Pain Management in Animals with Oncological Disease: Opioids as Influencers of Immune and Tumor Cellular Balance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Search Methodology
3. Mechanisms of Pain and Its Important Role in Animal Welfare
3.1. Concepts of Type Pain, Physiopathology, and Management Approach
3.2. Pathophysiology of Pain in the Oncologic Patient
4. Main Opioids Used for Pain Control in Oncological Patients
4.1. The Mechanisms of Action of Opioids Rely on the Body’s Receptors
4.2. Morphine, Methadone and Fentanyl as Pure Opioids Agonist
4.3. Buprenorphine, a Partial Opioid Agonist
4.4. Butorphanol, an Opioid Agonist-Antagonist
4.5. Tramadol, an Atypical Opioid
5. The “Conflicting Relationship” between Opioids and the Immune System
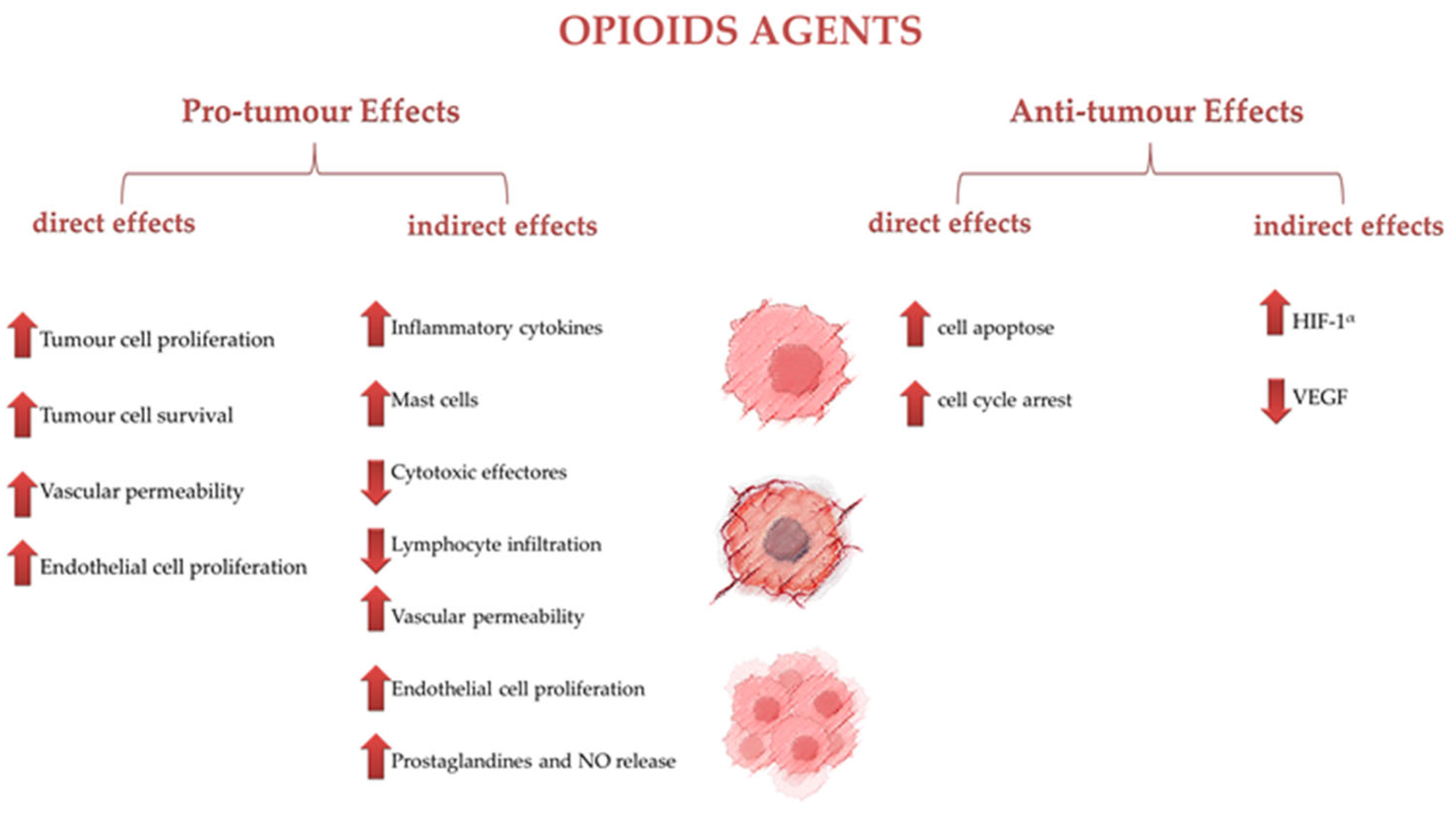
6. The Opioid Agents as Influencers of Tumor Survival
7. Limitations of Opioid Use for Cancer Pain Management in Dogs and Cats and Further Research
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Millman, S.T. Behavioral Responses of Cattle to Pain and Implications for Diagnosis, Management, and Animal Welfare. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 47–58. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Domínguez-Oliva, A.; Martínez-Burnes, J.; Casas-Alvarado, A.; Hernández-Ávalos, I. Euthanasia and Pain in Canine Patients with Terminal and Chronic-Degenerative Diseases: Ethical and Legal Aspects. Animals 2023, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.S. Control of Cancer Pain in Veterinary Patients. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1429–1448. [Google Scholar] [CrossRef]
- Looney, A. Oncology Pain in Veterinary Patients. Top. Companion Anim. Med. 2010, 25, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Inbar, S.; Neeman, E.; Avraham, R.; Benish, M.; Rosenne, E.; Ben-Eliyahu, S. Do Stress Responses Promote Leukemia Progression? An Animal Study Suggesting a Role for Epinephrine and Prostaglandin-E2 through Reduced NK Activity. PLoS ONE 2011, 6, e19246. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, Y.; Sorski, L.; Benish, M.; Levi, B.; Melamed, R.; Ben-Eliyahu, S. Improving Postoperative Immune Status and Resistance to Cancer Metastasis: A Combined Perioperative Approach of Immunostimulation and Prevention of Excessive Surgical Stress Responses. Ann. Surg. 2011, 253, 798–810. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic Stress Promotes Tumor Growth and Angiogenesis in a Mouse Model of Ovarian Carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Gültekin, Ç. Comparison of the Analgesic Effects of Morphine and Tramadol after Tumor Surgery in Dogs. Open Vet. J. 2021, 11, 613. [Google Scholar] [CrossRef]
- Lin, L.; Liu, C.; Tan, H.; Ouyang, H.; Zhang, Y.; Zeng, W. Anaesthetic Technique May Affect Prognosis for Ovarian Serous Adenocarcinoma: A Retrospective Analysis. Br. J. Anaesth. 2011, 106, 814–822. [Google Scholar] [CrossRef]
- Sacerdote, P.; Manfredi, B.; Mantegazza, P.; Panerai, A.E. Antinociceptive and Immunosuppressive Effects of Opiate Drugs: A Structure-Related Activity Study: Structure Related Immune Effects of Opiates. Br. J. Pharmacol. 1997, 121, 834–840. [Google Scholar] [CrossRef]
- Desborough, J.P. The Stress Response to Trauma and Surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef]
- Pinheiro, A.V.; Petrucci, G.N.; Dourado, A.; Pires, I. Anaesthesia in Veterinary Oncology: The Effects of Surgery, Volatile and Intravenous Anaesthetics on the Immune System and Tumour Spread. Animals 2023, 13, 3392. [Google Scholar] [CrossRef] [PubMed]
- Donati, P.A.; Tarragona, L.; Franco, J.V.A.; Kreil, V.; Fravega, R.; Diaz, A.; Verdier, N.; Otero, P.E. Efficacy of Tramadol for Postoperative Pain Management in Dogs: Systematic Review and Meta-Analysis. Vet. Anaesth. Analg. 2021, 48, 283–296. [Google Scholar] [CrossRef]
- Dourado, A.; Gomes, A.; Teixeira, P.; Lobo, L.; Azevedo, J.T.; Dias, I.R.; Pinelas, R. Antinociceptive Effect of a Sacro-Coccygeal Epidural of Morphine and Lidocaine in Cats Undergoing Ovariohysterectomy. Vet. Sci. 2022, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Self, I. BSAVA Guide to Pain Management in Small Animal Practice, 1st ed.; British Small Animal Veterinary Association: Gloucester, UK, 2019. [Google Scholar]
- Forget, P.; Aguirre, J.A.; Bencic, I.; Borgeat, A.; Cama, A.; Condron, C.; Eintrei, C.; Eroles, P.; Gupta, A.; Hales, T.G.; et al. How Anesthetic, Analgesic and Other Non-Surgical Techniques During Cancer Surgery Might Affect Postoperative Oncologic Outcomes: A Summary of Current State of Evidence. Cancers 2019, 11, 592. [Google Scholar] [CrossRef]
- Seymour, C.; Gleed, R. BSAVA Manual of Small Animal Anaesthesia & Analgesia, 2nd ed.; BSAVA: Gloucester, UK, 1999. [Google Scholar]
- Monteiro, B.P.; Lascelles, B.D.X.; Murrell, J.; Robertson, S.; Steagall, P.V.M.; Wright, B. 2022 WSAVA Guidelines for the Recognition, Assessment and Treatment of Pain. J. Small Anim. Pract. 2023, 64, 177–254. [Google Scholar] [CrossRef]
- Bell, A.; Helm, J.; Reid, J. Veterinarians’ Attitudes to Chronic Pain in Dogs. Vet. Rec. 2014, 175, 428. [Google Scholar] [CrossRef]
- Li, D.; Gao, L.; Ren, L.-Y.; Zeng, X.; Cui, E.-P.; Zhang, L.-J.; Wu, Q. Knowledge and Attitudes Regarding Cancer Pain Management among Oncology Nurses in China. J. Int. Med. Res. 2021, 49, 0300060520979448. [Google Scholar] [CrossRef]
- Farooqui, M.; Li, Y.; Rogers, T.; Poonawala, T.; Griffin, R.J.; Song, C.W.; Gupta, K. COX-2 Inhibitor Celecoxib Prevents Chronic Morphine-Induced Promotion of Angiogenesis, Tumour Growth, Metastasis and Mortality, without Compromising Analgesia. Br. J. Cancer 2007, 97, 1523–1531. [Google Scholar] [CrossRef]
- Sacerdote, P.; Bianchi, M.; Gaspani, L.; Manfredi, B.; Maucione, A.; Terno, G.; Ammatuna, M.; Panerai, A.E. The Effects of Tramadol and Morphine on Immune Responses and Pain After Surgery in Cancer Patients. Anesth. Analg. 2000, 90, 1411–1414. [Google Scholar] [CrossRef]
- Lamont, L.A. Multimodal Pain Management in Veterinary Medicine: The Physiologic Basis of Pharmacologic Therapies. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1173–1186. [Google Scholar] [CrossRef]
- Lamont, L.A. Adjunctive Analgesic Therapy in Veterinary Medicine. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- White, D.M.; Mair, A.R.; Martinez-Taboada, F. Opioid-Free Anaesthesia in Three Dogs. Open Vet. J. 2017, 7, 104. [Google Scholar] [CrossRef]
- Myles, P.S.; Peyton, P.; Silbert, B.; Hunt, J.; Rigg, J.R.A.; Sessler, D.I.; for the ANZCA Trials Group Investigators. Perioperative Epidural Analgesia for Major Abdominal Surgery for Cancer and Recurrence-Free Survival: Randomised Trial. Br. Med. J. 2011, 342, d1491. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Park, J.S.; Choi, G.-S.; Kim, H.J.; Kim, J.K.; Oh, J.; Park, S.Y. Comparison of the Analgesic Efficacy of Opioid-Sparing Multimodal Analgesia and Morphine-Based Patient-Controlled Analgesia in Minimally Invasive Surgery for Colorectal Cancer. World J. Surg. 2022, 46, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Haldar, R.; Ben-Eliyahu, S. Reducing the Risk of Post-Surgical Cancer Recurrence: A Perioperative Anti-Inflammatory Anti-Stress Approach. Future Oncol. 2018, 14, 1017–1021. [Google Scholar] [CrossRef]
- Pain Assessment in Dogs and Cats. Available online: https://todaysveterinarypractice.com/diagnostics/pain-assessment-in-dogs-and-cats/ (accessed on 23 January 2024).
- Foley, P.L.; Kendall, L.V.; Turner, P.V. Clinical Management of Pain in Rodents. Comp. Med. 2019, 69, 468–489. [Google Scholar] [CrossRef]
- AAHA/AAFP Pain Management Guidelines Task Force Members; Hellyer, P.; Rodan, I.; Brunt, J.; Downing, R.; Hagedorn, J.E.; Robertson, S.A. AAHA/AAFP Pain Management Guidelines for Dogs and Cats. J. Feline Med. Surg. 2007, 9, 466–480. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Davis, K.N.; Hellyer, P.W.; Carr, E.C.J.; Wallace, J.E.; Kogan, L.R. Qualitative Study of Owner Perceptions of Chronic Pain in Their Dogs. J. Am. Vet. Med. Assoc. 2019, 254, 88–92. [Google Scholar] [CrossRef]
- Faustino, L.C.; Lallo, M.A. Quality of Life and Pain in Dogs with Early-Stage Mammary Tumours. Acta Vet. Hung. 2015, 63, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Mellanby, R.J.; Herrtage, M.E.; Dobson, J.M. Owners’ assessments of Their Dog’s Quality of Life during Palliative Chemotherapy for Lymphoma. J. Small Anim. Pract. 2003, 44, 100–103. [Google Scholar] [CrossRef]
- Yazbek, K.V.B.; Fantoni, D.T. Validity of a Health-Related Quality-of-Life Scale for Dogs with Signs of Pain Secondary to Cancer. J. Am. Vet. Med. Assoc. 2005, 226, 1354–1358. [Google Scholar] [CrossRef]
- Treede, R.-D. The International Association for the Study of Pain Definition of Pain: As Valid in 2018 as in 1979, but in Need of Regularly Updated Footnotes. Pain Rep. 2018, 3, e643. [Google Scholar] [CrossRef] [PubMed]
- Loeser, J.D.; Treede, R.-D. The Kyoto Protocol of IASP Basic Pain Terminology. Pain 2008, 137, 473–477. [Google Scholar] [CrossRef]
- Reid, J.; Scott, E.M.; Calvo, G.; Nolan, A.M. Definitive Glasgow Acute Pain Scale for Cats: Validation and Intervention Level. Vet. Rec. 2017, 180, 449. [Google Scholar] [CrossRef] [PubMed]
- Helander, E.M.; Menard, B.L.; Harmon, C.M.; Homra, B.K.; Allain, A.V.; Bordelon, G.J.; Wyche, M.Q.; Padnos, I.W.; Lavrova, A.; Kaye, A.D. Multimodal Analgesia, Current Concepts, and Acute Pain Considerations. Curr. Pain Headache Rep. 2017, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.M.; Blake, C.; Staines, A.; Doody, C. Clinical Indicators of ‘Nociceptive’, ‘Peripheral Neuropathic’ and ‘Central’ Mechanisms of Musculoskeletal Pain A Delphi Survey of Expert Clinicians. Man. Ther. 2010, 15, 80–87. [Google Scholar] [CrossRef]
- Mathews, K.A. Pain Assessment and General Approach to Management. Vet. Clin. N. Am. Small Anim. Pract. 2000, 30, 729–755. [Google Scholar] [CrossRef]
- Gruen, M.E.; Lascelles, B.D.X.; Colleran, E.; Gottlieb, A.; Johnson, J.; Lotsikas, P.; Marcellin-Little, D.; Wright, B. 2022 AAHA Pain Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2022, 58, 55–76. [Google Scholar] [CrossRef]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. The Pain of Being Sick: Implications of Immune-to-Brain Communication for Understanding Pain. Annu. Rev. Psychol. 2000, 51, 29–57. [Google Scholar] [CrossRef]
- Hernández-Avalos, I.; Valverde, A.; Ibancovichi-Camarillo, J.A.; Sánchez-Aparicio, P.; Recillas-Morales, S.; Osorio-Avalos, J.; Rodríguez-Velázquez, D.; Miranda-Cortés, A.E. Clinical Evaluation of Postoperative Analgesia, Cardiorespiratory Parameters and Changes in Liver and Renal Function Tests of Paracetamol Compared to Meloxicam and Carprofen in Dogs Undergoing Ovariohysterectomy. PLoS ONE 2020, 15, e0223697. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leite, C.; Nunes, C.; Jamal, S.K.; Cuccovia, I.M.; Reis, S. Nonsteroidal Anti-Inflammatory Therapy: A Journey Toward Safety. Med. Res. Rev. 2017, 37, 802–859. [Google Scholar] [CrossRef]
- Volcheck, M.M.; Graham, S.M.; Fleming, K.C.; Mohabbat, A.B.; Luedtke, C.A. Central Sensitization, Chronic Pain, and Other Symptoms: Better Understanding, Better Management. CCJM 2023, 90, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Sivanesan, E.; Guan, Y. Central Sensitization, NMDA Receptors, and Human Experimental Pain Models: Bridging the Gap between Target Discovery and Drug Development. Anesthesiology 2019, 131, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.S.Y.; Kayani, K.; Whyte-Oshodi, D.; Whyte-Oshodi, A.; Nachiappan, N.; Gnanarajah, S.; Mohammed, R. Voltage Gated Sodium Channels as Therapeutic Targets for Chronic Pain. JPR 2019, 12, 2709–2722. [Google Scholar] [CrossRef] [PubMed]
- Harte, S.E.; Harris, R.E.; Clauw, D.J. The Neurobiology of Central Sensitization. J. Appl. Biobehav. Res. 2018, 23, e12137. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Bryson, J.; Tamber, A.; Seccareccia, D.; Zimmermann, C. Methadone for Treatment of Cancer Pain. Curr Oncol Rep. 2006, 8, 282–288. [Google Scholar] [CrossRef]
- Gebhart, G.F.; Bielefeldt, K. Physiology of Visceral Pain. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: New York, NY, USA, 2016; pp. 1609–1633. ISBN 978-0-470-65071-4. [Google Scholar]
- Greenwood-Van Meerveld, B.; Prusator, D.K.; Johnson, A.C. Animal Models of Gastrointestinal and Liver Diseases. Animal Models of Visceral Pain: Pathophysiology, Translational Relevance, and Challenges. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 308, G885–G903. [Google Scholar] [CrossRef]
- Davis, M.P. Drug Management of Visceral Pain: Concepts from Basic Research. Pain Res. Treat. 2012, 2012, 265605. [Google Scholar] [CrossRef] [PubMed]
- Hockley, J.R.F.; González-Cano, R.; McMurray, S.; Tejada-Giraldez, M.A.; McGuire, C.; Torres, A.; Wilbrey, A.L.; Cibert-Goton, V.; Nieto, F.R.; Pitcher, T.; et al. Visceral and Somatic Pain Modalities Reveal Na V 1.7-independent Visceral Nociceptive Pathways. J. Physiol. 2017, 595, 2661–2679. [Google Scholar] [CrossRef]
- Schmidt, B.L. The Neurobiology of Cancer Pain. Neuroscientist 2014, 20, 546–562. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, G.; Villano, I.; Ilardi, C.R.; Messina, A.; Monda, V.; Iodice, A.C.; Porro, C.; Panaro, M.A.; Chieffi, S.; Messina, G.; et al. Mechanisms of Transmission and Processing of Pain: A Narrative Review. Int. J. Env. Res. Public Health 2023, 20, 3064. [Google Scholar] [CrossRef]
- Haroun, R.; Wood, J.N.; Sikandar, S. Mechanisms of Cancer Pain. Front. Pain Res. 2023, 3, 1030899. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W.; Clohisy, D.R.; Koltzenburg, M.; Hunt, S.P. Molecular Mechanisms of Cancer Pain. Nat. Rev. Cancer 2002, 2, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, Q.; Lu, Z.; Wang, L.; Ding, L.; Xia, L.; Zhang, H.; Wang, M.; Chen, Y.; Li, G. Role of the Nervous System in Cancers: A Review. Cell Death Discov. 2021, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Santoni, A.; Santoni, M.; Arcuri, E. Chronic Cancer Pain: Opioids within Tumor Microenvironment Affect Neuroinflammation, Tumor and Pain Evolution. Cancers 2022, 14, 2253. [Google Scholar] [CrossRef]
- Deng, M.; Chen, S.-R.; Pan, H.-L. Presynaptic NMDA Receptors Control Nociceptive Transmission at the Spinal Cord Level in Neuropathic Pain. Cell Mol. Life Sci. 2019, 76, 1889–1899. [Google Scholar] [CrossRef]
- Winkler, F.; Venkatesh, H.S.; Amit, M.; Batchelor, T.; Demir, I.E.; Deneen, B.; Gutmann, D.H.; Hervey-Jumper, S.; Kuner, T.; Mabbott, D.; et al. Cancer Neuroscience: State of the Field, Emerging Directions. Cell 2023, 186, 1689–1707. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chang, M.C. Chronic Pain: Structural and Functional Changes in Brain Structures and Associated Negative Affective States. Int. J. Mol. Sci. 2019, 20, 3130. [Google Scholar] [CrossRef]
- Nishigami, T.; Manfuku, M.; Lahousse, A. Central Sensitization in Cancer Survivors and Its Clinical Implications: State of the Art. J. Clin. Med. 2023, 12, 4606. [Google Scholar] [CrossRef] [PubMed]
- Viet, C.T.; Schmidt, B.L. Biologic Mechanisms of Oral Cancer Pain and Implications for Clinical Therapy. J. Dent. Res. 2012, 91, 447–453. [Google Scholar] [CrossRef]
- Gupta, K.; Harvima, I.T. Mast Cell-Neural Interactions Contribute to Pain and Itch. Immunol. Rev. 2018, 282, 168–187. [Google Scholar] [CrossRef]
- Niscola, P.; Tendas, A.; Scaramucci, L.; Giovaninni, M.; Cupelli, L.; De Sanctis, V.; Brunetti, G.A.; Bondanini, F.; Palumbo, R.; Lamanda, M.; et al. Pain in Malignant Hematology. Expert. Rev. Hematol. 2011, 4, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.F.; Costa-Pereira, A.; Mendonça, L.; Dias, C.C.; Castro-Lopes, J.M. A Population-Based Study on Chronic Pain and the Use of Opioids in Portugal. Pain 2013, 154, 2844–2852. [Google Scholar] [CrossRef]
- Plante, G.E.; VanItallie, T.B. Opioids for Cancer Pain: The Challenge of Optimizing Treatment. Metabolism 2010, 59, S47–S52. [Google Scholar] [CrossRef]
- Aldred, E.M.; Buck, C.; Vall, K. Chapter 32—Analgesia and Relief of Pain. In Pharmacology; Aldred, E.M., Buck, C., Vall, K., Eds.; Churchill Livingstone: Edinburgh, UK, 2009; pp. 247–254. ISBN 978-0-443-06898-0. [Google Scholar]
- Vanderah, T.W. Delta and Kappa Opioid Receptors as Suitable Drug Targets for Pain. Clin. J. Pain 2010, 26, S10. [Google Scholar] [CrossRef]
- Stein, C. Opioid Receptors. Annu. Rev. Med. 2016, 67, 433–451. [Google Scholar] [CrossRef]
- Ruíz-López, P.; Navarrete-Calvo, R.; Morgaz, J.; Domínguez, J.M.; Quirós-Carmona, S.; Muñoz-Rascón, P.; Gómez-Villamandos, R.J.; Fernández-Sarmiento, J.A.; Granados, M.M. Determination of Acute Tolerance and Hyperalgesia to Remifentanil Constant Rate Infusion in Dogs Undergoing Sevoflurane Anaesthesia. Vet. Anaesth. Analg. 2020, 47, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.T.; Steagall, P.V. The Present and Future of Opioid Analgesics in Small Animal Practice. Vet. Pharm. Ther. 2017, 40, 315–326. [Google Scholar] [CrossRef]
- Welters, I.D.; Menzebach, A.; Goumon, Y.; Langefeld, T.W.; Teschemacher, H.; Hempelmann, G.; Stefano, G.B. Morphine Suppresses Complement Receptor Expression, Phagocytosis, and Respiratory Burst in Neutrophils by a Nitric Oxide and M3 Opiate Receptor-Dependent Mechanism. J. Neuroimmunol. 2000, 111, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Risdahl, J.M.; Peterson, P.K.; Chao, C.C.; Pijoan, C.; Molitor, T.W. Effects of Morphine Dependence on the Pathogenesis of Swine Herpesvirus Infection. J. Infect. Dis. 1993, 167, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Afsharimani, B.; Cabot, P.J.; Parat, M.-O. Morphine Use in Cancer Surgery. Front. Pharmacol. 2011, 2, 46. [Google Scholar] [CrossRef]
- Perry, J.A.; Douglas, H. Immunomodulatory Effects of Surgery, Pain, and Opioids in Cancer Patients. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 981–991. [Google Scholar] [CrossRef]
- Page, G.G.; Blakely, W.P.; Ben-Eliyahu, S. Evidence That Postoperative Pain Is a Mediator of the Tumor-Promoting Effects of Surgery in Ratsq. Pain 2001, 90, 191–199. [Google Scholar] [CrossRef]
- Pypendop, B.H.; Siao, K.T.; Pascoe, P.J.; Ilkiw, J.E. Effects of Epidurally Administered Morphine or Buprenorphine on the Thermal Threshold in Cats. AJVR 2008, 69, 983–987. [Google Scholar] [CrossRef]
- Jones, R.S. Epidural Analgesia in the Dog and Cat. Vet. J. 2001, 161, 123–131. [Google Scholar] [CrossRef]
- Mastrocinque, S.; Fantoni, D.T. A Comparison of Preoperative Tramadol and Morphine for the Control of Early Postoperative Pain in Canine Ovariohysterectomy. Vet. Anaesth. Analg. 2003, 30, 220–228. [Google Scholar] [CrossRef]
- Almeida, R.M.; Escobar, A.; Maguilnik, S. Comparison of Analgesia Provided by Lidocaine, Lidocaine-Morphine or Lidocaine-Tramadol Delivered Epidurally in Dogs Following Orchiectomy. Vet. Anaesth. Analg. 2010, 37, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Downing, R. Pain Management for Veterinary Palliative Care and Hospice Patients. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 531–550. [Google Scholar] [CrossRef]
- Campoy, L. Development of Enhanced Recovery After Surgery (ERAS) Protocols in Veterinary Medicine through a One-Health Approach: The Role of Anesthesia and Locoregional Techniques. J. Am. Vet. Med. Assoc. 2022, 260, 1751–1759. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolomics of Methadone: Clinical and Forensic Toxicological Implications and Variability of Dose Response. Drug Metab. Rev. 2016, 48, 568–576. [Google Scholar] [CrossRef]
- Kreutzwiser, D.; Tawfic, Q.A. Methadone for Pain Management: A Pharmacotherapeutic Review. CNS Drugs 2020, 34, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Warne, L.N.; Beths, T.; Holm, M.; Bauquier, S.H. Comparison of Perioperative Analgesic Efficacy between Methadone and Butorphanol in Cats. J. Am. Vet. Med. Assoc. 2013, 243, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.D.; Yates, D.; Hunt, J.; Murrell, J.C. A Comparison between Methadone and Buprenorphine for Perioperative Analgesia in Dogs Undergoing Ovariohysterectomy. J. Small Anim. Pract. 2018, 59, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Bieberly, Z.D.; KuKanich, B.; KuKanich, K.S.; Berke, K.A.; Klocke, E.E.; Upchurch, D.A.; Comroe, A.J.; Jugan, M.C.; Mason, D.E.; Orchard, R.J.; et al. Long-Acting Injectable Methadone (Methadone-Fluconazole) Provides Safe and Effective Postoperative Analgesia in a Randomized Clinical Trial for Dogs Undergoing Soft Tissue Surgery. Am. J. Vet. Res. 2022, 83, ajvr.22.01.0014. [Google Scholar] [CrossRef]
- Bruera, E.; Sweeney, C. Methadone Use in Cancer Patients with Pain: A Review. J. Palliat. Med. 2002, 5, 127–138. [Google Scholar] [CrossRef]
- Mercadante, S.; Bruera, E. Methadone as a First-Line Opioid in Cancer Pain Management: A Systematic Review. J. Pain Symptom Manag. 2018, 55, 998–1003. [Google Scholar] [CrossRef]
- Santoro, F.; Debidda, P.; Franci, P. Single-Injection Caudal Thoracic Paravertebral Block Improves Pain Control and Recovery Quality in Female Dogs Undergoing Unilateral Radical Mastectomy: A Randomized Controlled Trial. J. Am. Vet. Med. Assoc. 2022, 260, S53–S58. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.E. A Review of the Use of Methadone for the Treatment of Chronic Noncancer Pain. Pain Res. Manag. 2005, 10, 133–144. [Google Scholar] [CrossRef]
- Gutiérrez-Bautista, Á.J.; Morgaz, J.; Granados, M.D.M.; Gómez-Villamandos, R.J.; Dominguez, J.M.; Fernandez-Sarmiento, J.A.; Aguilar-García, D.; Navarrete-Calvo, R. Evaluation and Comparison of Postoperative Analgesic Effects of Dexketoprofen and Methadone in Dogs. Vet. Anaesth. Analg. 2018, 45, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, B.; Almahrezi, A.; Schreier, G. Methadone in the Treatment of Neuropathic Pain. Pain Res. Manag. 2003, 8, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Blanco, E.; Victoria-Mora, J.M.; Ibancovichi-Camarillo, J.A.; Sauri-Arceo, C.H.; Bolio-González, M.E.; Acevedo-Arcique, C.M.; Marin-Cano, G.; Steagall, P.V. Postoperative Analgesic Effects of Either a Constant Rate Infusion of Fentanyl, Lidocaine, Ketamine, Dexmedetomidine, or the Combination Lidocaine-Ketamine-Dexmedetomidine after Ovariohysterectomy in Dogs. Vet. Anaesth. Analg. 2015, 42, 309–318. [Google Scholar] [CrossRef]
- Goutchtat, R.; Chetboun, M.; Wiart, J.-F.; Gaulier, J.-M.; Pattou, F.; Allorge, D.; Hubert, T. Long-Term Analgesia Following a Single Application of Fentanyl Transdermal Solution in Pigs. Eur. Surg. Res. 2021, 62, 115–120. [Google Scholar] [CrossRef]
- Linton, D.D.; Wilson, M.G.; Newbound, G.C.; Freise, K.J.; Clark, T.P. The Effectiveness of a Long-acting Transdermal Fentanyl Solution Compared to Buprenorphine for the Control of Postoperative Pain in Dogs in a Randomized, Multicentered Clinical Study. J. Vet. Pharmacol. Ther. 2012, 35, 53–64. [Google Scholar] [CrossRef]
- Driessen, B.; Reimann, W.; Giertz, H. Effects of the Central Analgesic Tramadol on the Uptake and Release of Noradrenaline and Dopamine in Vitro. Br. J. Pharmacol. 1993, 108, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Driessen, B.; Reimann, W. Interaction of the Central Analgesic, Tramadol, with the Uptake and Release of 5-Hydroxytryptamine in the Rat Brain in Vitro. Br. J. Pharmacol. 1992, 105, 147–151. [Google Scholar] [CrossRef]
- Domínguez-Oliva, A.; Casas-Alvarado, A.; Miranda-Cortés, A.E.; Hernández-Avalos, I. Clinical Pharmacology of Tramadol and Tapentadol, and Their Therapeutic Efficacy in Different Models of Acute and Chronic Pain in Dogs and Cats. J. Adv. Vet. Anim. Res. 2021, 8, 404. [Google Scholar] [CrossRef]
- Cagnardi, P.; Villa, R.; Zonca, A.; Gallo, M.; Beccaglia, M.; Luvoni, G.C.; Vettorato, E.; Carli, S.; Fonda, D.; Ravasio, G. Pharmacokinetics, Intraoperative Effect and Postoperative Analgesia of Tramadol in Cats. Res. Vet. Sci. 2011, 90, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.C.; Monteiro, E.R.; Campagnol, D.; Coelho, K.; Bressan, T.F.; Monteiro, B.S. Effects of Tramadol Alone, in Combination with Meloxicam or Dipyrone, on Postoperative Pain and the Analgesic Requirement in Dogs Undergoing Unilateral Mastectomy with or without Ovariohysterectomy. Vet. Anaesth. Analg. 2013, 40, 641–649. [Google Scholar] [CrossRef]
- Karrasch, N.M.; Lerche, P.; Aarnes, T.K.; Gardner, H.L.; London, C.A. The Effects of Preoperative Oral Administration of Carprofen or Tramadol on Postoperative Analgesia in Dogs Undergoing Cutaneous Tumor Removal. Can. Vet. J. 2015, 56, 817–822. [Google Scholar] [PubMed]
- Sacerdote, P. Opioids and the Immune System. Palliat. Med. 2006, 20, 9–15. [Google Scholar] [CrossRef]
- Franchi, S.; Panerai, A.E.; Sacerdote, P. Buprenorphine Ameliorates the Effect of Surgery on Hypothalamus–Pituitary–Adrenal Axis, Natural Killer Cell Activity and Metastatic Colonization in Rats in Comparison with Morphine or Fentanyl Treatment. Brain Behav. Immun. 2007, 21, 767–774. [Google Scholar] [CrossRef]
- Suzuki, M.; Sakurada, T.; Gotoh, K.; Watanabe, S.; Satoh, N. Correlation Between the Administration of Morphine or Oxycodone and the Development of Infections in Patients with Cancer Pain. Am. J. Hosp. Palliat. Med. 2013, 30, 712–716. [Google Scholar] [CrossRef]
- Shavit, Y.; Lewis, J.W.; Terman, G.W.; Gale, R.P.; Liebeskind, J.C. Opioid Peptides Mediate the Suppressive Effect of Stress on Natural Killer Cell Cytotoxicity. Science 1984, 223, 188–190. [Google Scholar] [CrossRef]
- DeClue, A.E.; Yu, D.-H.; Prochnow, S.; Axiak-Bechtel, S.; Amorim, J.; Tsuruta, K.; Donaldson, R.; Lino, G.; Monibi, F.; Honaker, A.; et al. Effects of Opioids on Phagocytic Function, Oxidative Burst Capacity, Cytokine Production and Apoptosis in Canine Leukocytes. Vet. J. 2014, 200, 270–275. [Google Scholar] [CrossRef]
- Eisenstein, T.K. Opioids and the Immune System: What Is Their Mechanism of Action? Br. J. Pharmacol. 2011, 164, 1826–1828. [Google Scholar] [CrossRef]
- McCarthy, L.; Wetzel, M.; Sliker, J.K.; Eisenstein, T.K.; Rogers, T.J. Opioids, Opioid Receptors, and the Immune Response. Drug Alcohol. Depend. 2001, 62, 111–123. [Google Scholar] [CrossRef]
- Rahim, R.T.; Meissler, J.J.; Zhang, L.; Adler, M.W.; Rogers, T.J.; Eisenstein, T.K. Withdrawal from Morphine in Mice Suppresses Splenic Macrophage Function, Cytokine Production, and Costimulatory Molecules. J. Neuroimmunol. 2003, 144, 16–27. [Google Scholar] [CrossRef]
- Borman, A.; Ciepielewski, Z.; Wrona, D.; Stojek, W.; Glac, W.; Leszkowicz, E.; Tokarski, J. Small Doses of Morphine Can Enhance NK Cell Cytotoxicity in Pigs. Int. Immunopharmacol. 2009, 9, 277–283. [Google Scholar] [CrossRef]
- Bataduwaarachchi, V.R.; Hansanie, S.; Rockwood, N.; D’Cruz, L.G. Immunomodulatory Properties of Morphine and the Hypothesised Role of Long-Term Opioid Use in the Immunopathogenesis of Tuberculosis. Front. Immunol. 2023, 14, 1265511. [Google Scholar] [CrossRef]
- Lam, C.-F.; Chang, P.-J.; Huang, Y.-S.; Sung, Y.-H.; Huang, C.-C.; Lin, M.-W.; Liu, Y.-C.; Tsai, Y.-C. Prolonged Use of High-Dose Morphine Impairs Angiogenesis and Mobilization of Endothelial Progenitor Cells in Mice. Anesth. Analg. 2008, 107, 686–692. [Google Scholar] [CrossRef]
- Monibi, F.A.; Dodam, J.R.; Axiak-Bechtel, S.M.; Amorim, J.; Zhang, Y.; Tsuruta, K.; Mann, F.A.; DeClue, A.E. Morphine and Buprenorphine Do Not Alter Leukocyte Cytokine Production Capacity, Early Apoptosis, or Neutrophil Phagocytic Function in Healthy Dogs. Res. Vet. Sci. 2015, 99, 70–76. [Google Scholar] [CrossRef]
- Qian, Y.-N.; Jin, W.-J.; Wang, L.; Wang, H.-J. Effect of Different Concentrations of Morphine and Tramadol on the Differentiation of Human Helper T Cells in Vitro. Br. J. Anaesth. 2005, 95, 277. [Google Scholar] [CrossRef]
- Martin, J.L.; Charboneau, R.; Barke, R.A.; Roy, S. Chronic Morphine Treatment Inhibits LPS-Induced Angiogenesis: Implications in Wound Healing. Cell. Immunol. 2010, 265, 139–145. [Google Scholar] [CrossRef]
- Tuerxun, H.; Cui, J. The Dual Effect of Morphine on Tumor Development. Clin. Transl. Oncol. 2019, 21, 695–701. [Google Scholar] [CrossRef]
- Axiak-Bechtel, S.M.; Tsuruta, K.; Amorim, J.; Donaldson, R.; Lino, G.; Honaker, A.; Monibi, F.; Dodam, J.; DeClue, A. Effects of Tramadol and O-Desmethyltramadol on Canine Innate Immune System Function. Vet. Anaesth. Analg. 2015, 42, 260–268. [Google Scholar] [CrossRef]
- Sacerdote, P.; Bianchi, M.; Manfredi, B.; Panerai, A.E. Effects of Tramadol on Immune Responses and Nociceptive Thresholds in Mice. Pain 1997, 72, 325–330. [Google Scholar] [CrossRef]
- Madurai, N.K.; Kitase, Y.; Hamimi, S.; Kirk, S.E.; Sevensky, R.; Ramachandra, S.; Muthukumar, S.; Vasan, V.; Ozen, M.; Gerner, G.; et al. Methadone Alters the Peripheral Inflammatory and Central Immune Landscape Following Prenatal Exposure in Rats. Adv. Drug Alcohol. Res. 2022, 2, 10792. [Google Scholar] [CrossRef]
- Lo-Dieguez, A.C.; Sahs, J.; Goetz, R.; Sadr, W.E.; Sorell, S.; Gorman, J. The Effect of Methadone on Immunological Parameters among HIV-Positive and HIV-Negative Drug Users. Am. J. Drug Alcohol. Abus. 1994, 20, 317–329. [Google Scholar] [CrossRef]
- McLachlan, C.; Crofts, N.; Wodak, A.; Crowe, S. The Effects of Methadone on Immune Function among Injecting Drug Users: A Review. Addiction 1993, 88, 257–263. [Google Scholar] [CrossRef]
- Martucci, C.; Panerai, A.E.; Sacerdote, P. Chronic Fentanyl or Buprenorphine Infusion in the Mouse: Similar Analgesic Profile but Different Effects on Immune Responses. Pain 2004, 110, 385–392. [Google Scholar] [CrossRef]
- Yardeni, I.Z.; Beilin, B.; Mayburd, E.; Alcalay, Y.; Bessler, H. Relationship between Fentanyl Dosage and Immune Function in the Postoperative Period. J. Opioid Manag. 2008, 4, 27–33. [Google Scholar] [CrossRef]
- Boland, J.W.; Pockley, A.G. Influence of Opioids on Immune Function in Patients with Cancer Pain: From Bench to Bedside. Br. J. Pharmacol. 2018, 175, 2726–2736. [Google Scholar] [CrossRef]
- Luan, G.; Pan, F.; Bu, L.; Wu, K.; Wang, A.; Xu, X. Butorphanol Promotes Macrophage Phenotypic Transition to Inhibit Inflammatory Lung Injury via κ Receptors. Front. Immunol. 2021, 12, 692286. [Google Scholar] [CrossRef]
- Boland, J.W. Effect of Opioids on Immunity in Patients with Cancer. In Handbook of Cancer and Immunology; Rezaei, N., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–18. ISBN 978-3-030-80962-1. [Google Scholar]
- Shavit, Y.; Ben-Eliyahu, S.; Zeidel, A.; Beilin, B. Effects of Fentanyl on Natural Killer Cell Activity and on Resistance to Tumor Metastasis in Rats. Neuroimmunomodulation 2004, 11, 255–260. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Hu, Y.; Wang, F. Butorphanol Protects PC12 Cells against OGD/R-Induced Inflammation and Apoptosis. Mol. Med. Rep. 2020, 22, 1969–1975. [Google Scholar] [CrossRef]
- Roy, S.; Wang, J.; Kelschenbach, J.; Koodie, L.; Martin, J. Modulation of Immune Function by Morphine: Implications for Susceptibility to Infection. J. Neuroimmune Pharmacol. 2006, 1, 77–89. [Google Scholar] [CrossRef]
- Krajnik, M.; Schäfer, M.; Sobanski, P.; Kowalewski, J.; Bloch-Boguslawska, E.; Zylicz, Z.; Mousa, S.A. Enkephalin, Its Precursor, Processing Enzymes, and Receptor as Part of a Local Opioid Network throughout the Respiratory System of Lung Cancer Patients. Hum. Pathol. 2010, 41, 632–642. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Urfer, S.R.; White, M.; Megquier, K.; Shrager, S.; The Dog Aging Project Consortium; Ruple, A. Lifetime Prevalence of Malignant and Benign Tumours in Companion Dogs: Cross-sectional Analysis of Dog Aging Project Baseline Survey. Vet. Comp. Oncol. 2022, 20, 797–804. [Google Scholar] [CrossRef]
- Fleming, J.M.; Creevy, K.E.; Promislow, D.E.L. Mortality in North American Dogs from 1984 to 2004: An Investigation into Age-, Size-, and Breed-Related Causes of Death: Mortality of Dogs in North America. J. Vet. Intern. Med. 2011, 25, 187–198. [Google Scholar] [CrossRef]
- Sottnik, J.L.; Rao, S.; Lafferty, M.H.; Thamm, D.H.; Morley, P.S.; Withrow, S.J.; Dow, S.W. Association of Blood Monocyte and Lymphocyte Count and Disease-Free Interval in Dogs with Osteosarcoma: CBC Is Prognostic in Osteosarcoma. J. Vet. Intern. Med. 2010, 24, 1439–1444. [Google Scholar] [CrossRef]
- Niu, D.-G.; Peng, F.; Zhang, W.; Guan, Z.; Zhao, H.-D.; Li, J.-L.; Wang, K.-L.; Li, T.-T.; Zhang, Y.; Zheng, F.-M.; et al. Morphine Promotes Cancer Stem Cell Properties, Contributing to Chemoresistance in Breast Cancer. Oncotarget 2015, 6, 3963–3976. [Google Scholar] [CrossRef]
- Poonawala, T.; Levay-Young, B.K.; Hebbel, R.P.; Gupta, K. Opioids Heal Ischemic Wounds in the Rat. Wound Repair. Regen. 2005, 13, 165–174. [Google Scholar] [CrossRef]
- Ustun, F.; Durmus-Altun, G.; Altaner, S.; Tuncbilek, N.; Uzal, C.; Berkarda, S. Evaluation of Morphine Effect on Tumour Angiogenesis in Mouse Breast Tumour Model, EATC. Med. Oncol. 2011, 28, 1264–1272. [Google Scholar] [CrossRef]
- Afsharimani, B.; Baran, J.; Watanabe, S.; Lindner, D.; Cabot, P.J.; Parat, M.-O. Morphine and Breast Tumor Metastasis: The Role of Matrix-Degrading Enzymes. Clin. Exp. Metastasis 2014, 31, 149–158. [Google Scholar] [CrossRef]
- Sasamura, T.; Nakamura, S.; Iida, Y.; Fujii, H.; Murata, J.; Saiki, I.; Nojima, H.; Kuraishi, Y. Morphine Analgesia Suppresses Tumor Growth and Metastasis in a Mouse Model of Cancer Pain Produced by Orthotopic Tumor Inoculation. Eur. J. Pharmacol. 2002, 441, 185–191. [Google Scholar] [CrossRef]
- Page, G.G.; Ben-Eliyahu, S.; Yirmiya, R.; Liebeskind, J.C. Morphine Attenuates Surgery-Induced Enhancement of Metastatic Colonization in Rats. Pain 1993, 54, 21–28. [Google Scholar] [CrossRef]
- Koodie, L.; Ramakrishnan, S.; Roy, S. Morphine Suppresses Tumor Angiogenesis through a HIF-1α/p38MAPK Pathway. Am. J. Pathol. 2010, 177, 984–997. [Google Scholar] [CrossRef]
- Ecimovic, P.; Mchugh, B.; Murray, D.; Doran, P.; Buggy, D.J. Effects of Sevoflurane on Breast Cancer Cell Function In Vitro. Anticancer. Res. 2013, 33, 4255–4260. [Google Scholar]
- Koodie, L.; Yuan, H.; Pumper, J.A.; Yu, H.; Charboneau, R.; Ramkrishnan, S.; Roy, S. Morphine Inhibits Migration of Tumor-Infiltrating Leukocytes and Suppresses Angiogenesis Associated with Tumor Growth in Mice. Am. J. Pathol. 2014, 184, 1073–1084. [Google Scholar] [CrossRef]
- Bratcher, N.A.; Frost, D.J.; Hickson, J.; Huang, X.; Medina, L.M.; Oleksijew, A.; Ferguson, D.C.; Bolin, S. Effects of Buprenorphine in a Preclinical Orthotopic Tumor Model of Ovarian Carcinoma in Female CB17 SCID Mice. J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 583–588. [Google Scholar] [CrossRef]
- Husmann, K.; Arlt, M.J.E.; Jirkof, P.; Arras, M.; Born, W.; Fuchs, B. Primary Tumour Growth in an Orthotopic Osteosarcoma Mouse Model Is Not Influenced by Analgesic Treatment with Buprenorphine and Meloxicam. Lab. Anim. 2015, 49, 284–293. [Google Scholar] [CrossRef]
- Theile, D.; Mikus, G. Methadone against Cancer: Lost in Translation. Int. J. Cancer 2018, 143, 1840–1848. [Google Scholar] [CrossRef]
- Michalska, M.; Katzenwadel, A.; Wolf, P. Methadone as a “Tumor Theralgesic” against Cancer. Front. Pharmacol. 2017, 8, 733. [Google Scholar] [CrossRef]
- Fiegl, H.; Hagenbuchner, J.; Kyvelidou, C.; Seeber, B.; Sopper, S.; Tsibulak, I.; Wieser, V.; Reiser, E.; Roessler, J.; Huhtinen, K.; et al. Dubious Effects of Methadone as an “Anticancer” Drug on Ovarian Cancer Cell-Lines and Patient-Derived Tumor-Spheroids. Gynecol. Oncol. 2022, 165, 129–136. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Sue, S.-H.; Wu, Z.-S.; Huang, S.-M.; Lee, S.-Y.; Wu, Z.-F. Antitumorigenic Effect of Tramadol and Synergistic Effect with Doxorubicin in Human Breast Cancer Cells. Front. Oncol. 2022, 12, 811716. [Google Scholar] [CrossRef]
- Fan, T.M. Pain Management in Veterinary Patients with Cancer. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 989–1001. [Google Scholar] [CrossRef]
- Shen, K.-F.; Crain, S.M. Ultra-Low Doses of Naltrexone or Etorphine Increase Morphine’s Antinociceptive Potency and Attenuate Tolerancerdependence in Mice. Brain Res. 1997, 757, 176–190. [Google Scholar] [CrossRef] [PubMed]
| Opioid Analgesic | Dogs | Cats | Route of Administration 2 | Main Indications for Use? | ||
|---|---|---|---|---|---|---|
| Dose 2 | Frequency 2 | Dose 2 | Frequency 2 | |||
| Morphine | 0.3 to 0.5 mg/kg | every 2 to 4 h | 0.2 to 0.3 mg/kg | every 4 to 6 h | IM, IV * | Moderate-to-severe pain ‡ |
| Methadone | 0.3 to 0.5 mg/kg | every 3 to 4 h | 0.2 to 0.3 mg/kg | every 4 h | IM, IV, oral transmucosal † | Moderate-to-severe pain |
| Fentanyl | 2 to 5 μg/kg 3 to 6 μg/kg/h | Bolus CRI | 1 to 3 μg/kg 2 to 3 μg/kg/h | Bolus CRI | IV | Severe pain |
| Remifentanil | 6 to 12 μg/kg/h | CRI | 4 to 6 μg/kg/h | CRI | IV | Severe pain |
| Buprenorphine | 0.01 to 0.02 mg/kg | every 4 to 8 h | 0.02 to 0.04 mg/kg | every 4 to 8 h | IM, IV, oral transmucosal † | Moderate pain |
| Butorphanol | 0.2 to 0.4 mg/kg | every 1 to 2 h | 0.2 to 0.4 mg/kg | every 1 to 2 h | IM, IV | Mild pain |
| Tramadol | NR ¥ | NR ¥ | 2 to 4 mg/kg | every 8 to 12 h | Orally, IM, IV | Chronic pain |
| Drug | Dose | Effect on Immune System | Study Model | References |
|---|---|---|---|---|
| Fentanyl | Low (1–5 μg/kg) and High (75–100 μg/kg) | Suppression of NK cell activity | Human (perioperative) | [134] |
| Chronic administration | Suppression of NK activity, lymphocyte proliferation, and cytokine production | Rodent | [129] | |
| Butorphanol | Low | Enhanced macrophage phagocytic activity; balanced cytokine production | Rodent | [135] |
| Moderate | Reduced IL-6 and TNF-α | Rodent | [132] | |
| High | Increased immunosuppressive effects; potential for reduced inflammatory responses | Rodent | [132] | |
| Buprenorphine | Low to Moderate | Modulation of T cell function; reduced proinflammatory cytokine production | Rodent | [120] |
| Chronic administration | Development of tolerance to immunosuppressive effects | Rodent | [129] | |
| Methadone | Low to Moderate | Suppresses lymphocyte proliferation; reduces cytokine production | Rodent | [136] |
| Tramadol | Low to Moderate | Minimal immunosuppressive effects; can enhance certain immune functions | Rodent | [125] |
| Morphine | Low | Suppresses NK cell activity and cytokine production; increases susceptibility to infections | Rodent | [136] |
| High | Significant immunosuppression; decreased macrophage function and cytokine production | Rodent | [136] | |
| Chronic administration | Development of tolerance to immunosuppressive effects; persistent analgesic effects | Rodent | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, A.V.; Petrucci, G.N.; Dourado, A.; Silva, F.; Pires, I. Pain Management in Animals with Oncological Disease: Opioids as Influencers of Immune and Tumor Cellular Balance. Cancers 2024, 16, 3015. https://doi.org/10.3390/cancers16173015
Pinheiro AV, Petrucci GN, Dourado A, Silva F, Pires I. Pain Management in Animals with Oncological Disease: Opioids as Influencers of Immune and Tumor Cellular Balance. Cancers. 2024; 16(17):3015. https://doi.org/10.3390/cancers16173015
Chicago/Turabian StylePinheiro, Ana Vidal, Gonçalo N. Petrucci, Amândio Dourado, Filipe Silva, and Isabel Pires. 2024. "Pain Management in Animals with Oncological Disease: Opioids as Influencers of Immune and Tumor Cellular Balance" Cancers 16, no. 17: 3015. https://doi.org/10.3390/cancers16173015








