Extended Synaptotagmins 1 and 2 Are Required for Store-Operated Calcium Entry, Cell Migration and Viability in Breast Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
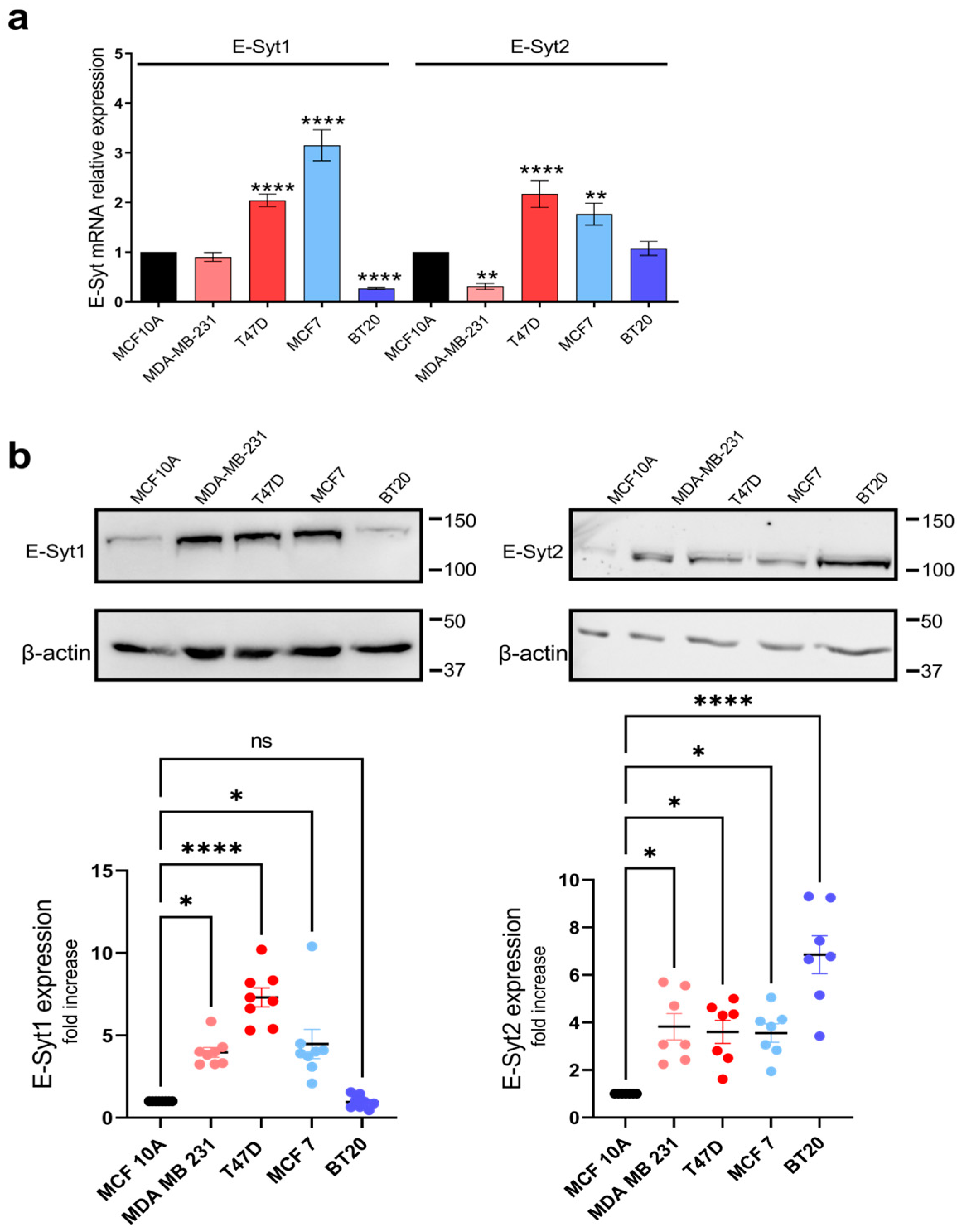
2.1. E-Syt Expression in Breast Cancer and Non-Tumoral Breast Epithelial Cells
2.2. Role of E-Syt1 and E-Syt2 in Thapsigargin-Induced Ca2+ Release and Entry in Breast Cancer and Non-Tumoral Breast Epithelial Cells
2.3. Functional Role of E-Syt1 and E-Syt2 in Breast Cancer and Non-Tumoral Breast Epithelial Cell Migration
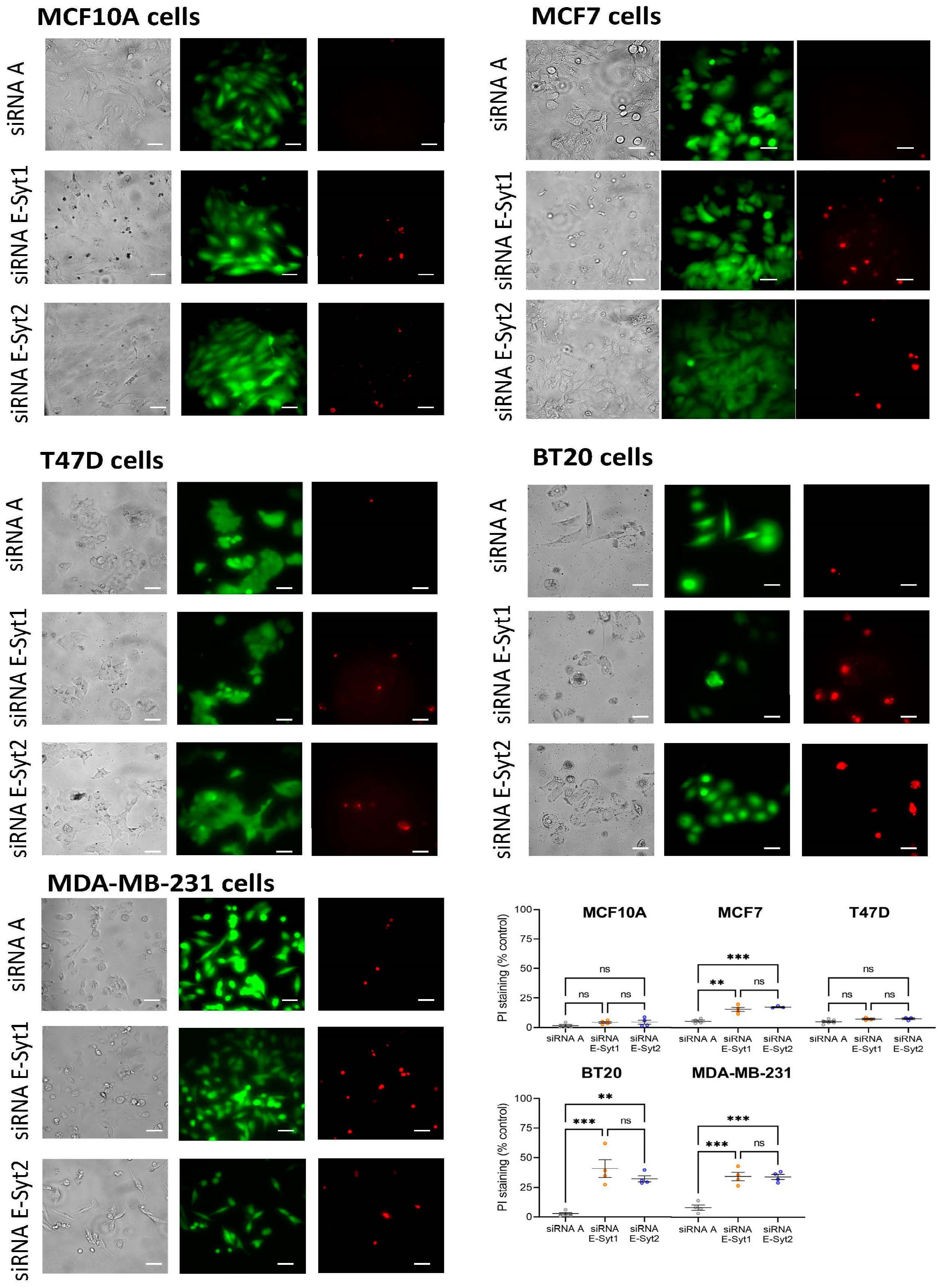
2.4. Functional Role of E-Syt1 in Cell Viability in ER+ Breast Cancer and Non-Tumoral Breast Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture and Transfections
4.3. Determination of Cytosolic Free-Ca2+ Concentration
4.4. Western Blotting
4.5. Wound Healing Assay
4.6. Determination of Cell Viability
4.7. Quantitative RT-PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emrich, S.M.; Yoast, R.E.; Xin, P.; Arige, V.; Wagner, L.E.; Hempel, N.; Gill, D.L.; Sneyd, J.; Yule, D.I.; Trebak, M. Omnitemporal choreographies of all five STIM/Orai and IP(3)Rs underlie the complexity of mammalian Ca2+ signaling. Cell Rep. 2021, 34, 108760. [Google Scholar] [CrossRef] [PubMed]
- Emrich, S.M.; Yoast, R.E.; Zhang, X.; Fike, A.J.; Wang, Y.H.; Bricker, K.N.; Tao, A.Y.; Xin, P.; Walter, V.; Johnson, M.T.; et al. Orai3 and Orai1 mediate CRAC channel function and metabolic reprogramming in B cells. eLife 2023, 12, e84708. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Janoshazi, A.; Janardhan, K.S.; Steinckwich, N.; D’Agostin, D.M.; Petranka, J.G.; Desai, P.N.; Roberts-Thomson, S.J.; Bird, G.S.; Tucker, D.K.; et al. Essential role of Orai1 store-operated calcium channels in lactation. Proc. Natl. Acad. Sci. USA 2015, 112, 5827–5832. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; DiGregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Yu, Y.; Roos, J.; Kozak, J.A.; Deerinck, T.J.; Ellisman, M.H.; Stauderman, K.A.; Cahalan, M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005, 437, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef]
- Mercer, J.C.; Dehaven, W.I.; Smyth, J.T.; Wedel, B.; Boyles, R.R.; Bird, G.S.; Putney, J.W., Jr. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J. Biol. Chem. 2006, 281, 24979–24990. [Google Scholar] [CrossRef]
- Peinelt, C.; Vig, M.; Koomoa, D.L.; Beck, A.; Nadler, M.J.; Koblan-Huberson, M.; Lis, A.; Fleig, A.; Penner, R.; Kinet, J.P. Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat. Cell Biol. 2006, 8, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Feske, S.; Gwack, Y.; Srikanth, S.; Rao, A.; Hogan, P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature 2006, 443, 230–233. [Google Scholar] [CrossRef]
- Hoglinger, C.; Grabmayr, H.; Maltan, L.; Horvath, F.; Krobath, H.; Muik, M.; Tiffner, A.; Renger, T.; Romanin, C.; Fahrner, M.; et al. Defects in the STIM1 SOARalpha2 domain affect multiple steps in the CRAC channel activation cascade. Cell. Mol. Life Sci. 2021, 78, 6645–6667. [Google Scholar] [CrossRef]
- Yoast, R.E.; Emrich, S.M.; Zhang, X.; Xin, P.; Johnson, M.T.; Fike, A.J.; Walter, V.; Hempel, N.; Yule, D.I.; Sneyd, J.; et al. The native ORAI channel trio underlies the diversity of Ca2+ signaling events. Nat. Commun. 2020, 11, 2444. [Google Scholar] [CrossRef] [PubMed]
- Emrich, S.M.; Yoast, R.E.; Xin, P.; Zhang, X.; Pathak, T.; Nwokonko, R.; Gueguinou, M.F.; Subedi, K.P.; Zhou, Y.; Ambudkar, I.S.; et al. Cross-talk between N-terminal and C-terminal domains in stromal interaction molecule 2 (STIM2) determines enhanced STIM2 sensitivity. J. Biol. Chem. 2019, 294, 6318–6332. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Ahuja, M.; Maleth, J.; Moreno, C.M.; Yuan, J.P.; Kim, M.S.; Muallem, S. The STIM1 CTID domain determines access of SARAF to SOAR to regulate Orai1 channel function. J. Cell Biol. 2013, 202, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.J.; Albarran, L.; Gomez, L.J.; Smani, T.; Salido, G.M.; Rosado, J.A. Molecular modulators of store-operated calcium entry. Biochim. Biophys. Acta 2016, 1863, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Min, S.W.; Chang, W.P.; Sudhof, T.C. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc. Natl. Acad. Sci. USA 2007, 104, 3823–3828. [Google Scholar] [CrossRef] [PubMed]
- Saheki, Y.; Bian, X.; Schauder, C.M.; Sawaki, Y.; Surma, M.A.; Klose, C.; Pincet, F.; Reinisch, K.M.; De Camilli, P. Control of plasma membrane lipid homeostasis by the extended synaptotagmins. Nat. Cell Biol. 2016, 18, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Tong, C.; Liu, J.J. E-Syt1 Regulates Neuronal Activity-Dependent Endoplasmic Reticulum-Plasma Membrane Junctions and Surface Expression of AMPA Receptors. Contact 2023, 6, 25152564231185011. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.; Sun, Z.; Srikanth, S.; Gwack, Y. The short isoform of extended synaptotagmin-2 controls Ca2+ dynamics in T cells via interaction with STIM1. Sci. Rep. 2020, 10, 14433. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ong, H.L.; Saadi, H.; Son, G.Y.; Shokatian, Z.; Terry, L.E.; Trebak, M.; Yule, D.I.; Ambudkar, I. Functional communication between IP(3)R and STIM2 at subthreshold stimuli is a critical checkpoint for initiation of SOCE. Proc. Natl. Acad. Sci. USA 2022, 119, e2114928118. [Google Scholar] [CrossRef]
- Maleth, J.; Choi, S.; Muallem, S.; Ahuja, M. Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nat. Commun. 2014, 5, 5843. [Google Scholar] [CrossRef]
- Smani, T.; Shapovalov, G.; Skryma, R.; Prevarskaya, N.; Rosado, J.A. Functional and physiopathological implications of TRP channels. Biochim. Biophys. Acta 2015, 1853, 1772–1782. [Google Scholar] [CrossRef]
- Motiani, R.K.; Abdullaev, I.F.; Trebak, M. A novel native store-operated calcium channel encoded by Orai3: Selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J. Biol. Chem. 2010, 285, 19173–19183. [Google Scholar] [CrossRef]
- Jardin, I.; Diez-Bello, R.; Lopez, J.J.; Redondo, P.C.; Salido, G.M.; Smani, T.; Rosado, J.A. TRPC6 Channels Are Required for Proliferation, Migration and Invasion of Breast Cancer Cell Lines by Modulation of Orai1 and Orai3 Surface Exposure. Cancers 2018, 10, 331. [Google Scholar] [CrossRef]
- Sanchez-Collado, J.; Lopez, J.J.; Jardin, I.; Camello, P.J.; Falcon, D.; Regodon, S.; Salido, G.M.; Smani, T.; Rosado, J.A. Adenylyl Cyclase Type 8 Overexpression Impairs Phosphorylation-Dependent Orai1 Inactivation and Promotes Migration in MDA-MB-231 Breast Cancer Cells. Cancers 2019, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Vashisht, A.; Tanwar, J.; Motiani, R.K. Regulation of proto-oncogene Orai3 by miR18a/b and miR34a. Cell Calcium 2018, 75, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Collado, J.; Lopez, J.J.; Gonzalez-Gutierrez, L.; Cantonero, C.; Jardin, I.; Salido, G.M.; Rosado, J.A. Functional role of TRPC6 and STIM2 in cytosolic and endoplasmic reticulum Ca2+ content in resting estrogen receptor-positive breast cancer cells. Biochem. J. 2020, 477, 3183–3197. [Google Scholar] [CrossRef] [PubMed]
- Jardin, I.; Alvarado, S.; Jimenez-Velarde, V.; Nieto-Felipe, J.; Lopez, J.J.; Salido, G.M.; Smani, T.; Rosado, J.A. Orai1alpha and Orai1beta support calcium entry and mammosphere formation in breast cancer stem cells. Sci. Rep. 2023, 13, 19471. [Google Scholar] [CrossRef]
- Putney, J.W. Store-Operated Calcium Entry: An Historical Overview. Adv. Exp. Med. Biol. 2017, 981, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, H.; Jin, F.; Fang, M.; Huang, M.; Yang, C.S.; Chen, T.; Fu, L.; Pan, Z. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget 2014, 5, 3455–3471. [Google Scholar] [CrossRef]
- Asghar, M.Y.; Lassila, T.; Paatero, I.; Nguyen, V.D.; Kronqvist, P.; Zhang, J.; Slita, A.; Lof, C.; Zhou, Y.; Rosenholm, J.; et al. Stromal interaction molecule 1 (STIM1) knock down attenuates invasion and proliferation and enhances the expression of thyroid-specific proteins in human follicular thyroid cancer cells. Cell. Mol. Life Sci. 2021, 78, 5827–5846. [Google Scholar] [CrossRef]
- Perez-Riesgo, E.; Hernando-Perez, E.; Feijoo, V.; Tajada, S.; Nunez, L.; Villalobos, C. Transcriptional Basis of Ca2+ Remodeling Reversal Induced by Polyamine Synthesis Inhibition in Colorectal Cancer Cells. Cancers 2023, 15, 1600. [Google Scholar] [CrossRef] [PubMed]
- Alhamed, A.S.; Alqinyah, M.; Alsufayan, M.A.; Alhaydan, I.A.; Alassmrry, Y.A.; Alnefaie, H.O.; Algahtani, M.M.; Alghaith, A.F.; Alhamami, H.N.; Albogami, A.M.; et al. Blockade of store-operated calcium entry sensitizes breast cancer cells to cisplatin therapy via modulating inflammatory response. Saudi Pharm. J. 2023, 31, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Collado, J.; Lopez, J.J.; Cantonero, C.; Jardin, I.; Regodon, S.; Redondo, P.C.; Gordillo, J.; Smani, T.; Salido, G.M.; Rosado, J.A. Orai2 Modulates Store-Operated Ca2+ Entry and Cell Cycle Progression in Breast Cancer Cells. Cancers 2021, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Bassett, J.J.; Robitaille, M.; Peters, A.A.; Bong, A.H.L.; Taing, M.W.; Wood, I.A.; Sadras, F.; Roberts-Thomson, S.J.; Monteith, G.R. ORAI1 regulates sustained cytosolic free calcium fluctuations during breast cancer cell apoptosis and apoptotic resistance via a STIM1 independent pathway. FASEB J. 2022, 36, e22108. [Google Scholar] [CrossRef] [PubMed]
- Girault, A.; Peretti, M.; Badaoui, M.; Hemon, A.; Morjani, H.; Ouadid-Ahidouch, H. The N and C-termini of SPCA2 regulate differently Kv10.1 function: Role in the collagen 1-induced breast cancer cell survival. Am. J. Cancer Res. 2021, 11, 251–263. [Google Scholar] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Aka, J.A.; Lin, S.X. Comparison of functional proteomic analyses of human breast cancer cell lines T47D and MCF7. PLoS ONE 2012, 7, e31532. [Google Scholar] [CrossRef]
- Radde, B.N.; Ivanova, M.M.; Mai, H.X.; Salabei, J.K.; Hill, B.G.; Klinge, C.M. Bioenergetic differences between MCF-7 and T47D breast cancer cells and their regulation by oestradiol and tamoxifen. Biochem. J. 2015, 465, 49–61. [Google Scholar] [CrossRef]
- Mooney, L.M.; Al-Sakkaf, K.A.; Brown, B.L.; Dobson, P.R. Apoptotic mechanisms in T47D and MCF-7 human breast cancer cells. Br. J. Cancer 2002, 87, 909–917. [Google Scholar] [CrossRef]
- Jardin, I.; Diez-Bello, R.; Falcon, D.; Alvarado, S.; Regodon, S.; Salido, G.M.; Smani, T.; Rosado, J.A. Melatonin downregulates TRPC6, impairing store-operated calcium entry in triple-negative breast cancer cells. J. Biol. Chem. 2021, 296, 100254. [Google Scholar] [CrossRef]
- Jardin, I.; Nieto-Felipe, J.; Alvarado, S.; Diez-Bello, R.; Lopez, J.J.; Salido, G.M.; Smani, T.; Rosado, J.A. SARAF and EFHB Modulate Store-Operated Ca2+ Entry and Are Required for Cell Proliferation, Migration and Viability in Breast Cancer Cells. Cancers 2021, 13, 4160. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Strohmer, D.; Feng, S.; Zhang, G.; Cui, H.; Song, Y. The role of extended synaptotagmin at membrane contact sites in cancer research. Front. Cell Dev. Biol. 2023, 11, 1291506. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, P.; Szalai, P.; Olesen, C.; Praetorius, H.A.; Nissen, P.; Christensen, S.B.; Engedal, N.; Moller, J.V. Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by thapsigargin analogs induces cell death via ER Ca2+ depletion and the unfolded protein response. J. Biol. Chem. 2017, 292, 19656–19673. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.G.; Moss, T. Loss of all 3 Extended Synaptotagmins does not affect normal mouse development, viability or fertility. Cell Cycle 2016, 15, 2360–2366. [Google Scholar] [CrossRef]
- Herdman, C.; Tremblay, M.G.; Mishra, P.K.; Moss, T. Loss of Extended Synaptotagmins ESyt2 and ESyt3 does not affect mouse development or viability, but in vitro cell migration and survival under stress are affected. Cell Cycle 2014, 13, 2616–2625. [Google Scholar] [CrossRef]


| Protein | Forward Primer | Reverse Primer |
|---|---|---|
| E-Syt1 | TCGCAAGACTAGGCAACCTC | CCAAATACACAGGTATCAGCACCA |
| E-Syt2 | CCTGAGAAAGACAGTGACAGGAAG | GCCCAGCCTACTTTAACTGCT |
| E-Syt3 | CTCGAGCTTGGGAGACAGATG | CCCAGGTAGCCAGCTAGGTA |
| GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redondo, P.C.; Lopez, J.J.; Alvarado, S.; Jardin, I.; Nieto-Felipe, J.; Macias-Diaz, A.; Jimenez-Velarde, V.; Salido, G.M.; Rosado, J.A. Extended Synaptotagmins 1 and 2 Are Required for Store-Operated Calcium Entry, Cell Migration and Viability in Breast Cancer Cells. Cancers 2024, 16, 2518. https://doi.org/10.3390/cancers16142518
Redondo PC, Lopez JJ, Alvarado S, Jardin I, Nieto-Felipe J, Macias-Diaz A, Jimenez-Velarde V, Salido GM, Rosado JA. Extended Synaptotagmins 1 and 2 Are Required for Store-Operated Calcium Entry, Cell Migration and Viability in Breast Cancer Cells. Cancers. 2024; 16(14):2518. https://doi.org/10.3390/cancers16142518
Chicago/Turabian StyleRedondo, Pedro C., Jose J. Lopez, Sandra Alvarado, Isaac Jardin, Joel Nieto-Felipe, Alvaro Macias-Diaz, Vanesa Jimenez-Velarde, Gines M. Salido, and Juan A. Rosado. 2024. "Extended Synaptotagmins 1 and 2 Are Required for Store-Operated Calcium Entry, Cell Migration and Viability in Breast Cancer Cells" Cancers 16, no. 14: 2518. https://doi.org/10.3390/cancers16142518
APA StyleRedondo, P. C., Lopez, J. J., Alvarado, S., Jardin, I., Nieto-Felipe, J., Macias-Diaz, A., Jimenez-Velarde, V., Salido, G. M., & Rosado, J. A. (2024). Extended Synaptotagmins 1 and 2 Are Required for Store-Operated Calcium Entry, Cell Migration and Viability in Breast Cancer Cells. Cancers, 16(14), 2518. https://doi.org/10.3390/cancers16142518









