The Aberrant Expression of Biomarkers and Risk Prediction for Neoplastic Changes in Barrett’s Esophagus–Dysplasia
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Cell Cycle Regulatory Genes
1.2. Proliferation Markers
1.3. Cell Adhesion Markers
1.4. Genomic Instability
2. Materials and Methods
2.1. Study Design
2.2. Immunohistochemistry Study of Biomarkers
2.3. Fluorescent In Situ Hybridization (FISH)
2.4. Statistical Methods
3. Results
3.1. Accurate Diagnosis of Barret’s Esophagus
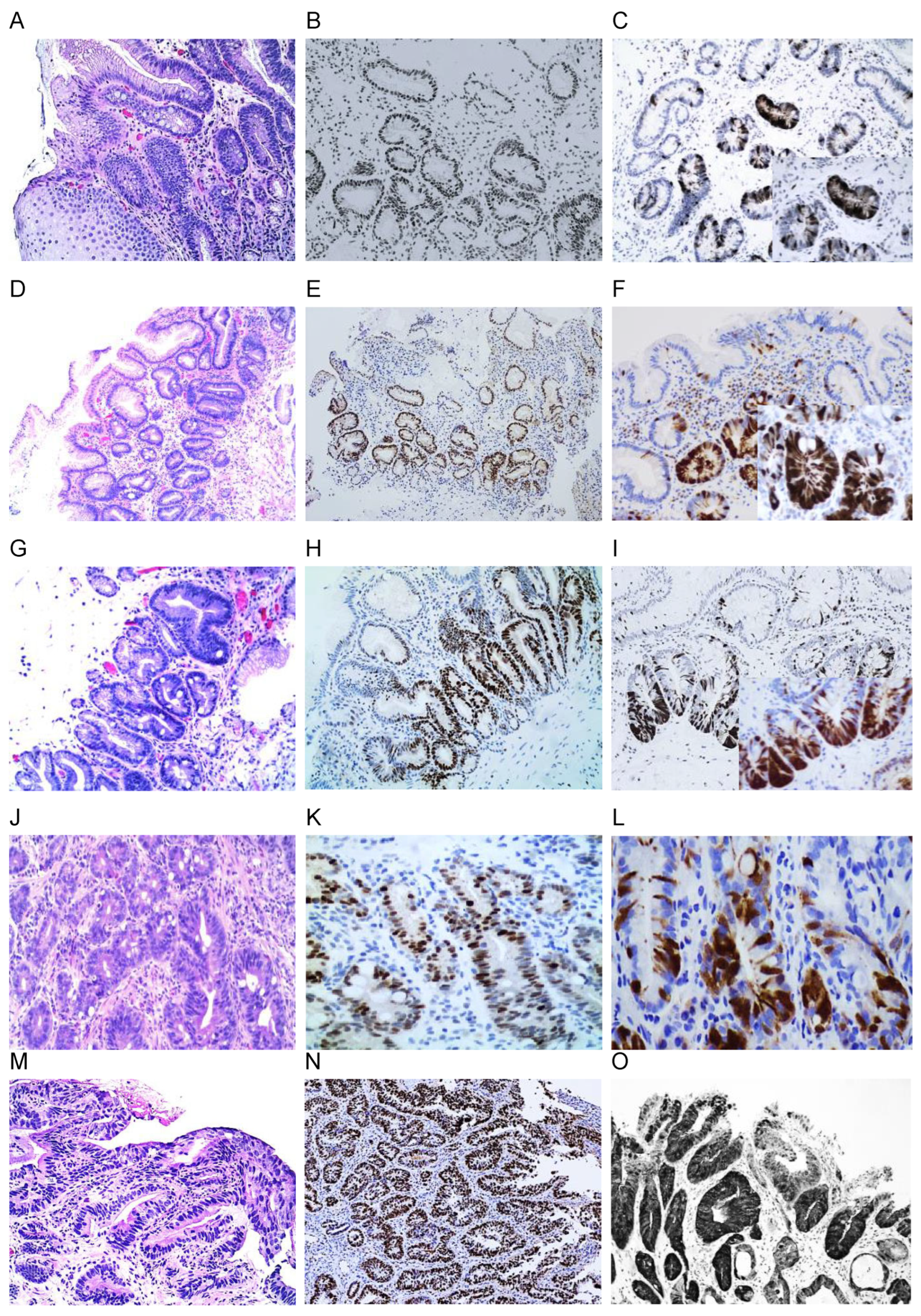
3.2. The Rate of Aberrant Expression of Biomarkers in Barret’s Esophagus and BE-Associated Dysplasia
3.2.1. p16 Expression
3.2.2. p53 Expression
3.2.3. Alternate Loss or Overexpression of p53 or p16
3.2.4. MCM2, Cyclin DI, and Ki-67 Expression
3.2.5. Beta-Catenin Expression
3.3. Fluorescent In Situ Hybridization (FISH)
Concurrent Chromosomal Alterations by FISH and Aberrant p53 and p16 Protein Expression
3.4. Significant Association of Aberrant Expression of Biomarkers and Relative Risk of Neoplastic Changes in BE–Dysplasia
3.5. Aberrant Expression of p53 and p16 in LGD and BE-IND Progressors and Non-Progressor Cohorts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wani, S.; Falk, G.W.; Post, J.; Yerian, L.; Hall, M.; Wang, A.; Gupta, N.; Gaddam, S.; Singh, M.; Singh, V.; et al. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology 2011, 141, 1179–1186.e1. [Google Scholar] [CrossRef]
- Duits, L.C.; van der Wei, M.; Cotton, C.C.; Phoa, K.N.; ten Kate, F.J.; Seldenrijk, C.A.; Offerhaus, J.A.; Visser, M.; Meijer, S.L.; Mallant-Hent, R.C.; et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 2017, 152, 993–1001. [Google Scholar] [CrossRef]
- Song, K.Y.; Henn, A.J.; Mesa, A.A.; Sultan, S.; Shaheen, N.J.; Shaukat, A.; Hanson, B.J. Persistent confirmed low-grade dysplasia in Barrett’s esophagus is a risk factor for progression to high-grade dysplasia and adenocarcinoma in a US Veterans cohort. Dis. Esophagus 2020, 33, doz061. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shaheen, N.J.; Katzka, D.; Bergman, J. AGA clinical practice update on endoscopic treatment of Barrett’s esophagus with dysplasia and/or early cancer: Expert review. Gastroenterology 2020, 158, 760–769. [Google Scholar] [CrossRef]
- Dam, A.N.; Klapman, J. A narrative review of Barrett’s esophagus in 2020: Molecular and clinical update. Ann. Trans. Med. 2020, 8, 1107. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Falk, G.W.; Lyer, P.G.; Souza, R.F.; Yadlapati, R.H.; Sauer, B.; Wani, S. Diagnosis and management of Barrett’s esophagus: An updated ACG Guideline. Am. J. Gastroenterol. 2022, 117, 559–587. [Google Scholar] [CrossRef] [PubMed]
- Mejza, M.; Malecka-Wojciesko, E. Diagnosis and management of Barrett’s esophagus. J. Clin. Med. 2023, 12, 2141. [Google Scholar] [CrossRef]
- Illig, R.; Klieser, E.; Kiesslich, T.; Neureiter, D. GERD-Barrett-Adenocarcinoma: Do we have suitable prognostic and predictive molecular markers? Gastroenterol. Res. Pract. 2013, 2013, 643084. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.M.; Mostafa, B.; Yehia, R.; El-Khayat, H. Biomarkers of Barrett’s esophagus. World J. Gastrointest. Pathophysiol. 2014, 15, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Staehler, M.D.; Camarda, N.D.; Deitrick, C.; Kim, A.; Agoston, A.T.; Odze, R.D.; Hornick, J.L.; Nag, A.; Thorner, A.R.; Ducar, M.; et al. Detection of mutations in Barrett’s esophagus adenocarcinoma. Gastroenterology 2018, 155, 156–167. [Google Scholar] [CrossRef]
- Maslyonkina, K.S.; Konyukova, A.; Alexeeva, D.Y.; Sinelnikov, M.Y.; Mikhaleva, L. Barrett’s esophagus: The pathomorphological and molecular genetic keystones of neoplastic progression. Cancer Med. 2022, 11, 447–478. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.T.; Lauwers, G.Y.; Montgomery, E.A. Utility of ancillary studies in the diagnosis and risk assessment of Barrett’s esophagus and dysplasia. Mod. Pathol. 2022, 35, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Stawinski, P.M.; Dziadkowiec, K.N.; Kuo, L.A.; Echavarria, J.; Saligrarn, S. Barrett’s Esophagus: An Updated Review. Diagnostics 2023, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Naini, B.V.; Souza, R.F.; Odze, R.D. Barrett’s Esophagus: A comprehensive and contemporary review for pathologists. Am. J. Surg. Pathol. 2016, 40, e45–e66. [Google Scholar] [CrossRef] [PubMed]
- Panarelli, N.C.; Yantiss, R.K. Do ancillary studies aid detection and classification of Barrett esophagus? Am. J. Surg. Pathol. 2016, 40, e83–e93. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.; Mansour, N.; White, D.; Sisson, A.; El-Serag, H.; Thrift, A. Systematic review with meta- analysis: Prevalence of prior and concurrent Barrett’s esophagus in esophageal adenocarcinoma patients. Aliment. Pharmacol. Ther. 2020, 52, 20–36. [Google Scholar] [CrossRef]
- Sawas, T.; Azad, N.; Killcoyne, S.; Iyer, P.; Wang, K.; Fitzgerald, R.; Katzka, F. Comparison of phenotypes and risk factors for esophageal adenocarcinoma at present vs. prior decades. Clin. Gastroenterol. Hepatol. 2020, 18, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Vajravelu, R.K.; Kolb, J.M.; Thanawala, S.U.; Scott, F.; Han, S.; Singal, A.; Falk, G.; Katzka, D.; Wani, S. Characterization of prevalent, post-endoscopy, and incident esophageal cancer in the United States: A large retrospective cohort study. Clin. Gastroenterol. Hepatol. 2022, 20, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.E.; Odze, R.D. High rate of missed barrett’s esophagus when screening with forceps biopsies. Esophagus 2023, 20, 43–149. [Google Scholar] [CrossRef]
- Dhaliwal, L.; Codipilly, D.C.; Gandhi, P.; Johnson, M.L.; Lansing, R.; Wang, K.; Leggett, C.; Katzka, D.; Lyer, P.G. Neoplasia detection rate in Barrett’s esophagus and its impact on missed dysplasia: Results from a large population-based database. Clin. Gastroenterol. Hepatol. 2020, 19, 922–929. [Google Scholar] [CrossRef]
- Bhat, S.K.; McManus, D.T.; Coleman, H.G.; Johnston, B.; Cardwell, C.; McMenamin, U.; Bannon, F.; Hicks, B.; Murray, L.J. Esophageal adenocarcinoma and prior diagnosis of Barrett’s esophagus: A population-based study. Gut 2015, 64, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Arul, G.S.; Moorghen, M.; Myerscough, N.; Alderson, D.; Spicer, R.; Corfield, A. Mucin gene expression in Barrett’s esophagus: An in situ hybridization and immunohistochemical study. Gut 2000, 47, 753–761. [Google Scholar] [CrossRef]
- McIntire, M.G.; Soucy, G.; Vaughan, T.; Shahsafaei, A.; Odze, R. MUC2 is a highly specific marker of goblet cell metaplasia in, the distal esophagus and gastroesophageal junction. Am. J. Surg. Pathol. 2011, 35, 1007–1013. [Google Scholar] [CrossRef]
- Zhou, Z.; Kalatskaya, L.; Russell, D.; Marcon, N.; Cirocco, M.; Krzyzanowski, P.; Streutker, C.; Liang, H.; Litle, V.; Godfrey, T.; et al. Combined EsophaCap cytology and MUC2 immunohistochemistry for screening of intestinal metaplasia, dysplasia and carcinoma. Clin. Exp. Gastroenterol. 2019, 12, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Lao-Sirieix, P.; Boussioutas, A.; Kadri, S.R.; O’Donovan, M.; Debiram, L.; Das, M.; Harihar, L.; Fitzgerald, R. Non-endoscopic screening biomarkers for Barrett’s esophagus: From microarray analysis to the clinic. Gut 2009, 58, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Ross-Innes, C.S.; Debiram-Beecham, I.; O’Donovan, M.; Walker, E.; Varghese, S.; Lao-Sirieix, P.; Griffin, M.; Ragunath, K.; Haidry, R.; Lovat, L.; et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: A multi-center case–control study. PLoS Med. 2015, 12, e1001780. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.C.; di Pietro, M.; O’Donovan, M.; Maroni, R.; Muldrew, B.; Debiram-Beecham, I.; Gehrung, M.; Offinan, J.; Tripathi, M.; Smith, S.; et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: A multicenter, pragmatic, randomized controlled trial. Lancet 2020, 396, 333–344. [Google Scholar] [CrossRef]
- Paterson, A.L.; Gehrung, M.; Fitzgerald, R.C.; O’Donovan, M. Role ofTFF3 as an adjunct in the diagnosis of Barrett’s esophagus using a minimally invasive esophageal sampling device—The Cytosponge. Diagn. Cytopathol. 2020, 48, 253–264. [Google Scholar] [CrossRef]
- Wani, S.; Rubenstein, J.H.; Vieth, M.; Bergman, J. Diagnosis and management of low-grade dysplasia in Barrett’s esophagus: Expert review from the clinical practice updates committee of the American Gastroenterological Association. Gastroenterology 2016, 151, 822–835. [Google Scholar] [CrossRef]
- Skacel, M.; Petras, R.E.; Rybicki, L.A.; Gramlich, T.; Richter, J.; Falk, G.; Goldblum, J. p53 expression in low grade dysplasia in Barrett’s esophagus: Correlation with interobserver agreement and disease progression. Am. J. Gastroenterol. 2002, 97, 2508–2513. [Google Scholar] [CrossRef]
- Vennalaganti, P.; Kanakadandi, V.; Goldblum, J.R.; Mathur, S.; Patil, D.; Offerhaus, G.; Meijer, S.; Vieth, M.; Odze, R.; Shreyas, S.; et al. Discordance among pathologists in the United States and Europe in diagnosis of low-grade dysplasia for patients with Barrett’s esophagus. Gastroenterology 2017, 152, 564–570.e4. [Google Scholar] [CrossRef]
- Falk, G.W. Current management of low-grade dysplasia in Barrett esophagus. Gastroenterol. Hepatol. 2017, 13, 221–225. [Google Scholar]
- Duits, L.C.; Phoa, K.N.; Curvers, W.L.; ten Kate, F.; Meijer, G.; Seldenrijk, C.; Offerhaus, J.; Bergman, J.J. Barrett’s esophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015, 64, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Curvers, W.; ten Kate, F.J.; Krishnadath, K.K.; Visser, M.; Elzer, B.; Baak, L.; Bohmer, C.; Mallant-Hent, R.; Bergman, J.J. Low-grade dysplasia in Barrett’s esophagus: Over diagnosed and underestimated. Am. J. Gastroenterol. 2010, 105, 1523–1530. [Google Scholar] [CrossRef]
- Sangle, N.A.; Taylor, S.L.; Emond, M.J.; Overholt, B.F.; Bronner, M.P. and on behalf of international photodynamic group for high grade dysplasia in Barrett’s esophagus. Overdiagnosis of high-grade dysplasia in Barrett’s esophagus: A multicenter, international study. Mod. Pathol. 2015, 28, 758–765. [Google Scholar] [CrossRef] [PubMed]
- van der Wel, M.J.; Coleman, H.G.; Bergman, J.; Jansen, M.; Meijer, S.L. BOLERO working group. Histopathologist features predictive of diagnostic concordance at expert level among a large international sample of pathologists diagnosing Barrett’s dysplasia using digital pathology. Gut 2020, 69, 811–822. [Google Scholar] [CrossRef]
- Bennett, C.; Moayyedi, P.; Corley, D.A.; DeCaestecker, J.; Falck-Ytter, Y.; Falk, G.; Vakil, N.; Sanders, S.; Vieth, M.; Inadomi, J.; et al. BOB CAT: A Large-scale review and Delphi consensus for management of Barrett’s esophagus with no dysplasia, indefinite for or low-grade dysplasia. Am. J. Gastroenterol. 2015, 110, 662–682, quiz 683. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Falk, G.; Lyer, P.G.; Gerson, L.B. ACG clinical guideline: Diagnosis and management of Barrett’s esophagus. Am. J. Gastroenterol. 2016, 111, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Weusten, B.; Bisschops, R.; Coron, E.; Dinis-Ribeiro, M.; Dumonceau, J.; Esteban, J.; Hassan, C.; Pech, O.; Repici, A. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017, 49, 191–198. [Google Scholar] [CrossRef]
- Kaye, P.V.; Haider, S.A.; Ilyas, M.; James, P.; Soomro, I.; Faisal, W.; Catton, J.; Parsons, S.; Ragunath, K. Barrett’s dysplasia and the Vienna classification: Reproducibility, prediction of progression and impact of consensus reporting and p53 immunohistochemistry. Histopathology 2009, 54, 699–712. [Google Scholar] [CrossRef]
- Kaye, P.V.; Jlyas, M.; Soomro, I.; Haider, S.; Atwal, G.; Menon, S.; Gill, S.; Richards, C.; Harrison, R.; West, K.; et al. Dysplasia in Barrett’s oesophagus: p53 immunostaining is more reproducible than hematoxylin and eosin diagnosis and improves overall reliability, while grading is poorly reproducible. Histopathology 2016, 69, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Kaz, A.M.; Grady, W.M.; Staehler, M.D.; Bass, A. Genetic and epigenetic alterations in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterol. Clin. N. Am. 2015, 44, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Gokulan, R.C.; Garcia-Buitrago, M.T.; Zaika, A.I. From genetics to signaling pathways: Molecular pathogenesis of esophageal adenocarcinoma. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Grady, W.; Yu, M.; Markowitz, S.; Chak, A. Barratts’s esophagus and esophageal adenocarcinoma biomarkers. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2486–2494. [Google Scholar] [CrossRef]
- Dulak, A.M.; Stojanov, P.; Peng, S.; Lawrence, M.; Fox, C.; Stewart, C.; Bandla, S.; Imamura, Y.; Steven, E.; Schumacher, S.; et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat. Genet. 2013, 45, 478–486. [Google Scholar] [CrossRef]
- Ross-Innes, C.S.; Becq, J.; Warren, A.; Cheetham, R.K.; Northen, H.; O’Donovan, M.; Malhotra, S.; di Pietro, M.; Ivakhno, S.; He, M.; et al. Whole-genome sequencing provides new insights into the clonal architecture of Barrett’s esophagus and esophageal adenocarcinoma. Nat. Genet. 2015, 47, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, F.; Biermann, K.; Steyerberg, E.W.; Verheij, J.; Kalisvaart, M.; Looijenga, L.H.J.; Stoop, H.A.; Walter, L.; Kuipers, E.J.; Spaander, M.C.W.; et al. Aberrant p53 protein expression is associated with an increased risk of neoplastic progression in patients with Barrett’s oesophagus. Gut 2013, 62, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Janmaat, V.T.; van Olphen, S.H.; Biermann, K.E.; Leendert, H.; Looijenga, J.; Bruno, M.; Spaander, M. Use of immunohistochemical biomarkers as independent predictor of neoplastic progression in Barrett’s esophagus surveillance: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186305. [Google Scholar] [CrossRef]
- Redston, M.; Noffsinger, A.; Kim, A.; Akarca, F.; Rara, M.; Stapleton, D.; Nowden, L.; Lash, R.; Bass, A.; Staehler, M. Abnormal TP53 predicts risk of progression in patients with Barrett’s esophagus regardless of a diagnosis of dysplasia. Gastroenterology 2022, 162, 468–481. [Google Scholar] [CrossRef]
- Snyder, P.; Dunbar, K.; Cipher, D.J.; Souza, R.F.; Spechler, S.; Vani, J.A.; Konda, V. Aberrant p53 immunostaining in Barrett’s esophagus predicts neoplastic progression: Systematic review and meta-analyses. Dig. Dis. Sci. 2019, 64, 1089–1097. [Google Scholar] [CrossRef]
- Ishida, H.; Kasajima, A.; Fujishima, F.; Akaishil, R.; Ueki, S.; Yamazaki, Y.; Onodera, Y.; Gao, X.; Okamoto, H.; Taniyama, Y.; et al. p16 in highly malignant esophageal carcinomas: The correlation with clinicopathological factors and human papillomavirus infection. Virchows Arch. 2012, 478, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Bani-Hani, K.; Martin, L.G.; Hardie, L.; Forman, H.; Wild, C.P. Prospective study of cyclin DI overexpression in Barrett’s esophagus: Association with increased risk of adenocarcinoma. J. Natl. Cancer Inst. 2000, 92, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Going, J.J.; Keith, W.N.; Neilson, L.; Stoeber, K.; Stuart, R.; Williams, G. Aberrant expression of minichromosome maintenance proteins 2 and 5, and Ki-67 in dysplastic squamous oesophageal epithelium and Barrett’s mucosa. Gut 2002, 50, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Alastal, H.N.; Rasheed, R. Molecular biomarkers of progression from Barrett’s esophagus to esophageal adenocarcinoma. Front. Gastroenterol. 2023, 2, 1007456. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Zhang, J.; Cheng, A.; Yu, J.; To, K.; Kang, W. MCM family in gastrointestinal and other malignancies: From functional characterization to clinical implication. BEA-Rev. Cancer 2020, 1874, 188415. [Google Scholar] [CrossRef] [PubMed]
- Sirieix, P.O.; O’ Donovan, M.; Brown, J.; Save, V.; Coleman, N.; Fitzgerald, R. Surface of minichromosome maintenance proteins provides a novel method for detecting patients at risk for developing adenocarcinoma in Barrett’s esophagus. Clin. Cancer Res. 2003, 9, 2560–2566. [Google Scholar] [PubMed]
- Torres-Rendon, A.; Roy, S.; Craig, G.T.; Speight, P.M. Expression ofMcm2, geminin and Ki67 in normal oral mucosa, oral epithelial dysplasia and their corresponding squamous-cell carcinomas. Br. Cancer 2009, 100, 1128–1134. [Google Scholar] [CrossRef]
- Ahire, M.S.; Nagar, S.R.; D’souza, Z.; Tupkari, J.; Dalvi, S. Expression of minichromosome maintenance protein 2 (MCM2) in oral epithelial dysplasia and oral squamous cell carcinoma: A systematic review. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, M.; Kerkhof, M.; Steyerberg, E.W.; Kusters, J.; van Strien, P.; Looman, C.; van Dekken, H.; Siersema, P.; Kuipers, E. Aneuploidy and overexpression of Ki67 and p53 as markers for neoplastic progression in Barrett’s esophagus: A case–control study. Gastroenterology 2009, 104, 2673–2680. [Google Scholar] [CrossRef]
- Altaf, K.; Xiong, J.J.; Iglesia, D.K.; Hickey, L.; Kaul, A. Meta-analysis of biomarkers predicting risk of malignant progression in Barrett’s esophagus. Br. J. Sur. 2017, 104, 493–502. [Google Scholar] [CrossRef]
- Osterheld, M.C.; Bian, Y.S.; Bosman, F.T.; Benhattar, J.; Fontolliet, C. Beta-catenin expression and its association with prognostic factors in adenocarcinoma developed in Barrett esophagus. Am. J. Clin. Pathol. 2002, 117, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.S.; Osterheld, M.C.; Bosman, F.T.; Fontolliet, C.; Benhattar, J. Nuclear accumulation of beta- catenin is a common and early event during neoplastic progression of Barrett esophagus. Am. J. Clin. Pathol. 2000, 114, 583–590. [Google Scholar] [CrossRef]
- Moyes, L.H.; Oien, K.A.; Foulis, A.K.; Fullarton, G.; Going, J. Prevalent low-grade dysplasia: The strongest predictor of malignant progression in Barrett’s columnar-lined oesophagus. Gut 2016, 65, 360–361. [Google Scholar] [CrossRef]
- Brankley, S.M.; Fritcher, E.G.B.; Smyrk, T.C.; Keeney, M.E.; Campion, M.B.; Voss, J.S.; Clayton, A.C.; Wang, K.K.; Lutzke, L.S.; Kipp, B.R.; et al. Fluorescence in situ hybridization mapping of esophagectomy specimens from patients with Barrett’s esophagus with high-grade dysplasia or adenocarcinoma. Hum. Pathol. 2012, 43, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Fritcher, E.G.; Brankley, S.M.; Kipp, B.R.; Voss, J.; Campion, M.; Morrison, L.; Halling, K.C. A comparison of conventional cytology, DNA ploidy analysis, and fluorescence in situ hybridization for the detection of dysplasia and adenocarcinoma in patients with Barrett’s esophagus. Hum. Pathol. 2008, 39, 1128–1135. [Google Scholar] [CrossRef]
- Timmer, M.R.; Brankley, S.M.; Gorospe, E.C.; Sun, G.; Lutzke, L.S.; Iyer, P.G.; Halling, K.C.; Krishnadath, K.K.; Wang, K.K. Prediction of response to endoscopic therapy of Barrett’s dysplasia by using genetic biomarkers. Gastrointest. Endosc. 2014, 80, 984–991. [Google Scholar] [CrossRef]
- Brankley, S.M.; Haling, J.C.; Jenkins, S.M.; Timmer, M.; Lyer, G.; Smyrk, T.; Barr Fritcher, E.; Voss, J.; Kipp, B.; Campion, M.; et al. Fluorescence in situ hybridization identifies high risk Barrett’s patients likely to develop esophageal adenocarcinoma. Dis. Esophagus 2016, 29, 513–519. [Google Scholar] [CrossRef]
- Poneros, J.M.; Faye, A.S.; Fritcher, E.G.B.; Sen, A.; Anandasabapathy, S.; Bresalier, R.S.; Marcon, N.; Turgeon, D.K.; Appelman, H.; Normolle, D.; et al. A multicenter study of fluorescence in situ hybridization probe set for diagnosing high-grade dysplasia and adenocarcinoma in Barrett’s esophagus. Dig. Dis. Sci. 2017, 62, 1216–1222. [Google Scholar] [CrossRef]
- Brankley, S.M.; Wang, K.K.; Harwood, A.R.; Miller, D.; Legator, M.; Lutzke, L.; Kipp, B.; Morrison, L.; Halling, K. The development of a fluorescence in situ for the detection of dysplasia and adenocarcinoma in Barrett’s esophagus. J. Mol. Diagn. 2006, 8, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Bhagwandeen, B.; Leong, A. p16, Cyclin DI, Ki-67, and AMACR as Markers for Dysplasia in Barrett Esophagus. Appl. Immunohistochem. Mol. Morphol. 2008, 16, 447–452. [Google Scholar] [CrossRef]
- Bian, Y.S.; Osterheld, M.C.; Fontolliet, C.; Bosman, F.T.; Benhattar, J. p16 inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in esophagus. Gastroenterology 2002, 122, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Paulson, T.; Galipeau, P.; Xu, L.; Kissel, H.; Li, X.; Blount, P.; Sanchez, C.; Odze, R.; Reid, B. pl6 Mutation Spectrum in the Premalignant Condition Barrett’s Esophagus. PLoS ONE 2008, 3, e3809. [Google Scholar] [CrossRef] [PubMed]
- Chueca, E.; Valeroc, A.; Hordnlerc, C.; Puertasc, A.; Carreraa, P.; Garcia-Gonziileza, M.; Strunkd, M.; Lanas, A.; Piazuelo, E. Quantitative analysis of pI 6 methylation in Barrett’s carcinogenesis. Ann. Diagn. Pathol. 2020, 47, 151554. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Brown, K.; Lauwers, G.Y.; Ergun, G.; Meriano, F.; Schmulen, C.; Barroso, A.; Ertan, A. p53 protein accumulation predicts malignant progression in Barrett’s metaplasia: A prospective study of 275 patients. Histopathology 2017, 71, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Davelaar, A.L.; Calpe, S.; Lau, L.; Timmer, M.; Visser, M.; ten Kate, F.; Parikh, K.; Meijer, S.; Bergman, J.; Fockens, P.; et al. Aberrant TP53 detected by combining immunohistochemistry and DNA- FISH improves Barrett’s esophagus progression prediction: A prospective follow-up study. Genes Chromosome Cancer 2014, 54, 82–90. [Google Scholar] [CrossRef]
- Horvath, B.; Singh, P.; Xie, H.; Thota, P.; Sun, X.; Liu, X. Expression of p53 predicts risk of prevalent and incident advanced neoplasia in patients with Barrett’s esophagus and epithelial changes indefinite for dysplasia. Gastroenterol. Rep. 2015, 4, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.P.; Banerjee, S.K.; Sharma, P.; Tran, T.; Richards, R.; Cherian, R. p53 protein overexpression in low grade dysplasia (LGD) in Barrett’s esophagus: Immunohistochemical marker predictive of progression. Am. Gastroenterol. 2001, 96, 1355–1362. [Google Scholar] [CrossRef]
- Ten Kate, F.J.; Nieboer, D.; ten Kate, F.J.; Doutkas, M.; Bruno, M.; Spaander, M.; Looijenga, L.; Biermann, K. Improved progression prediction in Barrett’s esophagus with low-grade dysplasia using specific histologic criteria. Amer. J. Surg. Pathol. 2018, 42, 918–926. [Google Scholar] [CrossRef]
- Duits, L.C.; Lao-Sirieix, P.; Wolf, W.A.; O’donovan, M.; Galeano-Dalmau, N.; Meijer, S.L.; Offerhaus, G.J.A.; Redman, J.; Crawte, J.; Zeki, S.; et al. A biomarker panel predicts progression of Barrett’s esophagus to esophageal adenocarcinoma. Dis. Esophagus 2018, 32, doy102. [Google Scholar] [CrossRef]
- Januszewicz, W.; Pilonis, N.; Sawas, T.; Phillips, R.; O’Donovan, M.; Miremadi, A.; Malhotra, S.; Tripathi, M. The utility of P53 immunohistochemistry in the diagnosis of Barrett’s oesophagus with indefinite for dysplasia. Histopathology 2022, 80, 1081–1090. [Google Scholar] [CrossRef]
- Helminen, O.; Melkko, J.; Saamio, J.; Sihvo, E.; Kuopio, T.; Ohtonen, P.; Kauppila, J.; Karttunen, T.; Huhta, H. Predictive value of p53, Ki67 and TLR5 in neoplastic progression of Barrett’s esophagus: Matched case–control study. Virchows Arch. 2022, 481, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Polkowski, W.; van Lanschot, J.J.; Ten Kate, F.J.; Baak, J.; Tytgat, G.; Obertop, H.; Voomij, W.; Offerhaus, D. The value of p53 and Ki67 as markers for tumor progression in the 171. Barrett’s dysplasia-carcinoma sequence. Surg. Oncol. 1995, 4, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Feith, M.; Stein, H.J.; Mueller, J.; Siewert, J.R. Malignant degeneration of Barrett’s esophagus: The role of the Ki-67 proliferation fraction, expression ofE-cadherin and p53. Dis. Esophagus 2004, 17, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Olvera, M.; Wickramasinghe, K.; Brynes, R.; Bu, X.; Chandrasoma, M.; Chandrasom, P. Ki67 expression in different epithelial types in columnar lined esophagus indicates varying levels of expanded and aberrant proliferative patterns. Histopathology 2005, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Lorinc, E.; Jakobsson, B.; Landberg, G.; Veress, B. Ki67 and p53 immunohistochemistry reduces interobserver variation in assessment of Barrett’s esophagus. Histopathology 2005, 46, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, M.; Steyerberg, E.W.; Kusters, J.G.; van Dekken, H.; van Vuuren, A.; Kuipers, E.; Siersema, P. Aneuploidy and high expression ofp53 and Ki67 is associated with neoplastic progression in Barrett esophagus. Cancer Biomark. 2008, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, H.; Hayat, U.; Manivel, J.; Iwamoto, C.; Peltola, J.; Hanson, B.; Larson, W.; Dachel, S.; Gravely, A.; Mesa, H. Surface Ki-67 expression improves reproducibility of dysplasia diagnosis in Barrett’s esophagus. Am. J. Clin. Pathol. 2020, 153, 695–704. [Google Scholar] [CrossRef] [PubMed]
- van Dekken, H.V.; Hop, W.C.; Tilanus, H.W.; Haringsma, J.; van der Valk, H.; Wink, J.; Vissers, K. Immunohistochemical evaluation of a panel of tumor cell markers during malignant progression in Barrett esophagus. Am. J. Clin. Pathol. 2008, 130, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Moyes, L.; McEwan, H.; Radulescu, S.; Pawlikowski, J.; Lamm, C.; Nixon, C.; Sansom, O.; Going, J.; Fullarton, G.; Adams, P. Activation of Wnt signalling promotes development of dysplasia in Barrett’s oesophagus. J. Pathol. 2012, 228, 99–112. [Google Scholar] [CrossRef]
- Waters, K.M.; Salimian, K.J.; Voltaggio, L.; Montgomery, E.A. Refined criteria for separating low-grade dysplasia and nondysplastic Barrett esophagus reduce equivocal diagnoses and improve prediction of patient outcome: A 10-Year Review. Am. J. Surg. Pathol. 2018, 42, 1723–1729. [Google Scholar] [CrossRef]
- Lyer, P.G.; Codipilly, C.D.; Chandar, A.K.; Agarwal, S.; Wang, K.; Leggett, C.; Latuche, L.; Schulte, P. Prediction of progression in Barrett’s esophagus using a tissue systems pathology test: A pooled analysis of international multicenter studies. Clin. Gastroenterol. Hepatol. 2022, 20, 2772–2779. [Google Scholar] [CrossRef]
- Frei, N.F.; Khoshiwal, A.M.; Konte, K.; Bossart, E.A.; Stebbins, K.; Zhang, Y.; Pouw, R.; ten Kate, F.; Seldenrijk, K.; Bergman, J.J. Tissue systems pathology test objectively risk stratifies Barrett’s esophagus patients with low-grad. dysplasia. Am. Gastroenterol. 2021, 116, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Critchley-Thome, R.J.; Duits, L.C.; Prichard, J.W.; Davison, J.; Jobe, B.; Campbell, B.; Zhang, Y.; Falk, G.W. A tissue systems pathology assay for high-risk Barrett’s esophagus. Cancer Epidemiol. Biomark. Prev. 2016, 25, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.C.; di Pietro, M.; Ragunath, K.; Ang, Y.; Kang, J.Y.; Watson, P.; Trudgill, N.; Kaye, P.V.; Sanders, S.; De Caestecker, J. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s esophagus. Gut 2014, 63, 7–42. [Google Scholar] [CrossRef] [PubMed]



| Histopathology | Types and Degree of p16 Reaction | Types and Degree of Beta-Catenin Reaction | ||||||
|---|---|---|---|---|---|---|---|---|
| C and N * | C or N | Loss | Negative | C and N * | Disrupted/Reduced | Loss | Negative | |
| % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | |
| BE | 10.7 (9/84) | 23.8 (20/84) | 0 (0/84) | 65.5 (55/84) | 0 (0/45) | 55.6 (25/45) | 0 (0/45) | 44.5 (20/45) |
| BE-IND | 23.1 (15/65) | 15.4 (10/65) | 1.5 (1/65) | 60.0 (39/65) | 4.5 (1/22) | 68.2 (15/22) | 4.5 (1/22) | 9.1 (2/22) |
| LGD | 64.4 (38/59) | 23.7 (14/59) | 11.9 (7/59) | 8.5 (5/59) | 42.2 (14/33) | 45.5 (15/33) | 9.1 (3/33) | 3.0 (1/33) |
| HGD | 71.4 (15/21) | 9.5 (2/21) | 14.3 (3/21) | 4.8 (1/21) | 50 (10) | 25.0 (5/20) | 25 (5/20) | 0 (0/20) |
| EAC | 83.3 (10/12) | 8.3 (1/12) | 8.3 (1/12) | 0 (0/12) | 66.6 (2/3) | 0 (0/3) | 33.3 (1/3) | 0 (0/3) |
| Histopathology | Degree of p53 Immunoreaction | Degree of MCM2 Immunoreaction | Degree of Cyclin D1 Immunoreaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4+ * | 3+ ** | Loss | Negative | 4+ * | 3+ ** | Loss | Negative | 4+ * | 3+ ** | 1–2+ *** | Loss | Negative | |
| % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | % (Case) | |
| BE | 8.7 (5/90) | 36.7 (33/90) | 0 (0/90) | 57.8 (52/90) | 7.4 (4/54) | 48.6 (24/54) | 0 (0/54) | 48.1 (26/54) | 0 (0/49) | 26.5 (13/49) | 40.8 (20/49) | 0 (0/49) | 32.7 (16/49) |
| BE-IND | 17.2 (14/79) | 44.3 (35/79) | 2.5 (2/79) | 41.7 (33/79) | 15.9 (7/44) | 52.3 (23/44) | 0 (0/44) | 31.8 (14/44) | 0 (0/39) | 56.4 (22/39) | 17.9 (7/39) | 0 (0/39) | 25.6 (10/39) |
| LGD | 63.5 (33/52) | 13.5 (7/52) | 23.1 (12/52) | 1.9 (1/52) | 80.0 (44/51) | 9.8 (5/51) | 3.9 (2/51) | 0 (0/51) | 69.8 (30/43) | 18.6 (8/43) | 4.7 (2/43) | 6.9 (3/43) | 0 (0/43) |
| HGD | 75 (9/12) | 8.3 (1/12) | 25 (3/12) | 16.7 (2/12) | 100 (11/11) | 0 (0/11) | 0 (0/11) | 0 (0/11) | 81.8 (9/11) | 18.2 (2/11) | 0 (0/11) | 0 (0/11) | 0 (0/11) |
| EAC | 85.7 (6/7) | 14.3 (1/7) | 14.3 (1/7) | 14.3 (1/7) | 90.1 (10/11)) | 0 (0/11) | 9.0 (1/11) | 0 (0/11) | 77.8 (7/9) | 0 (0/9) | 0 (0/9) | 22.2 (2/9) | 0 (0/9) |
| Biomarkers | Histology Category | Degree of Expression | RR (95% CI) | Sensitivity | Specificity |
|---|---|---|---|---|---|
| p53 | BE-IND | 4+ * | 2.0 (1.2–2.8) + | 33 | 91 |
| 3+ * | 1.3 (0.93–1.9) | 51 | 61 | ||
| LGD | 4 * | 47.7 (6.8–333.1) + | 98 | 91 | |
| 4+ ** | 25.1 (3.6–173.9) + | 98 | 67 | ||
| 3+ * | 9.3 (1.2–72.4) + | 88 | 61 | ||
| LGD/HGD/EAC | 4+ * | 12.98 (5.0–33.5) + | 94 | 91 | |
| 4+ ** | 7.4 (2.9–18.7) + | 94 | 67 | ||
| 3+ * | 3.0 (1.0–9.1) + | 69 | 61 | ||
| MCM2 | BE-IND | 4+ * | 1.8 (0.9–3.4) | 33 | 87 |
| 3+ * | 1.4 (0.8–2.3) | 62 | 52 | ||
| LGD | 4+ * | 24.8 (3.6–170.3) + | 83 | 87 | |
| 4+ ** | 13.1 (1.9–86.7) + | 98 | 67 | ||
| 3+ * | 4.6 (0.6–37.3) | 95 | 59 | ||
| LGD/HGD/EAC | 4+ * | 25.5 (3.7–174.6) + | 99 | 87 | |
| 4+ ** | 13.6 (2.0–90.5) + | 99 | 67 | ||
| 3+ * | 4.6 (0.6–37.3) | 83 | 52 | ||
| p16 | BE-IND | C&N and loss * | 1.62 (1.1–2.4) + | 29 | 87 |
| C or N | 1.01 (0.6–1.7) | 20 | 80 | ||
| LGD | C&N and loss * | 10.8 (4.6–25.4) + | 90 | 87 | |
| C&N and loss ** | 6.5 (2.8–15.0) + | 90 | 71 | ||
| C or N | 6.3 (2.5–15.8) + | 74 | 80 | ||
| LGD/HGD/EAC | C&N and loss * | 9.8 (4.6–21.1) + | 93 | 87 | |
| C&N and loss ** | 6.2 (2.9–13.1) + | 93 | 71 | ||
| C or N | 5.84 (2.5–13.4) + | 74 | 80 | ||
| Beta-catenin | BE-IND | C&N and loss * | 3.3 (1.1–10.2) + | 29 | 95 |
| disrupted/reduced | 1.87 (0.8–4.5) | 75 | 44 | ||
| LGD | C&N and loss * | 19.8 (2.9–134.7) + | 94 | 95 | |
| C&N and loss ** | 5.4 (0.9–32.2) | 94 | 71 | ||
| disrupted/reduced | 7.87 (1.1–55.6) + | 94 | 44 | ||
| LGD/HGD/EAC | C&N and loss * | 20.4 (3.0–130.4) + | 97 | 95 | |
| C&N and loss ** | 5.7 (0.9–34.0) | 97 | 71 | ||
| disrupted/reduced | 9.3 (1.3–64.9) + | 95 | 44 | ||
| Cyclin D1 | BE-IND | 4+ * | 1.3 (0.3–5.6) | 9 | 94 |
| 3+ * | 1.63 (0.9–2.8) | 69 | 55 | ||
| LGD | 4+ * | 16.5 (2.4–110.6) + | 97 | 94 | |
| 4+ ** | 16.7 (1.6–69.2) + | 97 | 91 | ||
| 3+ * | 6.5 (0.9–46.8) | 89 | 55 | ||
| LGD/HGD/EAC | 4+ * | 16.6 (2.5–111.7) + | 98 | 94 | |
| 4+ ** | 10.8 (1.7–69.9) + | 98 | 91 | ||
| 3+ * | 7.4 (1.0–52.4) + | 91 | 55 |
| Low-Grade Dysplasia to High-Grade Dysplasia | BE-IND * to Low-Grade Dysplasia | |||||||
|---|---|---|---|---|---|---|---|---|
| Biomarkers | Progressors (%) | Non-Progressors (%) | RR (95% CI) ** | Progressors (%) | Non-Progressors (%) | RR (95% CI) ** | ||
| p53 | ||||||||
| 4+ and loss | 12 (80.0) | 15 (39.5) | 3.1 (0.2–41.9) | 14 (26.9) | 2 (2.6) | 4.4 (2.7–7.1) + | ||
| 3+ | 3 (20.0) | 20 (52.6) | 1.01 (0.6–1.6) | 23 (44.2) | 15 (19.5) | 3.0 (1.8–5.1) + | ||
| Absent (ref) | 0 | 3 (7.9) | 15 (28.9) | 60 (77.9) | ||||
| 15 | 38 | 52 | 77 | |||||
| p16 | ||||||||
| C/N and loss | 12 (80) | 26 (72.2) | 3.5 (0.2–50.9) | 17 (33.3) | 8 (9.0) | 4.4 (2.5–7.6) + | ||
| C or N | 3 (20) | 5 (13.9) | 4.1 (0.3–67.2) | 20 (39.2) | 10 (11.4) | 4.3 (2.5–7.4) + | ||
| Absent (ref) | 0 | 5 (13.9) | 14 (27.5) | 76 (79.6) | ||||
| 15 | 36 | 51 | 88 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Bedford, A.; Pollack, S. The Aberrant Expression of Biomarkers and Risk Prediction for Neoplastic Changes in Barrett’s Esophagus–Dysplasia. Cancers 2024, 16, 2386. https://doi.org/10.3390/cancers16132386
Choi Y, Bedford A, Pollack S. The Aberrant Expression of Biomarkers and Risk Prediction for Neoplastic Changes in Barrett’s Esophagus–Dysplasia. Cancers. 2024; 16(13):2386. https://doi.org/10.3390/cancers16132386
Chicago/Turabian StyleChoi, Young, Andrew Bedford, and Simcha Pollack. 2024. "The Aberrant Expression of Biomarkers and Risk Prediction for Neoplastic Changes in Barrett’s Esophagus–Dysplasia" Cancers 16, no. 13: 2386. https://doi.org/10.3390/cancers16132386






