Tumor Cell Stemness and Stromal Cell Features Contribute to Oral Cancer Outcome Disparity in Black Americans
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Primary Cells from Oral Cavity Cancer Tissue
2.2. Limiting Dilution Analysis (LDA)
2.3. Tumor Cell Invasion Assay
2.4. Xenograft Tumor Establishment
2.5. Co-Culturing of Tumor Stromal Cells and T Cells for Immune Regulation Assay
2.6. Statistics
3. Results
3.1. Oral Cancers in Comparable TNM Stages and Histologic Grades Constitute Distinct Features of Cancer Stemness
3.2. TME Stromal Cells of Individual Oral Cancers Have Different Capabilities of Affecting CSC Stemness
3.3. TME Stromal Cells of Individual Oral Cancers Have Variable Potentials of Suppressing T Cell Functionality
3.4. Tumor Cell Stemness and Tumor Stromal Cell Property Are Linked to Oral Cancer Post-Surgery Recurrence and Metastasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Mezghani, N.; Yao, A.; Vasilyeva, D.; Kaplan, N.; Shackelford, A.; Yoon, A.; Phillipone, E.; Dubey, S.; Schwartz, G.K.; Taylor, A.M.; et al. Molecular Subtypes of Head and Neck Cancer in Patients of African Ancestry. Clin. Cancer Res. 2023, 29, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Keir, J.A.; Whiteside, O.J.; Winter, S.C.; Maitra, S.; Corbridge, R.C.; Cox, G.J. Outcomes in squamous cell carcinoma with advanced neck disease. Ann. R Coll. Surg. Engl. 2007, 89, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Pulte, D.; Brenner, H. Changes in survival in head and neck cancers in the late 20th and early 21st century: A period analysis. Oncologist 2010, 15, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.C.; Bhattacharyya, N. Racial differences in stage and survival in head and neck squamous cell carcinoma. Laryngoscope 2007, 117, 770–775. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Siegel, R.L.; Sauer, A.G.; Miller, K.D.; Fedewa, S.A.; Alcaraz, K.I.; Jemal, A. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J. Clin. 2016, 66, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Nishita, M.; Park, S.Y.; Nishio, T.; Kamizaki, K.; Wang, Z.; Tamada, K.; Takumi, T.; Hashimoto, R.; Otani, H.; Pazour, G.J.; et al. Ror2 signaling regulates Golgi structure and transport through IFT20 for tumor invasiveness. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Ko, H.C.; Chen, S.; Wieland, A.M.; Yu, M.; Baschnagel, A.M.; Hartig, G.K.; Harari, P.M.; Witek, M.E. Clinical outcomes for patients presenting with N3 head and neck squamous cell carcinoma: Analysis of the National Cancer Database. Head Neck 2017, 39, 2159–2170. [Google Scholar] [CrossRef]
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.A.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.B.; Osazuwa-Peters, O.L.; Okafor, S.I.; Boakye, E.A.; Kuziez, D.; Perera, C.; Simpson, M.C.; Barnes, J.M.; Bulbul, M.G.; Cannon, T.Y.; et al. Differential Outcomes Among Survivors of Head and Neck Cancer Belonging to Racial and Ethnic Minority Groups. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Reitzel, L.R.; Nguyen, N.; Zafereo, M.E.; Li, G.; Wei, Q.; Sturgis, E.M. Neighborhood deprivation and clinical outcomes among head and neck cancer patients. Health Place 2012, 18, 861–868. [Google Scholar] [CrossRef]
- McDonald, J.T.; Johnson-Obaseki, S.; Hwang, E.; Connell, C.; Corsten, M. The relationship between survival and socio-economic status for head and neck cancer in Canada. J. Otolaryngol. Head Neck Surg. 2014, 43, 2. [Google Scholar] [CrossRef] [PubMed]
- Megwalu, U.C. Impact of County-Level Socioeconomic Status on Oropharyngeal Cancer Survival in the United States. Otolaryngol. Head Neck Surg. 2017, 156, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Weizman, B.; Golan, N.; Ronen, O. Effect of socioeconomic status on survival in patients with head and neck cancer. Head Neck 2021, 43, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Kreso, A.; Dick, J.E. Evolution of the cancer stem cell model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, T.; Batlle, E.; Massague, J. Metastatic stem cells: Sources, niches, and vital pathways. Cell Stem Cell 2014, 14, 306–321. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.T.; Alferez, D.G.; Amant, F.; Annibali, D.; Arribas, J.; Biankin, A.V.; Bruna, A.; Budinska, E.; Caldas, C.; Chang, D.K.; et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 2017, 17, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.A.; Kessenbrock, K.; Davis, R.T.; Pervolarakis, N.; Werb, Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 2018, 20, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.Y.; Lee, K.W.; Shin, D.; An, S.; Cho, K.H.; Kim, S.H. A positive feedback loop bi-stably activates fibroblasts. Nat. Commun. 2018, 9, 3016. [Google Scholar] [CrossRef] [PubMed]
- Luskin, M.R.; Murakami, M.A.; Manalis, S.R.; Weinstock, D.M. Targeting minimal residual disease: A path to cure? Nat. Rev. Cancer 2018, 18, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Beroukhim, R.; Golub, T.R. Genomic evolution of cancer models: Perils and opportunities. Nat. Rev. Cancer 2019, 19, 97–109. [Google Scholar] [CrossRef]
- Celia-Terrassa, T.; Kang, Y. Metastatic niche functions and therapeutic opportunities. Nat. Cell Biol. 2018, 20, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Prager, B.C.; Xie, Q.; Bao, S.; Rich, J.N. Cancer Stem Cells: The Architects of the Tumor Ecosystem. Cell Stem Cell 2019, 24, 41–53. [Google Scholar] [CrossRef]
- Yuan, J.; Hu, Z.; Mahal, B.A.; Zhao, S.D.; Kensler, K.H.; Pi, J.; Hu, X.; Zhang, Y.; Wang, Y.; Jiang, J.; et al. Integrated Analysis of Genetic Ancestry and Genomic Alterations across Cancers. Cancer Cell 2018, 34, 549–560-e9. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Lawson, F.; Rodriguez-Torres, S.; Noordhuis, M.G.; Pirini, F.; Manuel, L.; Valle, B.L.; Hadar, T.; Rivera, B.; Folawiyo, O.; et al. JAK3 Variant, Immune Signatures, DNA Methylation, and Social Determinants Linked to Survival Racial Disparities in Head and Neck Cancer Patients. Cancer Prev. Res. 2019, 12, 255–270. [Google Scholar] [CrossRef]
- Carrot-Zhang, J.; Chambwe, N.; Damrauer, J.S.; Knijnenburg, T.A.; Robertson, A.G.; Yau, C.; Zhou, W.; Berger, A.C.; Huang, K.L.; Newberg, J.Y.; et al. Comprehensive Analysis of Genetic Ancestry and Its Molecular Correlates in Cancer. Cancer Cell 2020, 37, 639–654 e6. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Dam, V.; Ganguly, K.; Sharma, S.; Atri, P.; Chirravuri-Venkata, R.; Cox, J.L.; Sayed, Z.; Jones, D.T.; Ganti, A.K.; et al. Differential mutation spectrum and immune landscape in African Americans versus Whites: A possible determinant to health disparity in head and neck cancer. Cancer Lett. 2020, 492, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.J.; Zeng, P.Y.F.; Sorgini, A.; Shaikh, M.H.; Khan, H.; MacNeil, D.; Khan, M.I.; Mendez, A.; Yoo, J.; Fung, K.; et al. Tumor molecular differences associated with outcome disparities of Black patients with head and neck cancer. Head Neck 2022, 44, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Mirshahidi, S.; Simental, A.; Lee, S.C.; De Andrade Filho, P.A.; Peterson, N.R.; Duerksen-Hughes, P.; Yuan, X. Cancer stem cell self-renewal as a therapeutic target in human oral cancer. Oncogene 2019, 38, 5440–5456. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Smyth, G.K. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 2009, 347, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Soteriou, D.; Fuchs, Y. A matter of life and death: Stem cell survival in tissue regeneration and tumour formation. Nat. Rev. Cancer 2018, 18, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef]
- Lusby, R.; Dunne, P.; Tiwari, V.K. Tumour invasion and dissemination. Biochem. Soc. Trans. 2022, 50, 1245–1257. [Google Scholar] [CrossRef]
- Borriello, L.; Karagiannis, G.S.; Duran, C.L.; Coste, A.; Oktay, M.H.; Entenberg, D.; Condeelis, J.S. The role of the tumor microenvironment in tumor cell intravasation and dissemination. Eur. J. Cell Biol. 2020, 99, 151098. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [PubMed]
- Riggi, N.; Aguet, M.; Stamenkovic, I. Cancer Metastasis: A Reappraisal of Its Underlying Mechanisms and Their Relevance to Treatment. Annu. Rev. Pathol. 2018, 13, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A.; Kohn, E.C. The microenvironment of the tumour-host interface. Nature 2001, 411, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Calon, A.; Lonardo, E.; Berenguer-Llergo, A.; Espinet, E.; Hernando-Momblona, X.; Iglesias, M.; Sevillano, M.; Palomo-Ponce, S.; Tauriello, D.V.; Byrom, D.; et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015, 47, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, L.; Yao, H.H.; Zhu, Z.Q.; Ning, Z.L.; Huang, Q. Stromal Myofibroblasts Are Associated with Poor Prognosis in Solid Cancers: A Meta-Analysis of Published Studies. PLoS ONE 2016, 11, e0159947. [Google Scholar] [CrossRef] [PubMed]
- Gerling, M.; Buller, N.V.; Kirn, L.M.; Joost, S.; Frings, O.; Englert, B.; Bergstrom, A.; Kuiper, R.V.; Blaas, L.; Wielenga, M.C.; et al. Stromal Hedgehog signalling is downregulated in colon cancer and its restoration restrains tumour growth. Nat. Commun. 2016, 7, 12321. [Google Scholar] [CrossRef]
- Pallangyo, C.K.; Ziegler, P.K.; Greten, F.R. IKKbeta acts as a tumor suppressor in cancer-associated fibroblasts during intestinal tumorigenesis. J. Exp. Med. 2015, 212, 2253–2266. [Google Scholar] [CrossRef]
- Shin, K.; Lim, A.; Zhao, C.; Sahoo, D.; Pan, Y.; Spiekerkoetter, E.; Liao, J.C.; Beachy, P.A. Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer Cell 2014, 26, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Maris, P.; Blomme, A.; Palacios, A.P.; Costanza, B.; Bellahcene, A.; Bianchi, E.; Gofflot, S.; Drion, P.; Trombino, G.E.; Di Valentin, E.; et al. Asporin Is a Fibroblast-Derived TGF-beta1 Inhibitor and a Tumor Suppressor Associated with Good Prognosis in Breast Cancer. PLoS Med. 2015, 12, e1001871. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, X.; Knolhoff, B.L.; Hegde, S.; Lee, K.B.; Jiang, H.; Fields, R.C.; Pachter, J.A.; Lim, K.H.; DeNardo, D.G. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut 2020, 69, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.X.; Muller, S.; Keerthivasan, S.; Koeppen, H.; Hung, J.; Gierke, S.; Breart, B.; Foreman, O.; Bainbridge, T.W.; Castiglioni, A.; et al. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15(+) Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov. 2020, 10, 232–253. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479-e10. [Google Scholar] [CrossRef] [PubMed]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624 e24. [Google Scholar] [CrossRef] [PubMed]
- Galbo, P.M., Jr.; Zang, X.; Zheng, D. Molecular Features of Cancer-associated Fibroblast Subtypes and their Implication on Cancer Pathogenesis, Prognosis, and Immunotherapy Resistance. Clin. Cancer Res. 2021, 27, 2636–2647. [Google Scholar] [CrossRef]
- Hu, S.; Lu, H.; Xie, W.; Wang, D.; Shan, Z.; Xing, X.; Wang, X.M.; Fang, J.; Dong, W.; Dai, W.; et al. TDO2+ myofibroblasts mediate immune suppression in malignant transformation of squamous cell carcinoma. J. Clin. Invest. 2022, 132, e157649. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, B.S.; Jang, J.Y.; Lee, Y.S.; Kim, H.J.; Roh, J.; Shin, Y.S.; Woo, H.G.; Kim, C.H. Single-cell transcriptome profiling of the stepwise progression of head and neck cancer. Nat. Commun. 2023, 14, 1055. [Google Scholar] [CrossRef]
- Liu, J.C.; Egleston, B.L.; Blackman, E.; Ragin, C. Racial survival disparities in head and neck cancer clinical trials. J. Natl. Cancer Inst. 2023, 115, 288–294. [Google Scholar] [CrossRef]

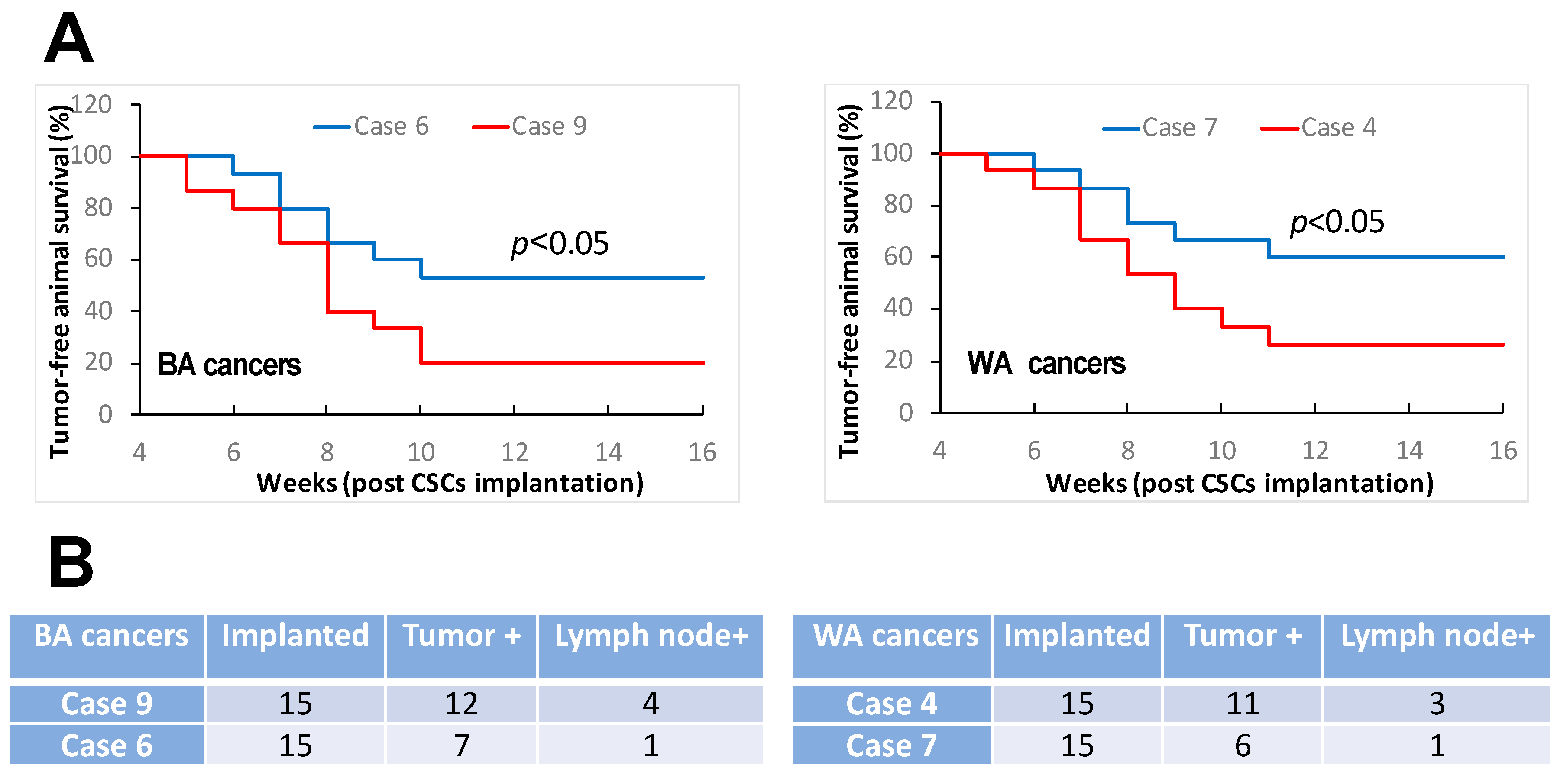

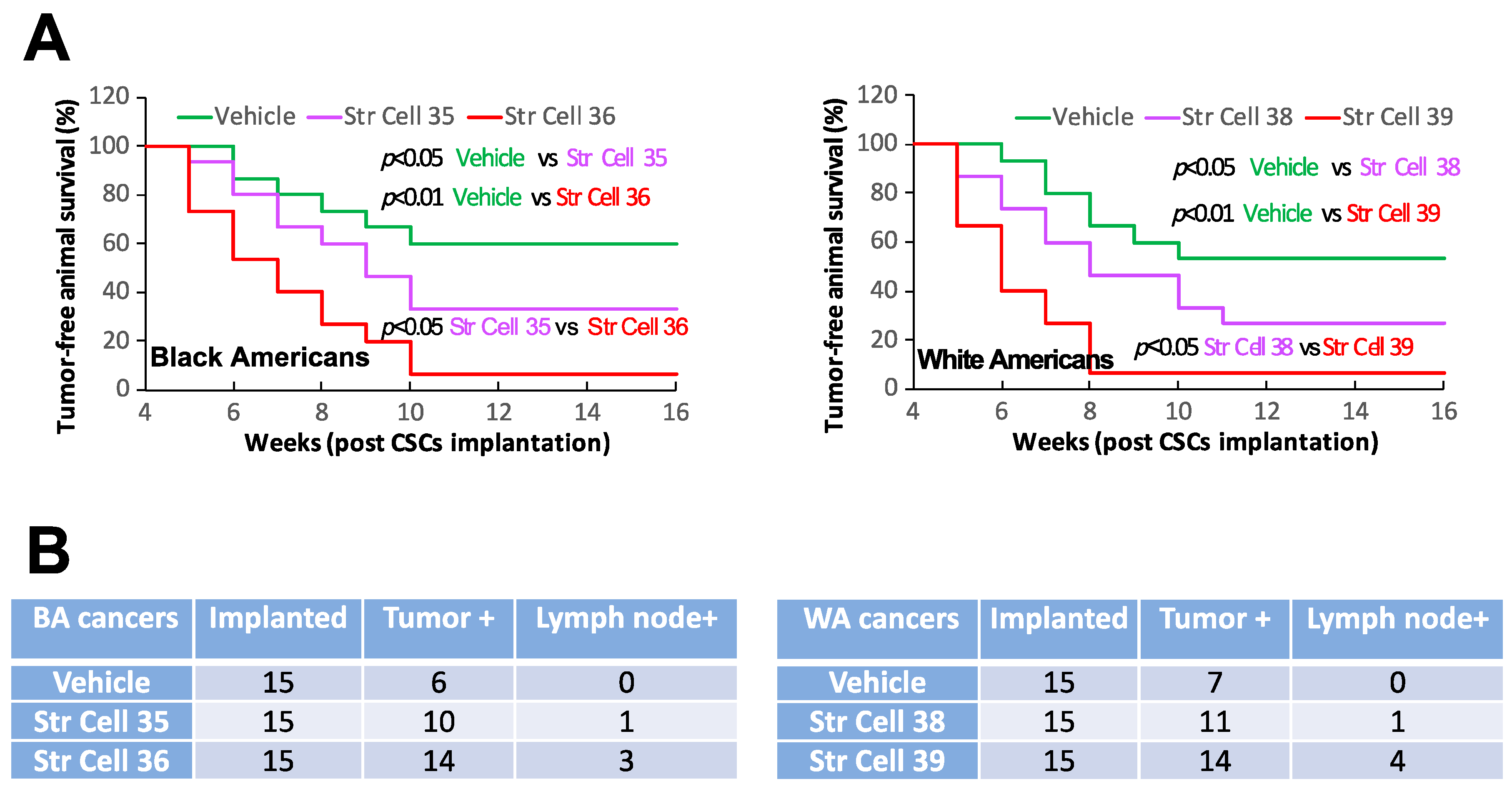

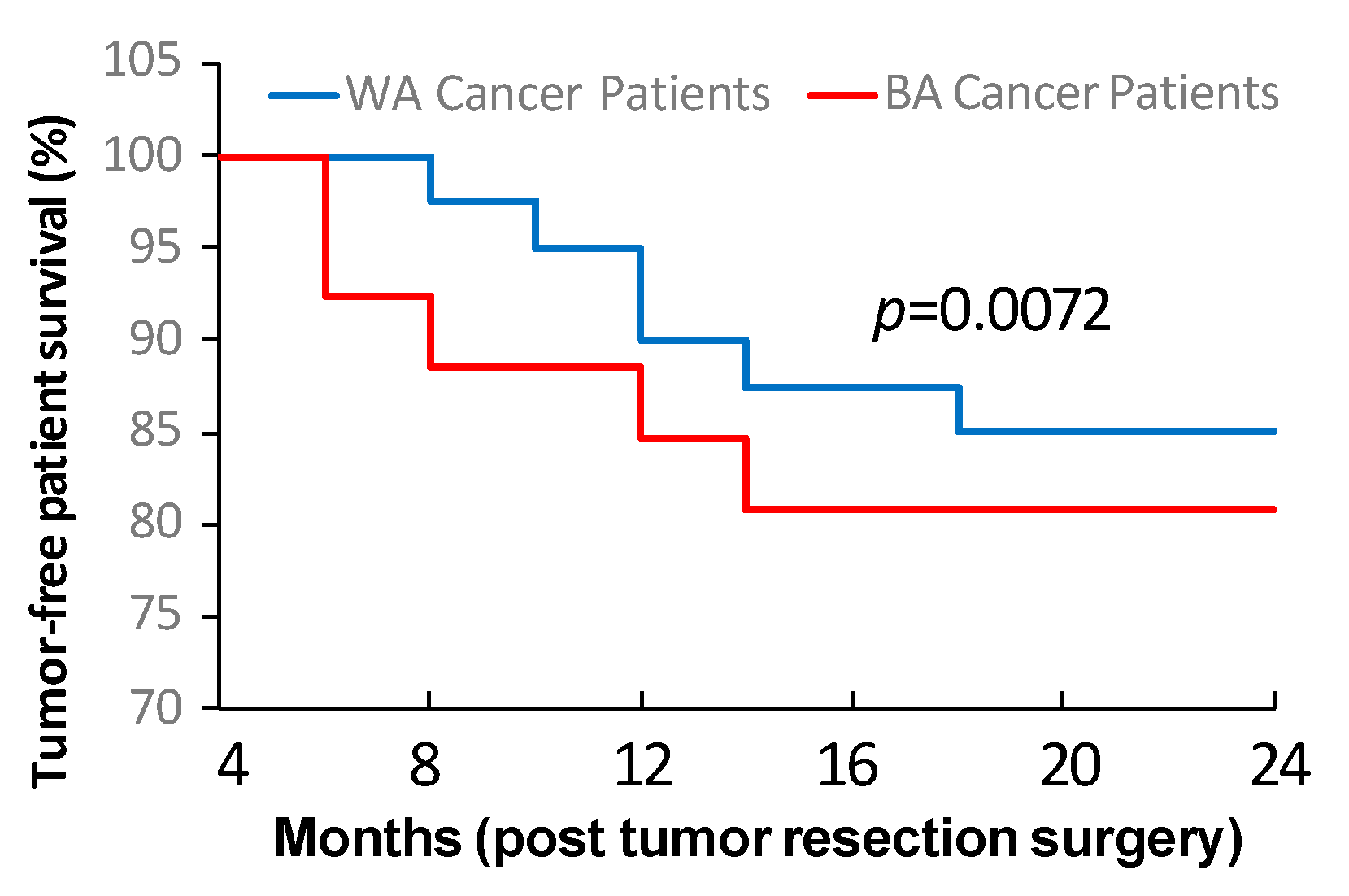
| Sample ID | Primary Site | TNM Stage | Tumor Grade | Race | Sex | Age at Surgery | Smoking History | HPV Status | Recurrence | Lymph Node |
|---|---|---|---|---|---|---|---|---|---|---|
| SCC2 | lip | T2N0M0 | G2 | BA | Male | 51 | Yes | Negative | No | No |
| SCC6 | gingival | T2N1M0 | G2 | BA | Male | 63 | No | Negative | No | No |
| SCC9 | tongue | T2N1M0 | G2 | BA | Female | 68 | Yes | Negative | Yes | Yes |
| SCC16 | floor of mouth | T3N0M0 | G3 | BA | Male | 66 | Yes | Negative | No | No |
| SCC17 | tongue | T2N0M0 | G2 | BA | Male | 49 | No | Negative | No | No |
| SCC19 | floor of mouth | T2N0M0 | G3 | BA | Male | 55 | No | Negative | Yes | No |
| SCC23 | buccal mucosa | T3N0M0 | G2 | BA | Female | 63 | Yes | Negative | No | No |
| SCC24 | gingival | T2N1M0 | G2 | BA | Male | 72 | Yes | Negative | Yes | Yes |
| SCC35 | gingival | T3N0M0 | G3 | BA | Male | 59 | No | Negative | No | No |
| SCC36 | tongue | T3N0M0 | G3 | BA | Male | 68 | Yes | Negative | Yes | Yes |
| SCC4 | floor of mouth | T2N1M0 | G3 | WA | Male | 53 | Yes | Negative | Yes | Yes |
| SCC7 | hard palate | T2N0M0 | G2 | WA | Female | 74 | No | Negative | No | No |
| SCC11 | gingival | T3N0M0 | G3 | WA | Male | 70 | Yes | Negative | No | No |
| SCC14 | buccal mucosa | T2N1M0 | G2 | WA | Male | 65 | Yes | Negative | No | No |
| SCC18 | tongue | T2N0M0 | G3 | WA | Male | 46 | No | Negative | No | No |
| SCC28 | tongue | T2N1M0 | G3 | WA | Female | 74 | Yes | Negative | Yes | Yes |
| SCC29 | lip | T2N0M0 | G2 | WA | Male | 81 | No | Negative | No | No |
| SCC38 | floor of mouth | T3N0M0 | G3 | WA | Female | 69 | Yes | Negative | No | No |
| SCC39 | gingival | T3N0M0 | G2 | WA | Male | 77 | Yes | Negative | Yes | No |
| Patients Cases | Cases with SFE 1:100–1:5000 | Cases with TICF 1:5000–1:500,000 | TNM Stage | Grade_ | Sex | Smoking History | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| >1:1000 | 1:500–1:1000 | <1:500 | >1:100,000 | 1:10,000 1:100,000 | <1:10,000 | T2 | T3 | G2 | G3 | Male | Female | Yes | No | |||
| Black American cancer | Recurrence/ metastasis − | 21 | 14 | 7 | 0 | 12 | 9 | 0 | 14 66.7% | 7 33.3% | 13 61.9% | 8 38.1% | 18 | 3 | 19 | 2 |
| Recurrence/ metastasis + | 5 | 0 | 1 | 4 | 0 | 2 | 3 | 3 60% | 2 40% | 3 60% | 2 40% | 4 | 1 | 5 | 0 | |
| White American cancer | Recurrence/ metastasis − | 34 | 29 | 5 | 0 | 27 | 7 | 0 | 23 67.6% | 11 32.4% | 22 64.7% | 12 35.3% | 28 | 6 | 30 | 4 |
| Recurrence/ metastasis + | 6 | 0 | 2 | 4 | 0 | 3 | 3 | 3 50% | 3 50% | 4 66.6% | 2 33.4% | 5 | 1 | 5 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirshahidi, S.; Yuan, I.J.; Chen, Z.; Simental, A.; Lee, S.C.; Andrade Filho, P.A.; Murry, T.; Zeng, F.; Duerksen-Hughes, P.; Wang, C.; et al. Tumor Cell Stemness and Stromal Cell Features Contribute to Oral Cancer Outcome Disparity in Black Americans. Cancers 2024, 16, 2730. https://doi.org/10.3390/cancers16152730
Mirshahidi S, Yuan IJ, Chen Z, Simental A, Lee SC, Andrade Filho PA, Murry T, Zeng F, Duerksen-Hughes P, Wang C, et al. Tumor Cell Stemness and Stromal Cell Features Contribute to Oral Cancer Outcome Disparity in Black Americans. Cancers. 2024; 16(15):2730. https://doi.org/10.3390/cancers16152730
Chicago/Turabian StyleMirshahidi, Saied, Isabella J. Yuan, Zhong Chen, Alfred Simental, Steve C. Lee, Pedro A. Andrade Filho, Thomas Murry, Feng Zeng, Penelope Duerksen-Hughes, Charles Wang, and et al. 2024. "Tumor Cell Stemness and Stromal Cell Features Contribute to Oral Cancer Outcome Disparity in Black Americans" Cancers 16, no. 15: 2730. https://doi.org/10.3390/cancers16152730
APA StyleMirshahidi, S., Yuan, I. J., Chen, Z., Simental, A., Lee, S. C., Andrade Filho, P. A., Murry, T., Zeng, F., Duerksen-Hughes, P., Wang, C., & Yuan, X. (2024). Tumor Cell Stemness and Stromal Cell Features Contribute to Oral Cancer Outcome Disparity in Black Americans. Cancers, 16(15), 2730. https://doi.org/10.3390/cancers16152730






