Serum CD133-Associated Proteins Identified by Machine Learning Are Connected to Neural Development, Cancer Pathways, and 12-Month Survival in Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Technological Innovations
- This study is the first to develop an approach to large-scale serum proteome data based on a single literature-validated protein (CD133) and a clinically meaningful endpoint (12-month OS).
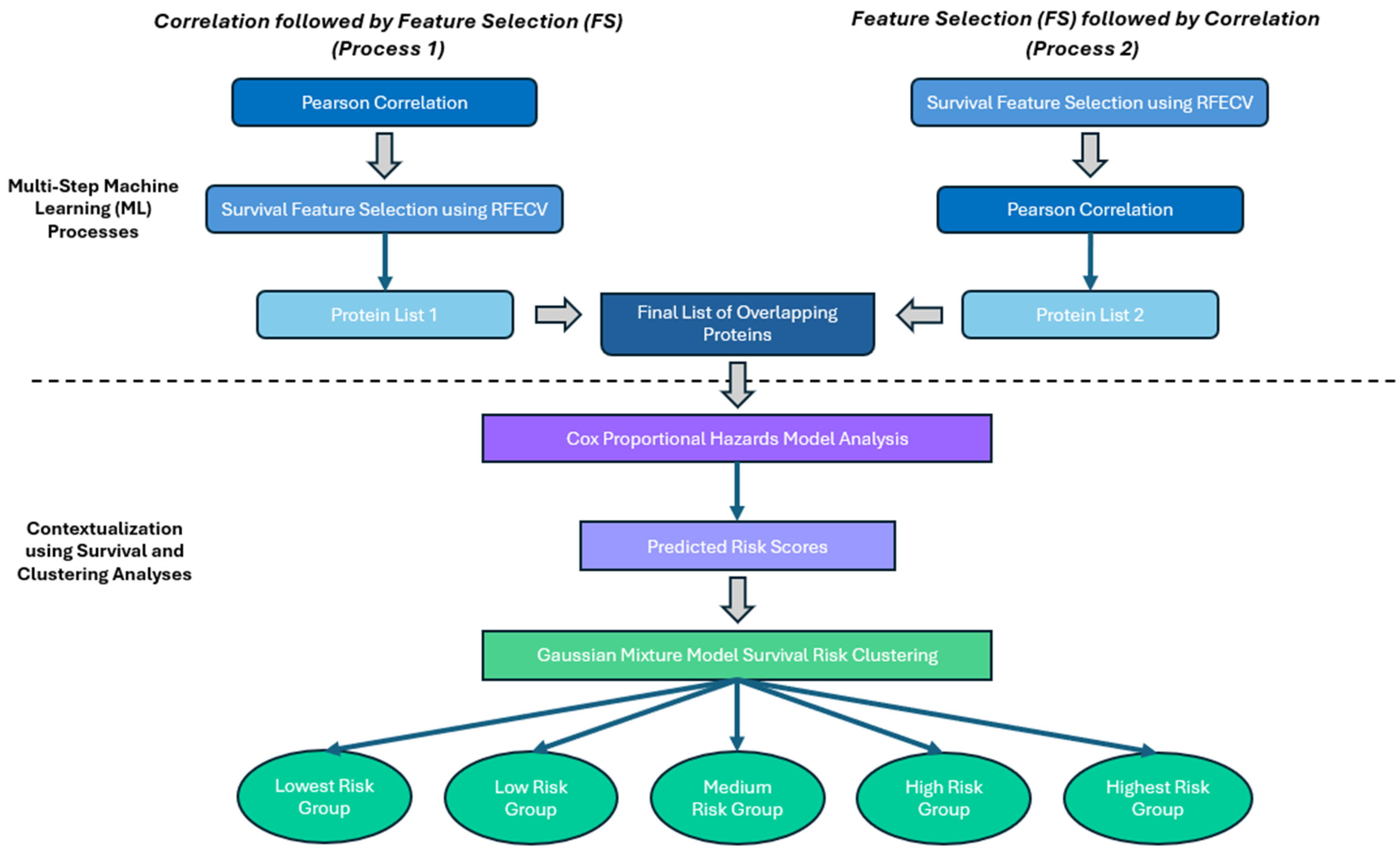
- The combined use of Pearson’s correlation analyses and Recursive Feature Selection, Cross-Validated (RFECV) for selecting serum proteins related to serum CD133 and 12-month OS in this study is novel.
- To ensure inclusive protein selection, we tested multiple FS methods on two base models with differing approaches to 12-month OS prediction.
- To validate our findings, we utilized two inverse but complementary processes involving correlation and FS to address the lack of large-scale data available for external validation.
- We used predicted hazard scores from the Cox Proportional Hazards Model for Gaussian Mixture Model clustering to categorize patients into risk groups based on their protein profiles.
1.2. Clinical Innovations
- This is the first study to investigate CD133 and its associations using serum proteome data.
- The findings enhance the understanding of conclusions derived from serum data while addressing whether these conclusions are transferable with other types of biospecimens.
- Almost all identified proteins are either expressed in the brain, involved in brain function, and/or related to cancer biology pathways, underscoring their likely involvement in, or alteration from, GBM.
- The results identify potentially harmful protein profiles that might predispose individuals to short survival with SOC management, warranting further validation.
- The list of identified proteins offers candidates that can potentially be used to monitor prognosis and/or treatment as new data emerge and reference ranges are validated.
2. Materials and Methods
2.1. Dataset
2.2. The Proposed Scheme
2.3. Pearson’s Correlation Analysis
2.4. Base Models
2.5. Feature Selection Methods
2.6. The Cox Proportional Hazards Model
2.7. Gaussian Mixture Model Clustering
3. Results
3.1. Correlation and Feature Selection Analysis
3.2. CD133 Serum
3.3. The Cox Proportional Hazards Model
3.4. Gaussian Mixture Model Clustering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma Multiforme |
| WHO | World Health Organization |
| SOC | Standard of Care |
| CRT | Chemoradiation Therapy |
| TMZ | Temozolomide |
| CSC | Cancer Stem Cell |
| PROM-1 | Prominin-1 |
| OS | Overall Survival |
| FS | Feature Selection |
| RFECV | Recursive Feature Selection, Cross-Validated |
| NIH | National Institutes of Health |
| IRB | Institutional Review Board |
| NIDAP | NIH Integrated Data Analysis Platform |
| ML | Machine Learning |
| FDR | False Discovery Rate |
| LR | Logistic Regression |
| RF | Random Forest |
| LASSO | Least Absolute Shrinkage and Selector Operator |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under Curve |
| BIC | Bayesian Information Criteria |
| RFU | Relative Fluorescence Unit |
References
- Nie, S.; Gurrea, M.; Zhu, J.; Thakolwiboon, S.; Heth, J.A.; Muraszko, K.M.; Fan, X.; Lubman, D.M. Tenascin-C: A novel candidate marker for cancer stem cells in glioblastoma identified by tissue microarrays. J. Proteome Res. 2015, 14, 814–822. [Google Scholar] [CrossRef]
- Baid, U.; Rane, S.U.; Talbar, S.; Gupta, S.; Thakur, M.H.; Moiyadi, A.; Mahajan, A. Overall Survival Prediction in Glioblastoma With Radiomic Features Using Machine Learning. Front. Comput. Neurosci. 2020, 14, 61. [Google Scholar] [CrossRef]
- Fernandes, C.; Costa, A.; Osório, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy; Codon Publications: Singapore, 2017; pp. 197–241. [Google Scholar]
- Sun, T.; Chen, G.; Li, Y.; Xie, X.; Zhou, Y.; Du, Z. Aggressive invasion is observed in CD133(-)/A2B5(+) glioma-initiating cells. Oncol. Lett. 2015, 10, 3399–3406. [Google Scholar] [CrossRef]
- Mia-Jan, K.; Jung, S.Y.; Kim, I.Y.; Oh, S.S.; Choi, E.; Chang, S.J.; Kang, T.Y.; Cho, M.Y. CD133 expression is not an independent prognostic factor in stage II and III colorectal cancer but may predict the better outcome in patients with adjuvant therapy. BMC Cancer 2013, 13, 166. [Google Scholar] [CrossRef]
- Joyce, T.; Jagasia, S.; Tasci, E.; Camphausen, K.; Krauze, A.V. An Overview of CD133 as a Functional Unit of Prognosis and Treatment Resistance in Glioblastoma. Curr. Oncol. 2023, 30, 8278–8293. [Google Scholar] [CrossRef]
- Tseng, Y.-J.; Huang, C.-E.; Wen, C.-N.; Lai, P.-Y.; Wu, M.-H.; Sun, Y.-C.; Wang, H.-Y.; Lu, J.-J. Predicting breast cancer metastasis by using serum biomarkers and clinicopathological data with machine learning technologies. Int. J. Med. Inform. 2019, 128, 79–86. [Google Scholar] [CrossRef]
- Huang, W.; Zhong, Z.; Luo, C.; Xiao, Y.; Li, L.; Zhang, X.; Yang, L.; Xiao, K.; Ning, Y.; Chen, L.; et al. The miR-26a/AP-2α/Nanog signaling axis mediates stem cell self-renewal and temozolomide resistance in glioma. Theranostics 2019, 9, 5497–5516. [Google Scholar] [CrossRef]
- Xie, Y.; Meng, W.-Y.; Li, R.-Z.; Wang, Y.-W.; Qian, X.; Chan, C.; Yu, Z.-F.; Fan, X.-X.; Pan, H.-D.; Xie, C.; et al. Early lung cancer diagnostic biomarker discovery by machine learning methods. Transl. Oncol. 2021, 14, 100907. [Google Scholar] [CrossRef]
- Müller Bark, J.; Kulasinghe, A.; Chua, B.; Day, B.W.; Punyadeera, C. Circulating biomarkers in patients with glioblastoma. Br. J. Cancer 2020, 122, 295–305. [Google Scholar] [CrossRef]
- Wang, J.; Bettegowda, C. Applications of DNA-Based Liquid Biopsy for Central Nervous System Neoplasms. J. Mol. Diagn. 2017, 19, 24–34. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Kudulaiti, N.; Zhou, Z.; Luo, C.; Zhang, J.; Zhu, F.; Wu, J. A nomogram for individualized prediction of overall survival in patients with newly diagnosed glioblastoma: A real-world retrospective cohort study. BMC Surg. 2021, 21, 238. [Google Scholar] [CrossRef] [PubMed]
- Bakas, S.; Sako, C.; Akbari, H.; Bilello, M.; Sotiras, A.; Shukla, G.; Rudie, J.D.; Santamaría, N.F.; Kazerooni, A.F.; Pati, S.; et al. The University of Pennsylvania glioblastoma (UPenn-GBM) cohort: Advanced MRI, clinical, genomics, & radiomics. Sci. Data 2022, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, W.; Wang, Y.; Guo, Y.; Cong, Z.; Du, F.; Song, B. MGMT promoter methylation in serum and cerebrospinal fluid as a tumor-specific biomarker of glioma. Biomed. Rep. 2015, 3, 543–548. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhi, F.; Shao, N.; Wang, R.; Deng, D.; Xue, L.; Wang, Q.; Zhang, Y.; Shi, Y.; Xia, X.; Wang, S.; et al. Identification of 9 serum microRNAs as potential noninvasive biomarkers of human astrocytoma. Neuro-Oncology 2015, 17, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shen, J.; Hodges, T.R.; Song, R.; Fuller, G.N.; Heimberger, A.B. Serum microRNA profiling in patients with glioblastoma: A survival analysis. Mol. Cancer 2017, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.; Walker, J.J.; Wilcox, S.K.; Williams, S. Advances in human proteomics at high scale with the SOMAscan proteomics platform. New Biotechnol. 2012, 29, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Palantir Foundry—The NIH Integrated Data Analysis Platform (NIDAP); NCI Center for Biomedical Informatics & Information Technology (CBIIT); Software Provided by Palantir Technologies Inc. Available online: https://www.palantir.com (accessed on 10 May 2024).
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Taylor, R. Interpretation of the Correlation Coefficient: A Basic Review. J. Diagn. Med. Sonogr. 1990, 6, 35–39. [Google Scholar] [CrossRef]
- Boateng, E.Y.; Abaye, D.A. A Review of the Logistic Regression Model with Emphasis on Medical Research. J. Data Anal. Inf. Process. 2019, 07, 190–207. [Google Scholar] [CrossRef]
- Rigatti, S.J. Random Forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef]
- MinMaxScaler. Available online: https://scikit-learn.org/stable/modules/generated/sklearn.preprocessing.MinMaxScaler.html (accessed on 10 May 2024).
- GridSearchCV. Available online: https://scikit-learn.org/stable/modules/generated/sklearn.model_selection.GridSearchCV.html (accessed on 10 May 2024).
- Muthukrishnan, R.; Rohini, R. LASSO: A feature selection technique in predictive modeling for machine learning. In Proceedings of the 2016 IEEE International Conference on Advances in Computer Applications (ICACA), Coimbatore, India, 24–24 October 2016. [Google Scholar]
- Liang, H.; Wu, J.; Zhang, H.; Yang, J. Two-Stage Short-Term Power Load Forecasting Based on RFECV Feature Selection Algorithm and a TCN–ECA–LSTM Neural Network. Energies 2023, 16, 1925. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Hoeting, J.A.; Jarnevich, C.S. The area under the precision-recall curve as a performance metric for rare binary events. Methods Ecol. Evol. 2019, 10, 565–577. [Google Scholar] [CrossRef]
- Bowers, A.J.; Zhou, X. Receiver Operating Characteristic (ROC) Area Under the Curve (AUC): A Diagnostic Measure for Evaluating the Accuracy of Predictors of Education Outcomes. J. Educ. Stud. Placed Risk (JESPAR) 2019, 24, 20–46. [Google Scholar] [CrossRef]
- RepeatedStratifiedKFold. Available online: https://scikit-learn.org/stable/modules/generated/sklearn.model_selection.RepeatedStratifiedKFold.html (accessed on 10 May 2024).
- CoxPHFitter. Available online: https://lifelines.readthedocs.io/en/latest/fitters/regression/CoxPHFitter.html (accessed on 10 May 2024).
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B Stat. Methodol. 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Gaussian Mixture Models. Available online: https://scikit-learn.org/stable/modules/mixture.html (accessed on 10 May 2024).
- Grosse-Gehling, P.; Fargeas, C.A.; Dittfeld, C.; Garbe, Y.; Alison, M.R.; Corbeil, D.; Kunz-Schughart, L.A. CD133 as a biomarker for putative cancer stem cells in solid tumours: Limitations, problems and challenges. J. Pathol. 2013, 229, 355–378. [Google Scholar] [CrossRef]
- Wu, B.; Sun, C.; Feng, F.; Ge, M.; Xia, L. Do relevant markers of cancer stem cells CD133 and Nestin indicate a poor prognosis in glioma patients? A systematic review and meta-analysis. J. Exp. Clin. Cancer Res. 2015, 34, 44. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Lv, S.; Yang, H. High CD133 Expression Is Associated with Worse Prognosis in Patients with Glioblastoma. Mol. Neurobiol. 2016, 53, 2354–2360. [Google Scholar] [CrossRef]
- Popescu, I.D.; Codrici, E.; Albulescu, L.; Mihai, S.; Enciu, A.-M.; Albulescu, R.; Tanase, C.P. Potential serum biomarkers for glioblastoma diagnostic assessed by proteomic approaches. Proteome Sci. 2014, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Nijaguna, M.B.; Schröder, C.; Patil, V.; Shwetha, S.D.; Hegde, A.S.; Chandramouli, B.A.; Arivazhagan, A.; Santosh, V.; Hoheisel, J.D.; Somasundaram, K. Definition of a serum marker panel for glioblastoma discrimination and identification of Interleukin 1β in the microglial secretome as a novel mediator of endothelial cell survival induced by C-reactive protein. J. Proteom. 2015, 128, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Linhares, P.; Carvalho, B.; Vaz, R.; Costa, B.M. Glioblastoma: Is There Any Blood Biomarker with True Clinical Relevance? Int. J. Mol. Sci. 2020, 21, 5809. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Pan, D.-L.; Xu, J.-H.; Chen, Y.; Xu, W.-F.; Chen, J.-Y.; Wu, Z.-Y.; Lin, Y.-X.; You, H.-H.; Ding, C.-Y.; et al. Serum Inflammatory Biomarkers Contribute to the Prognosis Prediction in High-Grade Glioma. Front. Oncol. 2022, 11, 754920. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Guo, L.; Zhang, Y.; Huang, B.; Chen, A.; Chen, W.; Liu, X.; Sun, S.; Wang, K.; Liu, A.; et al. Clinicopathological and Prognostic Significance of CD133 in Glioma Patients: A Meta-Analysis. Mol. Neurobiol. 2016, 53, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Abdoli Shadbad, M.; Hosseinkhani, N.; Asadzadeh, Z.; Brunetti, O.; Silvestris, N.; Baradaran, B. The Prognostic Value of CD133 in Predicting the Relapse and Recurrence Pattern of High-Grade Gliomas on MRI: A Meta-Analysis. Front. Oncol. 2021, 11, 722833. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.I.; Javed, G.; Laghari, A.A.; Bareeqa, S.B.; Farrukh, S.; Zahid, S.; Samar, S.S.; Aziz, K. CD133 Expression in Glioblastoma Multiforme: A Literature Review. Cureus 2018, 10, e3439. [Google Scholar] [CrossRef] [PubMed]
- Abdoli Shadbad, M.; Nejadi Orang, F.; Baradaran, B. CD133 significance in glioblastoma development: In silico and in vitro study. Eur. J. Med. Res. 2024, 29, 154. [Google Scholar] [CrossRef]
- Köhler, K.; Seitz, H. Validation Processes of Protein Biomarkers in Serum—A Cross Platform Comparison. Sensors 2012, 12, 12710–12728. [Google Scholar] [CrossRef]
- Gatto, L.; Franceschi, E.; Di Nunno, V.; Tosoni, A.; Lodi, R.; Brandes, A.A. Liquid Biopsy in Glioblastoma Management: From Current Research to Future Perspectives. Oncologist 2021, 26, 865–878. [Google Scholar] [CrossRef]
- Rakhshaninejad, M.; Fathian, M.; Shirkoohi, R.; Barzinpour, F.; Gandomi, A.H. Refining breast cancer biomarker discovery and drug targeting through an advanced data-driven approach. BMC Bioinform. 2024, 25, 33. [Google Scholar] [CrossRef] [PubMed]
- Golestan, A.; Tahmasebi, A.; Maghsoodi, N.; Faraji, S.N.; Irajie, C.; Ramezani, A. Unveiling promising breast cancer biomarkers: An integrative approach combining bioinformatics analysis and experimental verification. BMC Cancer 2024, 24, 155. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.; Tan, M.; Yang, Z.; Shi, S. Modeling of Feature Selection Based on Random Forest Algorithm and Pearson Correlation Coefficient. J. Phys. Conf. Ser. 2022, 2219, 012046. [Google Scholar] [CrossRef]
- Putro, I.H.; Ahmad, T. Feature Selection Using Pearson Correlation with Lasso Regression for Intrusion Detection System. In Proceedings of the 2024 12th International Symposium on Digital Forensics and Security (ISDFS), San Antonio, TX, USA, 29–30 April 2024. [Google Scholar]
- Sarkar, J.P.; Saha, I.; Sarkar, A.; Maulik, U. Machine learning integrated ensemble of feature selection methods followed by survival analysis for predicting breast cancer subtype specific miRNA biomarkers. Comput. Biol. Med. 2021, 131, 104244. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Morelli, D.; Littlejohns, T.J.; Clifton, D.A.; Clifton, L. Combining machine learning with Cox models to identify predictors for incident post-menopausal breast cancer in the UK Biobank. Sci. Rep. 2023, 13, 9221. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Qin, L.; Bay, C.; Chen, X.; Yu, K.H.; Miskin, N.; Li, A.; Xu, X.; Young, G. Deep Transfer Learning and Radiomics Feature Prediction of Survival of Patients with High-Grade Gliomas. Am. J. Neuroradiol. 2020, 41, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.; Chen, Y.; Li, Z.-C.; Li, Q.; Zhang, J.; Liu, J.; Zhai, G. A Deep Learning-Based Radiomics Model for Prediction of Survival in Glioblastoma Multiforme. Sci. Rep. 2017, 7, 10353. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yang, P.; Zhang, X.; Xu, L.; Wang, X.; Li, X.; Zhang, L.; Xie, R.; Yang, L.; Jing, Z.; et al. Sub-region based radiomics analysis for survival prediction in oesophageal tumours treated by definitive concurrent chemoradiotherapy. eBioMedicine 2019, 44, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Goyal, G.; Go, R.S.; Parikh, S.A.; Ngufor, C.G. Improved Interpretability of Machine Learning Model Using Unsupervised Clustering: Predicting Time to First Treatment in Chronic Lymphocytic Leukemia. JCO Clin. Cancer Inform. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Vivekanandan, T.; Narayanan, S.J. A Hybrid Risk Assessment Model for Cardiovascular Disease Using Cox Regression Analysis and a 2-means clustering algorithm. Comput. Biol. Med. 2019, 113, 103400. [Google Scholar] [CrossRef]
- Atlas, T.H.P. The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 10 May 2024).
- Grillet, N.; Dubreuil, V.; Dufour, H.D.; Brunet, J.F. Dynamic expression of RGS4 in the developing nervous system and regulation by the neural type-specific transcription factor Phox2b. J Neurosci 2003, 23, 10613–10621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Echizen, K.; Nakada, M.; Hayashi, T.; Sabit, H.; Furuta, T.; Nakai, M.; Koyama-Nasu, R.; Nishimura, Y.; Taniue, K.; Morishita, Y.; et al. PCDH10 is required for the tumorigenicity of glioblastoma cells. Biochem. Biophys. Res. Commun. 2014, 444, 13–18. [Google Scholar] [CrossRef]
- Mancia Leon, W.R.; Spatazza, J.; Rakela, B.; Chatterjee, A.; Pande, V.; Maniatis, T.; Hasenstaub, A.R.; Stryker, M.P.; Alvarez-Buylla, A. Clustered gamma-protocadherins regulate cortical interneuron programmed cell death. eLife 2020, 9, e55374. [Google Scholar] [CrossRef] [PubMed]
- Pischedda, F.; Ghirelli, A.; Tripathi, V.; Piccoli, G. Negr1-Derived Peptides Trigger ALK Degradation and Halt Neuroblastoma Progression In Vitro and In Vivo. Pharmaceutics 2023, 15, 2307. [Google Scholar] [CrossRef] [PubMed]
- Pischedda, F.; Piccoli, G. The IgLON Family Member Negr1 Promotes Neuronal Arborization Acting as Soluble Factor via FGFR2. Front. Mol. Neurosci. 2015, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, A.I.; González-Gómez, M.J.; Baladrón, V.; Laborda, J.; Nueda, M.L. Different Expression Levels of DLK2 Inhibit NOTCH Signaling and Inversely Modulate MDA-MB-231 Breast Cancer Tumor Growth In Vivo. Int. J. Mol. Sci. 2022, 23, 1554. [Google Scholar] [CrossRef] [PubMed]
- Nueda, M.L.; Naranjo, A.I.; Baladrón, V.; Laborda, J. The proteins DLK1 and DLK2 modulate NOTCH1-dependent proliferation and oncogenic potential of human SK-MEL-2 melanoma cells. Biochim. Biophys. Acta 2014, 1843, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, C.; Wang, Z.; Guo, Y.; Wang, Y.; Fan, X.; Jiang, T. Expression changes in ion channel and immunity genes are associated with glioma-related epilepsy in patients with diffuse gliomas. J. Cancer Res. Clin. Oncol. 2022, 148, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Zannikou, M.; Duffy, J.T.; Levine, R.N.; Seblani, M.; Liu, Q.; Presser, A.; Arrieta, V.A.; Chen, C.J.; Sonabend, A.M.; Horbinski, C.M.; et al. IL15 modification enables CAR T cells to act as a dual targeting agent against tumor cells and myeloid-derived suppressor cells in GBM. J. Immunother. Cancer 2023, 11, e006239. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Z.; Song, Y.; Fan, L.; Lei, T.; He, Y.; Hu, S. The Regulatory Network of CREB3L1 and Its Roles in Physiological and Pathological Conditions. Int. J. Med. Sci. 2024, 21, 123–136. [Google Scholar] [CrossRef]
- Liu, L.Q.; Feng, L.F.; Nan, C.R.; Zhao, Z.M. CREB3L1 and PTN expressions correlate with prognosis of brain glioma patients. Biosci. Rep. 2018, 38, BSR20170100. [Google Scholar] [CrossRef] [PubMed]
- Fagerving, A. Nervous System—Brain 1—Smart-Servier.png. 16 November 2023. adapted with presmission from Wikimedia Commons. Available online: https://commons.wikimedia.org/wiki/File:Nervous_system_-_Brain_1_--_Smart-Servier.png (accessed on 10 June 2024).
- Duck, U. Tumor MTK.jpg. 4 May 2022. Adapted with Permission from Wikimedia Commons. Available online: https://commons.wikimedia.org/w/index.php?search=tumor&title=Special:MediaSearch&go=Go&type=image (accessed on 10 June 2024).
- Cheng, X.; An, J.; Lou, J.; Gu, Q.; Ding, W.; Droby, G.N.; Wang, Y.; Wang, C.; Gao, Y.; Anand, J.R.; et al. Trans-lesion synthesis and mismatch repair pathway crosstalk defines chemoresistance and hypermutation mechanisms in glioblastoma. Nat. Commun. 2024, 15, 1957. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.E.; Baugh, E.H.; Thomas, A.; Bier, L.; Lippa, N.; Stong, N.; Mulhern, M.S.; Kushary, S.; Akman, C.I.; Heinzen, E.L.; et al. CSNK2B: A broad spectrum of neurodevelopmental disability and epilepsy severity. Epilepsia 2021, 62, e103–e109. [Google Scholar] [CrossRef] [PubMed]
- Borgo, C.; D’Amore, C.; Sarno, S.; Salvi, M.; Ruzzene, M. Protein kinase CK2: A potential therapeutic target for diverse human diseases. Signal Transduct. Target. Ther. 2021, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, E.; Pedrosa, L.; Bourmeau, G.; Anezo, O.; Noguera-Castells, A.; Esteve-Codina, A.; Passoni, L.; Matteoli, M.; de la Iglesia, N.; Seano, G.; et al. Dual Role of Integrin Alpha-6 in Glioblastoma: Supporting Stemness in Proneural Stem-Like Cells While Inducing Radioresistance in Mesenchymal Stem-Like Cells. Cancers 2021, 13, 3055. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Gao, S. Pan-Cancer Analysis of FURIN as a Potential Prognostic and Immunological Biomarker. Front. Mol. Biosci. 2021, 8, 648402. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, L.; Kuppe, A.; Damerau, A.; Wilantri, S.; Kirchner, M.; Mertins, P.; Strehl, C.; Buttgereit, F.; Gaber, T. Surface AMP deaminase 2 as a novel regulator modifying extracellular adenine nucleotide metabolism. FASEB J. 2021, 35, e21684. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.Z.; Qin, Y.; Wang, W.J.; Fei, B.J.; Han, W.F.; Jin, J.Q.; Gao, X. Overexpression of AMPD2 indicates poor prognosis in colorectal cancer patients via the Notch3 signaling pathway. World J. Clin. Cases 2020, 8, 3197–3208. [Google Scholar] [CrossRef] [PubMed]
- Tse, R.T.; Ding, X.; Wong, C.Y.; Cheng, C.K.; Chiu, P.K.; Ng, C.F. The Association between Spermidine/Spermine N(1)-Acetyltransferase (SSAT) and Human Malignancies. Int. J. Mol. Sci. 2022, 23, 5926. [Google Scholar] [CrossRef]
- Brett-Morris, A.; Wright, B.M.; Seo, Y.; Pasupuleti, V.; Zhang, J.; Lu, J.; Spina, R.; Bar, E.E.; Gujrati, M.; Schur, R.; et al. The polyamine catabolic enzyme SAT1 modulates tumorigenesis and radiation response in GBM. Cancer Res. 2014, 74, 6925–6934. [Google Scholar] [CrossRef]
- Akizu, N.; Cantagrel, V.; Schroth, J.; Cai, N.; Vaux, K.; McCloskey, D.; Naviaux, R.K.; Van Vleet, J.; Fenstermaker, A.G.; Silhavy, J.L.; et al. AMPD2 regulates GTP synthesis and is mutated in a potentially treatable neurodegenerative brainstem disorder. Cell 2013, 154, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Alkan, Z.; Duong, F.L.; Hawkes, W.C. Selenoprotein W controls epidermal growth factor receptor surface expression, activation and degradation via receptor ubiquitination. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 1087–1095. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Xu, F.; Fu, J.; Sun, J.; Gan, X.; Yang, C.; Mao, Z. ncRNAs-mediated high expression of TIMM8A correlates with poor prognosis and act as an oncogene in breast cancer. Cancer Cell Int. 2022, 22, 177. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Døssing, K.B.V.; Sloth, A.B.; He, X.; Rossing, M.; Kjaer, A. Quantitative Evaluation of Stem-like Markers of Human Glioblastoma Using Single-Cell RNA Sequencing Datasets. Cancers 2023, 15, 1557. [Google Scholar] [CrossRef]
- Bourgonje, A.M.; Verrijp, K.; Schepens, J.T.; Navis, A.C.; Piepers, J.A.; Palmen, C.B.; van den Eijnden, M.; Hooft van Huijsduijnen, R.; Wesseling, P.; Leenders, W.P.; et al. Comprehensive protein tyrosine phosphatase mRNA profiling identifies new regulators in the progression of glioma. Acta Neuropathol. Commun. 2016, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- Tasci, E.; Shah, Y.; Jagasia, S.; Zhuge, Y.; Shephard, J.; Johnson, M.O.; Elemento, O.; Joyce, T.; Chappidi, S.; Cooley Zgela, T.; et al. MGMT ProFWise: Unlocking a New Application for Combined Feature Selection and the Rank-Based Weighting Method to Link MGMT Methylation Status to Serum Protein Expression in Patients with Glioblastoma. Int. J. Mol. Sci. 2024, 25, 4082. [Google Scholar] [CrossRef]
- Krauze, A.V.; Sierk, M.; Nguyen, T.; Chen, Q.; Yan, C.; Hu, Y.; Jiang, W.; Tasci, E.; Zgela, T.C.; Sproull, M.; et al. Glioblastoma survival is associated with distinct proteomic alteration signatures post chemoirradiation in a large-scale proteomic panel. Front. Oncol. 2023, 13, 1127645. [Google Scholar] [CrossRef]
- Krauze, A.V.; Zhao, Y.; Li, M.C.; Shih, J.; Jiang, W.; Tasci, E.; Cooley Zgela, T.; Sproull, M.; Mackey, M.; Shankavaram, U.; et al. Revisiting Concurrent Radiation Therapy, Temozolomide, and the Histone Deacetylase Inhibitor Valproic Acid for Patients with Glioblastoma-Proteomic Alteration and Comparison Analysis with the Standard-of-Care Chemoirradiation. Biomolecules 2023, 13, 1499. [Google Scholar] [CrossRef]
- Amousey. Human-brain-vector.svg. 21 December 2022, Adapted with Permission from Wikimedia Commons. Available online: https://commons.wikimedia.org/wiki/File:Human-brain-vector.svg (accessed on 10 June 2024).
- Ebrambot. OpenMoji-color 1F489.svg. 24 May 2018, Adapted with Permission from Wikimedia Commons. Available online: https://commons.wikimedia.org/wiki/File:OpenMoji-color_1F489.svg (accessed on 10 June 2024).
- Fagerving, A. Equipment–Blood Test (8570)–Smart-Servier.png. 31 January 2023, Adapted with Permission from Wiki-media Commons. Available online: https://commons.wikimedia.org/wiki/File:Equipment_-_Blood_test_(8570)_--_Smart-Servier.png (accessed on 10 June 2024).







| Correlation Followed by Feature Selection (FS) (Process 1) | Feature selection (FS) Followed by Correlation (Process 2) | ||||
|---|---|---|---|---|---|
| Before FS | After FS | Before FS | After FS | ||
| Logistic Regression | LASSO | 0.77 | 0.77 | 0.82 | 0.73 |
| RFECV | 0.77 | 0.82 | 0.82 | 0.82 | |
| Random Forest | LASSO | 0.59 | 0.73 | 0.55 | 0.68 |
| RFECV | 0.59 | 0.73 | 0.55 | 0.68 | |
| Entrez Gene Symbol | Target Full Name |
|---|---|
| RPA2 | Replication protein A 32 kDa subunit |
| AMPD2 | AMP deaminase 2 |
| DLK2 | Protein delta homolog 2 |
| NEGR1 | Neuronal growth regulator 1 |
| PDCL2 | Phosducin-like protein 2 |
| POLI | DNA polymerase iota |
| CEACAM3 | Carcinoembryonic antigen-related cell adhesion molecule 3 |
| ITGA6 | Integrin alpha-6 |
| PCDHGA10 | Protocadherin gamma-A10 |
| SELENOW | Selenoprotein W |
| TIMM8A | Mitochondrial import inner membrane translocase subunit Tim8 A |
| CLN5 | Ceroid-lipofuscinosis neuronal protein 5: Lumenal domain |
| IL15RA | Interleukin-15 receptor subunit alpha |
| P3H1 | Prolyl 3-hydroxylase 1 |
| UTS2R | Urotensin-2 receptor |
| RGS4 | Regulator of G-protein signaling 4 |
| PTPRS | Receptor-type tyrosine-protein phosphatase S |
| FURIN | Furin |
| MSR1 | Macrophage scavenger receptor types I and II: Extracellular domain |
| CSNK2B | Casein kinase II subunit beta |
| SAT2 | Diamine acetyltransferase 2 |
| TRAPPC5 | Trafficking protein particle complex subunit 5 |
| CREB3L1 | Cyclic AMP-responsive element-binding protein 3-like protein 1 |
| SCN3B | Sodium channel subunit beta-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joyce, T.; Tasci, E.; Jagasia, S.; Shephard, J.; Chappidi, S.; Zhuge, Y.; Zhang, L.; Cooley Zgela, T.; Sproull, M.; Mackey, M.; et al. Serum CD133-Associated Proteins Identified by Machine Learning Are Connected to Neural Development, Cancer Pathways, and 12-Month Survival in Glioblastoma. Cancers 2024, 16, 2740. https://doi.org/10.3390/cancers16152740
Joyce T, Tasci E, Jagasia S, Shephard J, Chappidi S, Zhuge Y, Zhang L, Cooley Zgela T, Sproull M, Mackey M, et al. Serum CD133-Associated Proteins Identified by Machine Learning Are Connected to Neural Development, Cancer Pathways, and 12-Month Survival in Glioblastoma. Cancers. 2024; 16(15):2740. https://doi.org/10.3390/cancers16152740
Chicago/Turabian StyleJoyce, Thomas, Erdal Tasci, Sarisha Jagasia, Jason Shephard, Shreya Chappidi, Ying Zhuge, Longze Zhang, Theresa Cooley Zgela, Mary Sproull, Megan Mackey, and et al. 2024. "Serum CD133-Associated Proteins Identified by Machine Learning Are Connected to Neural Development, Cancer Pathways, and 12-Month Survival in Glioblastoma" Cancers 16, no. 15: 2740. https://doi.org/10.3390/cancers16152740
APA StyleJoyce, T., Tasci, E., Jagasia, S., Shephard, J., Chappidi, S., Zhuge, Y., Zhang, L., Cooley Zgela, T., Sproull, M., Mackey, M., Camphausen, K., & Krauze, A. V. (2024). Serum CD133-Associated Proteins Identified by Machine Learning Are Connected to Neural Development, Cancer Pathways, and 12-Month Survival in Glioblastoma. Cancers, 16(15), 2740. https://doi.org/10.3390/cancers16152740








