State-of-the-Art and New Treatment Approaches for Spinal Cord Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
| Grade 1 | These are the least malignant tumors and are usually associated with long-term survival. They grow slowly and have an almost normal appearance when viewed through a microscope. Surgery alone may be an effective treatment for this grade tumor. |
| Grade 2 | These tumors are slow-growing and look slightly abnormal under a microscope. Some can spread into nearby normal tissue and recur, sometimes as a higher-grade tumor. |
| Grade 3 | These tumors are, by definition, malignant, although there is not always a big difference between grade 2 and grade 3 tumors. The cells of a grade 3 tumor are actively reproducing abnormal cells, which grow into nearby normal brain tissue. These tumors tend to recur, often as a grade 4 tumors. |
| Grade 4 | These are the most malignant tumors. They reproduce rapidly, can have a bizarre appearance when viewed under a microscope, and easily grow into nearby normal brain tissue. These tumors form new blood vessels so they can maintain their rapid growth. They also have areas of dead cells in their centers. |
| Neuroepithelial tissue | |
| Paraspinal nerves | |
| Meninges | Meningothelial cells; mesenchymal; primary melanocytic lesions; other neoplasms |
| Lymphoma and hematopoietic neoplasms | |
| Germ cell tumors | |
| Metastatic tumors |
2. Diagnosis
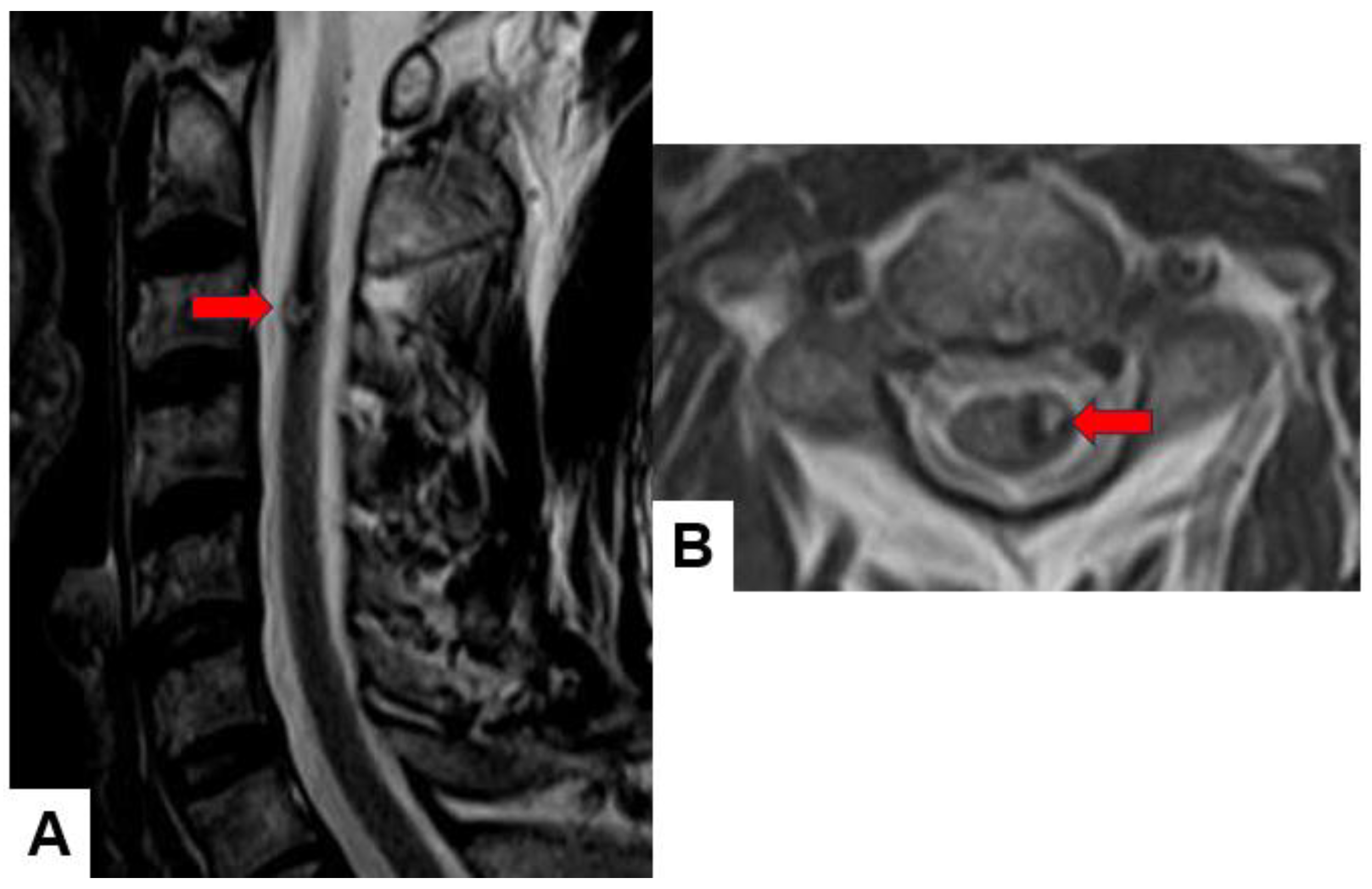
2.1. MRI and CT
| Tumor | Incidence |
|---|---|
| Ependymoma | 50–60% |
| -Myxopapillary | 20–30% |
| Astrocytoma | 15–30% |
| -Pilocytic | 10–45% |
| -High-grade | 10–33% |
| Hemangioblastoma | 3–11% |
| Cavernous angioma | 4–5% |
| Schwannoma | 1% |
| Metastasis | 1% |
| Tumor | Incidence |
|---|---|
| Astrocytoma | 41% |
| -Pilocytic | 6% |
| -High-grade | 26% |
| Ganglioglioma | 27% |
| Ependymoma | 12% |
| -Myxopapillary | 35% |
| Hemangioblastoma | 2% |
2.2. Molecular and Genetic Profiling
2.3. The Important Role of Biopsy and Deferential Diagnosis of Other Non-Tumor Conditions
3. Current Treatment Strategies and Their Limitations
3.1. Surgical Method
3.2. Radiotherapy
3.3. Systemic Therapy
4. Emerging Treatment Strategies
4.1. Immunotherapy
4.2. Neural Stem Cells
4.3. Cancer Vaccine
4.4. Tumor-Targeted Therapies (Nanotechnology)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grimm, S.; Chamberlain, M.C. Adult primary spinal cord tumors. Expert. Rev. Neurother. 2009, 9, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.K.; Geraghty, J.R.; Engelhard, H.H.; Linninger, A.A.; Mehta, A.I. Intramedullary spinal cord tumors: A review of current and future treatment strategies. Neurosurg. Focus 2015, 39, E14. [Google Scholar] [CrossRef]
- Toniutti, M.; Sasso, A.L.; Carai, A.; Colafati, G.S.; Piccirilli, E.; Del Baldo, G.; Mastronuzzi, A. Central nervous system tumours in neonates: What should the neonatologist know? Eur. J. Pediatr. 2024, 183, 1485–1497. [Google Scholar] [CrossRef]
- Raco, A.; Esposito, V.; Lenzi, J.; Piccirilli, M.; Delfini, R.; Cantore, G. Long-term follow-up of intramedullary spinal cord tumors: A series of 202 cases. Neurosurgery 2005, 56, 972–981. [Google Scholar]
- Parsa, A.T.; Lee, J.; Parney, I.F.; Weinstein, P.; McCormick, P.C.; Ames, C. Spinal cord and intradural-extraparenchymal spinal tumors: Current best care practices and strategies. J. Neuro-Oncol. 2004, 69, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Beall, D.P.; Googe, D.J.; Emery, R.L.; Thompson, D.B.; Campbell, S.E.; Ly, J.Q.; DeLone, D.; Smirniotopoulos, J.; Lisanti, C.; Currie, T.J. Extramedullary intradural spinal tumors: A pictorial review. Curr. Probl. Diagn. Radiol. 2007, 36, 185–198. [Google Scholar] [CrossRef]
- Ciftdemir, M.; Kaya, M.; Selcuk, E.; Yalniz, E. Tumors of the spine. World J. Orthop. 2016, 7, 109–116. [Google Scholar] [CrossRef]
- Das, J.M.; Hoang, S.; Mesfin, F.B. Intramedullary spinal cord tumors. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Arnautovic, K.; Arnautovic, A. Extramedullary intradural spinal tumors: A review of modern diagnostic and treatment options and a report of a series. Bosn. J. Basic Med. Sci. 2009, 9 (Suppl. S1), 40–45. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Tredway, T.L. Adult primary intradural spinal cord tumors: A review. Curr. Neurol. Neurosci. Rep. 2011, 11, 320–328. [Google Scholar] [CrossRef]
- Duong, L.M.; McCarthy, B.J.; McLendon, R.E.; Dolecek, T.A.; Kruchko, C.; Douglas, L.L.; Ajani, U.A. Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004–2007. Cancer 2012, 118, 4220–4227. [Google Scholar] [CrossRef]
- Milano, M.T.; Johnson, M.D.; Sul, J.; Mohile, N.A.; Korones, D.N.; Okunieff, P.; Walter, K.A. Primary spinal cord glioma: A surveillance, epidemiology, and end results database study. J. Neuro-Oncol. 2010, 98, 83–92. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Porras, J.L.; Pennington, Z.; Hung, B.; Hersh, A.; Schilling, A.; Goodwin, C.R.; Sciubba, D.M. Radiotherapy and Surgical Advances in the Treatment of Metastatic Spine Tumors: A Narrative Review. World Neurosurg. 2021, 151, 147–154. [Google Scholar] [CrossRef]
- Elsberg, C.A. Some aspects of the diagnosis and surgical treatment of tumors of the spinal cord: With a study of the end results in a series of 119 operations. Ann. Surg. 1925, 81, 1057–1073. [Google Scholar] [CrossRef]
- Swartz, A.M.; Shen, S.H.; Salgado, M.A.; Congdon, K.L.; Sanchez-Perez, L. Promising vaccines for treating glioblastoma. Expert Opin. Biol. Ther. 2018, 18, 1159–1170. [Google Scholar] [CrossRef]
- Berger, M.S.; Prados, M. Textbook of Neuro-Oncology, 1st ed.; Elsevier Saunders: Philadelphia, PA, USA, 2005. [Google Scholar]
- Hu, J.; Liu, T.; Han, B.; Tan, S.; Guo, H.; Xin, Y. Immunotherapy: A Potential Approach for High-Grade Spinal Cord Astrocytomas. Front. Immunol. 2021, 11, 582828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sumdani, H.; Aguilar-Salinas, P.; Avila, M.J.; Barber, S.R.; Dumont, T. Utility of Augmented Reality and Virtual Reality in Spine Surgery: A Systematic Review of the Literature. World Neurosurg. 2022, 161, e8–e17. [Google Scholar] [CrossRef] [PubMed]
- Grady, C.; Melnick, K.; Porche, K.; Dastmalchi, F.; Hoh, D.J.; Rahman, M.; Ghiaseddin, A. Glioma immunotherapy: Advances and challenges for spinal cord gliomas. Neurospine 2022, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Missenard, G.; Bouthors, C.; Fadel, E.; Court, C. Surgical strategies for primary malignant tumors of the thoracic and lumbar spine. Orthop. Traumatol. Surg. Res. 2020, 106, S53–S62. [Google Scholar] [CrossRef]
- Apostolov, G.; Kehayov, I.; Kitov, B. Clinical aspects of spinal meningiomas: A review. Folia Med. 2021, 63, 24–29. [Google Scholar] [CrossRef]
- Koeller, K.K.; Shih, R.Y. Intradural extramedullary spinal neoplasms: Radiologic-pathologic correlation. Radiographics 2019, 39, 468–490. [Google Scholar] [CrossRef] [PubMed]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical strategy for spinal metastases. Spine 2001, 26, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. Relat. Res. 1980, 153, 106–120. [Google Scholar] [CrossRef]
- Villanueva-Castro, E.; Meraz-Soto, J.M.; Hernández-Dehesa, I.A.; Tena-Suck, M.L.; Hernández-Reséndiz, R.; Mateo-Nouel, E.J.; Ponce-Gómez, J.A.; Arriada-Mendicoa, J.N. Spinal Ependymomas: An Updated WHO Classification and a Narrative Review. Cureus 2023, 15, e49086. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Jung, C.H. Metastatic spinal tumor. Asian Spine J. 2012, 6, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Maclean, F.M.; Soo, M.Y.; Ng, T. Chordoma: Radiological-pathological correlation. Australas. Radiol. 2005, 49, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, P.B.; Soundararajan, D.C.R.; Kanna, R.M.; Kuna, V.S.; Rajasekaran, S. Sporadic Intradural Extramedullary Hemangioblastoma of Cauda Equina with Large Peritumoral Cyst-A Rare Presentation. Indian J. Radiol. Imaging 2022, 31, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, K.; Tomita, H.; Hara, A. Peritumoral Edema in Gliomas: A Review of Mechanisms and Management. Biomedicines 2023, 11, 2731. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Dumrongpisutikul, N.; Intrapiromkul, J.; Yousem, D.M. Detection of intratumoral calcification in oligodendrogliomas by susceptibility-weighted MR imaging. Am. J. Neuroradiol. 2012, 33, 858–864. [Google Scholar] [CrossRef]
- Bönig, L.; Möhn, N.; Ahlbrecht, J.; Wurster, U.; Raab, P.; Puppe, W.; Sühs, K.W.; Stangel, M.; Skripuletz, T.; Schwenkenbecher, P. Leptomeningeal Metastasis: The Role of Cerebrospinal Fluid Diagnostics. Front. Neurol. 2019, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Abdel Razek, A.A.K.; Gamaleldin, O.A.; Elsebaie, N.A. Peripheral Nerve Sheath Tumors of Head and Neck: Imaging-Based Review of World Health Organization Classification. J. Comput. Assist. Tomogr. 2020, 44, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Eraky, A.M.; Beck, R.T.; Treffy, R.W.; Aaronson, D.M.; Hedayat, H. Role of Advanced MR Imaging in Diagnosis of Neurological Malignancies: Current Status and Future Perspective. J. Integr. Neurosci. 2023, 22, 73. [Google Scholar] [CrossRef] [PubMed]
- Lemay, A.; Gros, C.; Zhuo, Z.; Zhang, J.; Duan, Y.; Cohen-Adad, J.; Liu, Y. Automatic multiclass intramedullary spinal cord tumor segmentation on MRI with deep learning. Neuroimage Clin. 2021, 31, 102766. [Google Scholar] [CrossRef]
- Bhardwaj, A. Promise and Provisos of Artificial Intelligence and Machine Learning in Healthcare. J. Healthc. Leadersh. 2022, 14, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Jallo, G.I.; Freed, D.; Epstein, F. Intramedullary spinal cord tumors in children. Childs Nerv. Syst. 2003, 19, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Choi, Y.S.; Ahn, S.S.; Yi, S.; Kim, S.H.; Lee, S.K. Differentiation between spinal cord diffuse midline glioma with histone H3 K27M mutation and wild type: Comparative magnetic resonance imaging. Neuroradiology 2019, 61, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Anoosha, P.; Yesudhas, D.; Gromiha, M.M. Identification of potential driver mutations in glioblastoma using machine learning. Brief Bioinform. 2022, 23, bbac451. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Ebert, C.; von Haken, M.; Meyer-Puttlitz, B.; Wiestler, O.D.; Reifenberger, G.; Pietsch, T.; von Deimling, A. Molecular genetic analysis of ependymal tumors. NF2 mutations and chromosome 22q loss occur preferentially in intramedullary spinal ependymomas. Am. J. Pathol. 1999, 155, 627–632. [Google Scholar] [CrossRef]
- Dang, D.D.; Mugge, L.A.; Awan, O.K.; Gong, A.D.; Fanous, A.A. Spinal Meningiomas: A Comprehensive Review and Update on Advancements in Molecular Characterization, Diagnostics, Surgical Approach and Technology, and Alternative Therapies. Cancers 2024, 16, 1426. [Google Scholar] [CrossRef] [PubMed]
- Sestini, R.; Bacci, C.; Provenzano, A.; Genuardi, M.; Papi, L. Evidence of a four-hit mechanism involving SMARCB1 and NF2 in schwannomatosis-associated schwannomas. Hum. Mutat. 2008, 29, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, P.; Jiang, L.B.; Wang, H.L.; Hu, A.N.; Zhou, X.G.; Li, X.L.; Lin, H.; Wu, D.; Dong, J. Value of CT-guided Core Needle Biopsy in Diagnosing Spinal Lesions: A Comparison Study. Orthop. Surg. 2019, 11, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Rajeswaran, G.; Malik, Q.; Saifuddin, A. Image-guided percutaneous spinal biopsy. Skelet. Radiol. 2013, 42, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.P.; O’Neill, B.P.; Habermann, T.M.; Porter, A.B.; Keegan, B.M. Secondary intramedullary spinal cord non-Hodgkin’s lymphoma. J. Neuro-Oncol. 2012, 107, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.P. Autoimmune myelopathies. Handb. Clin. Neurol. 2016, 133, 327–351. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Gadol, A.A.; Zikel, O.M.; Miller, G.M.; Aksamit, A.J.; Scheithauer, B.W.; Krauss, W.E. Spinal cord biopsy: A review of 38 cases. Neurosurgery 2003, 52, 806–815; discussion 815–816. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, A.; Mercuri, S. Intradural spinal cysts. Acta Neurochir. 1983, 68, 289–314. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, R.H.; Odom, G.L. Spinal intradural cysts. In Tumors of the Spine and Spinal Cord, Part II. Handbook of Clinical Neurology; Vinkin, P.J., Bruyn, G.W., Eds.; North Holland Publishing: Amsterdam, The Netherlands, 1976; Volume 20, pp. 55–102. [Google Scholar]
- Savage, J.J.; Casey, J.N.; McNeill, I.T.; Sherman, J.H. Neurenteric cysts of the spine. J. Craniovertebral Junction Spine 2010, 1, 58–63. [Google Scholar] [CrossRef]
- De Moura Batista, L.; Acioly, M.A.; Carvalho, C.H.; Ebner, F.H.; Tatagiba, M. Cystic lesion of the ventriculus terminalis: Proposal for a new clinical classification. J. Neurosurg. Spine 2008, 8, 163–168. [Google Scholar] [CrossRef]
- Ganau, M.; Talacchi, A.; Cecchi, P.C.; Ghimenton, C.; Gerosa, M.; Faccioli, F. Cystic dilation of the ventriculus terminalis. J. Neurosurg. Spine 2012, 17, 86–92. [Google Scholar] [CrossRef]
- Guzel, N.; Eras, M.; Guzel, D.K. A child with spinal intramedullary abscess. Childs Nerv. Syst. 2003, 19, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Thurnher, M.M.; Bammer, R. Diffusion-weighted MR imaging (DWI) in spinal cord ischemia. Neuroradiology 2006, 48, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Yuh, W.T.; Marsh, E.E., 3rd; Wang, A.K.; Russell, J.W.; Chiang, F.; Koci, T.M.; Ryals, T.J. MR imaging of spinal cord and vertebral body infarction. Am. J. Neuroradiol. 1992, 13, 145–154. [Google Scholar] [PubMed]
- Patchana, T.; Savla, P.; Taka, T.M.; Ghanchi, H.; Wiginton, J., 4th; Schiraldi, M.; Cortez, V. Spinal Arteriovenous Malformation: Case Report and Review of the Literature. Cureus 2020, 12, e11614. [Google Scholar] [CrossRef] [PubMed]
- Hassler, W.; Thron, A.; Grote, E.H. Hemodynamics of spinal dural arteriovenous fistulas. An intraoperative study. J. Neurosurg. 1989, 70, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; El-Baba, C.; Elnaggar, M.H.; Elkholy, Y.O.; Mottawea, M.; Johar, D.; Al Shehabi, T.S.; Kobeissy, F.; Moussalem, C.; Massaad, E.; et al. Novel therapeutic strategies for spinal osteosarcomas. Semin. Cancer Biol. 2020, 64, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ottenhausen, M.; Ntoulias, G.; Bodhinayake, I.; Ruppert, F.H.; Schreiber, S.; Förschler, A.; Boockvar, J.A.; Jödicke, A. Intradural spinal tumors in adults—Update on management and outcome. Neurosurg. Rev. 2019, 42, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.G.; Dunn, I.F. Chondrosarcoma and Chordoma of the Skull Base and Spine: Implication of Tumor Location on Patient Survival. World Neurosurg. 2022, 162, e635–e639. [Google Scholar] [CrossRef]
- Ahangar, P.; Akoury, E.; Ramirez Garcia Luna, A.S.; Nour, A.; Weber, M.H.; Rosenzweig, D.H. Nanoporous 3D-Printed Scaffolds for Local Doxorubicin Delivery in Bone Metastases Secondary to Prostate Cancer. Materials 2018, 11, 1485. [Google Scholar] [CrossRef]
- Aoyama, R.; Anazawa, U.; Hotta, H.; Watanabe, I.; Takahashi, Y.; Matsumoto, S. The utility of augmented reality in spinal decompression surgery using CT/MRI fusion image. Cureus 2021, 13, e18187. [Google Scholar] [CrossRef] [PubMed]
- Burström, G.; Persson, O.; Edström, E.; Elmi-Terander, A. Augmented reality navigation in spine surgery: A systematic review. Acta Neurochir. 2021, 163, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Jud, L.; Fotouhi, J.; Andronic, O.; Aichmair, A.; Osgood, G.; Navab, N.; Farshad, M. Applicability of augmented reality in orthopedic surgery—A systematic review. BMC Musculoskelet. Disord. 2020, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Koyachi, M.; Koyama, Y.; Sugimoto, M.; Matsunaga, S.; Odaka, K.; Abe, S.; Katakura, A. Mixed reality and three dimensional printed models for resection of maxillary tumor: A case report. Quant. Imaging Med. Surg. 2021, 11, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, R.; Anazawa, U.; Hotta, H.; Watanabe, I.; Takahashi, Y.; Matsumoto, S. A Novel Technique of Mixed Reality Systems in the Treatment of Spinal Cord Tumors. Cureus 2022, 14, e23096. [Google Scholar] [CrossRef] [PubMed]
- Elmesallamy, W.A.E.A.; Yakout, H.; Hassanen, S.; Elshekh, M. The role of intraoperative ultrasound in management of spinal intradural mass lesions and outcome. Egypt. J. Neurosurg. 2023, 38, 38. [Google Scholar] [CrossRef]
- Prada, F.; Vetrano, I.G.; Filippini, A.; Del Bene, M.; Perin, A.; Casali, C.; Legnani, F.; Saini, M.; DiMeco, F. Intraoperative ultrasound in spinal tumor surgery. J. Ultrasound 2014, 17, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Selbekk, T.; Jakola, A.S.; Solheim, O.; Johansen, T.F.; Lindseth, F.; Reinertsen, I.; Unsgård, G. Ultrasound imaging in neurosurgery: Approaches to minimize surgically induced image artefacts for improved resection control. Acta Neurochir. 2013, 155, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.W.; Noh, S.H.; Park, J.Y.; Cho, Y.E.; Chin, D.K. Retrospective Study on Accuracy of Intraoperative Frozen Section Biopsy in Spinal Tumors. World Neurosurg. 2019, 129, e152–e157. [Google Scholar] [CrossRef]
- Roberts, D.W.; Hartov, A.; Kennedy, F.E.; Miga, M.I.; Paulsen, K.D. Intraoperative brain shift and deformation: A quantitative analysis of cortical displacement in 28 cases. Neurosurgery 1998, 43, 749–758; discussion 758–760. [Google Scholar] [CrossRef]
- Reinertsen, I.; Lindseth, F.; Askeland, C.; Iversen, D.H.; Unsgard, G. Intra-operative correction of brain-shift. Acta Neurochir. 2014, 156, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Lindner, D.; Trantakis, C.; Renner, C.; Arnold, S.; Schmitgen, A.; Schneider, J.; Meixensberger, J. Application of intraoperative 3D ultrasound during navigated tumor resection. Minim. Invasive Neurosurg. 2006, 49, 197–202. [Google Scholar] [CrossRef]
- Prada, F.; Del Bene, M.; Mattei, L.; Lodigiani, L.; DeBeni, S.; Kolev, V.; Vetrano, I.; Solbiati, L.; Sakas, G.; DiMeco, F. Preoperative magnetic resonance and intraoperative ultrasound fusion imaging for real-time neuronavigation in brain tumor surgery. Ultraschall Med. 2015, 36, 174–186. [Google Scholar] [CrossRef]
- Tyurikova, O.; Dembitskaya, Y.; Yashin, K.; Mishchenko, M.; Vedunova, M.; Medyanik, I.; Kazantsev, V. Perspectives in Intraoperative Diagnostics of Human Gliomas. Comput. Math. Methods Med. 2015, 2015, 479014. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Martirosyan, N.L.; Yagmurlu, K.; Miller, E.J.; Eschbacher, J.M.; Izadyyazdanabadi, M.; Bardonova, L.A.; Byvaltsev, V.A.; Nakaji, P.; Preul, M.C. Intraoperative Fluorescence Imaging for Personalized Brain Tumor Resection: Current State and Future Directions. Front. Surg. 2016, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Teixidor, P.; Arráez, M.Á.; Villalba, G.; Garcia, R.; Tardáguila, M.; González, J.J.; Rimbau, J.; Vidal, X.; Montané, E. Safety and Efficacy of 5-Aminolevulinic Acid for High Grade Glioma in Usual Clinical Practice: A Prospective Cohort Study. PLoS ONE 2016, 11, e0149244. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Stummer, W.; Sheehan, J.P. 5-ALA fluorescence-guided surgery of CNS tumors. J. Neuro-Oncol. 2019, 141, 477–478. [Google Scholar] [CrossRef]
- Mazurek, M.; Kulesza, B.; Stoma, F.; Osuchowski, J.; Mańdziuk, S.; Rola, R. Characteristics of Fluorescent Intraoperative Dyes Helpful in Gross Total Resection of High-Grade Gliomas-A Systematic Review. Diagnostics 2020, 10, 1100. [Google Scholar] [CrossRef]
- Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Della Pepa, G.M.; Offi, M.; Giordano, M.; Caccavella, V.M.; Falchetti, M.L.; Lauretti, L.; Pallini, R. 5-Aminolevulinic Acid (5-ALA)-Induced Protoporphyrin IX Fluorescence by Glioma Cells-A Fluorescence Microscopy Clinical Study. Cancers 2022, 14, 2844. [Google Scholar] [CrossRef]
- Takami, T.; Naito, K.; Yamagata, T.; Ohata, K. Surgical management of spinal intramedullary tumors: Radical and safe strategy for benign tumors. Neurol. Med. Chir. 2015, 55, 317–327. [Google Scholar] [CrossRef]
- Kucia, E.J.; Bambakidis, N.C.; Chang, S.W.; Spetzler, R.F. Surgical technique and outcomes in the treatment of spinal cord ependymomas, part 1: Intramedullary ependymomas. Neurosurgery 2011, 68 (Suppl. S1), 57–63; discussion 63. [Google Scholar] [CrossRef] [PubMed]
- Ohata, K.; Takami, T.; Gotou, T.; El-Bahy, K.; Morino, M.; Maeda, M.; Inoue, Y.; Hakuba, A. Surgical outcome of intramedullary spinal cord ependymoma. Acta Neurochir. 1999, 141, 341–346; discussion 346–347. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Ohata, K.; Takami, T.; Nishikawa, M.; Nishio, A.; Morino, M.; Tsuyuguchi, N.; Hara, M. Prevention of postoperative posterior tethering of spinal cord after resection of ependymoma. J. Neurosurg. 2003, 99 (Suppl. S2), 181–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, W.; Chen, H.; LoStracco, T.A.; Zhang, J.; Li, M.Y.; Germin, B.; Wang, H.Z. Advanced Neuroimaging in the Evaluation of Spinal Cord Tumors and Tumor Mimics: Diffusion Tensor and Perfusion-Weighted Imaging. Semin. Ultrasound CT MRI 2017, 38, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Jetty, S.N.; Badar, Z.; Drumsla, D.; Mangla, R. Clinical Significance of T2*gradient-recalled Echo/susceptibility-weighted Imaging Sequences in Evaluating Superficial Siderosis in the Setting of Intracerebral Tumors: Pilocytic Astrocytoma. J. Clin. Imaging Sci. 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Fuster-Garcia, E.; Tortajada, S.; García-Gómez, J.M.; Davies, N.; Natarajan, K.; Wilson, M.; Grundy, R.G.; Wesseling, P.; Monleón, D.; et al. Accurate classification of childhood brain tumours by in vivo 1H MRS—A multi-centre study. Eur. J. Cancer 2013, 49, 658–667. [Google Scholar] [CrossRef]
- Giammattei, L.; Penet, N.; Parker, F.; Messerer, M. Intramedullary ependymoma: Microsurgical resection technique. Neurochirurgie 2017, 63, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Parker, W.E.; Barzilai, O.; Bilsky, M.H. Surgical management of intramedullary spinal cord tumors. Neurosurg. Clin. N. Am. 2020, 31, 237–249. [Google Scholar]
- Abd-El-Barr, M.M.; Huang, K.T.; Moses, Z.B.; Iorgulescu, J.B.; Chi, J.H. Recent advances in intradural spinal tumors. Neuro-Oncology 2018, 20, 729–742. [Google Scholar] [CrossRef]
- Tendulkar, R.D.; Pai Panandiker, A.S.; Wu, S.; Kun, L.E.; Broniscer, A.; Sanford, R.A.; Merchant, T.E. Irradiation of pediatric high-grade spinal cord tumors. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1451–1456. [Google Scholar] [CrossRef]
- Mehta, A.I.; Mohrhaus, C.A.; Husain, A.M.; Karikari, I.O.; Hughes, B.; Hodges, T.; Gottfried, O.; Bagley, C.A. Dorsal column mapping for intramedullary spinal cord tumor resection decreases dorsal column dysfunction. J. Spinal Disord. Tech. 2012, 25, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.Y.; Viswanathan, A.N. Impact of Radiotherapy on Fertility, Pregnancy, and Neonatal Outcomes in Female Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Dea, N.; Gokaslan, Z.; Choi, D.; Fisher, C. Spine oncology–primary spine tumors. Neurosurgery 2017, 80 (Suppl. S3), S124–S130. [Google Scholar] [CrossRef]
- Purvis, T.E.; Goodwin, C.R.; Lubelski, D.; Laufer, I.; Sciubba, D.M. Review of stereotactic radiosurgery for intradural spine tumors. CNS Oncol. 2017, 6, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Sohn, M.J.; Kim, H.S.; Lee, D.J.; Jeon, S.R.; Hwang, Y.J.; Jho, E.H. Clinical analysis of spinal stereotactic radiosurgery in the treatment of neurogenic tumors. J. Neurosurg. Spine 2015, 23, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Gerszten, P.C.; Quader, M.; Novotny, J., Jr.; Flickinger, J.C. Radiosurgery for benign tumors of the spine: Clinical experience and current trends. Technol. Cancer Res. Treat. 2012, 11, 133–139. [Google Scholar] [CrossRef]
- Marchetti, M.; De Martin, E.; Milanesi, I.; Fariselli, L. Intradural extramedullary benign spinal lesions radiosurgery. Medium- to long-term results from a single institution experience. Acta Neurochir. 2013, 155, 1215–1222. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Chamberlain, M.C. Temozolomide for recurrent low-grade spinal cord gliomas in adults. Cancer 2008, 113, 1019–1024. [Google Scholar] [CrossRef]
- Kaley, T.J.; Mondesire-Crump, I.; Gavrilovic, I.T. Temozolomide or bevacizumab for spinal cord high-grade gliomas. J. Neuro-Oncol. 2012, 109, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Yoon, S.H.; Kim, C.Y.; Kim, K.J.; Lee, M.M.; Choe, G.; Kim, I.A.; Kim, J.H.; Kim, Y.J.; Kim, H.J. Temozolomide for malignant primary spinal cord glioma: An experience of six cases and a literature review. J. Neuro-Oncol. 2011, 101, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Menezes, A.H.; Torner, J.C. Role of resection and adjuvant therapy in long-term disease outcomes for low-grade pediatric intramedullary spinal cord tumors. J. Neurosurg. Pediatr. 2016, 18, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Etoposide for recurrent spinal cord ependymoma. Neurology 2002, 58, 1310–1311. [Google Scholar] [CrossRef] [PubMed]
- Fakhrai, N.; Neophytou, P.; Dieckmann, K.; Nemeth, A.; Prayer, D.; Hainfellner, J.; Marosi, C. Recurrent spinal ependymoma showing partial remission under Imatimib. Acta Neurochir. 2004, 146, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.A.; Afridi, S.K.; Evans, D.G.; Hensiek, A.E.; McCabe, M.G.; Kellett, M.; Halliday, D.; Pretorius, P.M.; Parry, A. The response of spinal cord ependymomas to bevacizumab in patients with neurofibromatosis Type 2. J. Neurosurg. Spine 2017, 26, 474–482. [Google Scholar] [CrossRef]
- Karajannis, M.A.; Legault, G.; Hagiwara, M.; Ballas, M.S.; Brown, K.; Nusbaum, A.O.; Hochman, T.; Goldberg, J.D.; Koch, K.M.; Golfinos, J.G.; et al. Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro-Oncology 2012, 14, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, M.; Stewart, C.F.; Blaney, S.M.; Onar-Thomas, A.; Schaiquevich, P.; Packer, R.J.; Gajjar, A.; Kun, L.E.; Boyett, J.M.; Gilbertson, R.J. Phase I trial of lapatinib in children with refractory CNS malignancies: A Pediatric Brain Tumor Consortium study. J. Clin. Oncol. 2010, 28, 4221–4227. [Google Scholar] [CrossRef]
- DeWire, M.; Fouladi, M.; Turner, D.C.; Wetmore, C.; Hawkins, C.; Jacobs, C.; Yuan, Y.; Liu, D.; Goldman, S.; Fisher, P.; et al. An open-label, two-stage, phase II study of bevacizumab and lapatinib in children with recurrent or refractory ependymoma: A collaborative ependymoma research network study (CERN). J. Neuro-Oncol. 2015, 123, 85–91. [Google Scholar] [CrossRef]
- Kringel, R.; Lamszus, K.; Mohme, M. Chimeric Antigen Receptor T Cells in Glioblastoma-Current Concepts and Promising Future. Cells 2023, 12, 1770. [Google Scholar] [CrossRef]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Wang, S.S.; Bandopadhayay, P.; Jenkins, M.R. Towards Immunotherapy for Pediatric Brain Tumors. Trends Immunol. 2019, 40, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, G.; De Chiara, A.; Parafioriti, A.; Armiraglio, E.; Fazioli, F.; Gallo, M.; Aversa, L.; Camerlingo, R.; Cacciatore, F.; Colella, G.; et al. Patient-derived organoids as a potential model to predict response to PD-1/PD-L1 checkpoint inhibitors. Br. J. Cancer 2019, 121, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Bobadilla, E.; Martin-Villalba, A. Adult NSC diversity and plasticity: The role of the niche. Curr. Opin. Neurobiol. 2017, 42, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Aboody, K.S.; Brown, A.; Rainov, N.G.; Bower, K.A.; Liu, S.; Yang, W.; Small, J.E.; Herrlinger, U.; Ourednik, V.; Black, P.M.; et al. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc. Natl. Acad. Sci. USA 2000, 97, 12846–12851, Erratum in Proc. Natl. Acad. Sci. USA 2001, 98, 777. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cargioli, T.G.; Machluf, M.; Yang, W.; Sun, Y.; Al-Hashem, R.; Kim, S.U.; Black, P.M.; Carroll, R.S. PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin. Cancer Res. 2005, 11, 5965–5970. [Google Scholar] [CrossRef] [PubMed]
- Aboody, K.; Capela, A.; Niazi, N.; Stern, J.H.; Temple, S. Translating stem cell studies to the clinic for CNS repair: Current state of the art and the need for a Rosetta stone. Neuron 2011, 70, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Ropper, A.E.; Zeng, X.; Haragopal, H.; Anderson, J.E.; Aljuboori, Z.; Han, I.; Abd-El-Barr, M.; Lee, H.J.; Sidman, R.L.; Snyder, E.Y.; et al. Targeted Treatment of Experimental Spinal Cord Glioma With Dual Gene-Engineered Human Neural Stem Cells. Neurosurgery 2016, 79, 481–491. [Google Scholar] [CrossRef]
- Teng, Y.D.; Abd-El-Barr, M.; Wang, L.; Hajiali, H.; Wu, L.; Zafonte, R.D. Spinal cord astrocytomas: Progresses in experimental and clinical investigations for developing recovery neurobiology-based novel therapies. Exp. Neurol. 2019, 311, 135–147. [Google Scholar] [CrossRef]
- Xu, S.; Tang, L.; Li, X.; Fan, F.; Liu, Z. Immunotherapy for glioma: Current management and future application. Cancer Lett. 2020, 476, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, X.; Li, Y.; Zhang, J.; Zong, Z.; Zhang, H. Current immunotherapies for glioblastoma multiforme. Front. Immunol. 2020, 11, 603911. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Weber, M.H.; Gokaslan, Z.; Wolinsky, J.-P.; Schmidt, M.; Rhines, L.; Fehlings, M.G.; Laufer, I.; Sciubba, D.M.; Clarke, M.J.; et al. Metastatic Spinal Cord Compression and Steroid Treatment: A Systematic Review. Clin. Spine Surg. 2017, 30, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.T.; Nie, Y.; Sun, S.N.; Lin, T.; Han, R.J.; Jiang, J.; Li, Z.; Li, J.Q.; Xiao, Y.P.; Fan, Y.Y.; et al. Tumor-associated antigen-based personalized dendritic cell vaccine in solid tumor patients. Cancer Immunol. Immunother. 2020, 69, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, K.; Akiyama, Y.; Iizuka, A.; Miyata, H.; Deguchi, S.; Hayashi, N.; Maeda, C.; Kondou, R.; Kanematsu, A.; Watanabe, K.; et al. Alpha-type-1 Polarized Dendritic Cell-based Vaccination in Newly Diagnosed High-grade Glioma: A Phase II Clinical Trial. Anticancer. Res. 2020, 40, 6473–6484. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.N.; Huang, Y.C.; Yang, D.M.; Kikuta, K.; Wei, K.J.; Kubota, T.; Yang, W.K. A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J. Clin. Neurosci. 2011, 18, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, L.; Sun, Z.; Song, D.; Cheng, Y. Targeted and intracellular delivery of protein therapeutics by a boronated polymer for the treatment of bone tumors. Bioact. Mater. 2022, 7, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, P.; Denyer, S.; Bhimani, A.D.; Arnone, G.D.; Esfahani, D.R.; Aguilar, T.; Zakrzewski, J.; Venugopal, I.; Habib, N.; Gallia, G.L.; et al. Magnetic Drug Targeting: A Novel Treatment for Intramedullary Spinal Cord Tumors. Sci. Rep. 2018, 8, 11417. [Google Scholar] [CrossRef]
- Ahmadi, D.; Zarei, M.; Rahimi, M.; Khazaie, M.; Asemi, Z.; Mir, S.M.; Sadeghpour, A.; Karimian, A.; Alemi, F.; Rahmati-Yamchi, M.; et al. Preparation and in-vitro evaluation of pH-responsive cationic cyclodextrin coated magnetic nanoparticles for delivery of methotrexate to the Saos-2 bone cancer cells. J. Drug Deliv. Sci. Technol. 2020, 57, 101584. [Google Scholar] [CrossRef]
- Huang, X.; Wu, W.; Jing, D.; Yang, L.; Guo, H.; Wang, L.; Zhang, W.; Pu, F.; Shao, Z. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J. Control Release 2022, 343, 107–117. [Google Scholar] [CrossRef]
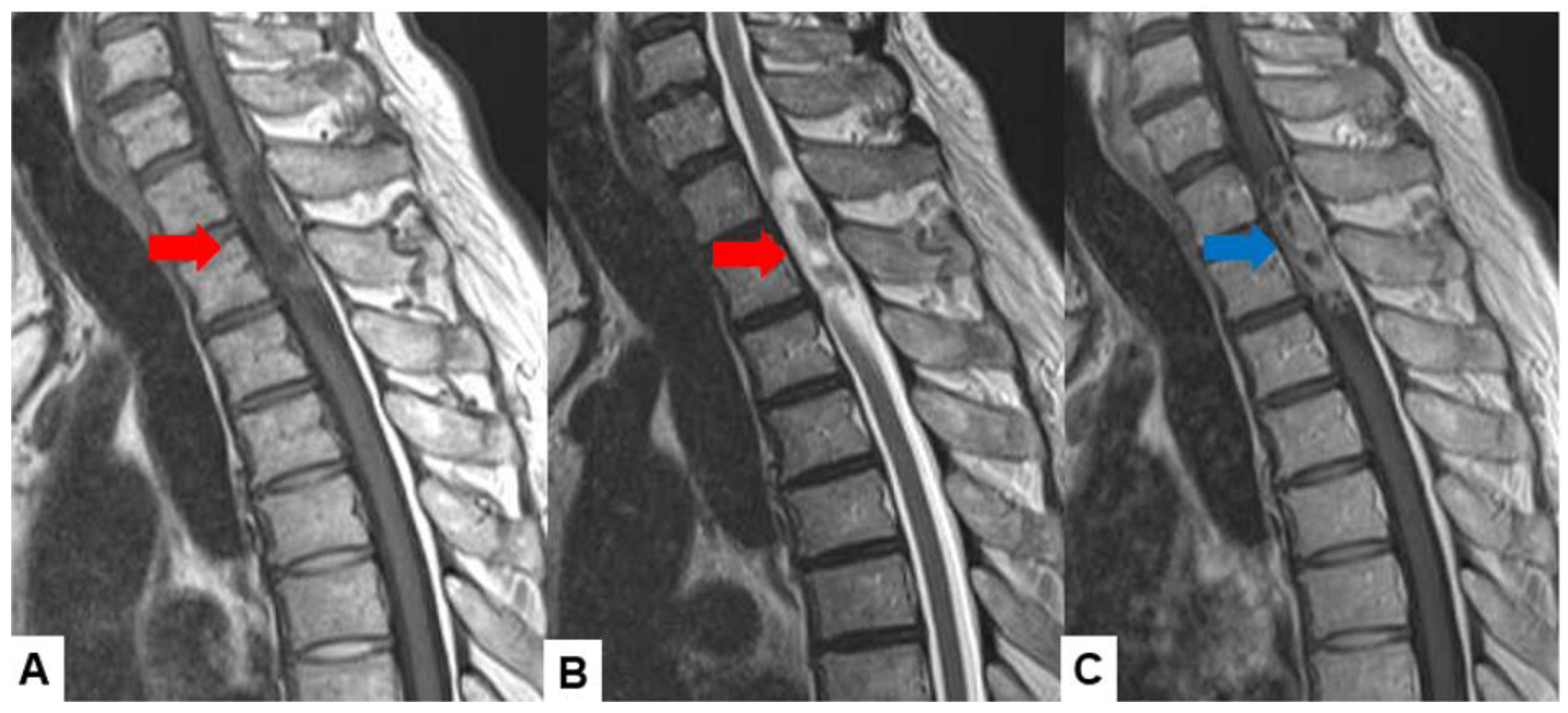

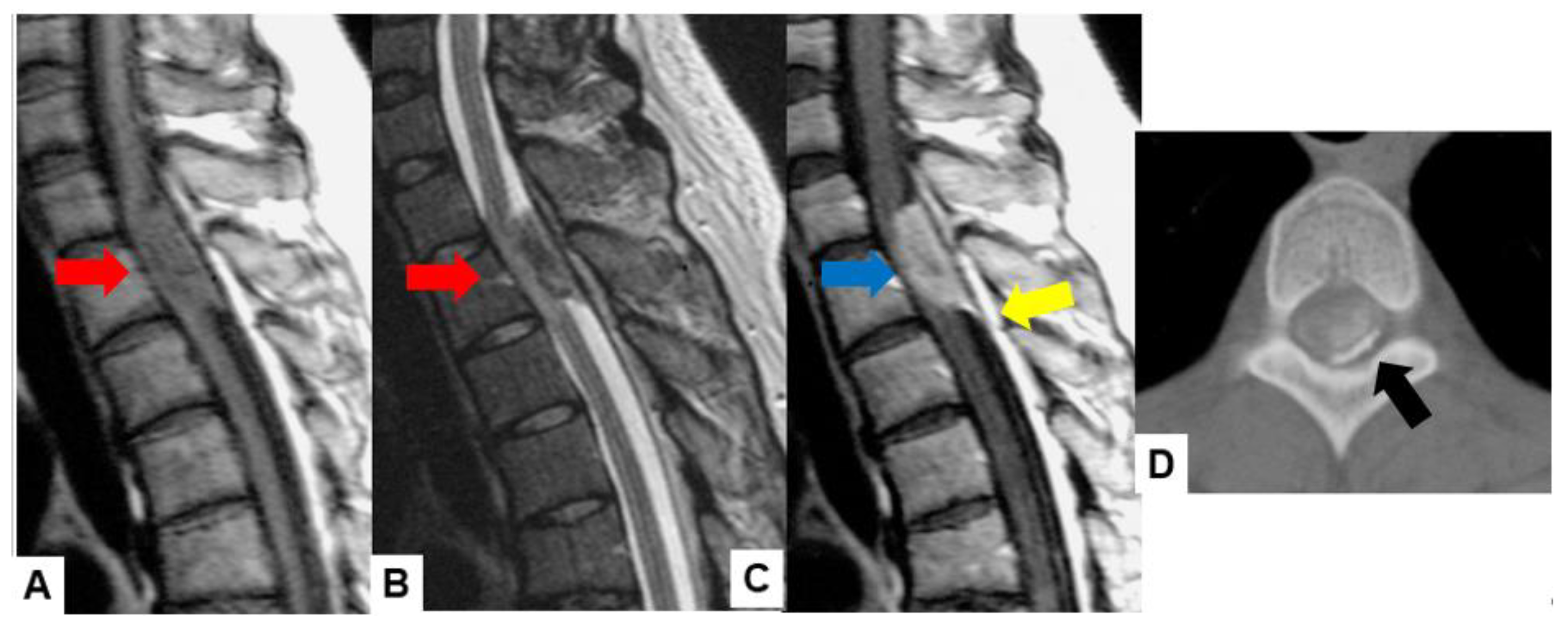
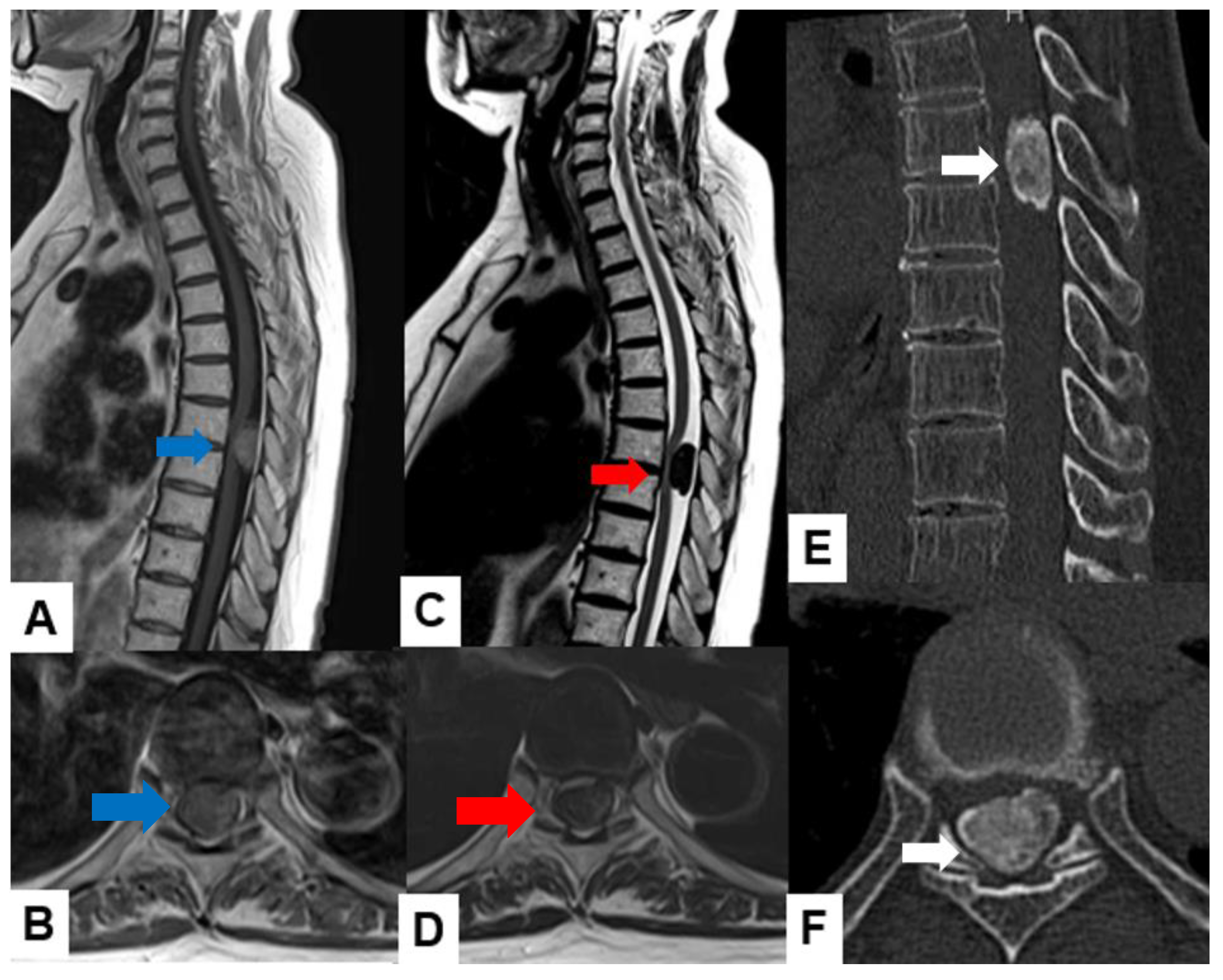
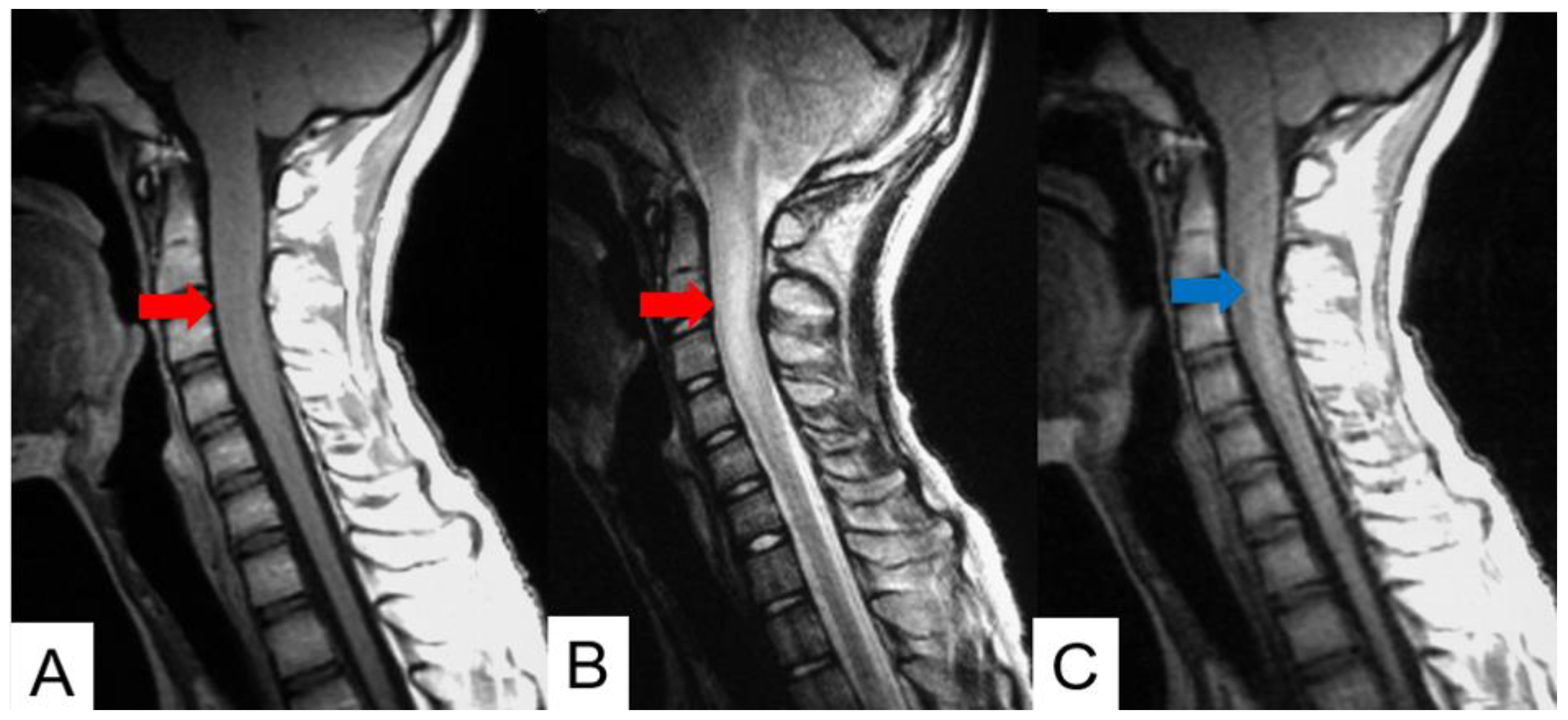


| Tumor location |
| MRI intensity and CT density |
| Enhancement pattern |
| Bone erosion |
| Accompanied findings (peritumoral cyst, edema, flow void, calcification, etc.) |
| Other studies (angiography, PET, CSF study, etc.) |
| Category | Meningioma | Schwannoma |
|---|---|---|
| T2-weighted MR imaging | Iso-low | High; heterogenous |
| Enhanced | Homogenous | Heterogenous |
| Location | Lateral | Posterior |
| Cyst | −~+ | ++ |
| Calcification | −~+ | − |
| Tumor angle | Dull | Sharp |
| Dural tail | + | − |
| Mobile tumor | − | + |
| Characteristics | Type A | Type B | Type C |
|---|---|---|---|
| Single layer of pseudostratified columnar or cuboidal cells mimicking respiratory or gastrointestinal epithelium | + | + | + |
| Complex invaginations with glandular organization; mucinous or serous production; and nerve ganglion, lymphoid, skeletal muscle, smooth muscle, fat, cartilage, and/or bone elements | − | + | − |
| Ependymal or glial tissue | − | − | + |
| Prognosis Parameter | Score |
|---|---|
| Patient condition Poor (performance status: 10–40%) Moderate (performance status: 50–70%) Good (performance status: 80–100%) | 0 1 2 |
| No. of bone metastases outside spine Poor (performance status: 10–40% Moderate (performance status: 50–70%) Good (performance status: 80–100%) | 0 1 2 |
| No. of bone metastases outside spine >2 1–2 0 | 0 1 2 |
| Metastasis to major organs Nonremovable Removable None | 0 1 2 |
| Primary site Lung; osteosarcoma; stomach; bladder; esophagus; pancreas Liver; gallbladder; unidentified Other Kidney; uterus Rectum Thyroid; breast; prostate; carcinoid tumor | 0 1 2 3 4 5 |
| Palsy Complete (Frankel A; B) Incomplete (Frankel C; D) None (Frankel E) | 0 1 2 |
| Intra-compartmental | Type 1 | Vertebral body |
| Type 2 | Pedicle extension | |
| Type 3 | Body-lamina extension | |
| Extra-compartmental | Type 4 | Epidural extension |
| Type 5 | Paravertebral extension | |
| Type 6 | 2-3 vertebrae | |
| Multiple | Type 7 | Multiple, more than 2 |
| Stage | Grade | Site | Metastasis |
|---|---|---|---|
| IA | Low (G1) | Intra-compartmental (T1) | No metastasis (M0) |
| IB | Low (G1) | Extra-compartmental (T2) | No metastasis (M0) |
| IIA | High (G2) | Intra-compartmental (T1) | No metastasis (M0) |
| IIB | High (G2) | Extra-compartmental (T2) | No metastasis (M0) |
| III | Any (G) | Any (T) | Regional or distant metastasis (M1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumawat, C.; Takahashi, T.; Date, I.; Tomita, Y.; Tanaka, M.; Arataki, S.; Komatsubara, T.; Flores, A.O.P.; Yu, D.; Jain, M. State-of-the-Art and New Treatment Approaches for Spinal Cord Tumors. Cancers 2024, 16, 2360. https://doi.org/10.3390/cancers16132360
Kumawat C, Takahashi T, Date I, Tomita Y, Tanaka M, Arataki S, Komatsubara T, Flores AOP, Yu D, Jain M. State-of-the-Art and New Treatment Approaches for Spinal Cord Tumors. Cancers. 2024; 16(13):2360. https://doi.org/10.3390/cancers16132360
Chicago/Turabian StyleKumawat, Chetan, Toshiyuki Takahashi, Isao Date, Yousuke Tomita, Masato Tanaka, Shinya Arataki, Tadashi Komatsubara, Angel O. P. Flores, Dongwoo Yu, and Mukul Jain. 2024. "State-of-the-Art and New Treatment Approaches for Spinal Cord Tumors" Cancers 16, no. 13: 2360. https://doi.org/10.3390/cancers16132360
APA StyleKumawat, C., Takahashi, T., Date, I., Tomita, Y., Tanaka, M., Arataki, S., Komatsubara, T., Flores, A. O. P., Yu, D., & Jain, M. (2024). State-of-the-Art and New Treatment Approaches for Spinal Cord Tumors. Cancers, 16(13), 2360. https://doi.org/10.3390/cancers16132360







