, A.J.; Colvin, E.K.; Scarlett, C.J.; Nguyen, N.Q.; Leong, R.W.L.; Cosman, P.H.; Kelly, M.I.; et al. Margin Clearance and Outcome in Resected Pancreatic Cancer. J. Clin. Oncol. 2009, 27, 2855–2862. [Google Scholar] [CrossRef]
- Gebauer, F.; Tachezy, M.; Vashist, Y.K.; Marx, A.H.; Yekebas, E.; Izbicki, J.R.; Bockhorn, M. Resection Margin Clearance in Pancreatic Cancer after Implementation of the Leeds Pathology Protocol (LEEPP): Clinically Relevant or Just Academic? World J. Surg. 2015, 39, 493–499. [Google Scholar] [CrossRef]
- Verbeke, C.S.; Leitch, D.; Menon, K.V.; McMahon, M.J.; Guillou, P.J.; Anthoney, A. Redefining the R1 Resection in Pancreatic Cancer. Br. J. Surg. 2006, 93, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Millikan, K.W.; Deziel, D.J.; Silverstein, J.C.; Kanjo, T.M.; Christein, J.D.; Doolas, A.; Prinz, R.A. Prognostic Factors Associated with Resectable Adenocarcinoma of the Head of the Pancreas. Am. Surg. 1999, 65, 618–623; discussion 623–624. [Google Scholar] [CrossRef]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Koniaris, L.; Kaushal, S.; Abrams, R.A.; Sauter, P.K.; Coleman, J.; Hruban, R.H.; Lillemoe, K.D. Resected Adenocarcinoma of the Pancreas-616 Patients: Results, Outcomes, and Prognostic Indicators. J. Gastrointest. Surg. 2000, 4, 567–579. [Google Scholar] [CrossRef]
- Sperti, C.; Pasquali, C.; Piccoli, A.; Pedrazzoli, S. Survival after Resection for Ductal Adenocarcinoma of the Pancreas. Br. J. Surg. 1996, 83, 625–631. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Gleisner, A.L.; Cameron, J.L.; Winter, J.M.; Assumpcao, L.; Lillemoe, K.D.; Wolfgang, C.; Hruban, R.H.; Schulick, R.D.; Yeo, C.J.; et al. Prognostic Relevance of Lymph Node Ratio Following Pancreaticoduodenectomy for Pancreatic Cancer. Surgery 2007, 141, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Riediger, H.; Keck, T.; Wellner, U.; zur Hausen, A.; Adam, U.; Hopt, U.T.; Makowiec, F. The Lymph Node Ratio Is the Strongest Prognostic Factor after Resection of Pancreatic Cancer. J. Gastrointest. Surg. 2009, 13, 1337–1344. [Google Scholar] [CrossRef]
- Strobel, O.; Hinz, U.; Gluth, A.; Hank, T.; Hackert, T.; Bergmann, F.; Werner, J.; Büchler, M.W. Pancreatic Adenocarcinoma: Number of Positive Nodes Allows to Distinguish Several N Categories. Ann. Surg. 2015, 261, 961–969. [Google Scholar] [CrossRef]
- Van den Broeck, A.; Sergeant, G.; Ectors, N.; Van Steenbergen, W.; Aerts, R.; Topal, B. Patterns of Recurrence after Curative Resection of Pancreatic Ductal Adenocarcinoma. Eur. J. Surg. Oncol. 2009, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.; Salvatore, L.; Fiorillo, C.; Bagalà, C.; Menghi, R.; Maria, B.; Cina, C.; Laterza, V.; Di Stefano, B.; Maratta, M.G.; et al. The Impact of the Multidisciplinary Tumor Board (MDTB) on the Management of Pancreatic Diseases in a Tertiary Referral Center. ESMO Open 2021, 6, 100010. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.; Fiorillo, C.; Menghi, R.; Cina, C.; Galiandro, F.; Longo, F.; Sofo, F.; Rosa, F.; Tortorelli, A.P.; Giustiniani, M.C.; et al. Total Mesopancreas Excision for Periampullary Malignancy: A Single-Center Propensity Score-Matched Comparison of Long-Term Outcomes. Langenbecks Arch. Surg. 2020, 405, 303–312. [Google Scholar] [CrossRef]
- Quero, G.; Fiorillo, C.; De Sio, D.; Laterza, V.; Menghi, R.; Cina, C.; Schena, C.A.; Rosa, F.; Galiandro, F.; Alfieri, S. The Role of Mesopancreas Excision for Ampullary Carcinomas: A Single Center Propensity-Score Matched Analysis. HPB 2021, 23, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.A.M.G.; Gouma, D.J.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; et al. Definition of a Standard Lymphadenectomy in Surgery for Pancreatic Ductal Adenocarcinoma: A Consensus Statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, C.S. Resection Margins in Pancreatic Cancer. Pathologe 2013, 34 (Suppl. 2), 241–247. [Google Scholar] [CrossRef]
- Niesen, W.; Hank, T.; Büchler, M.; Strobel, O. Local Radicality and Survival Outcome of Pancreatic Cancer Surgery. Ann. Gastroenterol. Surg. 2019, 3, 464–475. [Google Scholar] [CrossRef]
- Wittekind, C.; Compton, C.; Quirke, P.; Nagtegaal, I.; Merkel, S.; Hermanek, P.; Sobin, L.H. A Uniform Residual Tumor (R) Classification: Integration of the R Classification and the Circumferential Margin Status. Cancer 2009, 115, 3483–3488. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Warshaw, A.L.; Allen, J.N.; Blaszkowsky, L.S.; Castillo, C.F.-D.; Deshpande, V.; Hong, T.S.; Kwak, E.L.; Lauwers, G.Y.; Ryan, D.P.; et al. Pancreatic Ductal Adenocarcinoma: Is There a Survival Difference for R1 Resections versus Locally Advanced Unresectable Tumors? What Is a “True” R0 Resection? Ann. Surg. 2013, 257, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Gnerlich, J.L.; Luka, S.R.; Deshpande, A.D.; Dubray, B.J.; Weir, J.S.; Carpenter, D.H.; Brunt, E.M.; Strasberg, S.M.; Hawkins, W.G.; Linehan, D.C. Microscopic Margins and Patterns of Treatment Failure in Resected Pancreatic Adenocarcinoma. Arch. Surg. 2012, 147, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, N.B.; Foulis, A.K.; Oien, K.A.; Going, J.J.; Glen, P.; Dickson, E.J.; Imrie, C.W.; McKay, C.J.; Carter, R. Positive Mobilization Margins Alone Do Not Influence Survival Following Pancreatico-Duodenectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2010, 251, 1003–1010. [Google Scholar] [CrossRef]
- Kimbrough, C.W.; St Hill, C.R.; Martin, R.C.G.; McMasters, K.M.; Scoggins, C.R. Tumor-Positive Resection Margins Reflect an Aggressive Tumor Biology in Pancreatic Cancer. J. Surg. Oncol. 2013, 107, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Shaib, Y.; Davila, J.; Naumann, C.; El-Serag, H. The Impact of Curative Intent Surgery on the Survival of Pancreatic Cancer Patients: A U.S. Population-Based Study. Am. J. Gastroenterol. 2007, 102, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Dunn, J.A.; Almond, J.; Beger, H.G.; Pederzoli, P.; Bassi, C.; Dervenis, C.; Fernandez-Cruz, L.; Lacaine, F.; et al. Influence of Resection Margins on Survival for Patients with Pancreatic Cancer Treated by Adjuvant Chemoradiation and/or Chemotherapy in the ESPAC-1 Randomized Controlled Trial. Ann. Surg. 2001, 234, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Post, S.; Neuhaus, P.; Gellert, K.; Langrehr, J.; Ridwelski, K.; Schramm, H.; Fahlke, J.; Zuelke, C.; Burkart, C.; et al. Adjuvant Chemotherapy with Gemcitabine vs Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer: A Randomized Controlled Trial. JAMA 2007, 297, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Heh, V.; Pawlik, T.M.; Ejaz, A.; Dillhoff, M.; Tsung, A.; Williams, T.; Abushahin, L.; Bridges, J.F.P.; Santry, H. Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 1129. [Google Scholar] [CrossRef]
- Labori, K.J.; Bratlie, S.O.; Andersson, B.; Angelsen, J.-H.; Biörserud, C.; Björnsson, B.; Bringeland, E.A.; Elander, N.; Garresori, H.; Grønbech, J.E.; et al. Neoadjuvant FOLFIRINOX versus Upfront Surgery for Resectable Pancreatic Head Cancer (NORPACT-1): A Multicentre, Randomised, Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2024, 9, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Delpero, J.R.; Bachellier, P.; Regenet, N.; Le Treut, Y.P.; Paye, F.; Carrere, N.; Sauvanet, A.; Autret, A.; Turrini, O.; Monges-Ranchin, G.; et al. Pancreaticoduodenectomy for Pancreatic Ductal Adenocarcinoma: A French Multicentre Prospective Evaluation of Resection Margins in 150 Evaluable Specimens. HPB 2014, 16, 20–33. [Google Scholar] [CrossRef]
- Tseng, J.F.; Raut, C.P.; Lee, J.E.; Pisters, P.W.T.; Vauthey, J.-N.; Abdalla, E.K.; Gomez, H.F.; Sun, C.C.; Crane, C.H.; Wolff, R.A.; et al. Pancreaticoduodenectomy with Vascular Resection: Margin Status and Survival Duration. J. Gastrointest. Surg. 2004, 8, 935–949; discussion 949–950. [Google Scholar] [CrossRef] [PubMed]
- Pingpank, J.F.; Hoffman, J.P.; Ross, E.A.; Cooper, H.S.; Meropol, N.J.; Freedman, G.; Pinover, W.H.; LeVoyer, T.E.; Sasson, A.R.; Eisenberg, B.L. Effect of Preoperative Chemoradiotherapy on Surgical Margin Status of Resected Adenocarcinoma of the Head of the Pancreas. J. Gastrointest. Surg. 2001, 5, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Esposito, I.; Kleeff, J.; Bergmann, F.; Reiser, C.; Herpel, E.; Friess, H.; Schirmacher, P.; Büchler, M.W. Most Pancreatic Cancer Resections Are R1 Resections. Ann. Surg. Oncol. 2008, 15, 1651–1660. [Google Scholar] [CrossRef]
- Zhang, Y.; Frampton, A.E.; Cohen, P.; Kyriakides, C.; Bong, J.J.; Habib, N.A.; Spalding, D.R.C.; Ahmad, R.; Jiao, L.R. Tumor Infiltration in the Medial Resection Margin Predicts Survival after Pancreaticoduodenectomy for Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 1875–1882. [Google Scholar] [CrossRef]


| Variables | Study Population (n = 167) | R0 (n = 105) | R1 (n = 62) | p |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 78 (46.7) | 51 (48.6) | 27 (43.5) | 0.53 |
| Female | 89 (53.3) | 54 (51.4) | 35 (56.4) | |
| Age (years), median (QR) | 69 (60.5–75) | 69 (60.5–75.0) | 68 (59.5–73) | 0.15 |
| Tumor location, n (%) | ||||
| Head | 146 (87.4) | 89 (84.8) | 57 (91.9) | 0.48 |
| Isthmus | 4 (2.4) | 3 (2.9) | 1 (1.6) | |
| Uncinate process | 17 (10.2) | 13 (12.4) | 4 (6.4) | |
| Major complications, n (%) | 35 (21) | 23 (21.9) | 12 (19%) | 0.36 |
| Tumor grading, n (%) | ||||
| G1 | 3 (1.8) | 3 (2.9) | 0 (0) | 0.32 |
| G2 | 129 (77.2) | 81 (77.1) | 48 (77.4) | |
| G3 | 35 (21) | 21 (20) | 14 (22.6) | |
| Tumor dimension (mm), median (QR) | 30 (23–35) | 30 (22–35) | 28 (25–35.2) | 0.54 |
| T, n (%) | ||||
| T1a | 1 (0.6) | 1 (0.9) | 0 (0) | 0.004 |
| T1b | 0 (0) | 0 (0) | 0 (0) | |
| T1c | 25 (15) | 18 (17.1) | 7 (11.3) | |
| T2 | 124 (74.2) | 79 (75.2) | 45 (72.6) | |
| T3 | 17 (10.2) | 7 (6.7) | 10 (16.1) | |
| T4 | 0 (0) | 0 (0) | 0 (0) | |
| N, n (%) | ||||
| N0 | 56 (33.5) | 42 (40) | 14 (22.6) | 0.02 |
| N+ | 111 (66.5) | 63 (60) | 48 (77.4) | |
| Vascular resection, n (%) | 9 (5.4) | 6 (5.7) | 3 (4.8) | 0.2 |
| Harvested lymph nodes, median (QR) | 20 (16–28) | 19 (16–26.5) | 23 (15.7–28) | 0.28 |
| Positive lymph nodes, median (QR) | 2 (0–4) | 1 (0–4) | 2 (1–6) | 0.004 |
| Perineural invasion, n (%) | 152 (91) | 92 (87.6) | 60 (96.8) | 0.05 |
| Angio/lympho-vascular invasion, n (%) | 135 (80.8) | 81 (77.1) | 54 (87.1) | 0.03 |
| Adjuvant therapy, n (%) | 144 (86.2) | 88 (83.8) | 56 (90.3) | 0.24 |
| Patterns of Recurrence | Study Population (n = 167) | R0 (n = 105) | R1 (n = 62) | p |
|---|---|---|---|---|
| Recurrence, n (%) | 114 (68.3) | 68 (64.8) | 46 (74.2) | 0.04 |
| Time to recurrence (months), median (QR) | 10 (6–18) | 10 (6–18) | 9 (5–13) | 0.18 |
| Recurrence location, n (%) | 0.15 | |||
| Local | 21 (12.6) | 9 (8.6) | 12 (19.4) | |
| Distant | 61 (36.5) | 37 (35.2) | 24 (38.7) | |
| Local + distant | 32 (19.2) | 22 (21) | 10 (16.1) |
| Variable | OS | DFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Sex | 0.93 (0.61–1.41) | 0.73 | 0.78 (0.54–1.14) | 0.2 | ||||
| Age > 65 years | 1 (0.6–1.5) | 0.92 | 1 (0.7–1.4) | 0.95 | ||||
| Major complications | 1.2 (0.81–1.7) | 0.12 | 1.6 (0.92–3.5) | 0.07 | ||||
| Vascular resection | 0.81 (0.52–1.34) | 0.64 | 0.67 (0.41–1.01) | 0.4 | ||||
| R1 status | 1.8 (1.1–2.7) | 0.009 | 1.6 (1–2.5) | 0.03 | 1.7 (1.2–2.4) | 0.006 | 1.5 (1–2.1) | 0.04 |
| Multiple R1 | 0.7 (0.3–2.0) | 0.56 | 1.1 (0.5–2.4) | 0.87 | ||||
| T3 vs. T1–2 | 2.1 (1.2–3.8) | 0.01 | 1.6 (0.9–3) | 0.1 | 1.7 (1–3.1) | 0.02 | 1.5 (0.9–2.6) | 0.13 |
| N+ | 2 (1.3–3.3) | 0.004 | 1.7 (1–2.8) | 0.04 | 2 (1.3–3) | 0.001 | 1.8 (1.1–2.7) | 0.009 |
| Perineural invasion | 1.5 (0.7–3.2) | 0.24 | 1.5 (0.8–3) | 0.19 | ||||
| Angio/Lymphovascular invasion | 1.2 (0.7–2.1) | 0.46 | 1.2 (0.7–2) | 0.77 | ||||
| Adjuvant therapy | 1 (0.5–1.9) | 0.90 | 1.3 (0.7–2.3) | 0.42 | ||||
| Variables | R0N0 (n = 42) | R0N+ (n = 61) | R1N0 (n = 14) | R1N+ (n = 50) | p |
|---|---|---|---|---|---|
| Perineural infiltration, n (%) | 32 (76.2) | 58 (95.1) | 14 (100) | 48 (96) | 0.001 |
| Angio/lymphovascular invasion, n (%) | 21 (50) | 58 (95.1) | 12 (85.7) | 44 (88) | 0.0001 |
| G, n (%) | 0.09 | ||||
| 1 | 3 (7.1) | 0 | 0 | 0 | |
| 2 | 33 (78.6) | 46 (75.4) | 12 (85.7) | 38 (76) | |
| 3 | 6 (14.3) | 15 (24.6) | 2 (14.3) | 12 (24) | |
| Tumor dimension (mm), median (IQR) | 25 (20–35) | 31 (30–25) | 23 (18–25) | 30 (25–35) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quero, G.; De Sio, D.; Fiorillo, C.; Lucinato, C.; Panza, E.; Biffoni, B.; Langellotti, L.; Laterza, V.; Scaglione, G.; Taglioni, F.; et al. Resection Margin Status and Long-Term Outcomes after Pancreaticoduodenectomy for Ductal Adenocarcinoma: A Tertiary Referral Center Analysis. Cancers 2024, 16, 2347. https://doi.org/10.3390/cancers16132347
Quero G, De Sio D, Fiorillo C, Lucinato C, Panza E, Biffoni B, Langellotti L, Laterza V, Scaglione G, Taglioni F, et al. Resection Margin Status and Long-Term Outcomes after Pancreaticoduodenectomy for Ductal Adenocarcinoma: A Tertiary Referral Center Analysis. Cancers. 2024; 16(13):2347. https://doi.org/10.3390/cancers16132347
Chicago/Turabian StyleQuero, Giuseppe, Davide De Sio, Claudio Fiorillo, Chiara Lucinato, Edoardo Panza, Beatrice Biffoni, Lodovica Langellotti, Vito Laterza, Giulia Scaglione, Flavia Taglioni, and et al. 2024. "Resection Margin Status and Long-Term Outcomes after Pancreaticoduodenectomy for Ductal Adenocarcinoma: A Tertiary Referral Center Analysis" Cancers 16, no. 13: 2347. https://doi.org/10.3390/cancers16132347
APA StyleQuero, G., De Sio, D., Fiorillo, C., Lucinato, C., Panza, E., Biffoni, B., Langellotti, L., Laterza, V., Scaglione, G., Taglioni, F., Massimiani, G., Menghi, R., Rosa, F., Mezza, T., Alfieri, S., & Tondolo, V. (2024). Resection Margin Status and Long-Term Outcomes after Pancreaticoduodenectomy for Ductal Adenocarcinoma: A Tertiary Referral Center Analysis. Cancers, 16(13), 2347. https://doi.org/10.3390/cancers16132347





