Novel Detection of Pleomorphic Adenomas via Analysis of 68Ga-DOTATOC PET/CT Imaging
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Selection and Data Collection
2.2. Immunohistochemistry Protocol
2.3. IHC Scoring Methodology
2.4. Acquisition, Analysis and Interpretation of Images
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aro, K.; Valle, J.; Tarkkanen, J.; Mäkitie, A.; Atula, T. Repeatedly recurring pleomorphic adenoma: A therapeutic challenge. Acta Otorhinolaryngol. Ital. 2019, 39, 156–161. [Google Scholar] [CrossRef]
- Skálová, A.; Hyrcza, M.D.; Leivo, I. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Salivary Glands. Head Neck Pathol. 2022, 16, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Choi, J.; Hwang, I.; Cho, J.; Ko, Y.-H.; Jeong, H.-S. Comparative Longitudinal Analysis of Malignant Transformation in Pleomorphic Adenoma and Recurrent Pleomorphic Adenoma. J. Clin. Med. 2022, 11, 1808. [Google Scholar] [CrossRef] [PubMed]
- Key, S.; Chia, C.; Hasan, Z.; Sundaresan, P.; Dwivedi, R.C.; Riffat, F. Systematic review of prognostic factors in carcinoma ex pleomorphic adenoma. Oral Oncol. 2022, 133, 106052. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Li, W. Clinical parameters predictors of malignant transformation of recurrent parotid pleomorphic adenoma. Sci. Rep. 2023, 13, 4543. [Google Scholar] [CrossRef] [PubMed]
- Levyn, H.; Subramanian, T.; Eagan, A.; Katabi, N.; Lin, O.; Badillo, N.D.; Martinez, G.; Scholfield, D.W.; Wong, R.J.; Shah, J.P.; et al. Risk of Carcinoma in Pleomorphic Adenomas of the Parotid. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.; Ratnasingham, K. Metastasising pleomorphic adenoma: Systematic review. Int. J. Surg. 2015, 19, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Nouraei, S.A.R.; Ferguson, M.S.; Clarke, P.M.; Sandison, A.; Sandhu, G.S.; Michaels, L.; Rhys-Evans, P. Metastasizing pleomorphic salivary adenoma. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Alshagroud, R.; Kamoh, A.; Popat, S.R.; Brandwein-Weber, M.; Aguirre, A. Metastasizing Pleomorphic Adenoma Case Report and Review of the Literature. Head Neck Pathol. 2017, 11, 487–493. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.K.; Chan, J.K.; Rubin Grandis, J.; Slootweg, P.J. WHO Classification of Head and Neck Tumours; IARC Press: Lyon, France, 2017. [Google Scholar]
- Odell, E.W. Head and Neck Tumours; Forthcoming; International Agency for Research on Cancer: Lyon, France, 2024. [Google Scholar]
- Ihrler, S.; Stiefel, D.; Jurmeister, P.; Sandison, A.; Chaston, N.; Laco, J.; Zidar, N.; Brcic, L.; Stoehr, R.; Agaimy, A. Salivary carcinosarcoma: Insight into multistep pathogenesis indicates uniform origin as sarcomatoid variant of carcinoma ex pleomorphic adenoma with frequent heterologous elements. Histopathology 2023, 82, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Koochakzadeh, S.; Neskey, D.M.; Nguyen, S.A.; Lentsch, E.J. Carcinoma ex pleomorphic adenoma: A review of incidence, demographics, risk factors, and survival. Am. J. Otolaryngol. 2019, 40, 102279. [Google Scholar] [CrossRef] [PubMed]
- Ndotora, F.; Jackson, B. Concordance of fine needle aspiration cytology and final histology of salivary gland tumours. S. Afr. J. Surg. 2023, 61, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Buchman, C.; Stringer, S.P.; Mendenhall, W.M.; Parsons, J.T.; Jordan, J.R.; Cassisi, N.J. Pleomorphic adenoma: Effect of tumor spill and inadequate resection on tumor recurrence. Laryngoscope 1994, 104, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Kiciński, K.; Mikaszewski, B.; Stankiewicz, C. Risk factors for recurrence of pleomorphic adenoma. Otolaryngol. Pol. 2016, 70, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Colella, G.; Cannavale, R.; Chiodini, P. Meta-analysis of surgical approaches to the treatment of parotid pleomorphic adenomas and recurrence rates. J. Cranio Maxillofac. Surg. 2015, 43, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Witt, R.L.; Rejto, L. Pleomorphic adenoma: Extracapsular dissection versus partial superficial parotidectomy with facial nerve dissection. Del. Med. J. 2009, 81, 119–125. [Google Scholar]
- Almeslet, A.S. Pleomorphic Adenoma: A Systematic Review. Int. J. Clin. Pediatr. Dent. 2020, 13, 284–287. [Google Scholar] [CrossRef]
- Richie, A.J.; Mellonie, P. Sonological Evaluation of Major Salivary Gland Lesions with Histopathological Correlation. Int. J. Contemp. Med. Surg. Radiol. 2019, 4, B91–B94. [Google Scholar] [CrossRef]
- Shepherd, G.W. Sonographic Imaging of a Pleomorphic Adenoma of the Salivary Gland. J. Diagn. Med. Sonogr. 2008, 24, 299–302. [Google Scholar] [CrossRef]
- Harb, J.L.; Zaro, C.; Nassif, S.J.; Dhingra, J.K. Point-of-care ultrasound scan as the primary modality for evaluating parotid tumors. Laryngoscope Investig. Otolaryngol. 2022, 7, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Stanek, J.J.; Khariwala, S.S. What Is the Utility of Fine-Needle Aspiration in Parotid Gland Neoplasms? Laryngoscope 2019, 129, 1255–1256. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, J.; Lu, X.; Wang, Y.; Meng, F.; Zhao, J.; Guo, C.; Yu, L.; Zhu, Z.; Zhang, T. Radiomics-based comparison of MRI and CT for differentiating pleomorphic adenomas and Warthin tumors of the parotid gland: A retrospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 591–599. [Google Scholar] [CrossRef]
- Kato, H.; Kawaguchi, M.; Ando, T.; Mizuta, K.; Aoki, M.; Matsuo, M. Pleomorphic adenoma of salivary glands: Common and uncommon CT and MR imaging features. Jpn. J. Radiol. 2018, 36, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Laurens, S.T.; Netea-Maier, R.T.; Aarntzen, E.J. 68Ga-DOTA-TOC Uptake in Pleomorphic Adenoma. Clin. Nucl. Med. 2018, 43, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.; Hofauer, B.; Wirth, M.; Wollenberg, B.; Stögbauer, F.; Notohamiprodjo, S.; Haller, B.; Reschke, R.; Knopf, A.; Strassen, U. Novel Discovery of the Somatostatin Receptor (SSTR2) in Pleomorphic Adenomas via Immunohistochemical Analysis of Tumors of the Salivary Glands. Cancers 2023, 15, 3917. [Google Scholar] [CrossRef] [PubMed]
- Poeppel, T.D.; Boy, C.; Bockisch, A.; Kotzerke, J.; Buchmann, I.; Ezziddin, S.; Scheidhauer, K.; Krause, B.J.; Schmidt, D.; Amthauer, H.; et al. Peptide receptor radionuclide therapy for patients with somatostatin receptor expressing tumours. German Guideline (S1). Nuklearmedizin 2015, 54, 1–11; quiz N2. [Google Scholar] [PubMed]
- Memmert, S.; Damanaki, A.; Nokhbehsaim, M.; Nogueira, A.V.B.; Eick, S.; Cirelli, J.A.; Jäger, A.; Deschner, J. Regulation of somatostatin receptor 2 by proinflammatory, microbial and obesity-related signals in periodontal cells and tissues. Head Face Med. 2019, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Miederer, M.; Seidl, S.; Buck, A.; Scheidhauer, K.; Wester, H.-J.; Schwaiger, M.; Perren, A. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, K.; Mishra, A.; Singh, A.; Panda, P.; Mahapatra, A.; Lenka, A. Immunocytochemistry using Liquid-based Cytology: A Tool in Hormone Receptor Analysis of Breast Cancer. J. Cytol. 2018, 35, 260–264. [Google Scholar] [CrossRef]
- Konukiewitz, B.; Schlitter, A.M.; Jesinghaus, M.; Pfister, D.; Steiger, K.; Segler, A.; Agaimy, A.; Sipos, B.; Zamboni, G.; Weichert, W.; et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20%. Mod. Pathol. 2017, 30, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Boy, C.; Heusner, T.A.; Poeppel, T.D.; Redmann-Bischofs, A.; Unger, N.; Jentzen, W.; Brandau, W.; Mann, K.; Antoch, G.; Bockisch, A.; et al. 68Ga-DOTATOC PET/CT and somatostatin receptor (sst1–sst5) expression in normal human tissue: Correlation of sst2 mRNA and SUVmax. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1224–1236. [Google Scholar] [CrossRef]
- Özgüven, S.; Filizoğlu, N.; Kesim, S.; Öksüzoğlu, K.; Şen, F.; Öneş, T.; Inanır, S.; Turoğlu, H.T.; Erdil, T.Y. Physiological Biodistribution of 68Ga-DOTA-TATE in Normal Subjects. Mol. Imaging Radionucl. Ther. 2021, 30, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Cleeren, F.; Bormans, G.; Deroose, C.M. Somatostatin receptor PET ligands—The next generation for clinical practice. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 311–331. [Google Scholar] [PubMed]
- Walker, R.C.; Smith, G.T.; Liu, E.; Moore, B.; Clanton, J.; Stabin, M. Measured human dosimetry of68Ga-DOTATATE. J. Nucl. Med. 2013, 54, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Revheim, M.-E.; Raynor, W.; Zehetner, W.; Knoll, P.; Zandieh, S.; Alavi, A. 64Cu-DOTATOC PET-CT in Patients with Neuroendocrine Tumors. Oncol. Ther. 2020, 8, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Glas, A.S.; Vermey, A.; Hollema, H.; Robinson, P.H.; Roodenburg, J.L.; Nap, R.E.; Plukker, J.T.M. Surgical treatment of recurrent pleomorphic adenoma of the parotid gland: A clinical analysis of 52 patients. Head Neck 2001, 23, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Stueven, A.K.; Kayser, A.; Wetz, C.; Amthauer, H.; Wree, A.; Tacke, F.; Wiedenmann, B.; Roderburg, C.; Jann, H. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int. J. Mol. Sci. 2019, 20, 3049. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.R.; Li, T.; Ter-Minassian, M.S.; Yang, J.; Chan, J.A.; Brais, L.K.; Masugi, Y.; Thiaglingam, A.; Brooks, N.B.; Nishihara, R.; et al. Association Between Somatostatin Receptor Expression and Clinical Outcomes in Neuroendocrine Tumors. Pancreas 2016, 45, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.-H.; Arnold, R. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Phan, A.T.; Ćwikła, J.B.; Sedláčková, E.; Thanh, X.-M.T.; Wolin, E.M.; Ruszniewski, P. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: Final results of the CLARINET open-label extension study. Endocrine 2021, 71, 502–513. [Google Scholar] [CrossRef]
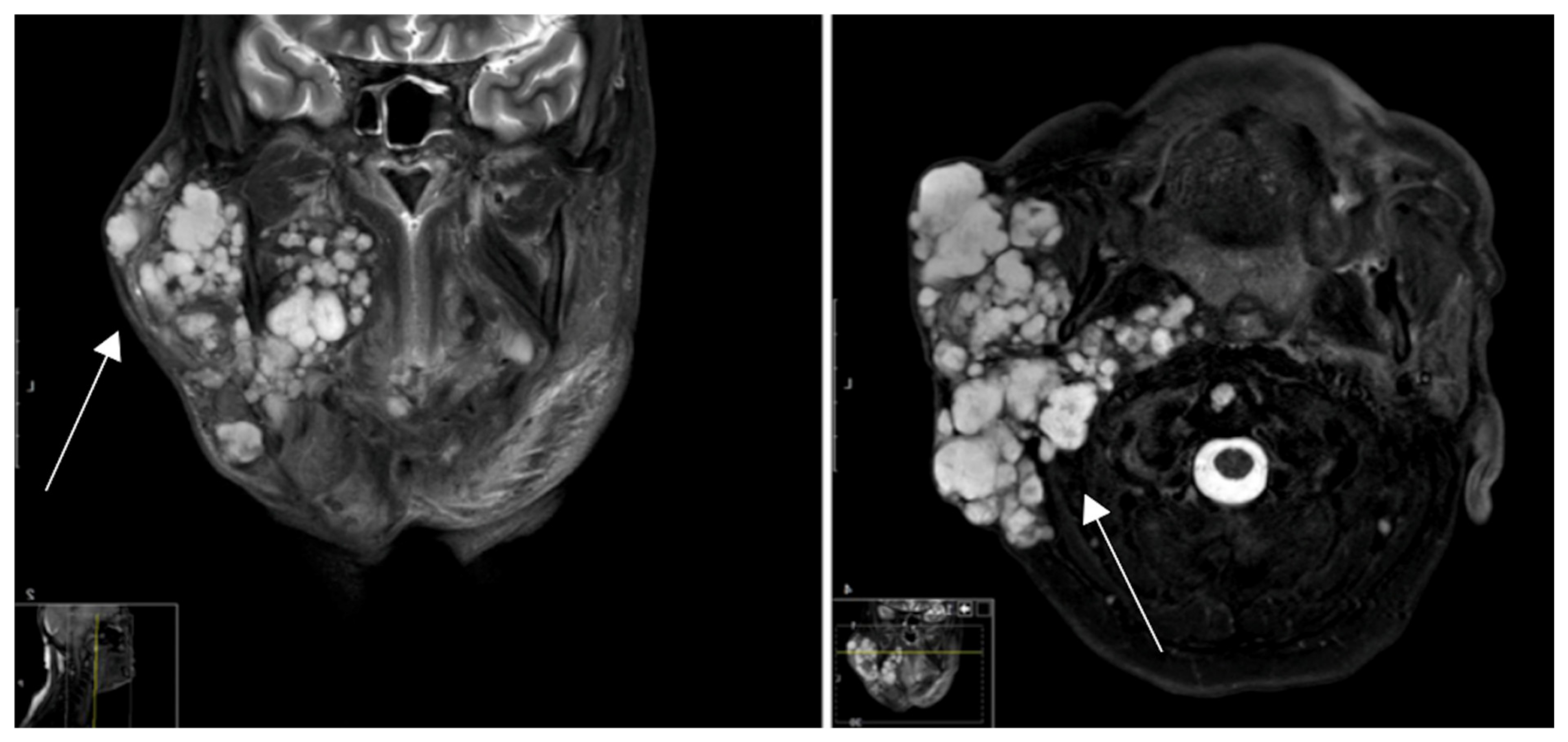










| Staining | Score | Evaluation |
|---|---|---|
| No staining observed, faint membrane staining in ≤10% of tumor cells | 0 | None |
| Incomplete, barely visible staining in >10% of tumor cells | 1 | Mild |
| Incomplete and/or weak circumferential staining in >10% of tumor cells, or complete, intense staining in ≤10% of tumor cells | 2 | Moderate |
| Complete, intense, staining in >10% of tumor cells | 3 | Strong |
| Patient | Entity | Percentage of Cells | Intensity of Staining | Tracer Uptake |
|---|---|---|---|---|
| 1 | PA | n/a * | n/a | Strong |
| 2 | PA | n/a | n/a | Moderate |
| 3 | PA | 55 | 3 | Weak |
| 4 | PA | 80 | 3 | Moderate |
| 5 | PA | 20 | 3 | Moderate |
| 6 | Warthin tumor | 0 | 0 | Weak |
| 7 | Warthin tumor | 0 | 0 | None |
| 8 | Warthin tumor | 0 | 0 | None |
| 9 | Oncocytoma | 0 | 0 | Weak |
| 10 | B-NHL | 20 | 2 | Weak |
| 11 | SCC | 0 | 0 | None |
| 12 | Granuloma | n/a | n/a | None |
| 13 | Chronic parotitis | 0 | 0 | None |
| SUVmax | SUVmean | |||||
|---|---|---|---|---|---|---|
| Tumor | Contralateral | p | Tumor | Contralateral | p | |
| Pleomorphic adenoma | 9.65 ± 6.34 | 1.53 ± 0.33 | 0.02 | 4.29 ± 2.38 | 1.15 ± 0.20 | 0.02 |
| Warthin tumor | 1.98 ± 0.63 | 1.81 ± 0.85 | 0.79 | 1.33 ± 0.68 | 1.33 ± 0.75 | 1.00 |
| Other | 3.67 ± 1.84 | 2.17 ± 0.44 | 0.11 | 2.61 ± 1.08 | 1.62 ± 0.25 | 0.08 |
| Liver Quotient | Blood Pool Quotient | |||||
|---|---|---|---|---|---|---|
| Tumor | Contralateral | p | Tumor | Contralateral | p | |
| Pleomorphic adenoma | 1.20 ± 0.85 | 0.18 ± 0.04 | 0.03 | 11.40 ± 8.28 | 1.73 ± 0.60 | 0.03 |
| Warthin tumor | 0.24 ± 0.02 | 0.22 ± 0.05 | 0.50 | 3.05 ± 1.08 | 2.77 ± 1.36 | 0.80 |
| Other | 0.46 ± 0.25 | 0.27 ± 0.08 | 0.15 | 4.92 ± 2.94 | 2.91 ± 1.04 | 0.19 |
| Ratio-SUVmax | Ratio-SUVmean | Ratio-Liver Quotient | Ratio-Blood Pool Quotient | |
|---|---|---|---|---|
| Pleomorphic adenoma | 6.26 ± 4.31 | 3.60 ± 1.93 | 1.20 ± 0.85 | 11.40 ± 8.28 |
| Warthin tumor | 1.15 ± 0.16 | 1.03 ± 0.20 | 0.24 ± 0.02 | 3.05 ± 1.08 |
| Other | 1.81 ± 1.13 | 1.67 ± 0.85 | 0.46 ± 0.25 | 4.92 ± 2.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, F.; Kloppenburg, M.; Hofauer, B.; Wollenberg, B.; Hoch, C.C.; Stögbauer, F.; Haller, B.; Knopf, A.; Strassen, U.; Notohamiprodjo, S. Novel Detection of Pleomorphic Adenomas via Analysis of 68Ga-DOTATOC PET/CT Imaging. Cancers 2024, 16, 2624. https://doi.org/10.3390/cancers16152624
Johnson F, Kloppenburg M, Hofauer B, Wollenberg B, Hoch CC, Stögbauer F, Haller B, Knopf A, Strassen U, Notohamiprodjo S. Novel Detection of Pleomorphic Adenomas via Analysis of 68Ga-DOTATOC PET/CT Imaging. Cancers. 2024; 16(15):2624. https://doi.org/10.3390/cancers16152624
Chicago/Turabian StyleJohnson, Felix, Marcel Kloppenburg, Benedikt Hofauer, Barbara Wollenberg, Cosima C. Hoch, Fabian Stögbauer, Bernhard Haller, Andreas Knopf, Ulrich Strassen, and Susan Notohamiprodjo. 2024. "Novel Detection of Pleomorphic Adenomas via Analysis of 68Ga-DOTATOC PET/CT Imaging" Cancers 16, no. 15: 2624. https://doi.org/10.3390/cancers16152624







