Surgical Interventions Following Radiotherapy in Spinal Metastases with Intermediate Instability: A Risk Factor Analysis: The Korean Society of Spinal Tumor Multicenter Study (KSST 2022-02)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Outcome Measures
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Chaichana, K.L.; Woodworth, G.F.; Sciubba, D.M.; McGirt, M.J.; Witham, T.J.; Bydon, A.; Wolinsky, J.P.; Gokaslan, Z. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery 2008, 62, 683–692, discussion 683–692. [Google Scholar] [CrossRef] [PubMed]
- Klimo, P., Jr.; Thompson, C.J.; Kestle, J.R.; Schmidt, M.H. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005, 7, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef] [PubMed]
- Fourney, D.R.; Frangou, E.M.; Ryken, T.C.; Dipaola, C.P.; Shaffrey, C.I.; Berven, S.H.; Bilsky, M.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; et al. Spinal instability neoplastic score: An analysis of reliability and validity from the spine oncology study group. J. Clin. Oncol. 2011, 29, 3072–3077. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.; Chang, S.Y.; Kim, H.; Chang, B.S. Treatment Strategy for Impending Instability in Spinal Metastases. Clin. Orthop. Surg. 2020, 12, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Pennington, Z.; Ahmed, A.K.; Westbroek, E.M.; Cottrill, E.; Lubelski, D.; Goodwin, M.L.; Sciubba, D.M. SINS Score and Stability: Evaluating the Need for Stabilization Within the Uncertain Category. World Neurosurg. 2019, 128, e1034–e1047. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.; Balboni, T.; Jones, J.; Lo, S.; Petit, J.; Rich, S.E.; Wong, R.; Hahn, C. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2017, 7, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Laufer, I.; Rubin, D.G.; Lis, E.; Cox, B.W.; Stubblefield, M.D.; Yamada, Y.; Bilsky, M.H. The NOMS framework: Approach to the treatment of spinal metastatic tumors. Oncologist 2013, 18, 744–751. [Google Scholar] [CrossRef]
- Lee, J.; Rhee, W.J.; Chang, J.S.; Chang, S.K.; Koom, W.S. Evaluation of predictive factors of vertebral compression fracture after conventional palliative radiotherapy for spinal metastasis from colorectal cancer. J. Neurosurg. Spine 2018, 28, 333–340. [Google Scholar] [CrossRef]
- Mantel, F.; Sweeney, R.A.; Klement, R.J.; Hawkins, M.A.; Belderbos, J.; Ahmed, M.; Toussaint, A.; Polat, B.; Flentje, M.; Guckenberger, M. Risk factors for vertebral compression fracture after spine stereotactic body radiation therapy: Long-term results of a prospective phase 2 study. Radiother. Oncol. 2019, 141, 62–66. [Google Scholar] [CrossRef]
- Lee, S.H.; Tatsui, C.E.; Ghia, A.J.; Amini, B.; Li, J.; Zavarella, S.M.; Tannir, N.M.; Brown, P.D.; Rhines, L.D. Can the spinal instability neoplastic score prior to spinal radiosurgery predict compression fractures following stereotactic spinal radiosurgery for metastatic spinal tumor?: A post hoc analysis of prospective phase II single-institution trials. J. Neurooncol 2016, 126, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Pyo, H.; Park, H.C.; Lim, D.H.; Yu, J.I.; Park, W.; Ahn, Y.C.; Choi, D.H.; Oh, D.; Noh, J.M.; et al. Clinical and dosimetric risk factors for vertebral compression fracture after single-fraction stereotactic body radiation therapy for spine metastases. J. Bone Oncol. 2021, 28, 100368. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.D.; Hertan, L.M.; Lam, T.C.; Skamene, S.; Chi, J.H.; Groff, M.; Cho, C.H.; Ferrone, M.L.; Harris, M.; Chen, Y.H.; et al. Assessing the utility of the spinal instability neoplastic score (SINS) to predict fracture after conventional radiation therapy (RT) for spinal metastases. Pract. Radiat. Oncol. 2018, 8, e285–e294. [Google Scholar] [CrossRef] [PubMed]
- Aiba, H.; Kimura, T.; Yamagami, T.; Watanabe, N.; Sakurai, H.; Kimura, H.; Shimozaki, S.; Yamada, S.; Otsuka, T. Prediction of skeletal-related events in patients with non-small cell lung cancer. Support. Care Cancer 2016, 24, 3361–3367. [Google Scholar] [CrossRef] [PubMed]
- Thibault, I.; Atenafu, E.G.; Chang, E.; Chao, S.; Ameen, A.O.; Zhou, S.; Boehling, N.; Balagamwala, E.H.; Cunha, M.; Cho, J.; et al. Risk of vertebral compression fracture specific to osteolytic renal cell carcinoma spinal metastases after stereotactic body radiotherapy: A multi-institutional study. J. Radiosurg SBRT 2015, 3, 297–305. [Google Scholar]
- Thibault, I.; Al-Omair, A.; Masucci, G.L.; Masson-Côté, L.; Lochray, F.; Korol, R.; Cheng, L.; Xu, W.; Yee, A.; Fehlings, M.G.; et al. Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: Analysis of outcomes and risk of vertebral compression fracture. J. Neurosurg. Spine 2014, 21, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, A.; Atenafu, E.G.; Chao, S.; Al-Omair, A.; Boehling, N.; Balagamwala, E.H.; Cunha, M.; Thibault, I.; Angelov, L.; Brown, P.; et al. Vertebral compression fracture after spine stereotactic body radiotherapy: A multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J. Clin. Oncol. 2013, 31, 3426–3431. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.V.; Al-Omair, A.; Atenafu, E.G.; Masucci, G.L.; Letourneau, D.; Korol, R.; Yu, E.; Howard, P.; Lochray, F.; da Costa, L.B.; et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): Analysis of predictive factors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e343–e349. [Google Scholar] [CrossRef] [PubMed]
- Faruqi, S.; Tseng, C.L.; Whyne, C.; Alghamdi, M.; Wilson, J.; Myrehaug, S.; Soliman, H.; Lee, Y.; Maralani, P.; Yang, V.; et al. Vertebral Compression Fracture After Spine Stereotactic Body Radiation Therapy: A Review of the Pathophysiology and Risk Factors. Neurosurgery 2018, 83, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Bilsky, M.H.; Laufer, I.; Fourney, D.R.; Groff, M.; Schmidt, M.H.; Varga, P.P.; Vrionis, F.D.; Yamada, Y.; Gerszten, P.C.; Kuklo, T.R. Reliability analysis of the epidural spinal cord compression scale. J. Neurosurg. Spine 2010, 13, 324–328. [Google Scholar] [CrossRef]
- Park, S.J.; Ma, C.H.; Lee, C.S.; Jeon, C.Y.; Shin, T.S.; Park, J.S. Survival and Functional Outcomes after Surgical Treatment for Spinal Metastasis in Patients with a Short Life Expectancy. J. Clin. Med. 2022, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Chang, B.S.; Kim, H.; Kang, D.H.; Chang, S.Y. An Updated Review on the Treatment Strategy for Spinal Metastasis from the Spine Surgeon’s Perspective. Asian Spine J. 2022, 16, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Ebata, K.; Yasuhara, Y.; Enomoto, A.; Saito, T. Outcomes and Prognosis of Neurological Decompression and Stabilization for Spinal Metastasis: Is Assessment with the Spinal Instability Neoplastic Score Useful for Predicting Surgical Results? Asian Spine J. 2018, 12, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Gonzalez, D.E.; Roblesgil-Medrano, A.; Villarreal-Espinosa, J.B.; Tellez-Garcia, E.; Bueno-Gutierrez, L.C.; Rodriguez-Barreda, J.R.; Flores-Villalba, E.; Martinez, H.R.; Benvenutti-Regato, M.; Figueroa-Sanchez, J.A. Minimally Invasive versus Open Surgery for Spinal Metastasis: A Systematic Review and Meta-Analysis. Asian Spine J. 2022, 16, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hong, J.T.; Lee, S.H.; Yi, S.; Sohn, M.J.; Kim, S.H.; Chung, C.K.; Korean Spine Oncology Research, S. Is the Spinal Instability Neoplastic Score Accurate and Reliable in Predicting Vertebral Compression Fractures for Spinal Metastasis? A Systematic Review and Qualitative Analysis. J. Korean Neurosurg. Soc. 2021, 64, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Lee, C.H.; Yang, S.H.; Hyun, S.J.; Kim, C.H.; Park, S.B.; Kim, K.J.; Chung, C.K. Accuracy and precision of the spinal instability neoplastic score (SINS) for predicting vertebral compression fractures after radiotherapy in spinal metastases: A meta-analysis. Sci. Rep. 2021, 11, 5553. [Google Scholar] [CrossRef] [PubMed]
- Bernard, V.; Bishop, A.J.; Allen, P.K.; Amini, B.; Wang, X.A.; Li, J.; Tatsui, C.E.; Rhines, L.D.; Brown, P.D.; Ghia, A.J. Heterogeneity in Treatment Response of Spine Metastases to Spine Stereotactic Radiosurgery Within "Radiosensitive" Subtypes. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, A.L.; van der Velden, J.M.; Verkooijen, H.M.; van Vulpen, M.; Oner, F.C.; Fisher, C.G.; Verlaan, J.J. The Effect of Introducing the Spinal Instability Neoplastic Score in Routine Clinical Practice for Patients With Spinal Metastases. Oncologist 2016, 21, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.H.; Kim, J.W.; Kim, Y.S.; Lee, S.H.; Cho, Y.B.; Lee, H.K.; Kim, Y.G.; Jeong, W.S.; Kim, K.B. Negligible pharmacokinetic interaction of red ginseng and antihypertensive agent amlodipine in Sprague-Dawley rats. J. Toxicol. Environ. Health A 2014, 77, 1372–1383. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.H.; Kim, K.; Kim, H.J.; Chie, E.K.; Shin, K.H.; Wu, H.G.; Kim, I.H. The Feasibility of Spinal Stereotactic Radiosurgery for Spinal Metastasis with Epidural Cord Compression. Cancer Res. Treat. 2019, 51, 1324–1335. [Google Scholar] [CrossRef]
- Rades, D.; Segedin, B.; Conde-Moreno, A.J.; Ferrer-Albiach, C.; Metz, M.; Polat, B.; Badakhshi, H.; Schreiber, A.; Nitsche, M.; Cacicedo, J.; et al. Patient-Reported Outcomes-Secondary Analysis of the SCORE-2 Trial Comparing 4 Gy x 5 to 3 Gy x 10 for Metastatic Epidural Spinal Cord Compression. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Fareed, M.M.; Pike, L.R.G.; Bang, A.; Huynh, M.A.; Taylor, A.; Spektor, A.; Awad, M.M.; Ott, P.A.; Krishnan, M.; Balboni, T.A.; et al. Palliative Radiation Therapy for Vertebral Metastases and Metastatic Cord Compression in Patients Treated With Anti-PD-1 Therapy. Front. Oncol. 2019, 9, 199. [Google Scholar] [CrossRef] [PubMed]
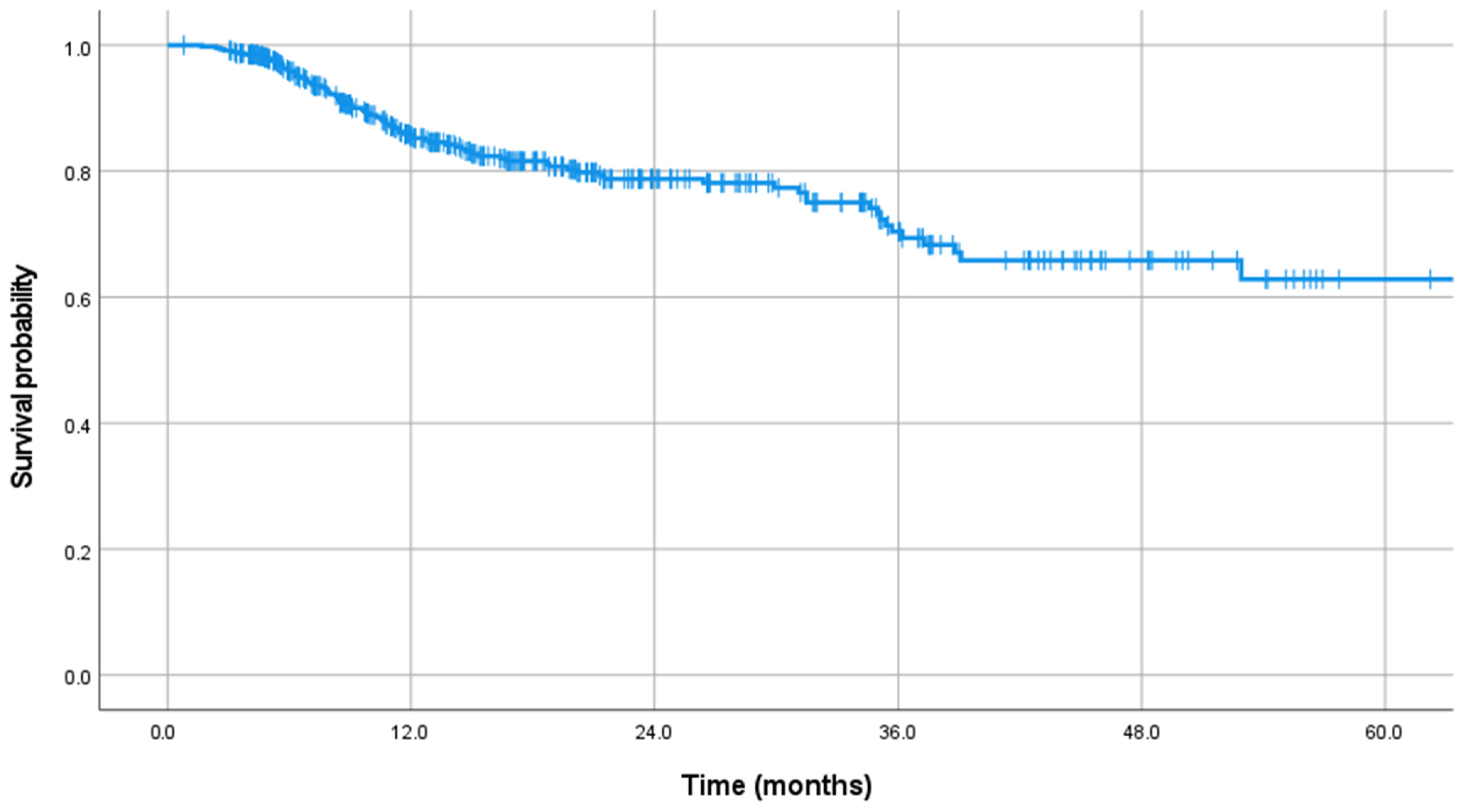
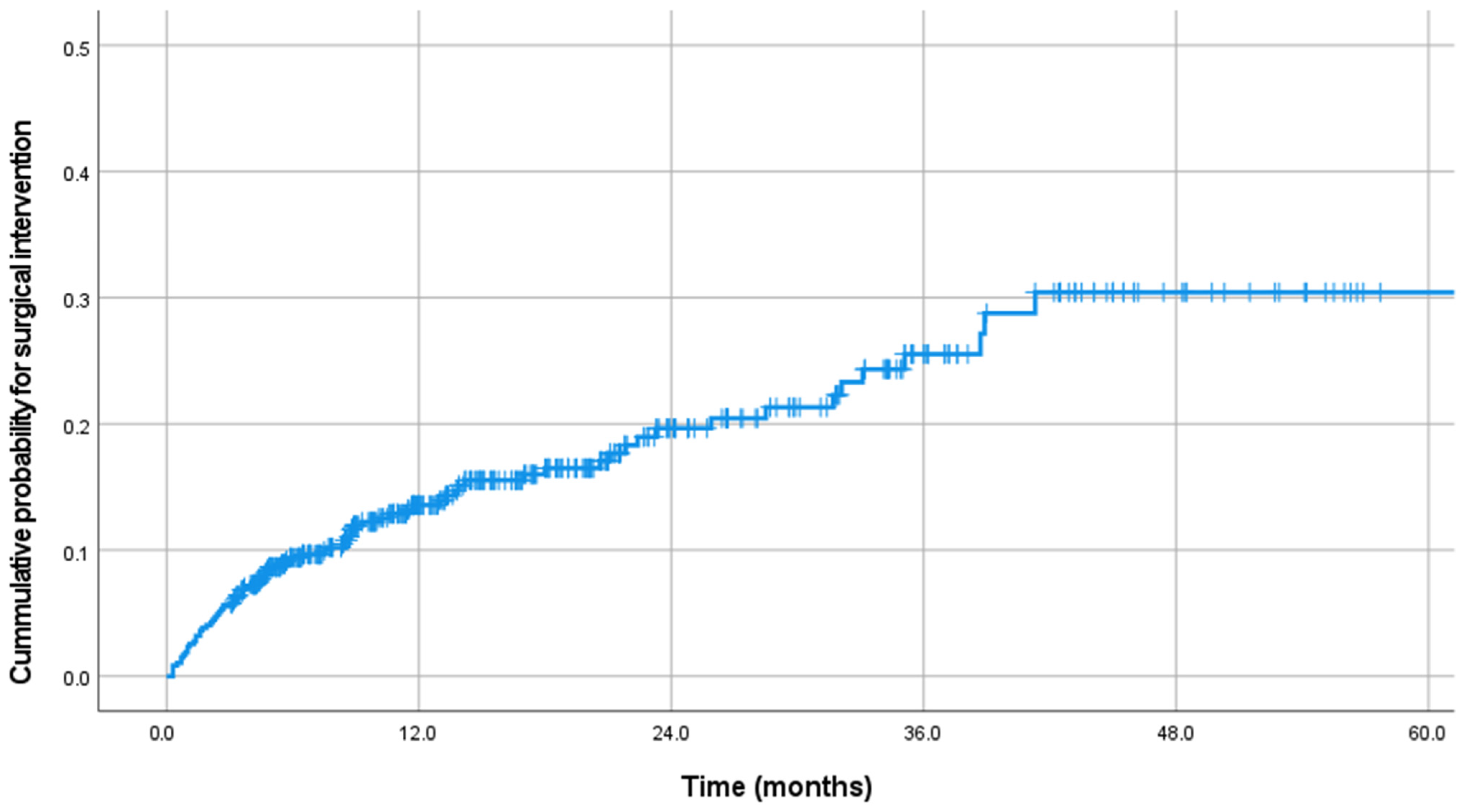

| Parameter | Total | Group S | Group NS | p Value |
|---|---|---|---|---|
| Age, years | 59.9 ± 12.7 | 58.5 ± 9.9 | 60.2 ± 13.1 | 0.309 |
| Male, n (%) | 271 (57.8%) | 51 (64.6%) | 220 (56.4%) | 0.212 |
| Primary tumor site, n (%) | 0.022 | |||
| Lung, n (%) | 123 (26.2%) | 25 (31.6%) | 98 (25.1%) | |
| Liver, n (%) | 72 (15.4%) | 15 (19.0%) | 57 (14.6%) | |
| Breast, n (%) | 63 (13.4%) | 3 (3.8%) | 60 (15.4%) | |
| Kidney, n (%) | 32 (6.8%) | 11 (13.9%) | 21 (5.4%) | |
| Colorectum, n (%) | 27 (5.8%) | 5 (6.3%) | 22 (5.6%) | |
| Prostate, n (%) | 21 (4.6%) | 2 (2.5%) | 19 (4.9%) | |
| Sarcoma, n (%) | 21 (4.6%) | 3 (3.8%) | 18 (4.6%) | |
| Nasopharynx, n (%) | 11 (2.3%) | 3 (3.8%) | 8 (2.1%) | |
| Pancreas, n (%) | 10 (2.2%) | 2 (2.5%) | 8 (2.1%) | |
| Others, n (%) | 89 (19.0%) | 10 (12.7%) | 79 (20.3%) | |
| Presence of visceral metastases | 0.796 | |||
| Yes, n (%) | 221 (48.4%) | 35 (50.0%) | 186 (48.1%) | |
| No, n (%) | 236 (51.6%) | 35 (50.0%) | 201 (51.9%) | |
| Bilsky grade for ESCC | <0.001 | |||
| Grade 0, n (%) | 113 (24.7%) | 5 (7.1%) | 108 (27.9%) | |
| Grade 1, n (%) | 206 (45.1%) | 29 (41.4%) | 177 (45.7%) | |
| Grade 2, n (%) | 105 (23.0%) | 22 (31.4%) | 83 (21.4%) | |
| Grade 3, n (%) | 33 (7.2%) | 14 (20.0%) | 19 (4.9%) |
| Parameter | Statistics |
|---|---|
| Follow-up duration, months | 18.2 ± 13.4 |
| Survival data | n = 469 |
| Number of alive patients, n (%) | 379 (80.8%) |
| Estimated median survival time after radiotherapy (months) | 43.6 ± 1.3 |
| Further treatment after radiotherapy | n = 469 |
| No further local treatment, n (%) | 310 (66.1%) |
| Re-radiotherapy, n (%) | 71 (15.1%) |
| Vertebroplasty, n (%) | 9 (1.9%) |
| Surgery, n (%) | 75 (16.0%) |
| Surgery recommended but not performed, n (%) | 4 (0.9%) |
| Surgical intervention | n = 79 |
| Mean time from radiotherapy, months | 10.3 ± 12.1 |
| Main symptoms of surgical intervention | |
| Pain, n (%) | 30 (40.0%) |
| Neurologic deficit, n (%) | 17 (21.5%) |
| Pain and neurologic deficit, n (%) | 32 (40.5%) |
| Types of surgical treatment | |
| Stabilization only, n (%) | 12 (15.2%) |
| Decompressive laminectomy with stabilization, n (%) | 49 (62.0%) |
| Vertebrectomy with stabilization, n (%) | 18 (22.8%) |
| SINS Category | Total | Group S | Group NS | p Value |
|---|---|---|---|---|
| Total sum of SINS, mean ± SD | 9.0 ± 1.5 | 9.1 ± 1.7 | 8.9 ± 1.5 | 0.430 |
| Location | 0.794 | |||
| Junctional, n (%) | 235 (50.1%) | 38 (48.1%) | 197 (50.5%) | |
| Mobile, n (%) | 135 (28.8%) | 22 (27.8%) | 113 (29.0%) | |
| Semi-rigid, n (%) | 97 (20.7%) | 19 (24.1%) | 78 (20.0%) | |
| Rigid, n (%) | 2 (0.4%) | 0 (0%) | 2 (0.5%) | |
| Pain | 0.015 | |||
| Yes, n (%) | 124 (26.4%) | 31 (39.2%) | 93 (23.8%) | |
| Occasional pain but not mechanical, n (%) | 330 (70.4%) | 45 (57.0%) | 285 (73.1%) | |
| Pain-free lesion, n (%) | 15 (3.2%) | 3 (3.8%) | 12 (3.1%) | |
| Bone lesion | 0.030 | |||
| Lytic, n (%) | 322 (68.7%) | 64 (81.0%) | 258 (66.2%) | |
| Mixed (lytic/blastic), n (%) | 130 (27.7%) | 14 (17.7%) | 116 (29.7%) | |
| Blastic, n (%) | 17 (3.6%) | 1 (1.3%) | 16 (4.1%) | |
| Spinal alignment | 0.367 | |||
| Subluxation/translation present, n (%) | 1 (0.2%) | 0 (0%) | 1 (0.3%) | |
| De novo deformity, n (%) | 68 (14.5%) | 7 (8.9%) | 61 (15.6%) | |
| Normal alignment, n (%) | 400 (85.3%) | 72 (91.1%) | 328 (84.1%) | |
| Vertebral body collapse | 0.034 | |||
| >50% collapse, n (%) | 39 (8.4%) | 13 (16.5%) | 26 (6.7%) | |
| <50% collapse, n (%) | 158 (33.8%) | 25 (31.6%) | 133 (34.3%) | |
| No collapse with >50% body involved, n (%) | 164 (35.1%) | 27 (34.2%) | 137 (35.3%) | |
| None of the above, n (%) | 106 (22.7%) | 14 (17.7%) | 92 (23.7%) | |
| Posterolateral involvement | 0.143 | |||
| Bilateral, n (%) | 90 (19.2%) | 21 (26.6%) | 69 (17.7%) | |
| Unilateral, n (%) | 238 (50.7%) | 39 (49.4%) | 199 (51.0%) | |
| None of the above, n (%) | 141 (30.1%) | 19 (24.1%) | 122 (31.3%) |
| Parameter | Total | Group S | Group NS | p Value |
|---|---|---|---|---|
| Under systemic treatment | 0.796 | |||
| Yes, n (%) | 306 (65.2%) | 53 (17.3%) | 26 (16.0%) | |
| No, n (%) | 163 (34.8%) | 253 (82.7%) | 137 (84.0%) | |
| Use of antiresorptive agent | 0.123 | |||
| Yes, n (%) | 39 (8.3%) | 3 (7.7%) | 76 (17.7%) | |
| No, n (%) | 430 (91.7%) | 36 (92.3%) | 354 (82.3%) | |
| Number of fractionations | 5.0 ± 3.7 | 4.9 ± 3.7 | 5.4 ± 3.6 | 0.303 |
| SBRT | 1.000 | |||
| Yes, n (%) | 179 (38.2%) | 30 (38.0%) | 149 (38.2%) | |
| No, n (%) | 290 (61.8%) | 49 (62.0%) | 241 (61.8%) | |
| Radiation dose per fraction, Gy | 8.2 ± 6.5 | 7.8 ± 6.5 | 8.2 ± 6.5 | 0.601 |
| Total dose, Gy | 23.8 ± 7.5 | 25.5 ± 10.3 | 23.4 ± 6.6 | 0.054 |
| EQD210, Gy | 31.2 ± 17.3 | 34.7 ± 20.1 | 30.5 ± 16.6 | 0.049 |
| Number of vertebral bodies irradiated | 0.082 | |||
| 1, n (%) | 198 (42.2%) | 37 (46.8%) | 161 (41.3%) | |
| 2, n (%) | 68 (14.5%) | 16 (20.3%) | 52 (13.3%) | |
| ≥3, n (%) | 203 (43.3%) | 26 (32.9%) | 177 (45.4%) |
| Variable | OR (95% CI) | p Value |
|---|---|---|
| Primary tumor site | 0.043 | |
| Others | Reference | |
| Lung | 2.871 (1.138–6.441) | 0.023 |
| Liver | 2.891 (1.127–6.512) | 0.034 |
| Breast | 0.542 (0.135–2.174) | 0.387 |
| Kidney | 5.588 (1.731–14.919) | 0.003 |
| Colorectum | 1.797 (0.491–6.582) | 0.376 |
| Prostate | 1.007 (0.195–5.752) | 0.994 |
| Sarcoma | 1.033 (0.285–5.330) | 0.806 |
| Nasopharynx | 3.212 (0.708–16.015) | 0.145 |
| Pancreas | 3.568 (0.497–19.314) | 0.176 |
| Bilsky grade for ESCC | <0.001 | |
| Grade 0 | Reference | |
| Grade 1 | 3.602 (1.299–9.650) | 0.014 |
| Grade 2 | 6.374 (2.144–17.600) | 0.001 |
| Grade 3 | 19.966 (5.662–60.038) | <0.001 |
| SINS pain | 0.119 | |
| No pain | Reference | |
| Occasional pain | 2.156 (0.486–9.558) | 0.312 |
| Mechanical pain | 1.200 (0.278–5.170) | 0.807 |
| SINS bone lesion | 0.068 | |
| Blastic | Reference | |
| Mixed | 1.957 (0.216–17.745) | 0.210 |
| Lytic | 2.175 (1.117–4.143) | 0.020 |
| SINS-ollapse | 0.613 | |
| No collapse | Reference | |
| Body involvement >50% | 1.142 (0.256–2.480) | |
| Collapse <50% | 0.950 (0.432–2.088) | |
| Collapse >50% | 1.749 (0.640–4.781) | |
| EQD210 | 1.014 (1.001–1.028) | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-J.; Kim, J.H.; Ahn, Y.C.; Koom, W.S.; Byun, H.K.; Kim, Y.-H.; Kim, S.-I.; Kang, D.-H.; on behalf of the Korean Society of Spinal Tumor. Surgical Interventions Following Radiotherapy in Spinal Metastases with Intermediate Instability: A Risk Factor Analysis: The Korean Society of Spinal Tumor Multicenter Study (KSST 2022-02). Cancers 2024, 16, 2554. https://doi.org/10.3390/cancers16142554
Park S-J, Kim JH, Ahn YC, Koom WS, Byun HK, Kim Y-H, Kim S-I, Kang D-H, on behalf of the Korean Society of Spinal Tumor. Surgical Interventions Following Radiotherapy in Spinal Metastases with Intermediate Instability: A Risk Factor Analysis: The Korean Society of Spinal Tumor Multicenter Study (KSST 2022-02). Cancers. 2024; 16(14):2554. https://doi.org/10.3390/cancers16142554
Chicago/Turabian StylePark, Se-Jun, Jin Ho Kim, Yong Chan Ahn, Woong Sub Koom, Hwa Kyung Byun, Young-Hoon Kim, Sang-Il Kim, Dong-Ho Kang, and on behalf of the Korean Society of Spinal Tumor. 2024. "Surgical Interventions Following Radiotherapy in Spinal Metastases with Intermediate Instability: A Risk Factor Analysis: The Korean Society of Spinal Tumor Multicenter Study (KSST 2022-02)" Cancers 16, no. 14: 2554. https://doi.org/10.3390/cancers16142554





