Emerging Therapeutic Targets and Future Directions in Advanced Gastric Cancer: A Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Anti-Her2 Treatments
3. Targeting Pd-L1 and MSI: The Role of Immune Checkpoint Inhibitors
4. Anti-VEGF Treatments
5. Targeting Claudin 18.2
6. New Frontiers in Metastatic Gastric Cancer
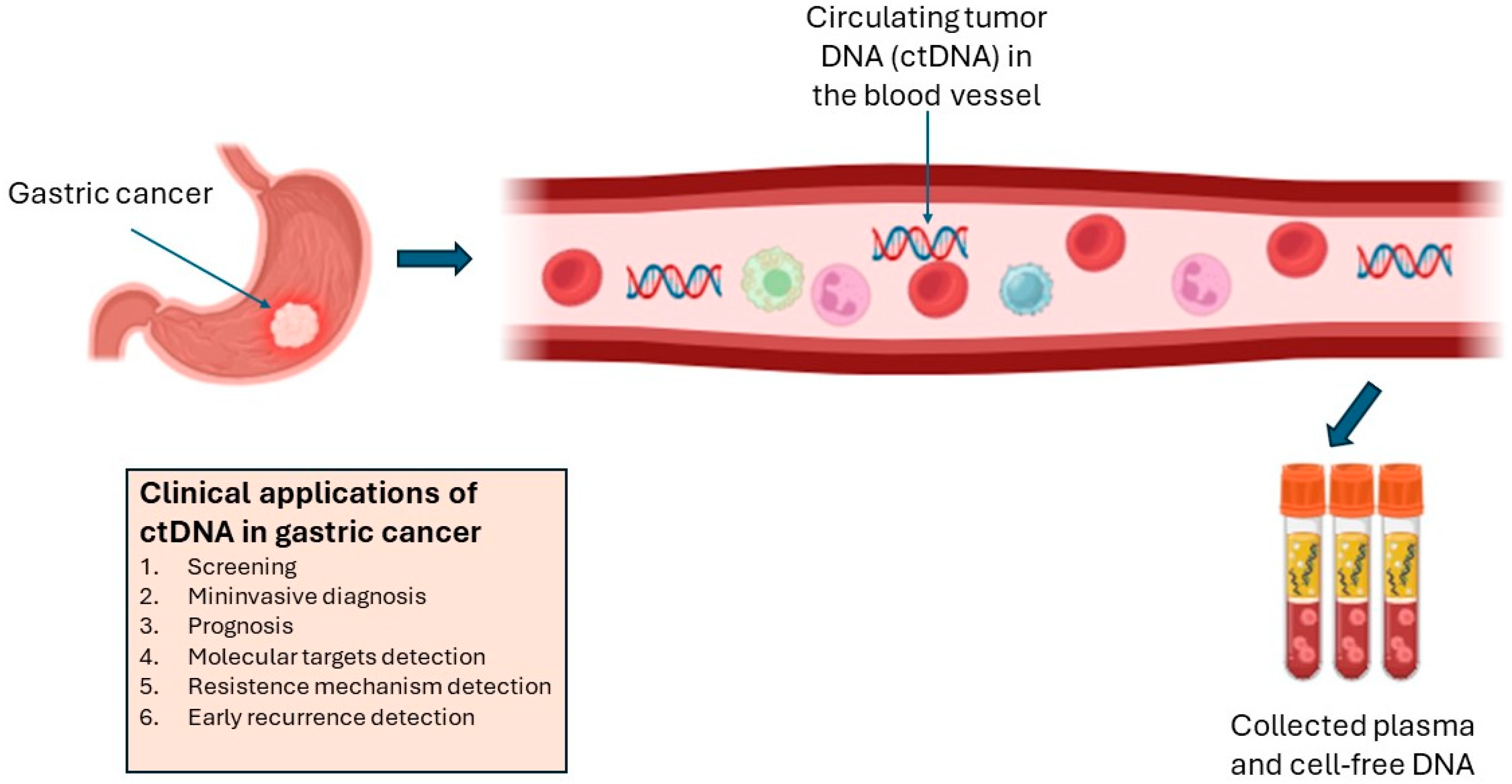
6.1. Potential Applications of ctDNA
6.2. Promising Molecular Targets: FGFR and MET
6.3. Role of Homologous Recombination Deficiency
6.4. CAR-T Cells
6.5. Cancer Vaccines
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C. Gastric Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; Van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van De Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Ratti, M.; Orlandi, E.; Hahne, J.C.; Vecchia, S.; Citterio, C.; Anselmi, E.; Toscani, I.; Ghidini, M. Targeting FGFR Pathways in Gastrointestinal Cancers: New Frontiers of Treatment. Biomedicines 2023, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, K.T.; Shin, S.-J.; Cheong, J.-H.; Choi, Y.Y. Genomic and Evolutionary Characteristics of Metastatic Gastric Cancer by Routes. Br. J. Cancer 2023, 129, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-L.; He, Y.; Xu, R.-H. Gastric Cancer Treatment: Recent Progress and Future Perspectives. J. Hematol. Oncol. 2023, 16, 57. [Google Scholar] [CrossRef]
- Jin, X.; Liu, Z.; Yang, D.; Yin, K.; Chang, X. Recent Progress and Future Perspectives of Immunotherapy in Advanced Gastric Cancer. Front. Immunol. 2022, 13, 948647. [Google Scholar] [CrossRef]
- Scheck, M.K.; Hofheinz, R.D.; Lorenzen, S. HER2-Positive Gastric Cancer and Antibody Treatment: State of the Art and Future Developments. Cancers 2024, 16, 1336. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in Combination with Chemotherapy versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Bang, Y.-J.; Qin, S.K.; Chung, H.C.; Xu, J.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in Combination with Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2–Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC—A Randomized Phase III Trial. JCO 2016, 34, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Kulukian, A.; Lee, P.; Taylor, J.; Rosler, R.; De Vries, P.; Watson, D.; Forero-Torres, A.; Peterson, S. Preclinical Activity of HER2-Selective Tyrosine Kinase Inhibitor Tucatinib as a Single Agent or in Combination with Trastuzumab or Docetaxel in Solid Tumor Models. Mol. Cancer Ther. 2020, 19, 976–987. [Google Scholar] [CrossRef]
- Park, H.; Bekaii-Saab, T.S.; Kim, S.S.; Kamath, S.D.; Pishvaian, M.J.; Chen, C.; Zhen, D.B.; Mayor, J.G.; Tan, Q.; Strickler, J.H. Phase 1b/2, Open-Label, Dose-Escalation and Expansion Trial of Tucatinib in Combination with Trastuzumab with and without Oxaliplatin-Based Chemotherapy or Pembrolizumab in Patients with Unresectable or Metastatic HER2+ Gastrointestinal Cancers (Trial in Progress). JCO 2022, 40 (Suppl. S4), TPS376. [Google Scholar] [CrossRef]
- Strickler, J.H.; Nakamura, Y.; Yoshino, T.; Catenacci, D.V.T.; Janjigian, Y.Y.; Barzi, A.; Bekaii-Saab, T.S.; Lenz, H.-J.; Lee, J.; Van Cutsem, E.; et al. MOUNTAINEER-02: Phase II/III Study of Tucatinib, Trastuzumab, Ramucirumab, and Paclitaxel in Previously Treated HER2+ Gastric or Gastroesophageal Junction Adenocarcinoma—Trial in Progress. JCO 2021, 39 (Suppl. S3), TPS252. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Di Bartolomeo, M.; Smyth, E.; Chau, I.; Park, H.; Siena, S.; Lonardi, S.; Wainberg, Z.A.; Ajani, J.; Chao, J.; et al. Trastuzumab Deruxtecan in Patients in the USA and Europe with HER2-Positive Advanced Gastric or Gastroesophageal Junction Cancer with Disease Progression on or after a Trastuzumab-Containing Regimen (DESTINY-Gastric02): Primary and Updated Analyses from a Single-Arm, Phase 2 Study. Lancet Oncol. 2023, 24, 744–756. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Oh, D.-Y.; Rha, S.Y.; Lee, K.W.; Steeghs, N.; Chao, Y.; Di Bartolomeo, M.; Díez Garcia, M.; Haj Mohammad, N.; Stein, A.; et al. Dose-Escalation and Dose-Expansion Study of Trastuzumab Deruxtecan (T-DXd) Monotherapy and Combinations in Patients (Pts) with Advanced/Metastatic HER2+ Gastric Cancer (GC)/Gastroesophageal Junction Adenocarcinoma (GEJA): DESTINY-Gastric03. JCO 2022, 40 (Suppl. S4), 295. [Google Scholar] [CrossRef]
- Shitara, K.; Barlaskar, F.; Franke, F.; Kawaguchi, Y.; Shen, L.; Kamio, T.; Meinhardt, G.; Tabernero, J. P-159 Trastuzumab Deruxtecan (T-DXd) in Patients with HER2-Positive Gastric Cancer (GC) or Gastroesophageal Junction (GEJ) Adenocarcinoma Who Have Progressed on or after a Trastuzumab-Containing Regimen (DESTINY-Gastric04): A Randomized Phase 3 Study. Ann. Oncol. 2022, 33, S306–S307. [Google Scholar] [CrossRef]
- Nordstrom, J.L.; Gorlatov, S.; Zhang, W.; Yang, Y.; Huang, L.; Burke, S.; Li, H.; Ciccarone, V.; Zhang, T.; Stavenhagen, J.; et al. Anti-Tumor Activity and Toxicokinetics Analysis of MGAH22, an Anti-HER2 Monoclonal Antibody with Enhanced Fcγ Receptor Binding Properties. Breast Cancer Res. 2011, 13, R123. [Google Scholar] [CrossRef]
- Bang, Y.J.; Giaccone, G.; Im, S.A.; Oh, D.Y.; Bauer, T.M.; Nordstrom, J.L.; Li, H.; Chichili, G.R.; Moore, P.A.; Hong, S.; et al. First-in-Human Phase 1 Study of Margetuximab (MGAH22), an Fc-Modified Chimeric Monoclonal Antibody, in Patients with HER2-Positive Advanced Solid Tumors. Ann. Oncol. 2017, 28, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Kang, Y.-K.; Park, H.; Uronis, H.E.; Lee, K.-W.; Ng, M.C.H.; Enzinger, P.C.; Park, S.H.; Gold, P.J.; Lacy, J.; et al. Margetuximab plus Pembrolizumab in Patients with Previously Treated, HER2-Positive Gastro-Oesophageal Adenocarcinoma (CP-MGAH22–05): A Single-Arm, Phase 1b–2 Trial. Lancet Oncol. 2020, 21, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Kang, Y.-K.; Yoon, H.H.; Shim, B.Y.; Kim, S.T.; Oh, D.-Y.; Spira, A.I.; Ulahannan, S.V.; Avery, E.J.; Boland, P.M.; et al. Margetuximab with Retifanlimab as First-Line Therapy in HER2+/PD-L1+ Unresectable or Metastatic Gastroesophageal Adenocarcinoma: MAHOGANY Cohort A. ESMO Open 2022, 7, 100563. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Beeram, M.; Hamilton, E.; Oh, D.-Y.; Hanna, D.L.; Kang, Y.-K.; Elimova, E.; Chaves, J.; Goodwin, R.; Lee, J.; et al. Zanidatamab, a Novel Bispecific Antibody, for the Treatment of Locally Advanced or Metastatic HER2-Expressing or HER2-Amplified Cancers: A Phase 1, Dose-Escalation and Expansion Study. Lancet Oncol. 2022, 23, 1558–1570. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Chaves, J.; Oh, D.-Y.; Lee, J.; Kang, Y.-K.; Hamilton, E.; Mayordomo, J.; Cobleigh, M.; Vaklavas, C.; Elimova, E.; et al. Abstract B001: Safety and Efficacy of ZW25, a HER2-Targeted Bispecific Antibody, in Combination with Chemotherapy in Patients with Locally Advanced and/or Metastatic HER2-Expressing Gastroesophageal Cancer. Mol. Cancer Ther. 2019, 18 (Suppl. S12), B001. [Google Scholar] [CrossRef]
- Elimova, E.; Ajani, J.A.; Burris Iii, H.A.; Denlinger, C.S.; Iqbal, S.; Kang, Y.-K.; Kim, Y.H.H.; Lee, K.-W.; Lin, B.; Mehta, R.; et al. Zanidatamab + Chemotherapy as First-Line Treatment for HER2-Expressing Metastatic Gastroesophageal Adenocarcinoma (mGEA). JCO 2023, 41 (Suppl. S4), 347. [Google Scholar] [CrossRef]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef] [PubMed]
- Chaganty, B.K.R.; Qiu, S.; Gest, A.; Lu, Y.; Ivan, C.; Calin, G.A.; Weiner, L.M.; Fan, Z. Trastuzumab Upregulates PD-L1 as a Potential Mechanism of Trastuzumab Resistance through Engagement of Immune Effector Cells and Stimulation of IFNγ Secretion. Cancer Lett. 2018, 430, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Bang, Y.-J.; Fuchs, C.S.; Qin, S.-K.; Satoh, T.; Shitara, K.; Tabernero, J.; Van Cutsem, E.; Alsina, M.; Cao, Z.A.; et al. First-Line Pembrolizumab/Placebo Plus Trastuzumab and Chemotherapy in Her2-Positive Advanced Gastric Cancer: KEYNOTE-811. Future Oncol. 2021, 17, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Paschold, L.; Tintelnot, J.; Goekkurt, E.; Henkes, S.-S.; Simnica, D.; Schultheiss, C.; Willscher, E.; Bauer, M.; Wickenhauser, C.; et al. Efficacy of Ipilimumab vs FOLFOX in Combination with Nivolumab and Trastuzumab in Patients with Previously Untreated ERBB2 -Positive Esophagogastric Adenocarcinoma: The AIO INTEGA Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1150–1158. [Google Scholar] [CrossRef]
- Ghosh, C.; Luong, G.; Sun, Y. A Snapshot of the PD-1/PD-L1 Pathway. J. Cancer 2021, 12, 2735–2746. [Google Scholar] [CrossRef]
- Cheng, R.; Li, B.; Wang, H.; Zeng, Y. Immune Checkpoint Inhibitors and Cellular Immunotherapy for Advanced Gastric, Gastroesophageal Cancer: A Long Pathway. Clin. Transl. Oncol. 2023, 25, 3122–3138. [Google Scholar] [CrossRef]
- Nevo, Y.; Ferri, L. Current Management of Gastric Adenocarcinoma: A Narrative Review. J. Gastrointest. Oncol. 2023, 14, 1933–1948. [Google Scholar] [CrossRef] [PubMed]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.-H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.-P.; Li, Z.; Kim, S.-B.; et al. Pembrolizumab plus Chemotherapy versus Chemotherapy Alone for First-Line Treatment of Advanced Oesophageal Cancer (KEYNOTE-590): A Randomised, Placebo-Controlled, Phase 3 Study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef]
- Rha, S.Y.; Oh, D.-Y.; Yañez, P.; Bai, Y.; Ryu, M.-H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.-K.; et al. Pembrolizumab plus Chemotherapy versus Placebo plus Chemotherapy for HER2-Negative Advanced Gastric Cancer (KEYNOTE-859): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-Line Nivolumab plus Chemotherapy versus Chemotherapy Alone for Advanced Gastric, Gastro-Oesophageal Junction, and Oesophageal Adenocarcinoma (CheckMate 649): A Randomised, Open-Label, Phase 3 Trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. JCO 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Shan, F.; Miao, R.; Xue, K.; Li, Z.; Li, Z.; Bu, Z.; Wu, A.; Zhang, L.; Wu, X.; Zong, X.; et al. Controlling Angiogenesis in Gastric Cancer: A Systematic Review of Anti-Angiogenic Trials. Cancer Lett. 2016, 380, 598–607. [Google Scholar] [CrossRef]
- Folkman, J. Proceedings: Tumor Angiogenesis Factor. Cancer Res. 1974, 34, 2109–2113. [Google Scholar]
- Folkman, J.; Watson, K.; Ingber, D.; Hanahan, D. Induction of Angiogenesis during the Transition from Hyperplasia to Neoplasia. Nature 1989, 339, 58–61. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S. Tumor Angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a Therapeutic Target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Kasper, S.; Schuler, M. Targeted Therapies in Gastroesophageal Cancer. Eur. J. Cancer 2014, 50, 1247–1258. [Google Scholar] [CrossRef]
- Poole, R.M.; Vaidya, A. Ramucirumab: First Global Approval. Drugs 2014, 74, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Greig, S.L.; Keating, G.M. Ramucirumab: A Review in Advanced Gastric Cancer. BioDrugs 2015, 29, 341–351. [Google Scholar] [CrossRef]
- Spratlin, J.L.; Mulder, K.E.; Mackey, J.R. Ramucirumab (IMC-1121B): A Novel Attack on Angiogenesis. Future Oncol. 2010, 6, 1085–1094. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.-Y.; et al. Ramucirumab plus Paclitaxel versus Placebo plus Paclitaxel in Patients with Previously Treated Advanced Gastric or Gastro-Oesophageal Junction Adenocarcinoma (RAINBOW): A Double-Blind, Randomised Phase 3 Trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D. R et al. Ramucirumab Monotherapy for Previously Treated Advanced Gastric or Gastro-Oesophageal Junction Adenocarcinoma (REGARD): An International, Randomised, Multicentre, Placebo-Controlled, Phase 3 Trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Lorenzen, S.; Thuss-Patience, P.; Pauligk, C.; Gökkurt, E.; Ettrich, T.; Lordick, F.; Stahl, M.; Reichardt, P.; Sökler, M.; Pink, D.; et al. FOLFIRI plus Ramucirumab versus Paclitaxel plus Ramucirumab as Second-Line Therapy for Patients with Advanced or Metastatic Gastroesophageal Adenocarcinoma with or without Prior Docetaxel—Results from the Phase II RAMIRIS Study of the German Gastric Cancer Study Group at AIO. Eur. J. Cancer 2022, 165, 48–57. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Gastric Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 28 June 2024).
- Fuchs, C.S.; Shitara, K.; Di Bartolomeo, M.; Lonardi, S.; Al-Batran, S.-E.; Van Cutsem, E.; Ilson, D.H.; Alsina, M.; Chau, I.; Lacy, J.; et al. Ramucirumab with Cisplatin and Fluoropyrimidine as First-Line Therapy in Patients with Metastatic Gastric or Junctional Adenocarcinoma (RAINFALL): A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 420–435. [Google Scholar] [CrossRef]
- Yoon, H.H.; Bendell, J.C.; Braiteh, F.S.; Firdaus, I.; Philip, P.A.; Cohn, A.L.; Lewis, N.; Anderson, D.M.; Arrowsmith, E.; Schwartz, J.D.; et al. Ramucirumab Combined with FOLFOX as Front-Line Therapy for Advanced Esophageal, Gastroesophageal Junction, or Gastric Adenocarcinoma: A Randomized, Double-Blind, Multicenter Phase II Trial. Ann. Oncol. 2016, 27, 2196–2203. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Muro, K.; Shitara, K.; Oh, D.-Y.; Kang, Y.-K.; Chung, H.C.; Kudo, T.; Chin, K.; Kadowaki, S.; Hamamoto, Y.; et al. Effect of First-Line S-1 Plus Oxaliplatin with or Without Ramucirumab Followed by Paclitaxel Plus Ramucirumab on Advanced Gastric Cancer in East Asia: The Phase 2 RAINSTORM Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e198243. [Google Scholar] [CrossRef]
- LONSURF (Trifluridine and Tipiracil) Tablets, for Oral Use Initial U.S. Approval: 2015. Available online: www.accessdata.fda.gov/drugsatfda_docs/label/2019/207981s008lbl.pdf (accessed on 28 June 2024).
- Peeters, M.; Cervantes, A.; Moreno Vera, S.; Taieb, J. Trifluridine/Tipiracil: An Emerging Strategy for the Management of Gastrointestinal Cancers. Future Oncol. 2018, 14, 1629–1645. [Google Scholar] [CrossRef] [PubMed]
- Taiho Oncology, Inc. Announces FDA Approval of LONSURF® (Trifluridine and Tipiracil) for Refractory Metastatic Colorectal Cancer (mCRC). Available online: www.taihooncology.com/documents/32/lon-pm-us-0270-taiho-oncology-lonsurf_fda-approval-press-release_sept-22_final.pdf (accessed on 27 June 2024).
- Shitara, K.; Doi, T.; Dvorkin, M.; Mansoor, W.; Arkenau, H.-T.; Prokharau, A.; Alsina, M.; Ghidini, M.; Faustino, C.; Gorbunova, V.; et al. Trifluridine/Tipiracil versus Placebo in Patients with Heavily Pretreated Metastatic Gastric Cancer (TAGS): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2018, 19, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Taieb, J.; Prager, G.W.; Ciardiello, F.; Fakih, M.; Leger, C.; Fougeray, R.; Amellal, N.; Van Cutsem, E. Trifluridine/Tipiracil Plus Bevacizumab for Third-Line Management of Metastatic Colorectal Cancer: SUNLIGHT Study Design. Future Oncol. 2021, 17, 1977–1985. [Google Scholar] [CrossRef]
- Sakai, D.; Boku, N.; Kodera, Y.; Komatsu, Y.; Fujii, M.; Iwasa, S.; Oki, E.; Koizumi, W.; Gamoh, M.; Muro, K.; et al. An Intergroup Phase III Trial of Ramucirumab plus Irinotecan in Third or More Line beyond Progression after Ramucirumab for Advanced Gastric Cancer (RINDBeRG Trial). JCO 2018, 36 (Suppl. S15), TPS4138. [Google Scholar] [CrossRef]
- Kawazoe, A.; Ando, T.; Hosaka, H.; Fujita, J.; Koeda, K.; Nishikawa, K.; Amagai, K.; Fujitani, K.; Ogata, K.; Watanabe, K.; et al. Safety and Activity of Trifluridine/Tipiracil and Ramucirumab in Previously Treated Advanced Gastric Cancer: An Open-Label, Single-Arm, Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2021, 6, 209–217. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.-H.; Shen, L.; Xu, J.; Bai, Y.; Yang, L.; Deng, Y.; Chen, Z.; Zhong, H.; et al. Effect of Fruquintinib vs. Placebo on Overall Survival in Patients with Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018, 319, 2486. [Google Scholar] [CrossRef]
- Dasari, A.; Lonardi, S.; Garcia-Carbonero, R.; Elez, E.; Yoshino, T.; Sobrero, A.; Yao, J.; García-Alfonso, P.; Kocsis, J.; Cubillo Gracian, A.; et al. Fruquintinib versus Placebo in Patients with Refractory Metastatic Colorectal Cancer (FRESCO-2): An International, Multicentre, Randomised, Double-Blind, Phase 3 Study. Lancet 2023, 402, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shen, L.; Guo, W.; Liu, T.; Li, J.; Qin, S.; Bai, Y.; Chen, Z.; Wang, J.; Pan, Y.; et al. Fruquintinib plus Paclitaxel versus Placebo plus Paclitaxel for Gastric or Gastroesophageal Junction Adenocarcinoma: The Randomized Phase 3 FRUTIGA Trial. Nat. Med. 2024. [Google Scholar] [CrossRef]
- Doyle, C. Fruquintinib Plus Paclitaxel Under Study as Second-Line Treatment of Gastroesophageal Cancer. The ASCO Post 2024. Available online: https://ascopost.com/issues/march-25-2024/fruquintinib-plus-paclitaxel-under-study-as-second-line-treatment-of-gastroesophageal-cancer/#:~:text=The%20combination%20of%20the%20small,Plenary%20Series%3A%20February%202024%20Session (accessed on 27 June 2024).
- Nakayama, I.; Qi, C.; Chen, Y.; Nakamura, Y.; Shen, L.; Shitara, K. Claudin 18.2 as a Novel Therapeutic Target. Nat. Rev. Clin. Oncol. 2024, 21, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Türeci, O.; Sahin, U.; Schulze-Bergkamen, H.; Zvirbule, Z.; Lordick, F.; Koeberle, D.; Thuss-Patience, P.; Ettrich, T.; Arnold, D.; Bassermann, F.; et al. A Multicentre, Phase IIa Study of Zolbetuximab as a Single Agent in Patients with Recurrent or Refractory Advanced Adenocarcinoma of the Stomach or Lower Oesophagus: The MONO Study. Ann. Oncol. 2019, 30, 1487–1495. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: A Randomised Phase II Study of Zolbetuximab (IMAB362) plus EOX versus EOX Alone for First-Line Treatment of Advanced CLDN18.2-Positive Gastric and Gastro-Oesophageal Adenocarcinoma. Ann. Oncol. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.-H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in Patients with CLDN18.2-Positive, HER2-Negative, Untreated, Locally Advanced Unresectable or Metastatic Gastric or Gastro-Oesophageal Junction Adenocarcinoma (SPOTLIGHT): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma: The Randomized, Phase 3 GLOW Trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef]
- Khan, K.; Rata, M.; Cunningham, D.; Koh, D.-M.; Tunariu, N.; Hahne, J.C.; Vlachogiannis, G.; Hedayat, S.; Marchetti, S.; Lampis, A.; et al. Functional Imaging and Circulating Biomarkers of Response to Regorafenib in Treatment-Refractory Metastatic Colorectal Cancer Patients in a Prospective Phase II Study. Gut 2018, 67, 1484–1492. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.U. Clinical Circulating Tumor DNA Testing for Precision Oncology. Cancer Res. Treat. 2023, 55, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Klempner, S.J.; Lee, K.-W.; Shitara, K.; Metges, J.-P.; Lonardi, S.; Ilson, D.H.; Fazio, N.; Kim, T.Y.; Bai, L.-Y.; Moran, D.; et al. ILUSTRO: Phase II Multicohort Trial of Zolbetuximab in Patients with Advanced or Metastatic Claudin 18.2–Positive Gastric or Gastroesophageal Junction Adenocarcinoma. Clin. Cancer Res. 2023, 29, 3882–3891. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shi, Z.; Wang, P.; Wang, C.; Yang, L.; Du, G.; Zhang, H.; Shi, B.; Jia, J.; Li, Q.; et al. Claudin18.2-Specific Chimeric Antigen Receptor Engineered T Cells for the Treatment of Gastric Cancer. JNCI J. Natl. Cancer Inst. 2019, 111, 409–418. [Google Scholar] [CrossRef]
- Qi, C.; Gong, J.; Li, J.; Liu, D.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; Zhou, J.; Cao, Y.; et al. Claudin18.2-Specific CAR T Cells in Gastrointestinal Cancers: Phase 1 Trial Interim Results. Nat. Med. 2022, 28, 1189–1198. [Google Scholar] [CrossRef]
- Mathias-Machado, M.C.; De Jesus, V.H.F.; Jácome, A.; Donadio, M.D.; Aruquipa, M.P.S.; Fogacci, J.; Cunha, R.G.; Da Silva, L.M.; Peixoto, R.D. Claudin 18.2 as a New Biomarker in Gastric Cancer—What Should We Know? Cancers 2024, 16, 679. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Bardelli, A. Liquid Biopsies: Genotyping Circulating Tumor DNA. JCO 2014, 32, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Mencel, J.; Slater, S.; Cartwright, E.; Starling, N. The Role of ctDNA in Gastric Cancer. Cancers 2022, 14, 5105. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, H.; Li, X.; Ge, K.; Wu, J. Circulating Tumor DNA Detection and Its Application Status in Gastric Cancer: A Narrative Review. Transl. Cancer Res. TCR 2021, 10, 529–536. [Google Scholar] [CrossRef]
- Moati, E.; Taly, V.; Garinet, S.; Didelot, A.; Taieb, J.; Laurent-Puig, P.; Zaanan, A. Role of Circulating Tumor DNA in Gastrointestinal Cancers: Current Knowledge and Perspectives. Cancers 2021, 13, 4743. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Chong, W.; Shang, L.; Jing, C.; Li, L. Liquid Biopsy in Gastric Cancer: Predictive and Prognostic Biomarkers. Cell Death Dis. 2022, 13, 903. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, H. Liquid Biopsy in Tumors: Opportunities and Challenges. Ann. Transl. Med. 2018, 6 (Suppl. S1), S89. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Zhang, D.-Y.; Zhu, J.-M.; Dong, L. Clinical Applications and Perspectives of Circulating Tumor DNA in Gastric Cancer. Cancer Cell Int. 2024, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.R.; Mojtahed, A.; Schneider, J.L.; Kanter, K.; Van Seventer, E.E.; Fetter, I.J.; Thabet, A.; Fish, M.G.; Teshome, B.; Fosbenner, K.; et al. Serial ctDNA Monitoring to Predict Response to Systemic Therapy in Metastatic Gastrointestinal Cancers. Clin. Cancer Res. 2020, 26, 1877–1885. [Google Scholar] [CrossRef]
- Alese, O.B.; Cook, N.; Ortega-Franco, A.; Ulanja, M.B.; Tan, L.; Tie, J. Circulating Tumor DNA: An Emerging Tool in Gastrointestinal Cancers. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 279–298. [Google Scholar] [CrossRef]
- Maron, S.B.; Chase, L.M.; Lomnicki, S.; Kochanny, S.; Moore, K.L.; Joshi, S.S.; Landron, S.; Johnson, J.; Kiedrowski, L.A.; Nagy, R.J.; et al. Circulating Tumor DNA Sequencing Analysis of Gastroesophageal Adenocarcinoma. Clin. Cancer Res. 2019, 25, 7098–7112. [Google Scholar] [CrossRef] [PubMed]
- Grizzi, G.; Salati, M.; Bonomi, M.; Ratti, M.; Holladay, L.; De Grandis, M.C.; Spada, D.; Baiocchi, G.L.; Ghidini, M. Circulating Tumor DNA in Gastric Adenocarcinoma: Future Clinical Applications and Perspectives. Int. J. Mol. Sci. 2023, 24, 9421. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Zhang, M.; Li, B.; Niu, Y.; Chen, L.; Yang, J.; Lu, S.; Gao, J.; Shen, L. Chromosomal Instability of Circulating Tumor DNA Reflect Therapeutic Responses in Advanced Gastric Cancer. Cell Death Dis. 2019, 10, 697. [Google Scholar] [CrossRef]
- Gravalos, C.; Jimeno, A. HER2 in Gastric Cancer: A New Prognostic Factor and a Novel Therapeutic Target. Ann. Oncol. 2008, 19, 1523–1529. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Manca, P.; Bellomo, S.E.; Corso, S.; Raimondi, A.; Berrino, E.; Morano, F.; Migliore, C.; Niger, M.; Castagnoli, L.; et al. HER2 Copy Number and Resistance Mechanisms in Patients with HER2-Positive Advanced Gastric Cancer Receiving Initial Trastuzumab-Based Therapy in JACOB Trial. Clin. Cancer Res. 2023, 29, 571–580. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, D.-L.; Wang, F.; Yang, C.; Chen, X.-X.; You, J.; Huang, J.-S.; Shao, Y.; Zhu, D.-Q.; Ouyang, Y.-M.; et al. The Predicting Role of Circulating Tumor DNA Landscape in Gastric Cancer Patients Treated with Immune Checkpoint Inhibitors. Mol. Cancer 2020, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.-M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive Molecular Characterization of Clinical Responses to PD-1 Inhibition in Metastatic Gastric Cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; He, C.; Lu, S.; Guan, W.; Wang, F.; Wang, X.; Jin, Y.; Wang, F.; Li, Y.; Shao, J.; et al. Prospective Observation: Clinical Utility of Plasma Epstein–Barr Virus DNA Load in EBV-associated Gastric Carcinoma Patients. Int. J. Cancer 2020, 146, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Jogo, T.; Nakamura, Y.; Shitara, K.; Bando, H.; Yasui, H.; Esaki, T.; Terazawa, T.; Satoh, T.; Shinozaki, E.; Nishina, T.; et al. Circulating Tumor DNA Analysis Detects FGFR2 Amplification and Concurrent Genomic Alterations Associated with FGFR Inhibitor Efficacy in Advanced Gastric Cancer. Clin. Cancer Res. 2021, 27, 5619–5627. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.T.; Kim, K.; Lee, H.; Kozarewa, I.; Mortimer, P.G.S.; Odegaard, J.I.; Harrington, E.A.; Lee, J.; Lee, T.; et al. Tumor Genomic Profiling Guides Patients with Metastatic Gastric Cancer to Targeted Treatment: The VIKTORY Umbrella Trial. Cancer Discov. 2019, 9, 1388–1405. [Google Scholar] [CrossRef] [PubMed]
- Frankell, A.M.; Smyth, E.C. ctDNA in Gastric and Gastroesophageal Cancer: Prognostic, Predictive, or Preliminary? Clin. Cancer Res. 2019, 25, 6893–6895. [Google Scholar] [CrossRef] [PubMed]
- Nagatsuma, A.K.; Aizawa, M.; Kuwata, T.; Doi, T.; Ohtsu, A.; Fujii, H.; Ochiai, A. Expression Profiles of HER2, EGFR, MET and FGFR2 in a Large Cohort of Patients with Gastric Adenocarcinoma. Gastric Cancer 2015, 18, 227–238. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bang, Y.-J.; Mansoor, W.; Petty, R.D.; Chao, Y.; Cunningham, D.; Ferry, D.R.; Smith, N.R.; Frewer, P.; Ratnayake, J.; et al. A Randomized, Open-Label Study of the Efficacy and Safety of AZD4547 Monotherapy versus Paclitaxel for the Treatment of Advanced Gastric Adenocarcinoma with FGFR2 Polysomy or Gene Amplification. Ann. Oncol. 2017, 28, 1316–1324. [Google Scholar] [CrossRef]
- Sootome, H.; Fujita, H.; Ito, K.; Ochiiwa, H.; Fujioka, Y.; Ito, K.; Miura, A.; Sagara, T.; Ito, S.; Ohsawa, H.; et al. Futibatinib Is a Novel Irreversible FGFR 1–4 Inhibitor That Shows Selective Antitumor Activity against FGFR-Deregulated Tumors. Cancer Res. 2020, 80, 4986–4997. [Google Scholar] [CrossRef]
- Javle, M.; King, G.; Spencer, K.; Borad, M.J. Futibatinib, an Irreversible FGFR1-4 Inhibitor for the Treatment of FGFR-Aberrant Tumors. Oncologist 2023, 28, 928–943. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Bahleda, R.; Hierro, C.; Sanson, M.; Bridgewater, J.; Arkenau, H.-T.; Tran, B.; Kelley, R.K.; Park, J.O.; Javle, M.; et al. Futibatinib, an Irreversible FGFR1–4 Inhibitor, in Patients with Advanced Solid Tumors Harboring FGF/FGFR Aberrations: A Phase I Dose-Expansion Study. Cancer Discov. 2022, 12, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Shitara, K.; Kojima, T.; Kuboki, Y.; Matsubara, N.; Bando, H.; Yoh, K.; Naito, Y.; Hirai, H.; Kurokawa, Y.; et al. Phase I Study of the Irreversible Fibroblast Growth Factor Receptor 1–4 Inhibitor Futibatinib in Japanese Patients with Advanced Solid Tumors. Cancer Sci. 2023, 114, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Rasco, D.; Lee, J.; Rha, S.Y.; Lee, K.-W.; Bang, Y.J.; Bendell, J.; Enzinger, P.; Marina, N.; Xiang, H.; et al. Phase I Escalation and Expansion Study of Bemarituzumab (FPA144) in Patients with Advanced Solid Tumors and FGFR2b-Selected Gastroesophageal Adenocarcinoma. JCO 2020, 38, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.A.; Enzinger, P.C.; Kang, Y.-K.; Qin, S.; Yamaguchi, K.; Kim, I.-H.; Saeed, A.; Oh, S.C.; Li, J.; Turk, H.M.; et al. Bemarituzumab in Patients with FGFR2b-Selected Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FIGHT): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Study. Lancet Oncol. 2022, 23, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Kang, Y.-K.; Saeed, A.; Yamaguchi, K.; Qin, S.; Lee, K.-W.; Kim, I.-H.; Oh, S.C.; Li, J.; Turk, H.M.; et al. FIGHT: A Randomized, Double-Blind, Placebo-Controlled, Phase II Study of Bemarituzumab (Bema) Combined with Modified FOLFOX6 in 1L FGFR2b+ Advanced Gastric/Gastroesophageal Junction Adenocarcinoma (GC). JCO 2021, 39 (Suppl. S15), 4010. [Google Scholar] [CrossRef]
- Van Herpe, F.; Van Cutsem, E. The Role of cMET in Gastric Cancer—A Review of the Literature. Cancers 2023, 15, 1976. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Cho, J.-Y.; Tan, I.B.; Tebbutt, N.C.; Yen, C.-J.; Kang, A.; Shames, D.S.; Bu, L.; Kang, Y.-K. A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction. Oncologist 2016, 21, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Tebbutt, N.C.; Davidenko, I.; Murad, A.M.; Al-Batran, S.-E.; Ilson, D.H.; Tjulandin, S.; Gotovkin, E.; Karaszewska, B.; Bondarenko, I.; et al. Rilotumumab plus Epirubicin, Cisplatin, and Capecitabine as First-Line Therapy in Advanced MET-Positive Gastric or Gastro-Oesophageal Junction Cancer (RILOMET-1): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1467–1482. [Google Scholar] [CrossRef]
- Doi, T.; Kang, Y.-K.; Muro, K.; Jiang, Y.; Jain, R.K.; Lizambri, R. A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study of Rilotumumab in Combination with Cisplatin and Capecitabine (CX) as First-Line Therapy for Asian Patients (Pts) with Advanced MET-Positive Gastric or Gastroesophageal Junction (G/GEJ) Adenocarcinoma: The RILOMET-2 Trial. JCO 2015, 33 (Suppl. S3), TPS226. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Muro, K.; Ryu, M.-H.; Yasui, H.; Nishina, T.; Ryoo, B.-Y.; Kamiya, Y.; Akinaga, S.; Boku, N. A Phase II Trial of a Selective C-Met Inhibitor Tivantinib (ARQ 197) Monotherapy as a Second- or Third-Line Therapy in the Patients with Metastatic Gastric Cancer. Investig. New Drugs 2014, 32, 355–361. [Google Scholar] [CrossRef]
- Hong, D.S.; LoRusso, P.; Hamid, O.; Janku, F.; Kittaneh, M.; Catenacci, D.V.T.; Chan, E.; Bekaii-Saab, T.; Gadgeel, S.M.; Loberg, R.D.; et al. Phase I Study of AMG 337, a Highly Selective Small-Molecule MET Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Wainberg, Z.A.; Catenacci, D.V.T.; Hochster, H.S.; Ford, J.; Kunz, P.; Lee, F.-C.; Kallender, H.; Cecchi, F.; Rabe, D.C.; et al. Phase II Study Evaluating 2 Dosing Schedules of Oral Foretinib (GSK1363089), cMET/VEGFR2 Inhibitor, in Patients with Metastatic Gastric Cancer. PLoS ONE 2013, 8, e54014. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.D.; Merino Vega, D.; Arend, R.C.; Baden, J.F.; Barbash, O.; Beaubier, N.; Collins, G.; French, T.; Ghahramani, N.; Hinson, P.; et al. Homologous Recombination Deficiency: Concepts, Definitions, and Assays. Oncologist 2022, 27, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T. Putting Poly (ADP-Ribose) Polymerase and Other DNA Repair Inhibitors into Clinical Practice. Curr. Opin. Oncol. 2013, 25, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Chen, A. PARP Inhibitors: Its Role in Treatment of Cancer. Chin. J. Cancer 2011, 30, 463–471. [Google Scholar] [CrossRef]
- Petrelli, A.; Rizzolio, S.; Pietrantonio, F.; Bellomo, S.E.; Benelli, M.; De Cecco, L.; Romagnoli, D.; Berrino, E.; Orrù, C.; Ribisi, S.; et al. BRCA2 Germline Mutations Identify Gastric Cancers Responsive to PARP Inhibitors. Cancer Res. 2023, 83, 1699–1710. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Huang, Y.; Xiong, H.; Su, J.; Chen, R.; Zou, Y. PARP Inhibitors in Gastric Cancer: Beacon of Hope. J. Exp. Clin. Cancer Res. 2021, 40, 211. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ying, H.; Wu, X.; Chen, H.; Hu, Y.; Zhang, H.; Wu, L.; Yang, Y.; Mao, B.; Zheng, L. The Mutational Pattern of Homologous Recombination (HR)-Associated Genes and Its Relevance to the Immunotherapeutic Response in Gastric Cancer. Cancer Biol. Med. 2020, 17, 1002–1013. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Xu, R.-H.; Chin, K.; Lee, K.-W.; Park, S.H.; Rha, S.Y.; Shen, L.; Qin, S.; Xu, N.; Im, S.-A.; et al. Olaparib in Combination with Paclitaxel in Patients with Advanced Gastric Cancer Who Have Progressed Following First-Line Therapy (GOLD): A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1637–1651. [Google Scholar] [CrossRef]
- Taieb, J.; Bennouna, J.; Penault-Llorca, F.; Basile, D.; Samalin, E.; Zaanan, A. Treatment of Gastric Adenocarcinoma: A Rapidly Evolving Landscape. Eur. J. Cancer 2023, 195, 113370. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Y.; Zhuang, Z.; Wang, D.; Ye, Z.; Jing, J.; Cheng, X. Advancements and Obstacles of PARP Inhibitors in Gastric Cancer. Cancers 2023, 15, 5114. [Google Scholar] [CrossRef]
- Christodoulidis, G.; Koumarelas, K.E.; Kouliou, M.N. Revolutionizing Gastric Cancer Treatment: The Potential of Immunotherapy. World J. Gastroenterol. 2024, 30, 286–289. [Google Scholar] [CrossRef]
- Li, Y.-N.; Xie, B.; Zhang, Y.; He, M.-H.; Xing, Y.; Mu, D.-M.; Wang, H.; Guo, R. Advances and Key Focus Areas in Gastric Cancer Immunotherapy: A Comprehensive Scientometric and Clinical Trial Review (1999–2023). World J. Gastroenterol. 2023, 29, 5593–5617. [Google Scholar] [CrossRef]
- Chen, T.; Wang, M.; Chen, Y.; Liu, Y. Current Challenges and Therapeutic Advances of CAR-T Cell Therapy for Solid Tumors. Cancer Cell Int. 2024, 24, 133. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Chen, P.; Gao, Y. From Anti-HER-2 to Anti-HER-2-CAR-T Cells: An Evolutionary Immunotherapy Approach for Gastric Cancer. JIR 2022, 15, 4061–4085. [Google Scholar] [CrossRef]
- Chen, S.; Zhan, S.; Ding, S.; Zhang, Q.; Xuan, H.; Zhang, X.; Cao, L. B7-H3 and CD47 Co-Expression in Gastric Cancer Is a Predictor of Poor Prognosis and Potential Targets for Future Dual-Targeting Immunotherapy. J. Cancer Res. Clin. Oncol. 2023, 149, 16609–16621. [Google Scholar] [CrossRef]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Ngosa Toka, F.; Jurka, P. Role of Cadherins in Cancer—A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef]
- Feng, Z.; He, X.; Zhang, X.; Wu, Y.; Xing, B.; Knowles, A.; Shan, Q.; Miller, S.; Hojnacki, T.; Ma, J.; et al. Potent Suppression of Neuroendocrine Tumors and Gastrointestinal Cancers by CDH17CAR T Cells without Toxicity to Normal Tissues. Nat. Cancer 2022, 3, 581–594. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness—A Literature Review. Cells 2023, 12, 2159. [Google Scholar] [CrossRef]
- Janes, M.E.; Gottlieb, A.P.; Park, K.S.; Zhao, Z.; Mitragotri, S. Cancer Vaccines in the Clinic. Bioeng. Transl. Med. 2024, 9, e10588. [Google Scholar] [CrossRef]
- Pallerla, S.; Abdul, A.U.R.M.; Comeau, J.; Jois, S. Cancer Vaccines, Treatment of the Future: With Emphasis on HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 779. [Google Scholar] [CrossRef]
- Tobias, J.; Garner-Spitzer, E.; Drinić, M.; Wiedermann, U. Vaccination against Her-2/Neu, with Focus on Peptide-Based Vaccines. ESMO Open 2022, 7, 100361. [Google Scholar] [CrossRef]
- Wiedermann, U.; Garner-Spitzer, E.; Chao, Y.; Maglakelidze, M.; Bulat, I.; Dechaphunkul, A.; Arpornwirat, W.; Charoentum, C.; Yen, C.-J.; Yau, T.C.; et al. Clinical and Immunologic Responses to a B-Cell Epitope Vaccine in Patients with HER2/Neu-Overexpressing Advanced Gastric Cancer—Results from Phase Ib Trial IMU.ACS.001. Clin. Cancer Res. 2021, 27, 3649–3660. [Google Scholar] [CrossRef]
- Maglakelidze, M.; Ryspayeva, D.E.; Andric, Z.; Petrovic, Z.; Bulat, I.; Nikolic, I.; Nagarkar, R.; Wiedermann, U.; Blumenstein, B.A.; Chong, L.M.O.; et al. HERIZON: A Phase 2 Study of HER-Vaxx (IMU-131), a HER2-Targeting Peptide Vaccine, plus Standard of Care Chemotherapy in Patients with HER2-Overexpressing Metastatic or Advanced Gastric/GEJ Adenocarcinoma— Overall Survival Analysis. JCO 2023, 41 (Suppl. S4), 289. [Google Scholar] [CrossRef]
- Banchereau, J.; Palucka, A.K. Dendritic Cells as Therapeutic Vaccines against Cancer. Nat. Rev. Immunol. 2005, 5, 296–306. [Google Scholar] [CrossRef]
- Ni, L. Advances in Human Dendritic Cell-Based Immunotherapy Against Gastrointestinal Cancer. Front. Immunol. 2022, 13, 887189. [Google Scholar] [CrossRef]
- Hato, L.; Vizcay, A.; Eguren, I.; Pérez-Gracia, J.L.; Rodríguez, J.; Gállego Pérez-Larraya, J.; Sarobe, P.; Inogés, S.; Díaz De Cerio, A.L.; Santisteban, M. Dendritic Cells in Cancer Immunology and Immunotherapy. Cancers 2024, 16, 981. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Peng, X.; Yang, Y.; Chen, Q.; Liu, J.; She, Q.; Tan, J.; Lou, C.; Liao, Z.; et al. mRNA Vaccine in Cancer Therapy: Current Advance and Future Outlook. Clin. Transl. Med. 2023, 13, e1384. [Google Scholar] [CrossRef]

| Study Name | Clinical Trial Number | Phase | Agent Tested | Outcome |
|---|---|---|---|---|
| ToGA | NCT01041404 | 3 | Trastuzumab | Improved OS in HER2-positive metastatic gastric and gastroesophageal junction adenocarcinoma resulted in establishing trastuzumab combined with chemotherapy as a standard first-line treatment for metastatic gastric cancer patients. |
| TRIO-013/LOGiC | NCT00680901 | 3 | Lapatinib | Lapatinib has been shown to improve PFS and OS when combined with chemotherapy. |
| SGNTUC-024 | NCT04430738 | 1b/2 | Combination of tucatinib with trastuzumab and oxaliplatin-based chemotherapy or pembrolizumab | Ongoing study—no results reported up to now. |
| MOUNTAINEER-02 | NCT04499924 | 3 | Combination of tucatinib with trastuzumab, ramucirumab, and paclitaxel for second-line treatment of HER2-positive metastatic gastric or gastroesophageal junction adenocarcinoma | Ongoing study—no results reported up to now. |
| DESTINY-Gastric01 | NCT03329690 | 2 | Trastuzumab deruxtecan | Improved median OS and PFS in HER2-positive metastatic gastric cancer. |
| DESTINY-Gastric02 | NCT04014075 | Trastuzumab deruxtecan | Improved median OS and PFS in HER2-positive advanced gastric cancer who progressed after trastuzumab treatment. | |
| DESTINY-Gastric03 | CT04379596 | Trastuzumab deruxtecan combined with immunotherapies | Ongoing study—no results reported up to now. | |
| DESTINY-Gastric04 | NCT04704934 | 3 | Comparing trastuzumab deruxtecan with ramucirumab plus paclitaxel | Ongoing study—no results reported up to now. |
| Safety study of MGAH22 in HER2-positive carcinomas | NCT01148849 | 1b | Margetuximab | Margetuximab exhibited greater cytotoxicity than trastuzumab in HER2-positive advanced gastric cancer. |
| CP-MGAH22-0 | NCT02689284 | 1b/2 | Margetuximab combined with pembrolizumab | Proof of concept of synergistic antitumor activity with the combination of an anti-HER2 agent along with anti-PD-1 checkpoint blockade in patients with high HER2 expression and PD-L1 positivity. |
| MAHOGANY | NCT04082364 | 2/3 | Margetuximab with retifanlimab | Promising antitumor activity and manageable toxicities in HER2-positive/PD-L1-positive unresectable or metastatic gastroesophageal adenocarcinoma. |
| Trial of ZW25 in Patients with advanced HER2-expressing cancers | NCT02892123 | 1 | Zanidatamab | Clinically meaningful antitumor effects of HER2-positive advanced gastric cancer. |
| First-Line Zanidatamab plus Chemotherapy for HER2-expressing metastatic gastroesophageal adenocarcinoma | NCT03929666 | 2 | Zanidatamab combined with standard chemotherapy | Improved PFS in HER2-positive metastatic gastric/gastroesophageal junction cancer; OS not yet reached. |
| Checkmate 649 | NCT02872116 | 3 | Nivolumab plus chemotherapy, nivolumab plus ipilimumab versus chemotherapy alone | Nivolumab plus chemotherapy achieved an improvement in OS compared to chemotherapy in patients with PD-L1 CPS ≥ 5. Survival benefit did not achieve statistical significance in tumors with CPS < 5, leading to restricted approval of combination therapy to CPS ≥ 5 population in HER2-negative gastric or gastro–oesophageal junction adenocarcinoma. |
| INTEGA | NCT03409848 | 2 | trastuzumab plus nivolumab with ipilimumab versus trastuzumab plus FOLFOX | The FOLFOX group had a higher 12-month OS in untreated HER2-positive gastric cancer. |
| Keynote 590 | NCC201807010 | 2 | standard first-line chemotherapy with cisplatin and 5-fluorouracil alone or in combination with pembrolizumab | Improved OS was seen in previously untreated metastatic esophageal and gastroesophageal junction tumors with PD-L1 CPS ≥ 10, but no benefit was seen for the addition of pembrolizumab in the PD-L1 CPS < 10 population. The trial led to the regulatory approval of pembrolizumab in combination with platinum and fluoropyrimidine-based chemotherapy restricted to PD-L1 CPS ≥ 10. |
| Keynote 859 | NCT03675737 | 3 | Combination of cisplatin and 5-fluorouracil (or capecitabine and oxaliplatin) with pembrolizumab | Participants with untreated metastatic gastric and gastroesophageal junction tumors in the pembrolizumab plus chemotherapy group had significantly improved PFS and ORR. |
| Keynote-158 | NCT02628067 | 2 | Pembrolizumab | Pembrolizumab monotherapy was highly active in pretreated microsatellite-high gastric cancer patients. |
| RAINBOW | NCT01170663 | 3 | Ramucirumab plus paclitaxel | Significant improvement in median OS in previously treated advanced gastric or gastro–oesophageal junction adenocarcinoma. Based on these results, ramucirumab became the first targeted therapy for angiogenesis in patients with advanced gastric cancer. |
| REGARD | NCT00917384 | 3 | Ramucirumab | Significant improvement in median OS in previously treated advanced gastric or gastro–oesophageal junction adenocarcinoma. Based on these results, ramucirumab became the first targeted therapy for angiogenesis in patients with advanced gastric cancer. |
| RAMIRIS | NCT03081143 | 2 | Ramucirumab plus FOLFIRI versus paclitaxel plus ramucirumab | Combination therapy with weekly paclitaxel and ramucirumab is recommended as the standard second-line chemotherapy for patients with advanced gastric cancer. |
| RAINFALL | NCT02314117 | 3 | Ramucirumab plus cisplatin and fluoropyrimidine | No survival benefit from the addition of ramucirumab to chemotherapy as first-line therapy was identified in previously untreated patients with advanced gastric cancer. |
| RINDBeRG | UMIN000023065 | 3 | Ramucirumab plus irinotecan in third or more line beyond progression after ramucirumab for advanced gastric cancer | Ongoing study—no results reported up to now. |
| RAINSTORM | NCT02539225 | 2 | Ramucirumab plus S-1 and oxaliplatin | No survival benefit from the addition of ramucirumab to chemotherapy was identified in previously untreated patients with advanced gastric cancer. |
| TAGS | NCT02500043 | 3 | Lonsurf | Increased OS against placebo with acceptable toxicity in patients with advanced gastric cancer who had previously been treated with at least two chemotherapy regimens for advanced disease. |
| Enhanced efficacy of anti-VEGFR2/taxane therapy after progression on immune checkpoint inhibition | JapicCTI-194596 | 2 | Lonsurf in combination with anti-angiogenic drugs (i.e., ramucirumab and bevacizumab) | Acceptable safety profile and clinical activity in patients with previously treated advanced gastric cancer regardless of previous ramucirumab exposure. |
| FRUTIGA | NCT03223376 | 3 | Fruquintinib plus paclitaxel versus paclitaxel alone | Improved PFS and ORR with fruquintinib plus paclitaxel in patients with advanced gastric and gastroesophageal junction adenocarcinoma after disease progression on fluoro-pyrimidine- or platinum-based first-line chemotherapy. |
| MONO | NCT01197885 | 2 | Zolbetuximab | Zolbetuximab monotherapy was well tolerated and exhibited antitumor activity in patients with CLDN18.2-positive advanced gastric and gastroesophageal junction adenocarcinoma. |
| FAST | NCT01630083 | 2b | Zolbetuximab plus epirubicin, oxaliplatin, and capecitabine (EOX) versus EOX alone | Significant survival benefits with zolbetuximab addition in HER2-negative metastatic gastric cancer. |
| SPOTLIGHT | NCT03504397 | 3 | Zolbetuximab plus mFOLFOX6 | Significantly increased median PFS and median OS in patients with CLDN18.2-positive and HER2-negative advanced gastric and gastroesophageal junction adenocarcinoma cancers. |
| GLOW | NCT03653507 | 3 | Zolbetuximab plus CAPOX as a first-line treatment | Zolbetuximab addition significantly improved median PFS and median OS in patients with CLDN18.2-positive, HER2-negative, locally advanced unresectable, or metastatic gastric or gastroesophageal junction adenocarcinoma cancer. |
| Chimeric antigen receptor T-cells targeting claudin18.2 in solid tumors | NCT03874897 | 1 | CLDN18.2-targeted CAR-T cells | CLDN18.2-targeted CAR-T cells showing promising efficacy with an acceptable safety profile in patients with heavily pretreated, CLDN18.2 positive gastric cancer. |
| VIKTORY | NCT02299648 | NA | Sequencing of ctDNA | ctDNA can be used for identifying targetable alterations for personalized treatment in the context of metastatic GC, and ctDNA prediction of treatment response based on ctDNA is earlier than radiological assessments. |
| SHINE | NCT01457846 | 2 | AZD4547 | AZD4547 failed to improve median PFS compared to paclitaxel. |
| YO28252 | NCT01590719 | 2 | Onartuzumab plus mFOLFOX6 versus mFOLFOX6 alone | Onartuzumab failed to demonstrate improved clinical outcomes when combined with mFOLFOX6 in metastatic HER2-negative gastric or gastroesophageal junction cancer. |
| FIGHT | NCT03343301 | 2 | Bemarituzumab plus mFOLFOX6 | Bemarituzumab did not significantly improve median PFS in combination with mFOLFOX6, but a post hoc analysis revealed prolonged median OS, particularly in patients with FGFR2b overexpression. |
| FORTITUDE-102 | NCT05111626 | 3 | Bemarituzumab plus chemotherapy and nivolumab | Ongoing study—no results reported up to now. |
| RILOMET-1 | NCT01697072 | 3 | Rilotumumab plus epirubicin, cisplatin, and capecitabine | Studies were prematurely terminated due to an observed increase in mortality among participants receiving rilotumumab. |
| RILOMET-2 | NCT02137343 | 3 | Rilotumumab plus cisplatin and capecitabine | Studies were prematurely terminated due to an observed increase in mortality among participants receiving rilotumumab. |
| A phase 3 randomized, double-blinded, placebo-controlled study of ARQ 197 plus erlotinib | NCT01377376 | 2 | ARQ 197 plus erlotinib | No significant clinical benefit in patients with metastatic gastric cancer. |
| A study of AMG 337 in subjects with advanced solid tumors | NCT01253707 | 1 | AMG 337 | No significant clinical benefit in patients with advanced solid tumors. |
| Study of GSK 1363089 in metastatic gastric cancer | NCT00725712 | 2 | GSK1363089 | No significant clinical benefit in patients with metastatic gastric cancer. |
| GOLD | NCT01924533 | 3 | Olaparib plus paclitaxel | No significant survival advantage from the addition of olaparib to taxane in the second-line treatment of advanced gastric cancer. |
| HERIZON | NCT02795988 | 2 | HER-Vaxx plus standard chemotherapy versus standard chemotherapy alone | HER-Vaxx is safe and provides relevant clinical benefit over standard chemotherapy in patients with HER2/neu overexpressing metastatic or advanced gastric and gastroesophageal junction cancer who were naïve to HER2 therapy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratti, M.; Orlandi, E.; Toscani, I.; Vecchia, S.; Anselmi, E.; Hahne, J.C.; Ghidini, M.; Citterio, C. Emerging Therapeutic Targets and Future Directions in Advanced Gastric Cancer: A Comprehensive Review. Cancers 2024, 16, 2692. https://doi.org/10.3390/cancers16152692
Ratti M, Orlandi E, Toscani I, Vecchia S, Anselmi E, Hahne JC, Ghidini M, Citterio C. Emerging Therapeutic Targets and Future Directions in Advanced Gastric Cancer: A Comprehensive Review. Cancers. 2024; 16(15):2692. https://doi.org/10.3390/cancers16152692
Chicago/Turabian StyleRatti, Margherita, Elena Orlandi, Ilaria Toscani, Stefano Vecchia, Elisa Anselmi, Jens Claus Hahne, Michele Ghidini, and Chiara Citterio. 2024. "Emerging Therapeutic Targets and Future Directions in Advanced Gastric Cancer: A Comprehensive Review" Cancers 16, no. 15: 2692. https://doi.org/10.3390/cancers16152692
APA StyleRatti, M., Orlandi, E., Toscani, I., Vecchia, S., Anselmi, E., Hahne, J. C., Ghidini, M., & Citterio, C. (2024). Emerging Therapeutic Targets and Future Directions in Advanced Gastric Cancer: A Comprehensive Review. Cancers, 16(15), 2692. https://doi.org/10.3390/cancers16152692








