Genotype–Phenotype Correlation in Neurofibromatosis Type 1: Evidence for a Mild Phenotype Associated with Splicing Variants Leading to In-Frame Skipping of NF1 Exon 24 [19a]
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
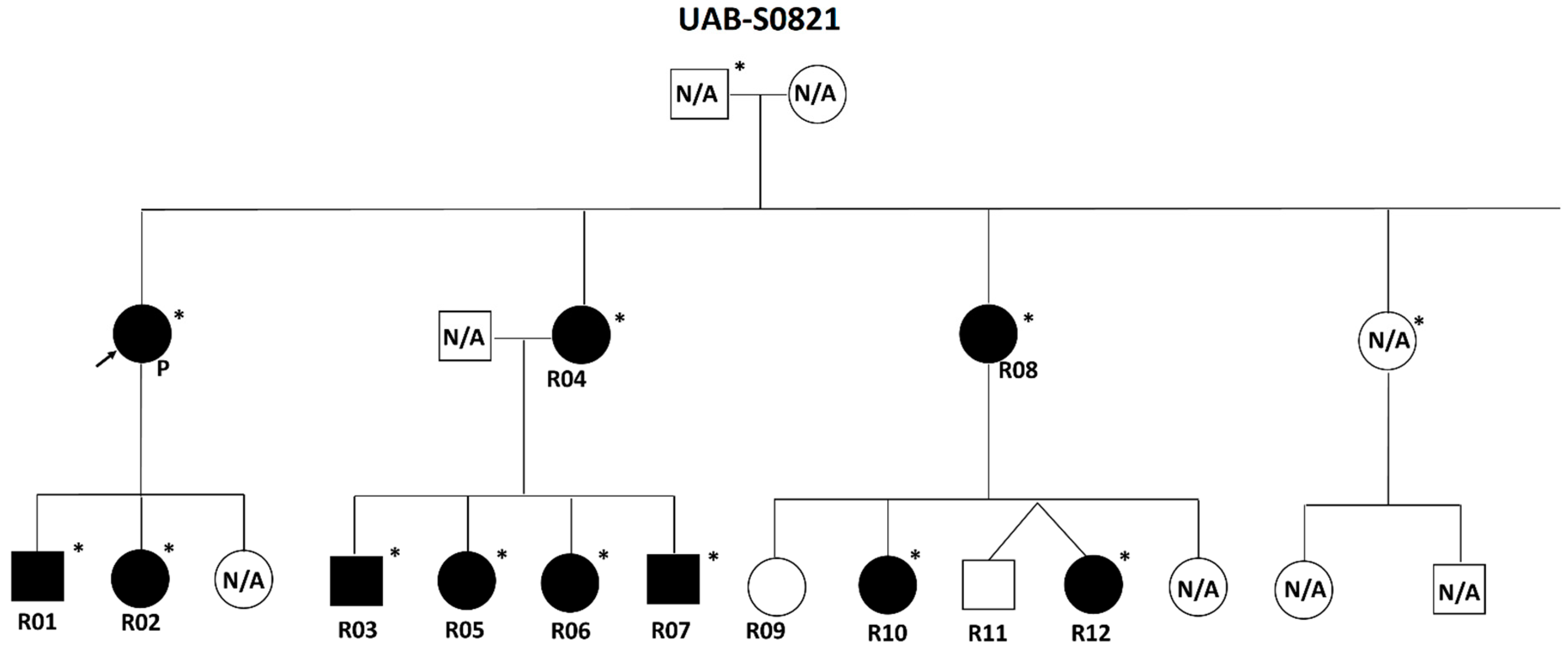
2.1. Individuals and Phenotypic Data
2.2. Comprehensive NF1 Molecular Analysis
2.3. Splicing Assessment for Twelve NF1 Variants at Seven Positions (c.3114-2, c.3114-1, c.3196, c.3197, c.3197+1, c.3197+2, c.3197+3), Which Might Be Associated with Exon 24 [19a] Skipping but Not Detected in the UAB Cohort
2.4. Effects of NF1 Exon 24 [19a] Skipping on 3D Structure of Neurofibromin
2.5. Assessment of Expression/Stability and Activity of Mouse Neurofibromin Lacking 28 Amino Acids Encoded by Exon 24 in Cells
2.6. Statistical Analysis
3. Results
3.1. Description of the Variants Leading to NF1 Exon 24 [19a] Skipping
3.2. Clinical Characterization of the Patient Cohort
3.3. Comparison of Clinical Features of the Studied Cohort with the Cohort of Individuals Heterozygous for NF1 p.Met992del, Cohorts of Individuals Carrying NF1 Missense Pathogenic Variants Affecting Codons 1809 and 844–848, and “Classic” NF1 Population
3.4. Assessment of NF1 Variants at c.3114-2, c.3114-1, c.3196, c.3197, c.3197+1, c.3197+2, c.3197+3, Which Potentially Lead to Exon 24 [19a] Skipping but Have Not Been Reported
3.5. 3D Structure Change of Neurofibromin Due to NF1 Exon 24 [19a] Skipping
3.6. Mutant Neurofibromin Levels and Ras Activity in HEK293 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lammert, M.; Friedman, J.M.; Kluwe, L.; Mautner, V.F. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch. Dermatol. 2005, 141, 71–74. [Google Scholar] [CrossRef]
- Evans, D.G.; Howard, E.; Giblin, C.; Clancy, T.; Spencer, H.; Huson, S.M.; Lalloo, F. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am. J. Med. Genet. A 2010, 152, 327–332. [Google Scholar] [CrossRef]
- Kallionpää, R.A.; Uusitalo, E.; Leppävirta, J.; Pöyhönen, M.; Peltonen, S.; Peltonen, J. Prevalence of neurofibromatosis type 1 in the Finnish population. Genet. Med. 2018, 20, 1082–1086. [Google Scholar] [CrossRef]
- Legius, E.; Messiaen, L.; Wolkenstein, P.; Pancza, P.; Avery, R.A.; Berman, Y.; Blakeley, J.; Babovic-Vuksanovic, D.; Cunha, K.S.; Ferner, R.; et al. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: An international consensus recommendation. Genet. Med. 2021, 23, 1506–1513. [Google Scholar] [CrossRef]
- Huson, S.M.; Compston, D.A.; Clark, P.; Harper, P.S. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J. Med. Genet. 1989, 26, 704–711. [Google Scholar] [CrossRef]
- Cnossen, M.H.; Smit, F.J.; de Goede-Bolder, A.; Frets, P.G.; Duivenvoorden, H.J.; Niermeijer, M.F. Diagnostic delay in neurofibromatosis type 1. Eur. J. Pediatr. 1997, 156, 482–487. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Colman, S.D.; Ho, V.T.; Abernathy, C.R.; Arn, P.H.; Weiss, L.; Schwartz, C.; A Saul, R.; Wallace, M.R. Constitutional and mosaic large NF1 gene deletions in neurofibromatosis type 1. J. Med. Genet. 1998, 35, 468–471. [Google Scholar] [CrossRef][Green Version]
- Kluwe, L.; Siebert, R.; Gesk, S.; Friedrich, R.E.; Tinschert, S.; Kehrer-Sawatzki, H.; Mautner, V. Screening 500 unselected neurofibromatosis 1 patients for deletions of the NF1 gene. Hum. Mutat. 2004, 23, 111–116. [Google Scholar] [CrossRef]
- Zhang, J.; Tong, H.; Fu, X.; Zhang, Y.; Liu, J.; Cheng, R.; Liang, J.; Peng, J.; Sun, Z.; Liu, H.; et al. Molecular Characterization of NF1 and Neurofibromatosis Type 1 Genotype-Phenotype Correlations in a Chinese Population. Sci. Rep. 2015, 5, 11291. [Google Scholar] [CrossRef]
- Kehrer-Sawatzki, H.; Mautner, V.F.; Cooper, D.N. Emerging genotype-phenotype relationships in patients with large NF1 deletions. Hum. Genet. 2017, 136, 349–376. [Google Scholar] [CrossRef]
- De Luca, A.; Bottillo, I.; Dasdia, M.C.; Morella, A.; Lanari, V.; Bernardini, L.; Divona, L.; Giustini, S.; Sinibaldi, L.; Novelli, A.; et al. Deletions of NF1 gene and exons detected by multiplex ligation-dependent probe amplification. J. Med. Genet. 2007, 44, 800–808. [Google Scholar] [CrossRef][Green Version]
- Mautner, V.-F.; Kluwe, L.; Friedrich, R.E.; Roehl, A.C.; Bammert, S.; Hogel, J.; Spori, H.; Cooper, D.N.; Kehrer-Sawatzki, H. Clinical characterisation of 29 neurofibromatosis type-1 patients with molecularly ascertained 1.4 Mb type-1 NF1 deletions. J. Med. Genet. 2010, 47, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Kehrer-Sawatzki, H.; Vogt, J.; Mußotter, T.; Kluwe, L.; Cooper, D.N.; Mautner, V.-F. Dissecting the clinical phenotype associated with mosaic type-2 NF1 microdeletions. Neurogenetics 2012, 13, 229–236. [Google Scholar] [CrossRef]
- Koczkowska, M.; Chen, Y.; Callens, T.; Gomes, A.; Sharp, A.; Johnson, S.; Hsiao, M.-C.; Chen, Z.; Balasubramanian, M.; Barnett, C.P.; et al. Genotype-Phenotype Correlation in NF1: Evidence for a More Severe Phenotype Associated with Missense Mutations Affecting NF1 Codons 844–848. Am. J. Hum. Genet. 2018, 102, 69–87. [Google Scholar] [CrossRef]
- Koczkowska, M.; Callens, T.; Chen, Y.; Gomes, A.; Hicks, A.D.; Sharp, A.; Johns, E.; Uhas, K.A.; Armstrong, L.; Bosanko, K.A.; et al. Clinical spectrum of individuals with pathogenic NF1 missense variants affecting p.Met1149, p.Arg1276, and p.Lys1423: Genotype-phenotype study in neurofibromatosis type 1. Hum. Mutat. 2020, 41, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, M.; Huson, S.M.; Davies, M.; Thomas, N.; Chuzhanova, N.; Giovannini, S.; Evans, D.G.; Howard, E.; Kerr, B.; Griffiths, S.; et al. An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970-2972 delAAT): Evidence of a clinically significant NF1 genotype-phenotype correlation. Am. J. Hum. Genet. 2007, 80, 140–151. [Google Scholar] [CrossRef]
- Pinna, V.; Lanari, V.; Daniele, P.; Consoli, F.; Agolini, E.; Margiotti, K.; Bottillo, I.; Torrente, I.; Bruselles, A.; Fusilli, C.; et al. p.Arg1809Cys substitution in neurofibromin is associated with a distinctive NF1 phenotype without neurofibromas. Eur. J. Hum. Genet. 2015, 23, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Rojnueangnit, K.; Xie, J.; Gomes, A.; Sharp, A.; Callens, T.; Chen, Y.; Liu, Y.; Cochran, M.; Abbott, M.-A.; Atkin, J.; et al. High Incidence of Noonan Syndrome Features Including Short Stature and Pulmonic Stenosis in Patients carrying NF1 Missense Mutations Affecting p.Arg1809: Genotype-Phenotype Correlation. Hum. Mutat. 2015, 36, 1052–1063. [Google Scholar] [CrossRef]
- Koczkowska, M.; Callens, T.; Gomes, A.; Sharp, A.; Chen, Y.; Hicks, A.D.; Aylsworth, A.S.; Azizi, A.A.; Basel, D.G.; Bellus, G.; et al. Expanding the clinical phenotype of individuals with a 3-bp in-frame deletion of the NF1 gene (c.2970_2972del): An update of genotype-phenotype correlation. Genet. Med. 2019, 21, 867–876. [Google Scholar] [CrossRef]
- Forde, C.; Burkitt-Wright, E.; Turnpenny, P.D.; Haan, E.; Ealing, J.; Mansour, S.; Holder, M.; Lahiri, N.; Dixit, A.; Procter, A.; et al. Natural history of NF1 c.2970_2972del p.(Met992del): Confirmation of a low risk of complications in a longitudinal study. Eur. J. Hum. Genet. 2022, 30, 291–297. [Google Scholar] [CrossRef]
- Ars, E.; Serra, E.; Garcia, J.; Kruyer, H.; Gaona, A.; Lázaro, C.; Estivill, X. Mutations affecting mRNA splicing are the most common molecular defects in patients with neurofibromatosis type 1. Hum. Mol. Genet. 2000, 9, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Messiaen, L.M.; Callens, T.; Mortier, G.; Beysen, D.; Vandenbroucke, I.; Van Roy, N.; Speleman, F.; Paepe, A.D. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum. Mutat. 2000, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, K.; Roca, X.; Beiglböck, H.; Callens, T.; Etzler, J.; Rao, A.R.; Krainer, A.R.; Fonatsch, C.; Messiaen, L. Extensive in silico analysis of NF1 splicing defects uncovers determinants for splicing outcome upon 5′ splice-site disruption. Hum. Mutat. 2007, 28, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Anna, A.; Monika, G. Splicing mutations in human genetic disorders: Examples, detection, and confirmation. J. Appl. Genet. 2018, 59, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Leier, A.; Moore, M.; Liu, H.; Daniel, M.; Hyde, A.M.; Messiaen, L.; Korf, B.R.; Selvakumaran, J.; Ciszewski, L.; Lambert, L.; et al. Targeted exon skipping of NF1 exon 17 as a therapeutic for neurofibromatosis type I. Mol. Ther. Nucleic Acids 2022, 28, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Koczkowska, M.; Chen, Y.; Xie, J.; Callens, T.; Gomes, A.; Wimmer, K.; Messiaen, L.M. Analysis of 200 unrelated individuals with a constitutional NF1 deep intronic pathogenic variant reveals that variants flanking the alternatively spliced NF1 exon 31 [23a] cause a classical neurofibromatosis type 1 phenotype while altering predominantly NF1 isoform type II. Hum. Genet. 2023, 142, 849–861. [Google Scholar]
- Messiaen, L.M.; Wimmer, K. Mutation analysis of the NF1 gene by cDNA-based sequencing of the coding region. In Advances in Neurofibromatosis Research; Cunha, K.S.G.a., Geller, M., Eds.; Nova Science: New York, NY, USA, 2012; pp. 89–108. [Google Scholar]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.C.; de la Hoya, M.; Wiggins, G.A.; Lindy, A.; Vincent, L.M.; Parsons, M.T.; Canson, D.M.; Bis-Brewer, D.; Cass, A.; Tchourbanov, A.; et al. Using the ACMG/AMP framework to capture evidence related to predicted and observed impact on splicing: Recommendations from the ClinGen SVI Splicing Subgroup. Am. J. Hum. Genet. 2023, 110, 1046–1067. [Google Scholar] [CrossRef]
- Jaganathan, K.; Panagiotopoulou, S.K.; McRae, J.F.; Darbandi, S.F.; Knowles, D.; Li, Y.I.; Kosmicki, J.A.; Arbelaez, J.; Cui, W.; Schwartz, G.B.; et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell 2019, 176, 535–548.e24. [Google Scholar] [CrossRef]
- Feurstein, S.K.; Luo, X.; Shah, M.; Walker, T.; Mehta, N.; Wu, D.; Godley, L.A. Revision of RUNX1 variant curation rules. Blood Adv. 2022, 6, 4726–4730. [Google Scholar] [CrossRef]
- Luo, X.; Maciaszek, J.L.; A Thompson, B.; Leong, H.S.; Dixon, K.; Sousa, S.; Anderson, M.; E Roberts, M.; Lee, K.; Spurdle, A.B.; et al. Optimising clinical care through CDH1-specific germline variant curation: Improvement of clinical assertions and updated curation guidelines. J. Med. Genet. 2023, 60, 568–575. [Google Scholar] [CrossRef]
- Naschberger, A.; Baradaran, R.; Rupp, B.; Carroni, M. The structure of neurofibromin isoform 2 reveals different functional states. Nature 2021, 599, 315–319. [Google Scholar] [CrossRef]
- Long, A.; Liu, H.; Liu, J.; Daniel, M.; Bedwell, D.M.; Korf, B.; Kesterson, R.A.; Wallis, D. Analysis of patient-specific NF1 variants leads to functional insights for Ras signaling that can impact personalized medicine. Hum. Mutat. 2022, 43, 30–41. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B-Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pros, E.; Gómez, C.; Martín, T.; Fábregas, P.; Serra, E.; Lázaro, C. Nature and mRNA effect of 282 different NF1 point mutations: Focus on splicing alterations. Hum. Mutat. 2008, 29, E173–E193. [Google Scholar] [CrossRef]
- Ekvall, S.; Sjörs, K.; Jonzon, A.; Vihinen, M.; Annerén, G.; Bondeson, M.L. Novel association of neurofibromatosis type 1-causing mutations in families with neurofibromatosis-Noonan syndrome. Am. J. Med. Genet. A. 2014, 164A, 579–587. [Google Scholar] [CrossRef]
- Nyström, A.M.; Ekvall, S.; Strömberg, B.; Holmström, G.; Thuresson, A.C.; Annerén, G.; Bondeson, M.L. A severe form of Noonan syndrome and autosomal dominant café-au-lait spots—evidence for different genetic origins. Acta Paediatr. 2009, 98, 693–698. [Google Scholar] [CrossRef]
- Santoro, C.; Maietta, A.; Giugliano, T.; Melis, D.; Perrotta, S.; Nigro, V.; Piluso, G. Arg1809 substitution in neurofibromin: Further evidence of a genotype-phenotype correlation in neurofibromatosis type 1. Eur. J. Hum. Genet. 2015, 23, 1460–1461. [Google Scholar] [CrossRef]
- Huson, S.M.; Harper, P.S.; Compston, D.A. Von Recklinghausen neurofibromatosis. A clinical and population study in south-east Wales. Brain 1988, 111, 1355–1381. [Google Scholar] [CrossRef] [PubMed]
- Huson, S.M.; Compston, D.A.; Harper, P.S. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. II. Guidelines for genetic counselling. J. Med. Genet. 1989, 26, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Listernick, R.; Charrow, J.; Greenwald, M.; Mets, M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: A longitudinal study. J. Pediatr. 1994, 125, 63–66. [Google Scholar] [CrossRef]
- Friedman, J.M.; Birch, P.H. Type 1 neurofibromatosis: A descriptive analysis of the disorder in 1,728 patients. Am. J. Med. Genet. 1997, 70, 138–143. [Google Scholar] [CrossRef]
- Bianchessi, D.; Ibba, M.C.; Saletti, V.; Blasa, S.; Langella, T.; Paterra, R.; Cagnoli, G.A.; Melloni, G.; Scuvera, G.; Natacci, F.; et al. Simultaneous Detection of NF1, SPRED1, LZTR1, and NF2 Gene Mutations by Targeted NGS in an Italian Cohort of Suspected NF1 Patients. Genes 2020, 11, 671. [Google Scholar] [CrossRef]
- Lupton, C.J.; Bayly-Jones, C.; D’Andrea, L.; Huang, C.; Schittenhelm, R.B.; Venugopal, H.; Whisstock, J.C.; Halls, M.L.; Ellisdon, A.M. The cryo-EM structure of the human neurofibromin dimer reveals the molecular basis for neurofibromatosis type 1. Nat. Struct. Mol. Biol. 2021, 28, 982–988. [Google Scholar] [CrossRef]
- Chaker-Margot, M.; Werten, S.; Dunzendorfer-Matt, T.; Lechner, S.; Ruepp, A.; Scheffzek, K.; Maier, T. Structural basis of activation of the tumor suppressor protein neurofibromin. Mol. Cell 2022, 82, 1288–1296.e5. [Google Scholar] [CrossRef] [PubMed]
- Sherekar, M.; Han, S.-W.; Ghirlando, R.; Messing, S.; Drew, M.; Rabara, D.; Waybright, T.; Juneja, P.; O’Neill, H.; Stanley, C.B.; et al. Biochemical and structural analyses reveal that the tumor suppressor neurofibromin (NF1) forms a high-affinity dimer. J. Biol. Chem. 2019, 295, 1105–1111. [Google Scholar] [CrossRef]
- Cui, Y.; Morrison, H. Construction of cloning-friendly minigenes for mammalian expression of full-length human NF1 isoforms. Hum. Mutat. 2019, 40, 187–192. [Google Scholar] [CrossRef]
- Wallis, D.; Li, K.; Lui, H.; Hu, K.; Chen, M.-J.; Li, J.; Kang, J.; Das, S.; Korf, B.R.; Kesterson, R.A. Neurofibromin (NF1) genetic variant structure-function analyses using a full-length mouse cDNA. Hum. Mutat. 2018, 39, 816–821. [Google Scholar] [CrossRef]
- Thomas, L.; Richards, M.; Mort, M.; Dunlop, E.; Cooper, D.N.; Upadhyaya, M. Assessment of the potential pathogenicity of missense mutations identified in the GTPase-activating protein (GAP)-related domain of the neurofibromatosis type-1 (NF1) gene. Hum. Mutat. 2012, 33, 1687–1696. [Google Scholar] [CrossRef]
- Douben, H.; Hoogeveen-Westerveld, M.; Nellist, M.; Louwen, J.; De Haan, M.K.; Punt, M.; Van Ommeren, B.; Van Unen, L.; Elfferich, P.; Kasteleijn, E.; et al. Functional assays combined with pre-mrna-splicing analysis improve variant classification and diagnostics for individuals with neurofibromatosis type 1 and legius syndrome. Hum. Mutat. 2023, 2023, 9628049. [Google Scholar] [CrossRef]
- Staedtke, V.; Anstett, K.; Bedwell, D.; Giovannini, M.; Keeling, K.; Kesterson, R.; Kim, Y.; Korf, B.; Leier, A.; McManus, M.L.; et al. Gene-targeted therapy for neurofibromatosis and schwannomatosis: The path to clinical trials. Clin. Trials 2024, 21, 51–66. [Google Scholar] [CrossRef] [PubMed]
- McGaughran, J.M.; Harris, D.I.; Donnai, D.; Teare, D.; MacLeod, R.; Westerbeek, R.; Kingston, H.; Super, M.; Harris, R.; Evans, D.G. A clinical study of type 1 neurofibromatosis in north west England. J. Med. Genet. 1999, 36, 197–203. [Google Scholar]
- Ferner, R.E.; Huson, S.M.; Thomas, N.; Moss, C.; Willshaw, H.; Evans, D.G.; Upadhyaya, M.; Towers, R.; Gleeson, M.; Steiger, C.; et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J. Med. Genet. 2007, 44, 81–88. [Google Scholar] [CrossRef]
- Tonsgard, J.H.; Kwak, S.M.; Short, M.P.; Dachman, A.H. CT imaging in adults with neurofibromatosis-1: Frequent asymptomatic plexiform lesions. Neurology 1998, 50, 1755–1760. [Google Scholar] [CrossRef]
- Ejerskov, C.; Farholt, S.; Nielsen, F.S.K.; Berg, I.; Thomasen, S.B.; Udupi, A.; Ågesen, T.; Licht, S.d.F.; Handrup, M.M. Clinical Characteristics and Management of Children and Adults with Neurofibromatosis Type 1 and Plexiform Neurofibromas in Denmark: A Nationwide Study. Oncol. Ther. 2023, 11, 97–110. [Google Scholar] [CrossRef]
- Thakkar, S.D.; Feigen, U.; Mautner, V.F. Spinal tumours in neurofibromatosis type 1: An MRI study of frequency, multiplicity and variety. Neuroradiology 1999, 41, 625–629. [Google Scholar] [CrossRef]
- Bai, R.-Y.; Esposito, D.; Tam, A.J.; McCormick, F.; Riggins, G.J.; Clapp, D.W.; Staedtke, V. Feasibility of using NF1-GRD and AAV for gene replacement therapy in NF1-associated tumors. Gene Ther. 2019, 26, 277–286. [Google Scholar] [CrossRef]
- Leier, A.; Bedwell, D.M.; Chen, A.T.; Dickson, G.; Keeling, K.M.; Kesterson, R.A.; Korf, B.R.; Lago, T.T.M.; Müller, U.F.; Popplewell, L.; et al. Mutation-Directed Therapeutics for Neurofibromatosis Type I. Mol. Ther. Nucleic Acids 2020, 20, 739–753. [Google Scholar] [CrossRef]

| Variant (cDNA Level) | Number of Patients in the UAB Cohort | Confirmed by RNA-Based Testing (Number of RNA Tests) | 1000G | gnomAD | LOVD | ClinVar | HGMD | Evidence | Classification of Pathogenicity |
|---|---|---|---|---|---|---|---|---|---|
| c.3114-2A>G | 7 | Yes (5; this study and PMID: 24789688) | 0 | 1 (in 1435698) | 2 (Pathogenic/NA) | Present (Likely pathogenic) | Present (DM) | PVS1 (RNA) a + PS4_strong b | Pathogenic |
| c.3114-1G>A | 4 | Yes (3; this study) | 0 | 0 | 0 | Present (Likely pathogenic) | 0 | PVS1 (RNA) + PS4_strong + PM2 e | Pathogenic |
| c.3196A>G | 5 | Yes (3; this study) | 0 | 1 (in 1455704) | 0 | Present (VUS) | 0 | PVS1 (RNA) + PS4_strong | Pathogenic |
| c.3197G>A | 2 | Yes (1; this study) | 0 | 0 | 4 (Pathogenic; including 1 de novo) | Present (Conflicting: Likely pathogenic/VUS) | Present (DM) | PVS1 (RNA) + PS4_moderate c + PM6 f | Pathogenic |
| c.3197G>T | 2 | Yes (1; this study) | 0 | 0 | 0 | Present (VUS) | 0 | PVS1 (RNA) + PS4_supporting d + PM2 | Pathogenic |
| c.3197+1G>A | 3 | Yes (5; this study and PMID: 10607834, 18546366) | 0 | 0 | 1 (Pathogenic; de novo) | Present (Pathogenic/Likely pathogenic) | Present (DM) | PVS1 (RNA) + PS4_strong + PM2 + PM6 | Pathogenic |
| c.3197+1G>T | 1 | Yes (1; PMID: 18546366) | 0 | 0 | 3 (Pathogenic; including 1 de novo) | Present (Pathogenic) | Present (DM) | PVS1 (RNA) + PM2 + PM6 | Pathogenic |
| c.3197+2T>C | 2 | Yes (1; this study) | 0 | 0 | 2 (Pathogenic) | Present (Pathogenic) | 0 | PVS1 (RNA) + PS4_suppoorting + PM2 | Pathogenic |
| c.3197+3A>T | 14 | Yes (2; this study) | 0 | 0 | 0 | Present (VUS) | 0 | PVS1 (RNA) + PS4_strong + PP1_strong g + PM2 | Pathogenic |
| NF1 Feature | N (%) | p Value (2-Tailed Fisher’s Exact Test) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Skipping Exon 24 [19a] | p.Met992del a | p.Arg1809 b | aa 844–848 c | Previously Reported NF1 Cohorts d | Skipping Exon 24 [19a] vs. p.Met992del | Skipping Exon 24 [19a] vs. p.Arg1809 | Skipping Exon 24 [19a] vs. aa 844–848 | Skipping Exon 24 [19a] vs. “Classic” NF1 | |
| ≥6 CALMs | 34/40 (85.0) | 165/182 (90.7) | 157/169 (92.9) | 130/157 (82.8) | 1537/1728 (89) | 0.2662 | 0.1211 | 0.8174 | 0.4415 |
| Skinfold freckling | 23/38 (60.5) | 105/171 (61.4) | 95/161 (59) | 104/144 (72.2) | 1403/1667 (84.2) | 1 | 1 | 0.1699 | 0.0005 ** ↘ |
| Lisch nodules | 0/23 (0) | 16/139 (11.5) | 12/120 (10) | 42/98 (42.9) | 729/1237 (58.9) | 0.1307 | 0.0768 | <0.0001 ** ↘ | <0.0001 ** ↘ |
| Major external plexiform neurofibromas e | 0/18 (0) | 0/125 (0) | 0/105 (0) | 36/92 (39.1) | 120/648 (18.5) | 1 | 1 | 0.0006 ** ↘ | 0.0554 |
| Cutaneous neurofibromas f | 0/8 (0) g | 0–1/57 (0–1.8) h | 0/57 (0) | 47/69 (68.1) | 656/723 (90.7) | 1 | 1 | 0.0003 ** ↘ | <0.0001 ** ↘ |
| Subcutaneous neurofibromas f | 0/8 (0) | 0–3/36 (0–8.3) h | 0–5/57 (0–8.8) h | 33/65 (50.8) | 297/515 (57.7) | 1 | 1 | 0.0068 * ↘ | 0.0011 ** ↘ |
| Symptomatic spinal neurofibromas | 0/33 (0) | 1/165 (0.6) | 0/76 (0) | 13/127 (10.2) | 36/2058 (1.8) | 1 | 1 | 0.0719 | 1 |
| Symptomatic OPGs i | 0/37 (0) | 0/170 (0) | 0/139 (0) | 12/136 (8.8) | 64/1650 (3.9) | 1 | 1 | 0.0721 | 0.3981 |
| Skeletal abnormalities | 4/36 (11.1) | 30/172 (17.4) | 21/126 (16.7) | 48/144 (33.3) | 144/948 (15.2) | 0.4609 | 0.6014 | 0.0076 * ↘ | 0.6386 |
| Scoliosis f | 2/36 (5.6) | 7/57 (12.3) | 6/48 (12.5) | 20/64 (31.3) | 51/236 (21.6) | 0.4742 | 0.4566 | 0.0025 * ↘ | 0.0229 ↘ |
| Cognitive impairment, developmental delay, and/or learning disabilities | 13/39 (33.3) | 58/176 (33) | 80/159 (50.3) | 56/138 (40.6) | 190/424 (44.8) | 1 | 0.0731 | 0.461 | 0.1811 |
| Noonan-like features j | 1/35 (2.9) | 19/166 (11.5) | 46/148 (31.1) | 10/134 (7.5) | 57/1683 (3.4) | 0.2099 | 0.0002 ** ↘ | 0.4629 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Fu, Y.; Koczkowska, M.; Callens, T.; Gomes, A.; Liu, J.; Bradley, W.; Brown, B.; Shaw, B.; D’Agostino, D.; et al. Genotype–Phenotype Correlation in Neurofibromatosis Type 1: Evidence for a Mild Phenotype Associated with Splicing Variants Leading to In-Frame Skipping of NF1 Exon 24 [19a]. Cancers 2024, 16, 2406. https://doi.org/10.3390/cancers16132406
Chen Y, Fu Y, Koczkowska M, Callens T, Gomes A, Liu J, Bradley W, Brown B, Shaw B, D’Agostino D, et al. Genotype–Phenotype Correlation in Neurofibromatosis Type 1: Evidence for a Mild Phenotype Associated with Splicing Variants Leading to In-Frame Skipping of NF1 Exon 24 [19a]. Cancers. 2024; 16(13):2406. https://doi.org/10.3390/cancers16132406
Chicago/Turabian StyleChen, Yunjia, Yulong Fu, Magdalena Koczkowska, Tom Callens, Alicia Gomes, Jian Liu, William Bradley, Bryce Brown, Brandon Shaw, Daniela D’Agostino, and et al. 2024. "Genotype–Phenotype Correlation in Neurofibromatosis Type 1: Evidence for a Mild Phenotype Associated with Splicing Variants Leading to In-Frame Skipping of NF1 Exon 24 [19a]" Cancers 16, no. 13: 2406. https://doi.org/10.3390/cancers16132406
APA StyleChen, Y., Fu, Y., Koczkowska, M., Callens, T., Gomes, A., Liu, J., Bradley, W., Brown, B., Shaw, B., D’Agostino, D., Fu, C., & Wallis, D. (2024). Genotype–Phenotype Correlation in Neurofibromatosis Type 1: Evidence for a Mild Phenotype Associated with Splicing Variants Leading to In-Frame Skipping of NF1 Exon 24 [19a]. Cancers, 16(13), 2406. https://doi.org/10.3390/cancers16132406





