Advancing Cancer Therapy: The Role of KIF20A as a Target for Inhibitor Development and Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. KIF20A Expression and Protein Structure
3. KIF20A Expression in Cancer: Implications for Prognosis and Therapeutic Strategies
4. Multiple Roles of KIF20A
4.1. Transport of Golgi Membranes and Associated Vesicles
4.2. Recruitment of PLK1 to the Central Spindle
4.3. CPC-Mediated Cytokinesis
4.4. Activation of the JAK/STAT3 Pathway
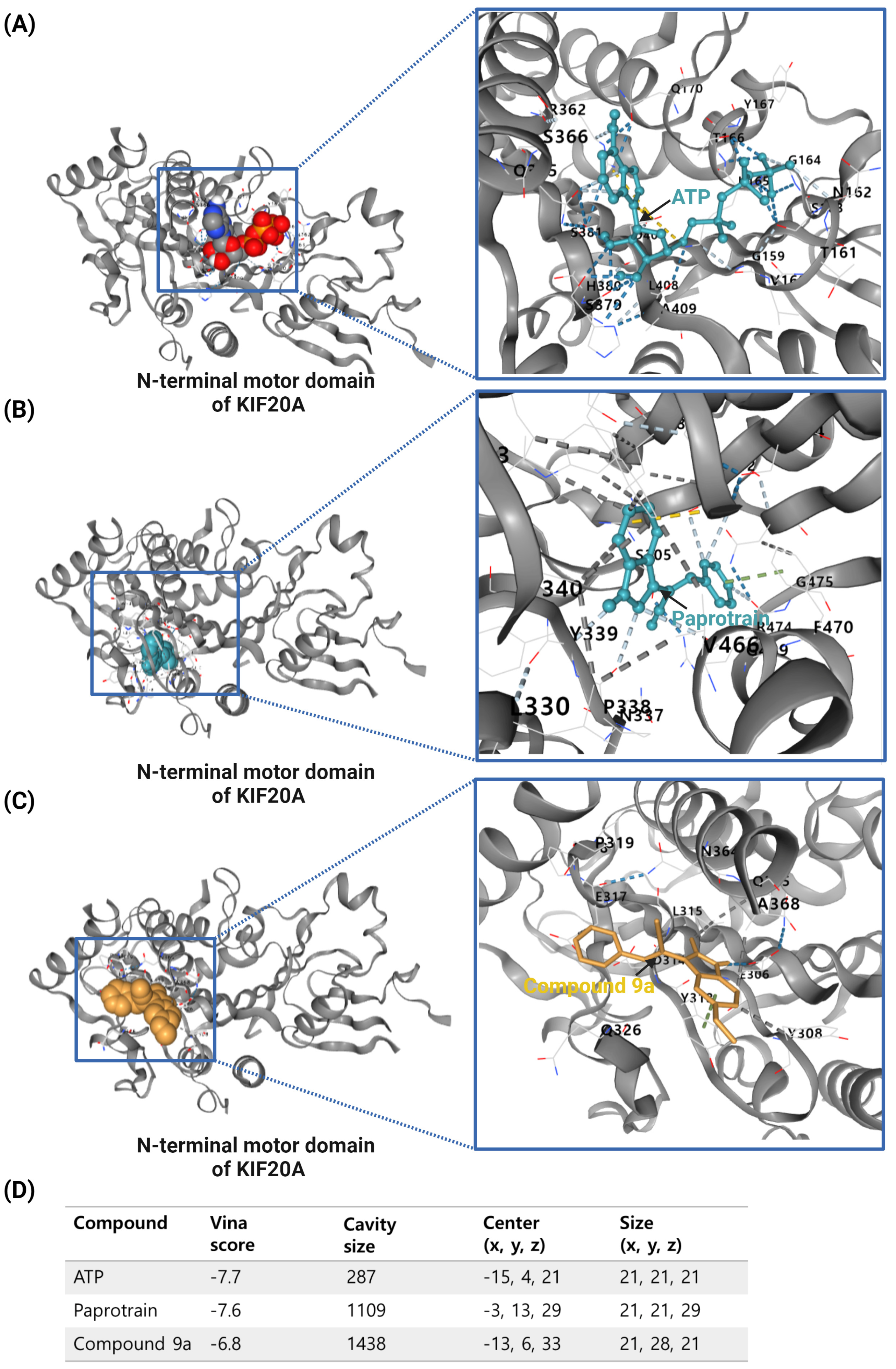
5. Types and Functions of KIF20A Inhibitors
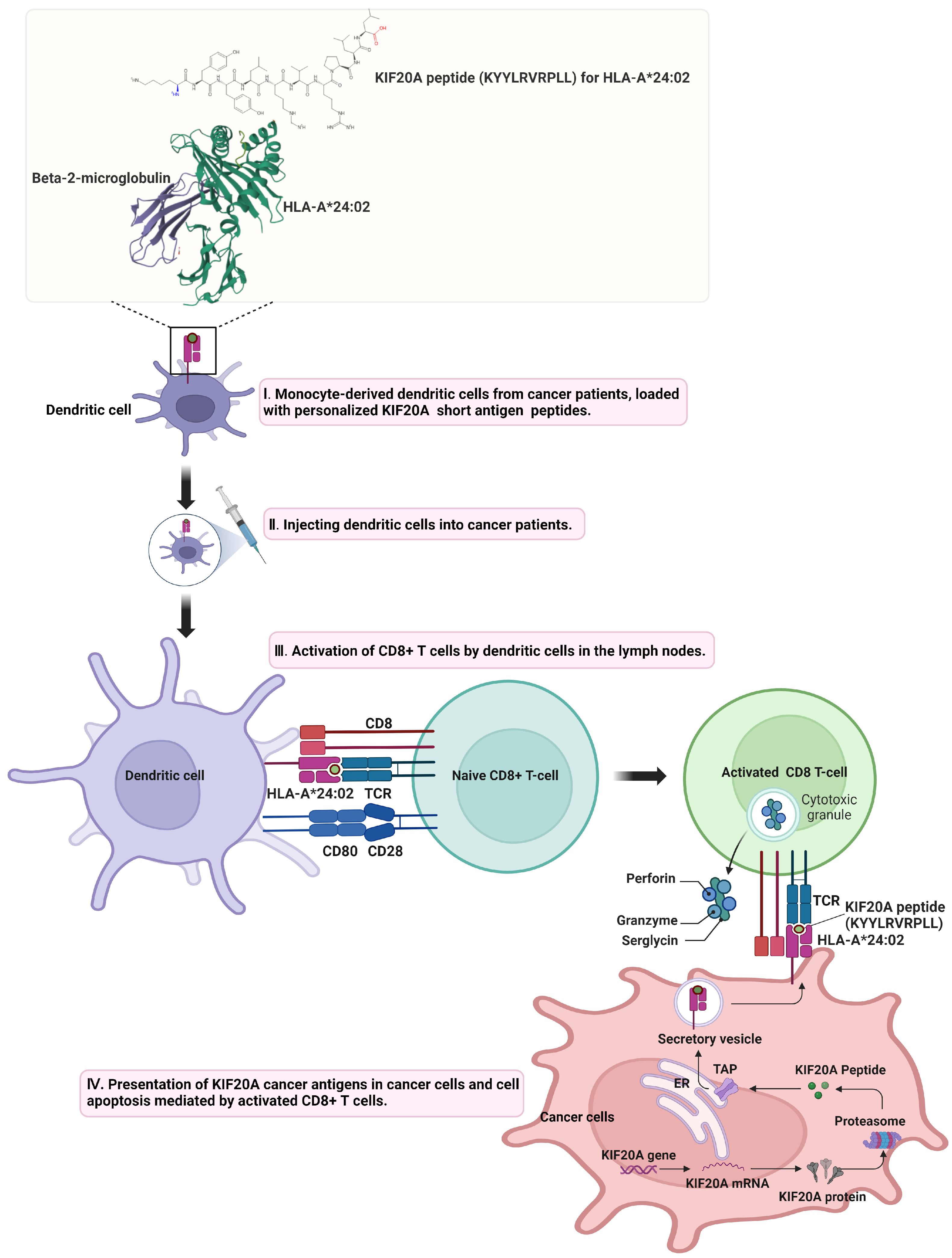
6. The Emergence of KIF20A as a Pivotal Target in Cancer Immunotherapy
7. Challenges and Future Directions in KIF20A-Targeted Cancer Therapy
7.1. Selectivity and Specificity
7.2. Resistance Mechanisms
7.3. Heterogeneity of Tumor Response
7.4. Delivery Challenges
7.5. Precision Medicine Approaches
7.6. Combination Therapies
7.7. Immune System Modulation
7.8. Comprehensive Understanding of KIF20A Function
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; Zhang, S.; Lin, R.; Cao, X.; Yuan, L. Anti-tumor target screening of sea cucumber saponin Frondoside A: A bioinformatics and molecular docking analysis. Front. Oncol. 2023, 13, 1307838. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, H.; Zhang, C.; Wang, L. An evaluation of KIF20A as a prognostic factor and therapeutic target for lung adenocarcinoma using integrated bioinformatics analysis. Front. Bioeng. Biotechnol. 2022, 10, 993820. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Yang, L.; Zhang, Z.; Yu, J.; Dai, L.; Gao, M.; Shang, Z.; Niu, Y. KIF20A Affects the Prognosis of Bladder Cancer by Promoting the Proliferation and Metastasis of Bladder Cancer Cells. Dis. Markers 2019, 2019, 4863182. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, W.; Yuan, H.; Dong, W.; Xiao, W.; Zhang, X. KDELR2-KIF20A axis facilitates bladder cancer growth and metastasis by enhancing Golgi-mediated secretion. Biol. Proced. Online 2022, 24, 12. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Z.; Guo, M.; Wang, W.; Zhang, W.; Liang, S.; Xu, Z.; Ye, J.; Zhu, G.; Zhang, C.; et al. FOXM1 modulates docetaxel resistance in prostate cancer by regulating KIF20A. Cancer Cell. Int. 2020, 20, 545. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Gong, M.; Liu, D.; Liang, S.; Li, Y.; Sang, W.; Zhu, R. FOXM1/KIF20A axis promotes clear cell renal cell carcinoma progression via regulating EMT signaling and affects immunotherapy response. Heliyon 2023, 9, e22734. [Google Scholar] [CrossRef]
- Ranaivoson, F.M.; Crozet, V.; Benoit, M.; Khalid, A.A.M.; Kikuti, C.; Sirkia, H.; Marjou, A.E.; Miserey-Lenkei, S.; Asenjo, A.B.; Sosa, H.; et al. Nucleotide-free structures of KIF20A illuminate atypical mechanochemistry in this kinesin-6. Open Biol. 2023, 13, 230122. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Baxevanis, C.N.; Perez, S.A.; Papamichail, M. Cancer immunotherapy. Crit. Rev. Clin. Lab. Sci. 2009, 46, 167–189. [Google Scholar] [CrossRef]
- Ragone, C.; Manolio, C.; Cavalluzzo, B.; Mauriello, A.; Tornesello, M.L.; Buonaguro, F.M.; Castiglione, F.; Vitagliano, L.; Iaccarino, E.; Ruvo, M.; et al. Identification and validation of viral antigens sharing sequence and structural homology with tumor-associated antigens (TAAs). J. Immunother. Cancer 2021, 9, e002694. [Google Scholar] [CrossRef]
- Lai, F.; Fernald, A.A.; Zhao, N.; Le Beau, M.M. cDNA cloning, expression pattern, genomic structure and chromosomal location of RAB6KIFL, a human kinesin-like gene. Gene 2000, 248, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 1998, 279, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Huang, D.; Lu, N.; Chen, D.; Zhang, M.; Yan, Y.; Deng, L.; Lu, Q.; Lu, H.; Luo, S. Aberrantly activated Gli2-KIF20A axis is crucial for growth of hepatocellular carcinoma and predicts poor prognosis. Oncotarget 2016, 7, 26206–26219. [Google Scholar] [CrossRef]
- Sicklick, J.K.; Li, Y.X.; Jayaraman, A.; Kannangai, R.; Qi, Y.; Vivekanandan, P.; Ludlow, J.W.; Owzar, K.; Chen, W.; Torbenson, M.S.; et al. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis 2006, 27, 748–757. [Google Scholar] [CrossRef]
- Briscoe, J.; Thérond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef]
- Gartel, A.L. FOXM1 in Cancer: Interactions and Vulnerabilities. Cancer Res. 2017, 77, 3135–3139. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.B.; Li, X.Z.; Zeng, S.; Liu, C.; Yang, S.M.; Yang, L.; Hu, C.J.; Bai, J.Y. Regulation of the master regulator FOXM1 in cancer. Cell Commun. Signal. 2018, 16, 57. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Sattler, U.G.; Mueller-Klieser, W. Lactate: A metabolic key player in cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef]
- Pennington, Z.; Goodwin, M.L.; Westbroek, E.M.; Cottrill, E.; Ahmed, A.K.; Sciubba, D.M. Lactate and cancer: Spinal metastases and potential therapeutic targets (part 2). Ann. Transl. Med. 2019, 7, 221. [Google Scholar] [CrossRef]
- Jung, Y.D.; Cho, J.H.; Park, S.; Kang, M.; Park, S.J.; Choi, D.H.; Jeong, M.; Park, K.C.; Yeom, Y.I.; Lee, D.C. Lactate Activates the E2F Pathway to Promote Cell Motility by Up-Regulating Microtubule Modulating Genes. Cancers 2019, 11, 274. [Google Scholar] [CrossRef]
- Jin, Z.; Peng, F.; Zhang, C.; Tao, S.; Xu, D.; Zhu, Z. Expression, regulating mechanism and therapeutic target of KIF20A in multiple cancer. Heliyon 2023, 9, e13195. [Google Scholar] [CrossRef]
- Atherton, J.; Yu, I.M.; Cook, A.; Muretta, J.M.; Joseph, A.; Major, J.; Sourigues, Y.; Clause, J.; Topf, M.; Rosenfeld, S.S.; et al. The divergent mitotic kinesin MKLP2 exhibits atypical structure and mechanochemistry. eLife 2017, 6, e27793. [Google Scholar] [CrossRef]
- Benoit, M.; Asenjo, A.B.; Paydar, M.; Dhakal, S.; Kwok, B.H.; Sosa, H. Structural basis of mechano-chemical coupling by the mitotic kinesin KIF14. Nat. Commun. 2021, 12, 3637. [Google Scholar] [CrossRef]
- Cao, L.; Wang, W.; Jiang, Q.; Wang, C.; Knossow, M.; Gigant, B. The structure of apo-kinesin bound to tubulin links the nucleotide cycle to movement. Nat. Commun. 2014, 5, 5364. [Google Scholar] [CrossRef]
- Guan, R.; Zhang, L.; Su, Q.P.; Mickolajczyk, K.J.; Chen, G.Y.; Hancock, W.O.; Sun, Y.; Zhao, Y.; Chen, Z. Crystal structure of Zen4 in the apo state reveals a missing conformation of kinesin. Nat. Commun. 2017, 8, 14951. [Google Scholar] [CrossRef]
- Miserey-Lenkei, S.; Bousquet, H.; Pylypenko, O.; Bardin, S.; Dimitrov, A.; Bressanelli, G.; Bonifay, R.; Fraisier, V.; Guillou, C.; Bougeret, C.; et al. Coupling fission and exit of RAB6 vesicles at Golgi hotspots through kinesin-myosin interactions. Nat. Commun. 2017, 8, 1254. [Google Scholar] [CrossRef]
- Wu, W.D.; Yu, K.W.; Zhong, N.; Xiao, Y.; She, Z.Y. Roles and mechanisms of Kinesin-6 KIF20A in spindle organization during cell division. Eur. J. Cell Biol. 2019, 98, 74–80. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, C.; Xu, G.; Liang, T.; Yu, C.; Liao, S.; Zhang, Z.; Lu, Z.; Wang, Z.; Chen, J.; et al. Identification of Hub Genes Associated With Melanoma Development by Comprehensive Bioinformatics Analysis. Front. Oncol. 2021, 11, 621430. [Google Scholar] [CrossRef]
- Nakamura, M.; Takano, A.; Thang, P.M.; Tsevegjav, B.; Zhu, M.; Yokose, T.; Yamashita, T.; Miyagi, Y.; Daigo, Y. Characterization of KIF20A as a prognostic biomarker and therapeutic target for different subtypes of breast cancer. Int. J. Oncol. 2020, 57, 277–288. [Google Scholar] [CrossRef]
- Zhang, W.; He, W.; Shi, Y.; Gu, H.; Li, M.; Liu, Z.; Feng, Y.; Zheng, N.; Xie, C.; Zhang, Y. High Expression of KIF20A Is Associated with Poor Overall Survival and Tumor Progression in Early-Stage Cervical Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0167449. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Sun, X.; Chen, J.; Li, Y.; Niu, C.; Xu, B.; Zhang, Y. Overexpression of kinesin family member 20A is associated with unfavorable clinical outcome and tumor progression in epithelial ovarian cancer. Cancer Manag. Res. 2018, 10, 3433–3450. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, W.; Hong, B.; Jiang, X.; Sun, R.; Yan, Q.; Zhang, S.; Lu, M.; Wang, S.; Zhang, Z.; et al. Upregulation of KIF20A correlates with poor prognosis in gastric cancer. Cancer Manag. Res. 2018, 10, 6205–6216. [Google Scholar] [CrossRef]
- Duan, J.; Huang, W.; Shi, H. Positive expression of KIF20A indicates poor prognosis of glioma patients. Onco. Targets Ther. 2016, 9, 6741–6749. [Google Scholar] [CrossRef]
- Qiu, R.; Wu, J.; Gudenas, B.; Northcott, P.A.; Wechsler-Reya, R.J.; Lu, Q. Depletion of kinesin motor KIF20A to target cell fate control suppresses medulloblastoma tumour growth. Commun. Biol. 2021, 4, 552. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Yan, Y.; Li, L.; Wang, Z.; Pang, J.; Guan, X.; Yuan, Y.; Xia, Z.; Yi, W. A comprehensive analysis of the role of QPRT in breast cancer. Sci. Rep. 2023, 13, 15414. [Google Scholar] [CrossRef]
- Miserey-Lenkei, S.; Chalancon, G.; Bardin, S.; Formstecher, E.; Goud, B.; Echard, A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat. Cell Biol. 2010, 12, 645–654. [Google Scholar] [CrossRef]
- Echard, A.; Jollivet, F.; Martinez, O.; Lacapère, J.J.; Rousselet, A.; Janoueix-Lerosey, I.; Goud, B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science 1998, 279, 580–585. [Google Scholar] [CrossRef]
- Maliga, Z.; Junqueira, M.; Toyoda, Y.; Ettinger, A.; Mora-Bermúdez, F.; Klemm, R.W.; Vasilj, A.; Guhr, E.; Ibarlucea-Benitez, I.; Poser, I.; et al. A genomic toolkit to investigate kinesin and myosin motor function in cells. Nat. Cell Biol. 2013, 15, 325–334. [Google Scholar] [CrossRef]
- Grigoriev, I.; Splinter, D.; Keijzer, N.; Wulf, P.S.; Demmers, J.; Ohtsuka, T.; Modesti, M.; Maly, I.V.; Grosveld, F.; Hoogenraad, C.C.; et al. Rab6 regulates transport and targeting of exocytotic carriers. Dev. Cell 2007, 13, 305–314. [Google Scholar] [CrossRef]
- Brennan, I.M.; Peters, U.; Kapoor, T.M.; Straight, A.F. Polo-like kinase controls vertebrate spindle elongation and cytokinesis. PLoS ONE 2007, 2, e409. [Google Scholar] [CrossRef]
- Lowery, D.M.; Mohammad, D.H.; Elia, A.E.; Yaffe, M.B. The Polo-box domain: A molecular integrator of mitotic kinase cascades and Polo-like kinase function. Cell Cycle 2004, 3, 128–131. [Google Scholar] [CrossRef]
- Hadders, M.A.; Lens, S.M.A. Changing places: Chromosomal Passenger Complex relocation in early anaphase. Trends Cell Biol. 2022, 32, 165–176. [Google Scholar] [CrossRef]
- Klein, U.R.; Nigg, E.A.; Gruneberg, U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol. Biol. Cell 2006, 17, 2547–2558. [Google Scholar] [CrossRef]
- Carmena, M.; Wheelock, M.; Funabiki, H.; Earnshaw, W.C. The chromosomal passenger complex (CPC): From easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 2012, 13, 789–803. [Google Scholar] [CrossRef]
- Yang, J.; Zappacosta, F.; Annan, R.S.; Nurse, K.; Tummino, P.J.; Copeland, R.A.; Lai, Z. The catalytic role of INCENP in Aurora B activation and the kinetic mechanism of Aurora B/INCENP. Biochem. J. 2009, 417, 355–360. [Google Scholar] [CrossRef]
- Basant, A.; Lekomtsev, S.; Tse, Y.C.; Zhang, D.; Longhini, K.M.; Petronczki, M.; Glotzer, M. Aurora B kinase promotes cytokinesis by inducing centralspindlin oligomers that associate with the plasma membrane. Dev. Cell 2015, 33, 204–215. [Google Scholar] [CrossRef]
- Samejima, K.; Platani, M.; Wolny, M.; Ogawa, H.; Vargiu, G.; Knight, P.J.; Peckham, M.; Earnshaw, W.C. The Inner Centromere Protein (INCENP) Coil Is a Single α-Helix (SAH) Domain That Binds Directly to Microtubules and Is Important for Chromosome Passenger Complex (CPC) Localization and Function in Mitosis. J. Biol. Chem. 2015, 290, 21460–21472. [Google Scholar] [CrossRef]
- Vader, G.; Kauw, J.J.; Medema, R.H.; Lens, S.M. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep. 2006, 7, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bekier, M.E.; Mazur, T.; Rashid, M.S.; Taylor, W.R. Borealin dimerization mediates optimal CPC checkpoint function by enhancing localization to centromeres and kinetochores. Nat. Commun. 2015, 6, 6775. [Google Scholar] [CrossRef] [PubMed]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial-Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zhuang, K.; Luo, Y.; Lai, Q.; Luo, X.; Fang, Y.; Zhang, Y.; Li, A.; Liu, S. KIF20A promotes cellular malignant behavior and enhances resistance to chemotherapy in colorectal cancer through regulation of the JAK/STAT3 signaling pathway. Aging 2019, 11, 11905–11921. [Google Scholar] [CrossRef]
- Tcherniuk, S.; Skoufias, D.A.; Labriere, C.; Rath, O.; Gueritte, F.; Guillou, C.; Kozielski, F. Relocation of Aurora B and survivin from centromeres to the central spindle impaired by a kinesin-specific MKLP-2 inhibitor. Angew. Chem. Int. Ed. Engl. 2010, 49, 8228–8231. [Google Scholar] [CrossRef]
- Adriaans, I.E.; Hooikaas, P.J.; Aher, A.; Vromans, M.J.M.; van Es, R.M.; Grigoriev, I.; Akhmanova, A.; Lens, S.M.A. MKLP2 Is a Motile Kinesin that Transports the Chromosomal Passenger Complex during Anaphase. Curr. Biol. 2020, 30, 2628–2637.e9. [Google Scholar] [CrossRef]
- Copello, V.A.; Burnstein, K.L. The kinesin KIF20A promotes progression to castration-resistant prostate cancer through autocrine activation of the androgen receptor. Oncogene 2022, 41, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Kositza, J.; Nguyen, J.; Hong, T.; Mantwill, K.; Nawroth, R. Identification of the KIF and MCM protein families as novel targets for combination therapy with CDK4/6 inhibitors in bladder cancer. Urol. Oncol. 2023, 41, 253.e11–253.e20. [Google Scholar] [CrossRef]
- Schrock, M.S.; Scarberry, L.; Stromberg, B.R.; Sears, C.; Torres, A.E.; Tallman, D.; Krupinski, L.; Chakravarti, A.; Summers, M.K. MKLP2 functions in early mitosis to ensure proper chromosome congression. J. Cell. Sci. 2022, 135, jcs259560. [Google Scholar] [CrossRef]
- Labrière, C.; Talapatra, S.K.; Thoret, S.; Bougeret, C.; Kozielski, F.; Guillou, C. New MKLP-2 inhibitors in the paprotrain series: Design, synthesis and biological evaluations. Bioorg. Med. Chem. 2016, 24, 721–734. [Google Scholar] [CrossRef]
- Ferrero, H.; Corachán, A.; Quiñonero, A.; Bougeret, C.; Pouletty, P.; Pellicer, A.; Domínguez, F. Inhibition of KIF20A by BKS0349 reduces endometriotic lesions in a xenograft mouse model. Mol. Hum. Reprod. 2019, 25, 562–571. [Google Scholar] [CrossRef]
- Moussion, C.; Delamarre, L. Antigen cross-presentation by dendritic cells: A critical axis in cancer immunotherapy. Semin. Immunol. 2024, 71, 101848. [Google Scholar] [CrossRef]
- Abascal, J.; Oh, M.S.; Liclican, E.L.; Dubinett, S.M.; Salehi-Rad, R.; Liu, B. Dendritic Cell Vaccination in Non-Small Cell Lung Cancer: Remodeling the Tumor Immune Microenvironment. Cells 2023, 12, 2404. [Google Scholar] [CrossRef]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Yewdall, A.W.; Drutman, S.B.; Jinwala, F.; Bahjat, K.S.; Bhardwaj, N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS ONE 2010, 5, e11144. [Google Scholar] [CrossRef]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef]
- Melief, C.J.M.; Welters, M.J.P.; Vergote, I.; Kroep, J.R.; Kenter, G.G.; Ottevanger, P.B.; Tjalma, W.A.A.; Denys, H.; van Poelgeest, M.I.E.; Nijman, H.W.; et al. Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Sci. Transl. Med. 2020, 12, eaaz8235. [Google Scholar] [CrossRef]
- Andersen, M.H. Tumor microenvironment antigens. Semin. Immunopathol. 2023, 45, 253–264. [Google Scholar] [CrossRef]
- Aly, H.A. Cancer therapy and vaccination. J. Immunol. Methods 2012, 382, 1–23. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef]
- Bright, R.K.; Bright, J.D.; Byrne, J.A. Overexpressed oncogenic tumor-self antigens. Hum. Vaccin. Immunother. 2014, 10, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Margalit, A. Targeting tumor-associated antigens to the MHC class I presentation pathway. Endocr. Metab. Immune Disord. Drug Targets 2007, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Sprooten, J.; Ceusters, J.; Coosemans, A.; Agostinis, P.; De Vleeschouwer, S.; Zitvogel, L.; Kroemer, G.; Galluzzi, L.; Garg, A.D. Trial watch: Dendritic cell vaccination for cancer immunotherapy. Oncoimmunology 2019, 8, e1638212. [Google Scholar] [CrossRef]
- Pardo, J.; Aguilo, J.I.; Anel, A.; Martin, P.; Joeckel, L.; Borner, C.; Wallich, R.; Müllbacher, A.; Froelich, C.J.; Simon, M.M. The biology of cytotoxic cell granule exocytosis pathway: Granzymes have evolved to induce cell death and inflammation. Microbes Infect. 2009, 11, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Depraetere, V.; Golstein, P. Fas and other cell death signaling pathways. Semin. Immunol. 1997, 9, 93–107. [Google Scholar] [CrossRef]
- St Paul, M.; Ohashi, P.S. The Roles of CD8(+) T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef]
- Rabu, C.; Rangan, L.; Florenceau, L.; Fortun, A.; Charpentier, M.; Dupré, E.; Paolini, L.; Beauvillain, C.; Dupel, E.; Latouche, J.B.; et al. Cancer vaccines: Designing artificial synthetic long peptides to improve presentation of class I, class II T cell epitopes by dendritic cells. Oncoimmunology 2019, 8, e1560919. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Roosinovich, E.; Ma, B.; Hung, C.F.; Wu, T.C. Therapeutic HPV DNA vaccines. Immunol. Res. 2010, 47, 86–112. [Google Scholar] [CrossRef] [PubMed]
- Benteyn, D.; Heirman, C.; Bonehill, A.; Thielemans, K.; Breckpot, K. mRNA-based dendritic cell vaccines. Expert Rev. Vaccines 2015, 14, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Melief, C.J.; van der Burg, S.H. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat. Rev. Cancer 2008, 8, 351–360. [Google Scholar] [CrossRef]
- Shibata, H.; Xu, N.; Saito, S.; Zhou, L.; Ozgenc, I.; Webb, J.; Fu, C.; Zolkind, P.; Egloff, A.M.; Uppaluri, R. Integrating CD4(+) T cell help for therapeutic cancer vaccination in a preclinical head and neck cancer model. Oncoimmunology 2021, 10, 1958589. [Google Scholar] [CrossRef]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef]
- Hammerich, L.; Bhardwaj, N.; Kohrt, H.E.; Brody, J.D. In situ vaccination for the treatment of cancer. Immunotherapy 2016, 8, 315–330. [Google Scholar] [CrossRef]
- Imai, K.; Hirata, S.; Irie, A.; Senju, S.; Ikuta, Y.; Yokomine, K.; Harao, M.; Inoue, M.; Tomita, Y.; Tsunoda, T.; et al. Identification of HLA-A2-restricted CTL epitopes of a novel tumour-associated antigen, KIF20A, overexpressed in pancreatic cancer. Br. J. Cancer 2011, 104, 300–307. [Google Scholar] [CrossRef]
- Osawa, R.; Tsunoda, T.; Yoshimura, S.; Watanabe, T.; Miyazawa, M.; Tani, M.; Takeda, K.; Nakagawa, H.; Nakamura, Y.; Yamaue, H. Identification of HLA-A24-restricted novel T Cell epitope peptides derived from P-cadherin and kinesin family member 20A. J. Biomed. Biotechnol. 2012, 2012, 848042. [Google Scholar] [CrossRef]
- Tomita, Y.; Yuno, A.; Tsukamoto, H.; Senju, S.; Kuroda, Y.; Hirayama, M.; Irie, A.; Kawahara, K.; Yatsuda, J.; Hamada, A.; et al. Identification of promiscuous KIF20A long peptides bearing both CD4+ and CD8+ T-cell epitopes: KIF20A-specific CD4+ T-cell immunity in patients with malignant tumor. Clin. Cancer Res. 2013, 19, 4508–4520. [Google Scholar] [CrossRef] [PubMed]
- Asahara, S.; Takeda, K.; Yamao, K.; Maguchi, H.; Yamaue, H. Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J. Transl. Med. 2013, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hazama, S.; Iguchi, H.; Uesugi, K.; Tanaka, H.; Hirakawa, K.; Aruga, A.; Hatori, T.; Ishizaki, H.; Umeda, Y.; et al. Phase II clinical trial of peptide cocktail therapy for patients with advanced pancreatic cancer: VENUS-PC study. Cancer Sci. 2017, 108, 73–80. [Google Scholar] [CrossRef]
- Yatsuda, J.; Irie, A.; Harada, K.; Michibata, Y.; Tsukamoto, H.; Senju, S.; Tomita, Y.; Yuno, A.; Hirayama, M.; Abu Sayem, M.; et al. Establishment of HLA-DR4 transgenic mice for the identification of CD4+ T cell epitopes of tumor-associated antigens. PLoS ONE 2013, 8, e84908. [Google Scholar] [CrossRef]
- Okuyama, R.; Aruga, A.; Hatori, T.; Takeda, K.; Yamamoto, M. Immunological responses to a multi-peptide vaccine targeting cancer-testis antigens and VEGFRs in advanced pancreatic cancer patients. Oncoimmunology 2013, 2, e27010. [Google Scholar] [CrossRef]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J. Transl. Med. 2014, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Katsuda, M.; Maguchi, H.; Katanuma, A.; Ishii, H.; Ozaka, M.; Yamao, K.; Imaoka, H.; Kawai, M.; Hirono, S.; et al. Phase II clinical trial using novel peptide cocktail vaccine as a postoperative adjuvant treatment for surgically resected pancreatic cancer patients. Int. J. Cancer 2017, 140, 973–982. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Okada, K.; Omori, T.; Sugimura, K.; Miyata, H.; Ohue, M.; Kobayashi, S.; Takahashi, H.; Nakano, H.; Mochizuki, C.; et al. Multiple therapeutic peptide vaccines for patients with advanced gastric cancer. Int. J. Oncol. 2017, 50, 1655–1662. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Sugimura, K.; Miyata, H.; Omori, T.; Nakano, H.; Mochizuki, C.; Shimizu, K.; Saito, H.; Ashida, K.; Honjyo, S.; et al. A Pilot Study of Post-Operative Adjuvant Vaccine for Advanced Gastric Cancer. Yonago Acta Med. 2017, 60, 101–105. [Google Scholar] [CrossRef]
- Kikuchi, R.; Ueda, R.; Saito, K.; Shibao, S.; Nagashima, H.; Tamura, R.; Morimoto, Y.; Sasaki, H.; Noji, S.; Kawakami, Y.; et al. A Pilot Study of Vaccine Therapy with Multiple Glioma Oncoantigen/Glioma Angiogenesis-Associated Antigen Peptides for Patients with Recurrent/Progressive High-Grade Glioma. J. Clin. Med. 2019, 8, 263. [Google Scholar] [CrossRef]
- Murahashi, M.; Tsuruta, T.; Yamada, K.; Hijikata, Y.; Ogata, H.; Kishimoto, J.; Yoshimura, S.; Hikichi, T.; Nakanishi, Y.; Tani, K. Clinical Trial of a Cancer Vaccine Targeting VEGF and KIF20A in Advanced Biliary Tract Cancer. Anticancer Res. 2021, 41, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Schossig, P.; Coskun, E.; Arsenic, R.; Horst, D.; Sehouli, J.; Bergmann, E.; Andresen, N.; Sigler, C.; Busse, A.; Keller, U.; et al. Target Selection for T-Cell Therapy in Epithelial Ovarian Cancer: Systematic Prioritization of Self-Antigens. Int. J. Mol. Sci. 2023, 24, 2292. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, Y.; Hosono, A.; Yoshikawa, T.; Kaneda, H.; Nitani, C.; Hara, J.; Kinoshita, Y.; Kohashi, K.; Manabe, A.; Fukutani, M.; et al. Efficacy of the NCCV Cocktail-1 vaccine for refractory pediatric solid tumors: A phase I clinical trial. Cancer Sci. 2019, 110, 3650–3662. [Google Scholar] [CrossRef] [PubMed]
- Kida, A.; Mizukoshi, E.; Tamai, T.; Terashima, T.; Kitahara, M.; Arai, K.; Yamashita, T.; Fushimi, K.; Honda, M.; Kaneko, S. Immune responses against tumour-associated antigen-derived cytotoxic T lymphocyte epitopes in cholangiocarcinoma patients. Liver Int. 2018, 38, 2040–2050. [Google Scholar] [CrossRef]
- Shuel, S.L. Targeted cancer therapies: Clinical pearls for primary care. Can. Fam. Physician 2022, 68, 515–518. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Cree, I.A.; Charlton, P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer 2017, 17, 10. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Rübben, A.; Araujo, A. Cancer heterogeneity: Converting a limitation into a source of biologic information. J. Transl. Med. 2017, 15, 190. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Abd-Aziz, N.; Poh, C.L. Development of Peptide-Based Vaccines for Cancer. J. Oncol. 2022, 2022, 9749363. [Google Scholar] [CrossRef] [PubMed]


| RNA-Seq Data | ||||
|---|---|---|---|---|
| Tissue | Mann_Whitney.p. a | Fold.Change.Mean b | Fold.Change.Median c | |
| Blader Urothelial Carcinoma | 7.79 × 10−4 | 3.88 | 19.00 | |
| Breast invasive Carcinoma | 3.59 × 10−19 | 8.64 | 13.01 | |
| Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma | 1.81 × 10−1 | 38.88 | 74.08 | |
| Cholangiocarcinoma | 9.15 × 10−3 | 67.07 | 42.67 | |
| Colon Adenocarcinoma | 2.72 × 10−6 | 2.94 | 4.27 | |
| Esophageal Carcinoma | 1.41 × 10−2 | 5.10 | 3.64 | |
| Head and Neck Carcinoma | 1.69 × 10−6 | 2.72 | 2.79 | |
| Kidney Chromophobe | 2.34 × 10−2 | 3.90 | 1.38 | |
| Kidney Renal Clear Cell Carcinoma | 1.28 × 10−11 | 5.41 | 6.18 | |
| Kidney Renal Papillary Cell Carcinoma | 6.56 × 10−6 | 10.90 | 5.92 | |
| Liver Hepatocellular Carcinoma | 1.26 × 10−9 | 20.78 | 16.50 | |
| Lung Adenocarcinoma | 6.2 × 10−11 | 11.47 | 10.19 | |
| Lung Squamous Cell Carcinoma | 1.14 × 10−9 | 13.43 | 18.73 | |
| Pancreatic Adenocarcinoma | 1 × 10−1 | 2.21 | 2.02 | |
| Pheochromocytoma and Paraganglioma | 1.81 × 10−1 | 1.59 | 2.56 | |
| Prostate Adenocarcinoma | 9.67 × 10−8 | 2.84 | 2.73 | |
| Rectum Adenocarcinoma | 9.15 × 10−3 | 3.75 | 3.69 | |
| Sarcoma | 1 × 100 | 10.65 | 10.65 | |
| Gene chip data | ||||
| Breast | 9.16 × 10−6 | 2.00 | 1.93 | |
| CNS | 1.81 × 10−1 | 11.05 | 1.17 | |
| Colon | 1.16 × 10−23 | 2.32 | 2.64 | |
| Gastric | 4.78 × 10−38 | 2.57 | 2.59 | |
| Kidney | 2.9 × 10−32 | 5.22 | 6.87 | |
| Liver | 3.87 × 10−21 | 5.08 | 7.70 | |
| Lung | 9.62 × 10−41 | 4.26 | 6.16 | |
| Lymphoid | 3 × 10−2 | 0.39 | 0.26 | |
| Neural | 1.81 × 10−1 | 1.74 | 1.62 | |
| Esophageal | 2.52 × 10−8 | 4.85 | 4.91 | |
| Oral cavity | 1 × 10−1 | 2.20 | 2.33 | |
| Ovarian | 5.91 × 10−2 | 3.41 | 4.96 | |
| Pancreas | 1.58 × 10−8 | 2.61 | 2.58 | |
| Prostate | 1.71 × 10−2 | 1.74 | 1.57 | |
| Skin | 3.34 × 10−3 | 2.22 | 3.19 | |
| Soft tissue | 2.01 × 10−1 | 1.29 | 1.35 | |
| Thyroid | 2.01 × 10−1 | 1.29 | 1.35 | |
| Uterus | 2.01 × 10−1 | 1.29 | 1.35 | |
| Inhibitor Type | Model (Cell or Animal) | Inhibitor Concentration | Results | Ref. |
|---|---|---|---|---|
Paprotrain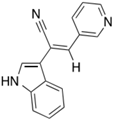 | HeLa Cells | 10 to 50 μM | Increased binucleated cells, disrupted chromosome passenger protein relocation, specific inhibition of KIF20A without affecting Kif4 or MKLP-1 | [54] |
| Paprotrain | HeLa Cells | 50 μM | Reduced motor activity and transport efficiency of KIF20A, decreased number and velocity of coreCPC complexes, and inhibited recruitment of coreCPC | [55] |
| Paprotrain | Castration-resistant prostate cancer cells | 500 nM | Reduced castration-resistant proliferation by inhibiting AR signaling activation driven by KIF20A. | [56] |
| Paprotrain | Bladder cancer cell lines (RT112, T24, UMUC3) | 0 to 20 μM | Combined with the drug palbociclib, it significantly enhances the effectiveness of the treatment, leading to a more substantial reduction in cancer cell survival. | [57] |
| Paprotrain | HeLa cells with fluorescently tagged histones | 0 to 11 μM | Induced significant mitotic arrest by disrupting chromosome congression, increased Aurora kinase activity, and led to chromosomal instability. | [58] |
Compound 9a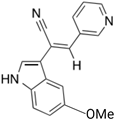 | Human tumor cell lines (e.g., MIA-PaCa-2, HCT116) | 0 to 50 μM | Demonstrated 2- to 10-fold higher potency than Paprotrain in inhibiting KIF20A ATPase activities; significant increase in antiproliferative effects across different tumor cell lines. | [59] |
| BKS0349 (unpublished) | Xenograft mouse model (endometriosis) | 200 mg/kg/week | Significantly reduced size of endometriotic lesions, decreased cell proliferation, increased apoptosis. | [60] |
| Products | Clinical Stage | Vaccine Targeting | Main Results | Ref. |
|---|---|---|---|---|
| KIF20A-2, KIF20A-8, KIF20A-28 peptides | Preclinical | KIF20A(+) HLA-A2(+) pancreatic cancer cells | Identified peptides induce HLA-A2-restricted CD8+ T-cells in transgenic mice and human CD8+ T-cells in vitro, targeting pancreatic cancer cells expressing KIF20A and HLA-A2 without causing autoimmunity. | [89] |
| KIF20A-10-66 peptide | Preclinical | HLA-A*2402+ cancer cells expressing KIF20A | KIF20A-10-66 peptide induces specific CD8+ T-cells that target and exhibit cytotoxic activity against cancer cells expressing KIF20A and HLA-A*2402, demonstrating potential for CD8+ T-cell-inducing cancer therapies. | [90] |
| KIF20A long peptides (KIF20A-LPs) | Preclinical | KIF20A+ HNMT expressing HLA-A2 or HLA-A24 | Identified KIF20A-LPs induce KIF20A-specific TH1 and CD8+ T-cell responses in vivo and in vitro, with significant TH1 responses in 50% of HNMT patients, associating with KIF20A expression in tumor tissues. | [91] |
| KIF20A-66 peptide | Phase I/II | Advanced pancreatic cancer | The vaccine was well tolerated, showed a disease control rate of 72%, and significantly prolonged survival compared to historical controls, indicating effective immunotherapy against advanced pancreatic cancer. | [92] |
| KIF20A peptide, VEGFR1 peptide, VEGFR2 peptide | Phase II | Advanced pancreatic cancer | No severe adverse effects observed; patients with peptide-specific CD8+ T-cell induction for KIF20A or VEGFR1 showed better prognosis; therapeutic peptide cocktail may be effective in patients with peptide-specific immune reactions. | [93] |
| KIF20A494-517 peptide | Preclinical | HLA-DR4 transgenic mice | Successful induction of CD4+ T cell responses to KIF20A and other TAAs in transgenic mice and HLA-DR4-positive human PBMCs, validating the approach for vaccine peptide screening. | [94] |
| KIF20A (KVYLRVRPLL) peptide | Phase I | Advanced pancreatic cancer | The KIF20A component of the multi-peptide vaccine was well-tolerated and induced specific T-cell responses, contributing to observed clinical benefits in patients. | [95] |
| KIF20A peptide | Phase I | Advanced biliary tract cancer | The vaccine was well-tolerated, induced peptide-specific T-cell responses, and stable disease was observed in 5 of 9 patients. Median PFS and OS were 3.4 and 9.7 months, respectively. | [96] |
| KIF20A peptide, VEGFR1 peptide, VEGFR2 peptide | Phase II | Resected pancreatic cancer | The vaccine was well-tolerated; median DFS was 15.8 months. Patients with KIF20A-specific CD8+ T-cell responses and/or KIF20A expression showed better DFS. | [97] |
| Peptide cocktail vaccine (including KIF20A peptide) | Phase II | Surgically resected pancreatic cancer patients | The vaccine was safe and induced specific immune responses. Patients showing KIF20A-specific CD8+ T-cell responses had significantly better disease-free survival, highlighting its potential as an effective postoperative adjuvant treatment. | [98] |
| KIF20A peptide (part of a cocktail vaccine) | Pilot study (Post-operative adjuvant) | Advanced gastric cancer | The combination of vaccine therapy and S-1 was safe and manageable as adjuvant therapy for stage III gastric cancer, achieving optimal relative dose intensity of S-1 with manageable injection-site reactions. | [99] |
| LY6K, DEPDC1, KIF20A, FOXM1, VEGFR1, VEGFR2 peptides | Pilot Study | Recurrent/progressive high-grade glioma (HGG) | Well-tolerated treatment inducing robust T-lymphocyte responses, with a median OS of 9.2 months and progression-free status in some patients for at least six months. | [100] |
| OCV-C01 (VEGFR1, VEGFR2, KIF20A peptides) | Phase II | Advanced biliary tract cancer | Induced vaccine-specific T-cell responses in patients, with observed contributions to prolonged overall survival. | [101] |
| KIF20A | Target Selection Study | Epithelial ovarian cancer | KIF20A identified as a high-priority antigen for T cell therapy due to its expression and prognostic relevance in EOC, supported by immunohistochemical staining and HLA-ligandome analysis. | [102] |
| NCCV Cocktail-1 (KOC1, FOXM1, KIF20A peptides) | Phase I | Pediatric refractory solid tumors | Well tolerated; induced peptide-specific CD8+ T-cells for KOC1, FOXM1, and KIF20A; associated with better progression-free survival in patients with high CD8+ T-cell frequencies. | [103] |
| KIF20A-derived epitopes | Preclinical | Cholangiocarcinoma | Identified KIF20A among epitopes stimulating specific immune responses in cholangiocarcinoma patients, with potential for immunotherapy. Higher lymphocyte counts correlated with TAA-specific response, and overall survival was significantly prolonged in patients with two or more TAA-specific CD8+ T-cell responses. | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, D.O. Advancing Cancer Therapy: The Role of KIF20A as a Target for Inhibitor Development and Immunotherapy. Cancers 2024, 16, 2958. https://doi.org/10.3390/cancers16172958
Moon DO. Advancing Cancer Therapy: The Role of KIF20A as a Target for Inhibitor Development and Immunotherapy. Cancers. 2024; 16(17):2958. https://doi.org/10.3390/cancers16172958
Chicago/Turabian StyleMoon, Dong Oh. 2024. "Advancing Cancer Therapy: The Role of KIF20A as a Target for Inhibitor Development and Immunotherapy" Cancers 16, no. 17: 2958. https://doi.org/10.3390/cancers16172958
APA StyleMoon, D. O. (2024). Advancing Cancer Therapy: The Role of KIF20A as a Target for Inhibitor Development and Immunotherapy. Cancers, 16(17), 2958. https://doi.org/10.3390/cancers16172958





