Prevention of Anthracyclines and HER2 Inhibitor-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Sources and Search Strategy
- ((Prevention) OR (Prolepsis)) AND (Anthracycline) AND ((Cardiotoxicity) OR (Cardiopathy) OR (Heart disease) OR (Cardiac damage)).
- ((Prevention) OR (Prolepsis)) AND ((Her2 antagonist) OR (Her2 inhibitor) OR (Human epidermal growth factor receptor 2 inhibitor)) AND ((Cardiotoxicity) OR (Cardiopathy) OR (Heart disease) OR (Cardiac damage)).
2.3. Screening and Data Extraction
2.4. Assessment of Risk of Bias
2.5. Statistical Analysis
3. Results
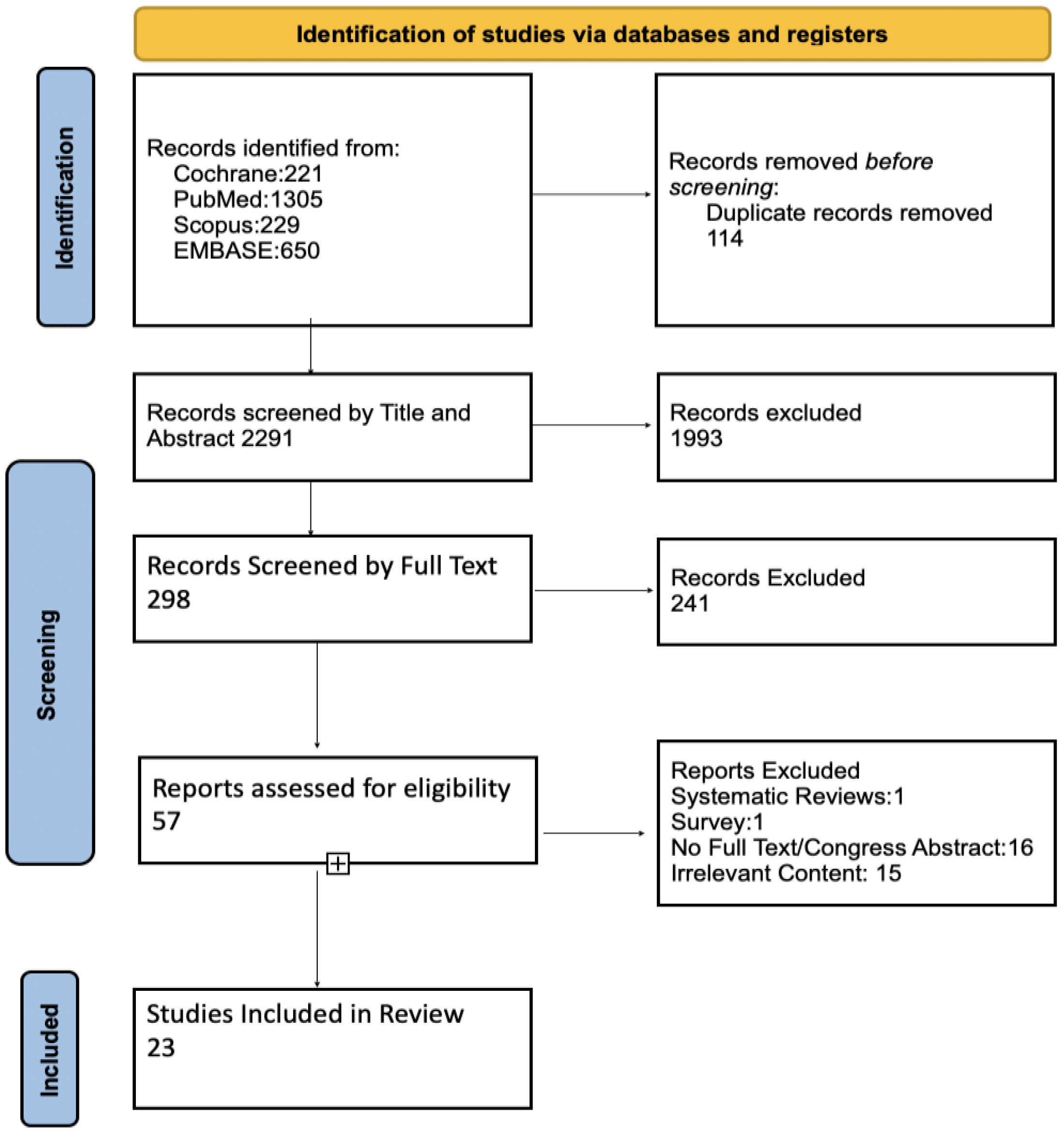
3.1. Study Selection
3.2. Study Characteristics
3.3. Assessment of Risk of Bias and Applicability
3.4. Systematic Review
3.5. Meta-Analysis
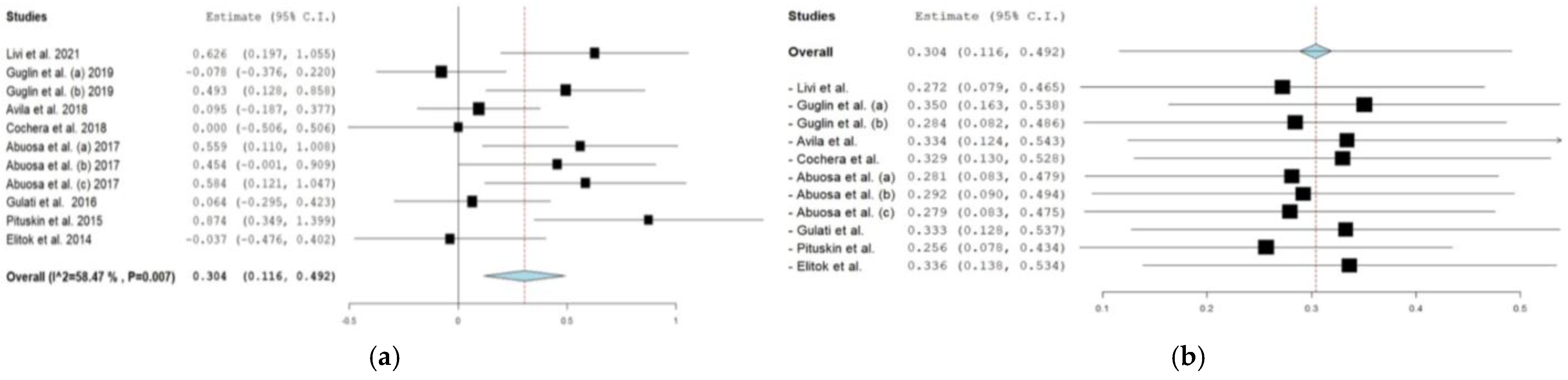
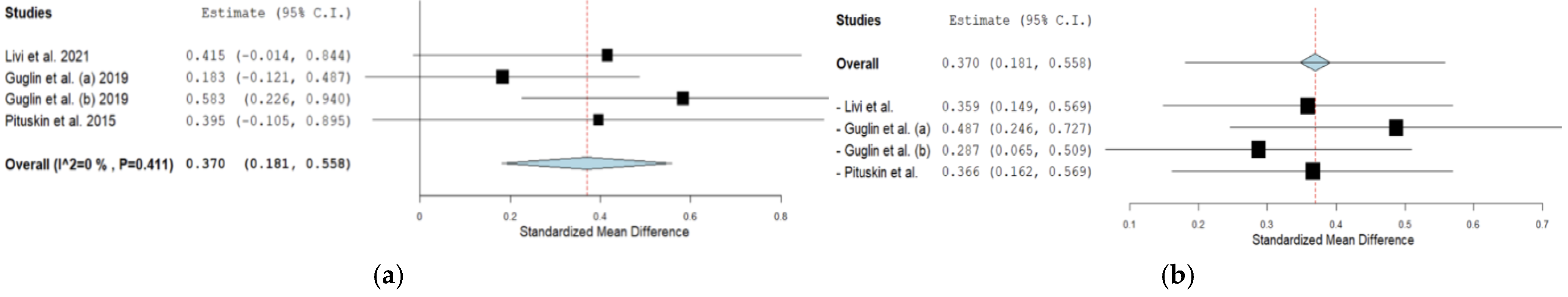
3.5.1. β-blockers
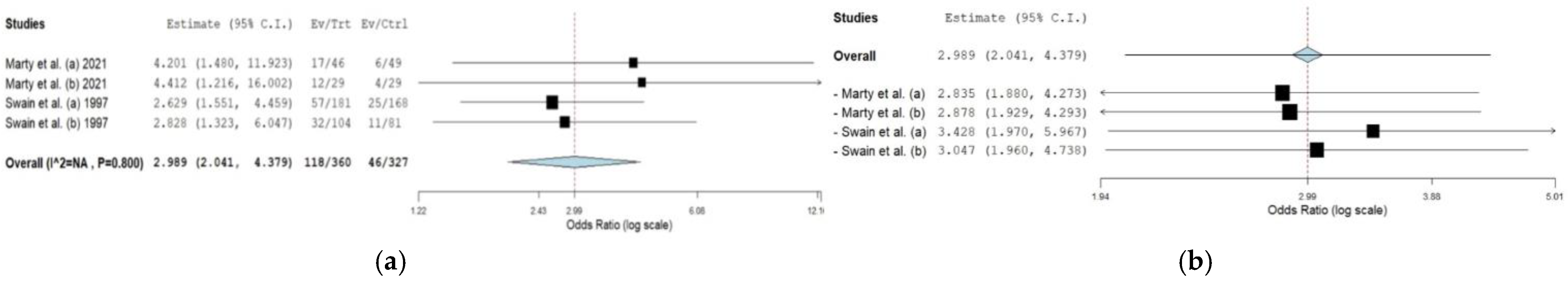
3.5.2. Dexrazoxane
3.5.3. ACEs
3.5.4. Funnel Plot
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dhingra, R.; Margulets, V.; Kirshenbaum, L.A. Molecular mechanisms underlying anthracycline cardiotoxicity: Challenges in cardio-oncology. In Cardio-Oncology; Academic Press: Cambridge, MA, USA, 2017; pp. 25–34. [Google Scholar] [CrossRef]
- Ben, D.D.; Palumbo, M.; Zagotto, G.; Capranico, G.; Moro, S. DNA Topoisomerase II Structures and Anthracycline Activity: Insights into Ternary Complex Formation. Curr. Pharm. Des. 2007, 13, 2766–2780. [Google Scholar] [CrossRef]
- Baselga, J.; Albanell, J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann. Oncol. 2001, 12, S35–S41. [Google Scholar] [CrossRef]
- Albanell, J.; Codony, J.; Rovira, A.; Mellado, B.; Gascon, P. Mechanism of action of Anti-Her2 monoclonal antibodies: Scientific update on trastuzumab and 2C4. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 253–268. [Google Scholar] [CrossRef]
- Barbieri, M.A.; Sorbara, E.E.; Cicala, G.; Santoro, V.; Cutroneo, P.M.; Franchina, T.; Spina, E. Adverse drug reactions with HER2-positive breast cancer treatment: An analysis from the Italian pharmacovigilance database. Drugs Real World Outcomes 2021, 9, 91–107. [Google Scholar] [CrossRef]
- Muntasell, A.; Cabo, M.; Servitja, S.; Tusquets, I.; Martínez-García, M.; Rovira, A.; Rojo, F.; Albanell, J.; López-Botet, M. Interplay between Natural Killer Cells and Anti-HER2 Antibodies: Perspectives for Breast Cancer Immunotherapy. Front. Immunol. 2017, 8, 1544. [Google Scholar] [CrossRef] [PubMed]
- Anthracycline Medications (Doxorubicin)—Statpearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551633/ (accessed on 22 June 2023).
- Huang, W.; Xu, R.; Zhou, B.; Lin, C.; Guo, Y.; Xu, H.; Guo, X. Clinical Manifestations, Monitoring, and Prognosis: A Review of Cardiotoxicity After Antitumor Strategy. Front. Cardiovasc. Med. 2022, 9, 912329. [Google Scholar] [CrossRef]
- Saour, J. Clinical Presentations of Doxorubicin-Induced Cardiotoxicity. Ann. Saudi Med. 1984, 4, 257–261. [Google Scholar] [CrossRef]
- A-Mohannadi, A.; Kunhoth, J.; Najeeb, A.; Al-Maadeed, S.; Sadasivuni, K. Conventional clinical methods for predicting heart disease. In Predicting Heart Failure; Wiley: Hoboken, NJ, USA, 2022; pp. 23–46. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ. Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Engel, J.M.; Stankowski, R.V.; Liang, H.; Berg, R.L.; Doi, S.A. High-sensitivity C-Reactive Protein (Hs-CRP) as a Biomarker for Trastuzumab-Induced Cardiotoxicity in HER2-Positive Early-Stage Breast Cancer: A Pilot Study. Breast Cancer Res. Treat. 2012, 134, 291–298. [Google Scholar] [CrossRef]
- Putt, M.; Hahn, V.S.; Januzzi, J.L.; Sawaya, H.; Sebag, I.A.; Plana, J.C.; Picard, M.H.; Carver, J.R.; Halpern, E.F.; Kuter, I.; et al. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin. Chem. 2015, 61, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.L.; Niu, J.; Zhang, N.; Giordano, S.H.; Chavez-MacGregor, M. Cardiotoxicity and cardiac monitoring among chemotherapy-treated breast cancer patients. JACC Cardiovasc. Imaging 2018, 11, 1084–1093. [Google Scholar] [CrossRef]
- Hashemipour, S.M.A.; Valizadeh, R.; Keshavarzian, E.; Sadighpour, T.; Mortazavizadeh, S.M.; Soltani, M.; Motevalipoor, A.F.; Khamas, S.S.; Moazen, M.; Kogani, M.; et al. Prophylactic Agents for Preventing Cardiotoxicity Induced Following Anticancer Agents: A Systematic Review and Meta-Analysis of Clinical Trials. Rev. Recent Clin. Trials 2023, 18, 112–122. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Gerber, M.C.; Weisberg, S.; York, M.; Spicer, D.; Jones, S.E.; Wadler, S.; Desai, A.; Vogel, C.; et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J. Clin. Oncol. 1997, 15, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.; Heck, S.L.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; Von Knobelsdorff-Brenkenhoff, F.; Bratland, A.; Storas, T.H.; et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): A 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016, 37, 1671–1680. [Google Scholar] [CrossRef]
- Guglin, M.E.; Krischer, J.; Tamura, R. Lisinopril or carvedilol for prevention of trastuzumab induced cardiotoxicity. In Proceedings of the 67th Annual American College of Cardiology Meeting, Orlando, FL, USA, 10–12 March 2018. Abstract 405. [Google Scholar]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef]
- Calvillo-Argüelles, O.; Michalowska, M.; Billia, F.; Suntheralingam, S.; Amir, E.; Thavendiranathan, P. Cardioprotective Effect of Statins in Patients with HER2-Positive Breast Cancer Receiving Trastuzumab Therapy. Can. J. Cardiol. 2019, 35, 153–159. [Google Scholar] [CrossRef] [PubMed]
- RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials, 19 December 2022. Available online: https://methods.cochrane.org (accessed on 3 January 2023).
- The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses, 19 December 2022. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 3 January 2023).
- Akpek, M.; Ozdogru, I.; Sahin, O.; Inanc, M.; Dogan, A.; Yazici, C.; Berk, V.; Karaca, H.; Kalay, N.; Oguzhan, A.; et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur. J. Heart Fail. 2014, 17, 81–89. [Google Scholar] [CrossRef]
- Seicean, S.; Seicean, A.; Plana, J.C.; Budd, G.T.; Marwick, T.H. Effect of Statin Therapy on the Risk for Incident Heart Failure in Patients with Breast Cancer Receiving Anthracycline Chemotherapy: An Observational Clinical Cohort Study. J. Am. Coll. Cardiol. 2012, 60, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Qadir, H.; Bobrowski, D.; Zhou, L.; Austin, P.C.; Calvillo-Argüelles, O.; Amir, E.; Lee, D.S.; Thavendiranathan, P. Statin Exposure and Risk of Heart Failure After Anthracycline- or Trastuzumab-Based Chemotherapy for Early Breast Cancer: A Propensity Score–Matched Cohort Study. J. Am. Hear Assoc. 2021, 10, e018393. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.S.; Ayub-Ferreira, S.A.; De Barros Wanderley, M.R.; Das Dores Cruz, F.; Goncalves Brandao, S.M.; Carvalho Rigaud, V.O.; Higuchi Dos Santos, M.H.; Hajjar, L.A.; Filho, R.K.; Hoff, P.M.; et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity. J. Am. Coll. Cardiol. 2018, 71, 2281–2290, (NCT 01724450). [Google Scholar] [CrossRef]
- Cochera, F.; Dinca, D.; Bordejevic, D.A.; Citu, I.M.; Mavrea, A.M.; Andor, M.; Trofenciuc, M.; Tomescu, M.C. Nebivolol effect on doxorubicin-induced cardiotoxicity in breast cancer. Cancer Manag. Res. 2018, 10, 2071–2081. [Google Scholar] [CrossRef]
- Abuosa, A.M.; Elshiekh, A.Y.; Qureshi, K.; Abrar, M.B.; Kholeif, M.A.; Kinsara, A.J.; Andejani, A.; Ahmed, A.H.; Cleland, J.G.F. Prophylactic use of carvedilol to prevent ventricular dysfunction in patients with cancer treated with doxorubicin. Indian Heart J. 2018, 70, S96–S100. [Google Scholar] [CrossRef] [PubMed]
- Wittayanukorn, S.; Qian, J.; Westrick, S.C.; Billor, N.; Johnson, B.; Hansen, R.A. Prevention of trastuzumab and anthracycline-induced cardiotoxicity using angiotensin-converting enzyme inhibitors or β-blockers in older adults with breast cancer. Am. J. Clin. Oncol. 2018, 41, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Boekhout, A.H.; Gietema, J.; Kerklaan, B.M.; Van Werkhoven, E.D.; Altena, R.; Honkoop, A.; Los, M.; Smit, W.M.; Nieboer, P.; Smorenburg, C.H.; et al. Angiotensin II–receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer. JAMA Oncol. 2016, 2, 1030, (NCT00459771). [Google Scholar] [CrossRef] [PubMed]
- Livi, L.; Barletta, G.; Martella, F.; Saieva, C.; Desideri, I.; Bacci, C.; Riccarda del Bene, M.; Airoldi, M.; Amoroso, D.; Coltelli, L.; et al. Cardioprotective strategy for patients with nonmetastatic breast cancer who are receiving an anthracycline-based chemotherapy. JAMA Oncol. 2021, 7, 1544. [Google Scholar] [CrossRef] [PubMed]
- Wihandono, A.; Azhar, Y.; Abdurahman, M.; Hidayat, S. The role of Lisinopril and bisoprolol to prevent anthracycline induced cardiotoxicity in locally advanced breast cancer patients. Asian Pac. J. Cancer Prev. 2021, 22, 2847–2853. [Google Scholar] [CrossRef] [PubMed]
- Guglin, M.; Krischer, J.; Tamura, R.; Flink, A.; Bello-Matricaria, L.; McCaskill-Stevens, W.; Munster, P. Randomized trial of Lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J. Am. Coll. Cardiol. 2019, 73, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Pituskin, E.; Mackey, J.; Koshman, S.; Jassal, D.; Pitz, M.; Haykowsky, M.; Pagano, J.; Chow, K.; Thompson, R.; Vos, L.; et al. Multidisciplinary approach to novel therapies in Cardio-Oncology Research (manticore 101–breast): A randomized trial for the prevention of Trastuzumab-associated cardiotoxicity. J. Clin. Oncol. 2017, 35, 870–877, (NCT01016886). [Google Scholar] [CrossRef]
- Marty, M.; Espie, M.; Llombart, A.; Monnier, A.; Rapoport, B.; Stahalova, V. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane®) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann. Oncol. 2006, 17, 614–622. [Google Scholar] [CrossRef]
- Venturini, M.; Michelotti, A.; Del Mastro, L.; Gallo, L.; Carnino, F.; Garrone, O.; Tibaldi, C.; Molea, N.; Bellina, R.C.; Pronzato, P.; et al. Multicenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancer. J. Clin. Oncol. 1996, 14, 3112–3120. [Google Scholar] [CrossRef]
- Getz, K.D.; Sung, L.; Alonzo, T.A.; Leger, K.J.; Gerbing, R.B.; Pollard, J.A.; Cooper, T.; Kolb, E.A.; Gamis, A.S.; Ky, B.; et al. Effect of dexrazoxane on left ventricular systolic function and treatment outcomes in patients with acute myeloid leukemia: A report from the Children’s Oncology Group. J. Clin. Oncol. 2020, 38, 2398–2406. [Google Scholar] [CrossRef]
- Filomena, D.; Versacci, P.; Cimino, S.; Mattiucci, C.; Maestrini, V.; Cantisani, D.; Petronilli, V.; Agati, L.; Schiavetti, A. Echocardiographic long-term follow-up of adult survivors of pediatric cancer treated with Dexrazoxane-Anthracyclines Association. Int. J. Cardiol. 2020, 299, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Park, E.S.; Kang, H.J.; Shin, H.J.; Noh, C.; Yun, S.J.; Ahn, H.S.; Choi, J.C. Dexrazoxane for preventing anthracycline cardiotoxicity in children with solid tumors. J. Korean Med. Sci. 2010, 25, 1336. [Google Scholar] [CrossRef]
- Elbl, L.; Hrstkova, H.; Tomaskova, I.; Blazek, B.; Michalek, J. Long-term serial echocardiographic examination of late anthracycline cardiotoxicity and its prevention by dexrazoxane in paediatric patients. Eur. J. Pediatr. 2005, 164, 678–684. [Google Scholar] [CrossRef]
- Silber, J.; Cnaan, A.; Paridon, S.; Chin, A.; Rychik, J.; Hogarty, A.; Cohen, M.; Barber, G.; Rutkowski, M.; Kimball, T.; et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to Anthracyclines. J. Clin. Oncol. 2004, 22, 820–828. [Google Scholar] [CrossRef]
- Elitok, A.; Oz, F.; Cizgici, A.; Kilic, L.; Ciftci, R.; Sen, F.; Bugra, Z.; Mercanoglu, F.; Oncul, A.; Oflaz, H. Effect of carvedilol on silent anthracycline-induced cardiotoxicity assessed by strain imaging: A prospective randomized controlled study with six-month follow-up. Cardiol. J. 2014, 21, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Daniyal, M.; Li, J.; Mao, Y. Preventive use of carvedilol for anthracycline-induced cardiotoxicity: A systematic review and meta-analysis of randomized controlled trials. Herz 2019, 45, 1–14. [Google Scholar] [CrossRef]
- Ma, Y.; Bai, F.; Qin, F.; Li, J.; Liu, N.; Li, D.; Li, T.; Xie, H.; Liu, D.; Zhou, S.; et al. Beta-blockers for the primary prevention of anthracycline-induced cardiotoxicity: A meta-analysis of randomized controlled trials. BMC Pharmacol. Toxicol. 2019, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, K.; Vaseghi, G.; Mansourian, M. Pharmacological interventions for preventing anthracycline-induced clinical and subclinical cardiotoxicity: A network meta-analysis of Metastatic Breast Cancer. J. Oncol. Pharm. Pract. 2020, 27, 414–427. [Google Scholar] [CrossRef]
- D’Amario, D.; Laborante, R.; Bianchini, E.; Galli, M.; Ciliberti, G.; Mennuni, M.; Patti, G. Statins as preventive therapy for Anthracycline Cardiotoxicity: A meta-analysis of randomized controlled trials. Int. J. Cardiol. 2023, 391, 131219. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, R.; Jiang, J.; Hu, Y.; Li, H.; Wang, Y. ACEI/ARB and beta-blocker therapies for preventing cardiotoxicity of antineoplastic agents in breast cancer: A systematic review and meta-analysis. Heart Fail. Rev. 2023, 28, 1405–1415. [Google Scholar] [CrossRef]
- Gujral, D.M.; Lloyd, G.; Bhattacharyya, S. Effect of prophylactic Betablocker or ACE inhibitor on cardiac dysfunction & heart failure during anthracycline chemotherapy ± trastuzumab. Breast 2018, 37, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Quinaglia, T.; Onoue, T.; Mahmood, S.S.; Drobni, Z.D.; Gilman, H.K.; Smith, A.; Heemelaar, J.C.; Brahmbhatt, P.; Ho, J.S.; et al. Atorvastatin for anthracycline-associated cardiac dysfunction. JAMA 2023, 330, 528. [Google Scholar] [CrossRef] [PubMed]
- Liesse, K.; Harris, J.; Chan, M.; Schmidt, M.; Chiu, B. Dexrazoxane significantly reduces anthracycline-induced cardiotoxicity in pediatric solid tumor patients: A systematic review. J. Pediatr. Hematol. Oncol. 2018, 40, 417–425. [Google Scholar] [CrossRef] [PubMed]
| Case-Control | Selection | Comparability | Outcome | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Case Definition Adequate | Representativeness of Cases | Selection of Controls | Definition of Controls | Of Cases and Controls Based on Design | Ascertainment of Exposure | Same Ascertainment | No Response Rate | |
| Arguelles et al., 2018 [20] | ★ | ★ | ★ | ★ ★ | ★ | ★ | 7/9 | ||
| Cohort | Selection | Comparability | Outcome | Total Score | |||||
| Author | Representativeness of exposed cohort | Selection of non-exposed | Ascertainment of exposure | Outcome not present at start of study | Of cohorts on basis of design | Assessment of outcome | Folllow-up time enough for outcomes to occur | Adequacy of follow-up | |
| Abdel et al., 2021 [25] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | 8/9 | |
| Wittayanukorn et al., 2017 [29] | ★ | ★ | ★★ | ★ | ★ | ★ | 7/9 | ||
| Seicean et al., 2012 [24] | ★ | ★ | ★ | ★ ★ | ★ | ★ | 7/9 | ||
| Elbl et al., 2005 [40] | ★ | ★ | ★ | ★ ★ | ★ | ★ | ★ | 8/9 | |
| Author | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Result Selection |
|---|---|---|---|---|---|
| Livi et al., 2021 [31] | 🟢 | 🟢 | 🟢 | 🟢 | 🟢 |
| Wihandono et al., 2021 [32] | 🟢 | 🟡 | 🟢 | 🟢 | 🟢 |
| Guglin et al., 2019 [33] | 🟢 | 🟢 | 🟢 | 🔴 | 🟢 |
| Avila et al., 2018 [26] | 🟢 | 🟡 | 🟢 | 🟢 | 🟢 |
| Cochera et al., 2018 [27] | 🟢 | 🟢 | 🟢 | 🟢 | 🟢 |
| Abuosa et al., 2017 [28] | 🟢 | 🟢 | 🟢 | 🟢 | 🟢 |
| Boekhout et al., 2016 [30] | 🟢 | 🟢 | 🟢 | 🟢 | 🟢 |
| Gulati et al., 2016 [17] | 🟢 | 🟢 | 🟢 | 🟢 | 🟢 |
| Pituskin et al., 2015 [34] | 🟢 | 🟢 | 🟢 | 🟢 | 🟡 |
| Akpek et al., 2014 [23] | 🟢 | 🟡 | 🟢 | 🟢 | 🟢 |
| Elitok et al., 2014 [42] | 🟡 | 🟢 | 🟢 | 🟢 | 🟢 |
| Marty et al., 2005 [35] | 🟡 | 🟢 | 🟢 | 🟢 | 🟢 |
| Getz et al., 2020 [37] | 🟡 | 🟢 | 🟢 | 🟢 | 🟢 |
| Silber et al., 2004 [41] | 🟢 | 🟢 | 🟢 | 🟢 | 🟢 |
| Swain et al., 1997 [16] | 🟢 | 🟢 | 🟢 | 🟢 | 🟢 |
| Venturini et al., 1996 [36] | 🟢 | 🟡 | 🟢 | 🟡 | 🟢 |
| Author | Confounding | Selection | Classification Measurement of Exposure | Departures from Exposure | Missing Data | Measurement of Outcome | Reported Results |
|---|---|---|---|---|---|---|---|
| Filomena et al., 2019 [38] | 🟠 | 🟡 | 🟡 | 🟡 | 🟡 | 🟡 | 🟡 |
| Choi et al., 2010 [39] | 🟢 | 🟡 | 🟡 | 🟡 | 🟢 | 🟡 | 🟡 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotiropoulou, I.M.; Manetas-Stavrakakis, N.; Kourek, C.; Xanthopoulos, A.; Magouliotis, D.; Giamouzis, G.; Skoularigis, J.; Briasoulis, A. Prevention of Anthracyclines and HER2 Inhibitor-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2419. https://doi.org/10.3390/cancers16132419
Sotiropoulou IM, Manetas-Stavrakakis N, Kourek C, Xanthopoulos A, Magouliotis D, Giamouzis G, Skoularigis J, Briasoulis A. Prevention of Anthracyclines and HER2 Inhibitor-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(13):2419. https://doi.org/10.3390/cancers16132419
Chicago/Turabian StyleSotiropoulou, Ioanna Myrto, Nikolaos Manetas-Stavrakakis, Christos Kourek, Andrew Xanthopoulos, Dimitrios Magouliotis, Grigorios Giamouzis, John Skoularigis, and Alexandros Briasoulis. 2024. "Prevention of Anthracyclines and HER2 Inhibitor-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis" Cancers 16, no. 13: 2419. https://doi.org/10.3390/cancers16132419






