Regulation of Tumor Microenvironment through YAP/TAZ under Tumor Hypoxia
Abstract
Simple Summary
Abstract
1. Introduction
2. The Protection of DNA Damage by YAP/TAZ under Hypoxia
- (1)
- Inhibition of apoptosisYAP/TAZ activation under hypoxia can also suppress apoptosis, allowing cells to survive despite DNA damage [14]. HIFs, which are activated under low oxygen conditions, can interact with YAP/TAZ to regulate the expression of anti-apoptotic genes [6]. By inhibiting apoptosis, YAP/TAZ contribute to cell survival, even in the presence of DNA damage, thereby protecting cells from undergoing programmed cell death [6,13,15]. For example, YAP can trigger apoptosis by binding p73 instead of TEAD, thereby upregulating the anti-apoptotic gene [16]. In addition, inhibition of YAP signaling can promote apoptosis in multiple pathways. Knockdown of YAP and TAZ can enhance apoptosis under hypoxic condition [17].
- (2)
- Promotion of cell cycle progressionYAP/TAZ activation under hypoxia can promote cell cycle progression, facilitating the proliferation of damaged cells [17]. This effect is mediated through the transcriptional regulation of cell cycle-related genes by YAP/TAZ [18]. By promoting cell cycle progression, YAP/TAZ contribute to the proliferation of cells with DNA damage, potentially leading to tumor progression and expansion despite the presence of hypoxia-induced genotoxic stress [14,17]. YAP activation was increased, and this facilitated cell cycle progression through RhoA and cytoskeletal dynamics. Increased YAP and TEAD activity lead to marked expansion of the neural progenitor population by facilitating cell cycle progression through induction of cyclin and cyclin dependent kinase [19,20].
- (3)
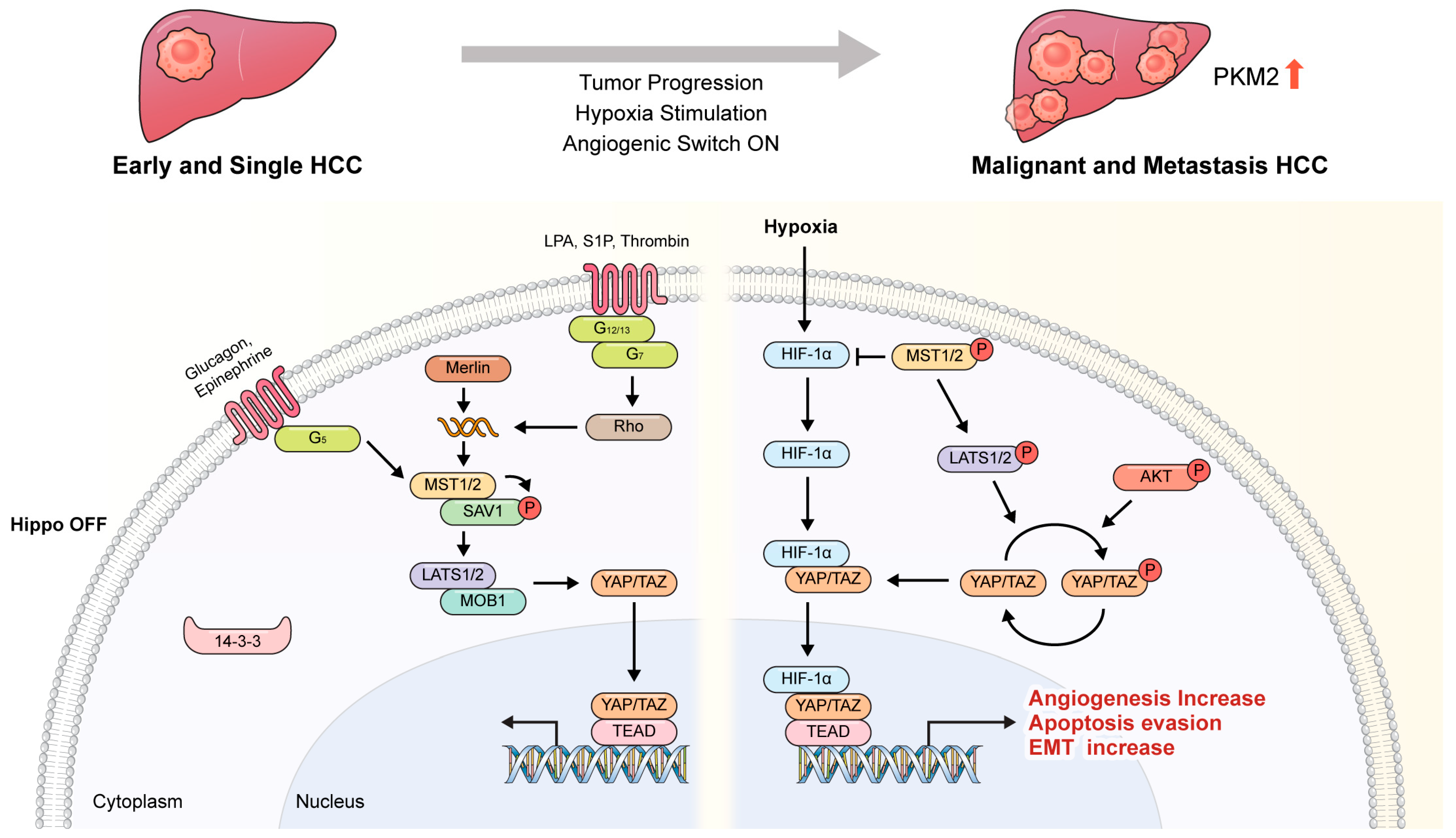
- Development of tumor angiogenesisTumor angiogenesis is activated by endothelial proliferation, collective cell migration, and cellular rearrangements under tumor hypoxia [21]. Angiogenic stimuli, such as VEGF and FGF, activate endothelial cells (ECs) to promote the formation of endothelial tip cells that migrate toward the proliferating tumor [22]. During development, the formation of new blood vessels is accompanied by a decrease in angiogenic growth factor levels, and the vessels mature through stabilized cell–cell junctions and recruiting endothelial cells to the walls of the vessels. In mature vessels, the induction of ECs is arrested and YAP/TAZ are inactivated in the ECs [22,23]. During wound healing, YAP/TAZ are activated as needed to induce angiogenesis in various inflammatory and wound conditions [7]. However, in the tumor microenvironment, the angiogenic process is to some extent similar to that of inflamed wound tissue, and, especially in the hypoxic phase [23], YAP/TAZ remain activated in the endothelial cells and the vasculature does not develop to a mature state; instead, immature and incomplete vessels are formed [24]. This cycle is repeated every time the tumor proliferates, and YAP/TAZ activity in the ECs is also maintained in an active phase without an inactive phase [7,21,22]. The resulting immature vessels repeatedly induce tumor proliferation and metastasis to other organs or tissues [25]. The development of these vessels is prominent in multivessel tumors such as liver cancer, and the activity of YAP/TAZ in the ECs is also affected by the hypoxic tumor microenvironment.
- (4)
- Interaction with other signaling pathwaysYAP/TAZ can interact with various signaling pathways involved in DNA damage response and repair, such as the p53 pathway and the ATM/ATR kinase pathway [26]. Under hypoxic conditions, YAP/TAZ may modulate the activity of these pathways to promote cell survival and DNA repair [27]. Additionally, YAP/TAZ can crosstalk with other hypoxia-responsive transcription factors, such as HIFs, to coordinate cellular responses to low oxygen levels and genotoxic stress [26,27]. And in phosphorylation-independent pathways, such as the Wnt and hormone signaling pathways, mechanical signals are transmitted to the nucleus via stress fibers and actin remodeling. Unphosphorylated YAP/TAZ can undergo nuclear translocation via Rho or beta-catenin [28].
3. HIF-1α Interacts with YAP and Promotes Nuclear Translocation
4. Differential Regulation of YAP and TAZ under Hypoxia
- (1)
- Regulatory Mechanisms:Under hypoxia, YAP can be regulated through both transcriptional and post-translational mechanisms. HIF-1α can directly interact with YAP, promoting its nuclear translocation and activation of target genes [11]. Additionally, hypoxia may affect the expression of upstream regulators of YAP, such as the Hippo pathway components MST1/2 and LATS1/2, leading to altered YAP activity [53,54]. On the other hand, TAZ regulation under hypoxia appears to involve similar mechanisms as YAP, including direct interaction with HIF-1α [55]. However, the specific regulatory pathways and the extent of TAZ activation under hypoxia may differ from YAP [55]. Additionally, TAZ may have distinct binding partners or post-translational modifications that influence its activity in response to hypoxic stress [56,57].
- (2)
- Transcriptional Targets:YAP can activate the expression of genes involved in promoting cell survival, proliferation, and angiogenesis, contributing to tumor growth and progression under hypoxia [7,17,52]. These target genes may include those encoding for angiogenic factors, glycolytic enzymes, and anti-apoptotic proteins [17]. TAZ shares many transcriptional targets with YAP and can similarly regulate genes involved in cell proliferation, survival, and tissue growth under hypoxic conditions [58]. However, TAZ may also have unique target genes or regulate gene expression in a context-dependent manner, leading to distinct cellular outcomes compared to YAP [59].
- (3)
- Cellular Localization:Under normoxic conditions, YAP is predominantly localized in the cytoplasm, where it undergoes phosphorylation-mediated inhibition by Hippo pathway kinases [59]. However, under hypoxia, YAP can translocate to the nucleus, where it interacts with transcription factors such as HIF-1α to regulate gene expression [11]. Similar to YAP, TAZ is regulated by phosphorylation and predominantly localized in the cytoplasm under normoxia conditions [56]. Upon hypoxic stimulation, TAZ can also translocate to the nucleus and participate in transcriptional regulation, potentially through interaction with HIF-1α or other nuclear factors [55].
- (4)
- Functional Roles:Not only YAP activation under hypoxia is associated with increased cell proliferation, survival, and angiogenesis, contributing to tumor growth and metastasis, but also TAZ activation under hypoxia likely plays a similar role in promoting cell survival, proliferation, and angiogenesis [52,55,56]. Although, its specific functions may vary depending on the cellular context and the repertoire of target genes regulated by TAZ [55,57,59].
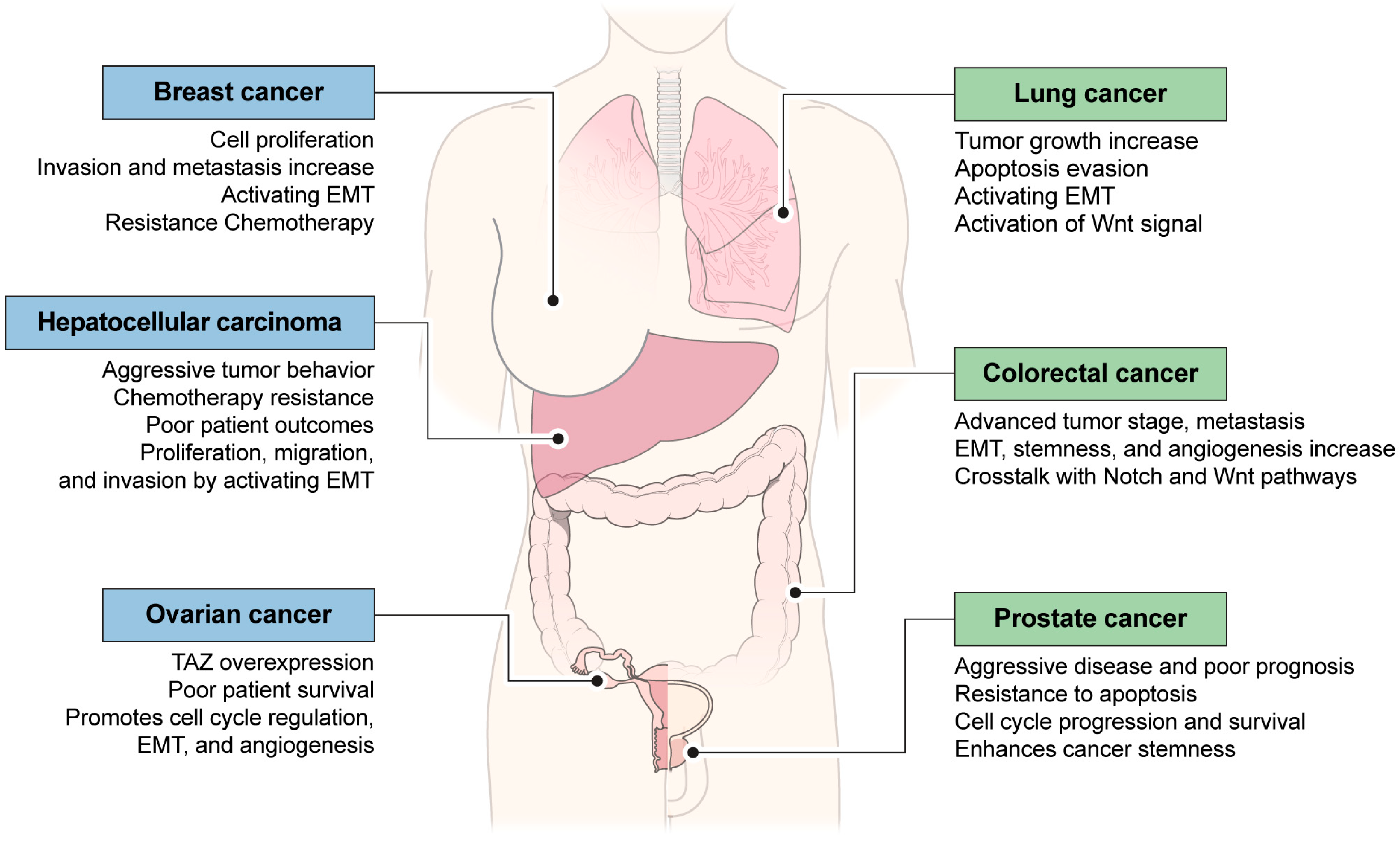
5. YAP or TAZ Is Functionally Involved in Other Cancer Cells
6. Hypoxic Conditions Had Opposing Roles in the Level of p-YAP and p-TAZ
- (1)
- YAP Phosphorylation: Hypoxia can lead to the stabilization and nuclear accumulation of YAP in some cellular contexts [87]. This is often mediated through the inactivation of the Hippo pathway, which normally phosphorylates YAP, leading to its cytoplasmic retention and degradation [7,88]. Under hypoxic conditions, decreased activity of the Hippo pathway kinases, such as LATS1/2 (Large Tumor Suppressor 1/2), may occur, resulting in reduced phosphorylation of YAP [89]. As a consequence, YAP is less likely to undergo degradation and more likely to translocate to the nucleus, where it acts as a transcriptional co-activator [90]. In certain cancer cells, hypoxia-induced YAP activation can promote cell survival, proliferation, and metastasis by regulating the expression of target genes involved in these processes [86,88,90]. YAP activation under hypoxia may thus contribute to tumor aggressiveness and therapy resistance [16,59].
- (2)
- TAZ Phosphorylation: Contrary to YAP, hypoxic conditions may lead to increased phosphorylation and cytoplasmic retention of TAZ in some cellular components [60]. This can occur through various mechanisms, including activation of the Hippo pathway or other signaling pathways that modulate TAZ phosphorylation. Hypoxia-induced TAZ phosphorylation may involve the activation of LATS1/2 or other kinases that directly phosphorylate TAZ, promoting its interaction with 14-3-3 proteins and sequestering it in the cytoplasm [91,92]. Cytoplasmic retention of phosphorylated TAZ under hypoxia prevents its nuclear translocation and transcriptional co-activation activity [44,93]. Consequently, the expression of TAZ target genes involved in cell proliferation, survival, and EMT may be downregulated [94]. In certain cellular contexts, hypoxia-induced inhibition of TAZ activity [19,95,96] may contribute to the suppression of tumorigenic processes, such as cell proliferation, invasion, and metastasis [94,97]. Therefore, the hypoxia phenomenon that occurs during tumor growth can itself affect tumor proliferation and metastasis through the HIF pathway but has a synergistic effect on the stabilization of tumor development and amplification of proliferation and metastasis by YAP pathway.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oh, J.H.; Jun, D.W. The latest global burden of liver cancer: A past and present threat. Clin. Mol. Hepatol. 2023, 29, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, W. Recent update on comprehensive therapy for advanced hepatocellular carcinoma. World J. Gastrointest. Oncol. 2021, 13, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Liu, H.N.; Qu, P.F.; Ma, X.; Ma, L.Y.; Chen, X.X.; Wang, Y.Q.; Qin, X.B.; Han, Z.X. Progress in the treatment of advanced hepatocellular carcinoma with immune combination therapy. World J. Gastrointest. Oncol. 2024, 16, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Park, J.Y. Regulation of the hypoxic tumor environment in hepatocellular carcinoma using RNA interference. Cancer Cell Int. 2017, 17, 3. [Google Scholar] [CrossRef]
- Zhao, B.; Tumaneng, K.; Guan, K.L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef]
- Fu, M.; Hu, Y.; Lan, T.; Guan, K.L.; Luo, T.; Luo, M. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct. Target. Ther. 2022, 7, 376. [Google Scholar] [CrossRef]
- Boopathy, G.T.K.; Hong, W. Role of Hippo Pathway-YAP/TAZ Signaling in Angiogenesis. Front. Cell Dev. Biol. 2019, 7, 49. [Google Scholar] [CrossRef]
- Paul, S.; Xie, S.; Yao, X.; Dey, A. Transcriptional Regulation of the Hippo Pathway: Current Understanding and Insights from Single-Cell Technologies. Cells 2022, 11, 2225. [Google Scholar] [CrossRef]
- Triner, D.; Shah, Y.M. Hypoxia-inducible factors: A central link between inflammation and cancer. J. Clin. Investig. 2016, 126, 3689–3698. [Google Scholar] [CrossRef]
- Rankin, E.B.; Giaccia, A.J. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008, 15, 678–685. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ma, Y.; Yang, L.; Wang, T.; Meng, X.; Zong, Z.; Sun, X.; Hua, X.; Li, H. Yes-associated protein (YAP) binds to HIF-1alpha and sustains HIF-1alpha protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. J. Exp. Clin. Cancer Res. 2018, 37, 216. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Qiu, Z.; Wang, Y.; Xi, C.; Zhang, G.; Sun, Z.; Luo, Q.; Shen, C. HIF-1alpha/YAP Signaling Rewrites Glucose/Iodine Metabolism Program to Promote Papillary Thyroid Cancer Progression. Int. J. Biol. Sci. 2023, 19, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Jang, J.W.; Bae, S.C. DNA binding partners of YAP/TAZ. BMB Rep. 2018, 51, 126–133. [Google Scholar] [CrossRef]
- Chang, H.A.; Ou Yang, R.Z.; Su, J.M.; Nguyen, T.M.H.; Sung, J.M.; Tang, M.J.; Chiu, W.T. YAP nuclear translocation induced by HIF-1alpha prevents DNA damage under hypoxic conditions. Cell Death Discov. 2023, 9, 385. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Cheng, Y.; Mao, M.; Lu, Y. The biology of YAP in programmed cell death. Biomark. Res. 2022, 10, 34. [Google Scholar] [CrossRef]
- Zhou, W.; Lim, A.; Edderkaoui, M.; Osipov, A.; Wu, H.; Wang, Q.; Pandol, S. Role of YAP Signaling in Regulation of Programmed Cell Death and Drug Resistance in Cancer. Int. J. Biol. Sci. 2024, 20, 15–28. [Google Scholar] [CrossRef]
- Zhao, Y.; Sheldon, M.; Sun, Y.; Ma, L. New Insights into YAP/TAZ-TEAD-Mediated Gene Regulation and Biological Processes in Cancer. Cancers 2023, 15, 5497. [Google Scholar] [CrossRef]
- Baroja, I.; Kyriakidis, N.C.; Halder, G.; Moya, I.M. Expected and unexpected effects after systemic inhibition of Hippo transcriptional output in cancer. Nat. Commun. 2024, 15, 2700. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Zhang, X.; Aubel, D.; Bai, Y.; Li, X.; Wei, Y.; Fussenegger, M.; Deng, X. Role of YAP/TAZ in Cell Lineage Fate Determination and Related Signaling Pathways. Front. Cell Dev. Biol. 2020, 8, 735. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, D.; Han, Y.S.; Baek, M.J. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann. Surg. Treat. Res. 2015, 89, 1–8. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Hooglugt, A.; van der Stoel, M.M.; Boon, R.A.; Huveneers, S. Endothelial YAP/TAZ Signaling in Angiogenesis and Tumor Vasculature. Front. Oncol. 2020, 10, 612802. [Google Scholar] [CrossRef]
- Rausch, V.; Hansen, C.G. The Hippo Pathway, YAP/TAZ, and the Plasma Membrane. Trends Cell Biol. 2020, 30, 32–48. [Google Scholar] [CrossRef]
- Maishi, N.; Hida, K. Tumor endothelial cells accelerate tumor metastasis. Cancer Sci. 2017, 108, 1921–1926. [Google Scholar] [CrossRef]
- Ferraiuolo, M.; Verduci, L.; Blandino, G.; Strano, S. Mutant p53 Protein and the Hippo Transducers YAP and TAZ: A Critical Oncogenic Node in Human Cancers. Int. J. Mol. Sci. 2017, 18, 961. [Google Scholar] [CrossRef] [PubMed]
- Pefani, D.E.; O’Neill, E. Hippo pathway and protection of genome stability in response to DNA damage. FEBS J. 2016, 283, 1392–1403. [Google Scholar] [CrossRef]
- Wei, Y.; Hui, V.L.Z.; Chen, Y.; Han, R.; Han, X.; Guo, Y. YAP/TAZ: Molecular pathway and disease therapy. MedComm (2020) 2023, 4, e340. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Mao, W.; Peng, H.; Wang, Q.; Jiao, L. YAP promotes sorafenib resistance in hepatocellular carcinoma by upregulating survivin. Cell Oncol 2021, 44, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Meng, L.; Liu, P.; Ji, R.; Su, X.; Xin, Y.; Jiang, X. YAP/TAZ: A promising target for squamous cell carcinoma treatment. Cancer Manag. Res. 2019, 11, 6245–6252. [Google Scholar] [CrossRef] [PubMed]
- Ghiso, E.; Migliore, C.; Ciciriello, V.; Morando, E.; Petrelli, A.; Corso, S.; De Luca, E.; Gatti, G.; Volante, M.; Giordano, S. YAP-Dependent AXL Overexpression Mediates Resistance to EGFR Inhibitors in NSCLC. Neoplasia 2017, 19, 1012–1021. [Google Scholar] [CrossRef]
- Wang, S.; Ma, K.; Chen, L.; Zhu, H.; Liang, S.; Liu, M.; Xu, N. TAZ promotes cell growth and inhibits Celastrol-induced cell apoptosis. Biosci. Rep. 2016, 36, e00386. [Google Scholar] [CrossRef]
- Di Agostino, S.; Sorrentino, G.; Ingallina, E.; Valenti, F.; Ferraiuolo, M.; Bicciato, S.; Piazza, S.; Strano, S.; Del Sal, G.; Blandino, G. YAP enhances the pro-proliferative transcriptional activity of mutant p53 proteins. EMBO Rep. 2016, 17, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Liu, W.; Yan, H.; Zhang, Y.; Cheung, A.H.K.; Zhang, J.; Chen, B.; Liang, L.; Zhou, Z.; et al. MCM6 is a critical transcriptional target of YAP to promote gastric tumorigenesis and serves as a therapeutic target. Theranostics 2022, 12, 6509–6526. [Google Scholar] [CrossRef]
- Sarmasti Emami, S.; Zhang, D.; Yang, X. Interaction of the Hippo Pathway and Phosphatases in Tumorigenesis. Cancers 2020, 12, 2438. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, N.; Bai, J.; Zhou, Q.; Mao, J.; Xu, L.; Liu, J.; Wei, H.; Ren, C.; Wu, X.; et al. Human telomerase reverse transcriptase is a novel target of Hippo-YAP pathway. FASEB J. 2020, 34, 4178–4188. [Google Scholar] [CrossRef]
- Wang, X.; Freire Valls, A.; Schermann, G.; Shen, Y.; Moya, I.M.; Castro, L.; Urban, S.; Solecki, G.M.; Winkler, F.; Riedemann, L.; et al. YAP/TAZ Orchestrate VEGF Signaling during Developmental Angiogenesis. Dev. Cell 2017, 42, 462–478 e467. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.T.; Andrade, J.; Armbruster, M.; Shi, C.; Castro, M.; Costa, A.S.H.; Sugino, T.; Eelen, G.; Zimmermann, B.; Wilhelm, K.; et al. A YAP/TAZ-TEAD signalling module links endothelial nutrient acquisition to angiogenic growth. Nat. Metab. 2022, 4, 672–682. [Google Scholar] [CrossRef]
- Nagasawa-Masuda, A.; Terai, K. Yap/Taz transcriptional activity is essential for vascular regression via Ctgf expression and actin polymerization. PLoS ONE 2017, 12, e0174633. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Zhang, J.; Cheng, A.S.L.; Yu, J.; To, K.F.; Kang, W. MCM family in gastrointestinal cancer and other malignancies: From functional characterization to clinical implication. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188415. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP and TAZ: A signalling hub of the tumour microenvironment. Nat. Rev. Cancer 2019, 19, 454–464. [Google Scholar] [CrossRef]
- Hagenbeek, T.J.; Webster, J.D.; Kljavin, N.M.; Chang, M.T.; Pham, T.; Lee, H.J.; Klijn, C.; Cai, A.G.; Totpal, K.; Ravishankar, B.; et al. The Hippo pathway effector TAZ induces TEAD-dependent liver inflammation and tumors. Sci. Signal 2018, 11, eaaj1757. [Google Scholar] [CrossRef]
- Matthaios, D.; Tolia, M.; Mauri, D.; Kamposioras, K.; Karamouzis, M. YAP/Hippo Pathway and Cancer Immunity: It Takes Two to Tango. Biomedicines 2021, 9, 1949. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Shi, Z.; Zhu, Y.; Yang, J.; Liu, X.; Que, R.; Lin, L.; Chen, Y.; Li, Y. Ginsenosides Regulates Innate Immunity to Affect Immune Microenvironment of AIH Through Hippo-YAP/TAZ Signaling Pathway. Front. Immunol. 2022, 13, 851560. [Google Scholar] [CrossRef]
- Janse van Rensburg, H.J.; Azad, T.; Ling, M.; Hao, Y.; Snetsinger, B.; Khanal, P.; Minassian, L.M.; Graham, C.H.; Rauh, M.J.; Yang, X. The Hippo Pathway Component TAZ Promotes Immune Evasion in Human Cancer through PD-L1. Cancer Res. 2018, 78, 1457–1470. [Google Scholar] [CrossRef]
- Pan, Z.; Tian, Y.; Cao, C.; Niu, G. The Emerging Role of YAP/TAZ in Tumor Immunity. Mol. Cancer Res. 2019, 17, 1777–1786. [Google Scholar] [CrossRef]
- Simsek, H.; Klotzsch, E. The solid tumor microenvironment-Breaking the barrier for T cells: How the solid tumor microenvironment influences T cells: How the solid tumor microenvironment influences T cells. Bioessays 2022, 44, e2100285. [Google Scholar] [CrossRef]
- Wang, J.; Shen, C.; Zhang, J.; Zhang, Y.; Liang, Z.; Niu, H.; Wang, Y.; Yang, X. TEAD4 is an Immune Regulating-Related Prognostic Biomarker for Bladder Cancer and Possesses Generalization Value in Pan-Cancer. DNA Cell Biol. 2021, 40, 798–810. [Google Scholar] [CrossRef]
- Marxsen, J.H.; Stengel, P.; Doege, K.; Heikkinen, P.; Jokilehto, T.; Wagner, T.; Jelkmann, W.; Jaakkola, P.; Metzen, E. Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases. Biochem. J. 2004, 381, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zeng, C.; Ye, S.; Dai, X.; He, Q.; Yang, B.; Zhu, H. Yes-associated protein (YAP) and transcriptional coactivator with a PDZ-binding motif (TAZ): A nexus between hypoxia and cancer. Acta Pharm. Sin. B 2020, 10, 947–960. [Google Scholar] [CrossRef]
- Talukdar, P.D.; Chatterji, U. Transcriptional co-activators: Emerging roles in signaling pathways and potential therapeutic targets for diseases. Signal Transduct. Target. Ther. 2023, 8, 427. [Google Scholar] [CrossRef]
- Luo, J.; Zou, H.; Guo, Y.; Tong, T.; Chen, Y.; Xiao, Y.; Pan, Y.; Li, P. The oncogenic roles and clinical implications of YAP/TAZ in breast cancer. Br. J. Cancer 2023, 128, 1611–1624. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Mottier-Pavie, V.; Plouffe, S.W.; Hansen, C.G.; Hong, A.W.; Park, H.W.; Mo, J.S.; Lu, W.; Lu, S.; et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat. Commun. 2015, 6, 8357. [Google Scholar] [CrossRef]
- Plouffe, S.W.; Meng, Z.; Lin, K.C.; Lin, B.; Hong, A.W.; Chun, J.V.; Guan, K.L. Characterization of Hippo Pathway Components by Gene Inactivation. Mol. Cell 2016, 64, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Gilkes, D.M.; Hu, H.; Luo, W.; Bullen, J.W.; Liang, H.; Semenza, G.L. HIF-1alpha and TAZ serve as reciprocal co-activators in human breast cancer cells. Oncotarget 2015, 6, 11768–11778. [Google Scholar] [CrossRef]
- He, M.; Zhou, Z.; Shah, A.A.; Hong, Y.; Chen, Q.; Wan, Y. New insights into posttranslational modifications of Hippo pathway in carcinogenesis and therapeutics. Cell Div. 2016, 11, 4. [Google Scholar] [CrossRef]
- Plouffe, S.W.; Lin, K.C.; Moore, J.L.; Tan, F.E., 3rd; Ma, S.; Ye, Z.; Qiu, Y.; Ren, B.; Guan, K.L. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J. Biol. Chem. 2018, 293, 11230–11240. [Google Scholar] [CrossRef]
- Ortega, A.; Vera, I.; Diaz, M.P.; Navarro, C.; Rojas, M.; Torres, W.; Parra, H.; Salazar, J.; De Sanctis, J.B.; Bermudez, V. The YAP/TAZ Signaling Pathway in the Tumor Microenvironment and Carcinogenesis: Current Knowledge and Therapeutic Promises. Int. J. Mol. Sci. 2021, 23, 430. [Google Scholar] [CrossRef]
- Piccolo, S.; Panciera, T.; Contessotto, P.; Cordenonsi, M. YAP/TAZ as master regulators in cancer: Modulation, function and therapeutic approaches. Nat. Cancer 2023, 4, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Ashayeri, N.; Baghaie, L.; Sambi, M.; Satari, K.; Baluch, N.; Bosykh, D.A.; Szewczuk, M.R.; Chakraborty, S. The Hippo Pathway Effectors YAP/TAZ-TEAD Oncoproteins as Emerging Therapeutic Targets in the Tumor Microenvironment. Cancers 2023, 15, 3468. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Jiang, N.; Zhou, B.; Liu, Q.; Du, C. TAZ regulates cell proliferation and epithelial-mesenchymal transition of human hepatocellular carcinoma. Cancer Sci. 2015, 106, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, M.; Tan, J.; Hou, J.; He, J.; Wang, F.; Cui, H.; Yi, L. Transcriptional co-activator with PDZ-binding motif overexpression promotes cell proliferation and transcriptional co-activator with PDZ-binding motif deficiency induces cell cycle arrest in neuroblastoma. Oncol. Lett. 2017, 13, 4295–4301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, X.; Lei, Q.Y. Regulation of TAZ in cancer. Protein Cell 2016, 7, 548–561. [Google Scholar] [CrossRef]
- Chen, G.; Xie, J.; Huang, P.; Yang, Z. Overexpression of TAZ promotes cell proliferation, migration and epithelial-mesenchymal transition in ovarian cancer. Oncol. Lett. 2016, 12, 1821–1825. [Google Scholar] [CrossRef]
- Cordenonsi, M.; Zanconato, F.; Azzolin, L.; Forcato, M.; Rosato, A.; Frasson, C.; Inui, M.; Montagner, M.; Parenti, A.R.; Poletti, A.; et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011, 147, 759–772. [Google Scholar] [CrossRef]
- Lo Sardo, F.; Strano, S.; Blandino, G. YAP and TAZ in Lung Cancer: Oncogenic Role and Clinical Targeting. Cancers 2018, 10, 137. [Google Scholar] [CrossRef]
- Lamar, J.M.; Stern, P.; Liu, H.; Schindler, J.W.; Jiang, Z.G.; Hynes, R.O. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl. Acad. Sci. USA 2012, 109, E2441–E2450. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.N.; Curtis, S.J.; Fillmore, C.M.; Rowbotham, S.P.; Mohseni, M.; Wagner, D.E.; Beede, A.M.; Montoro, D.T.; Sinkevicius, K.W.; Walton, Z.E.; et al. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. EMBO J. 2014, 33, 468–481. [Google Scholar] [CrossRef]
- Lin, M.; Bu, C.; He, Q.; Gu, J.; Wang, H.; Feng, N.; Jiang, S.W. TAZ is overexpressed in prostate cancers and regulates the proliferation, migration and apoptosis of prostate cancer PC3 cells. Oncol. Rep. 2020, 44, 747–756. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef]
- Marx, A.; Schumann, A.; Hoflmayer, D.; Bady, E.; Hube-Magg, C.; Moller, K.; Tsourlakis, M.C.; Steurer, S.; Buscheck, F.; Eichenauer, T.; et al. Up regulation of the Hippo signalling effector YAP1 is linked to early biochemical recurrence in prostate cancers. Sci. Rep. 2020, 10, 8916. [Google Scholar] [CrossRef] [PubMed]
- Koinis, F.; Chantzara, E.; Samarinas, M.; Xagara, A.; Kratiras, Z.; Leontopoulou, V.; Kotsakis, A. Emerging Role of YAP and the Hippo Pathway in Prostate Cancer. Biomedicines 2022, 10, 2834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, S.; Chen, X.; Stauffer, S.; Yu, F.; Lele, S.M.; Fu, K.; Datta, K.; Palermo, N.; Chen, Y.; et al. The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol. Cell Biol. 2015, 35, 1350–1362. [Google Scholar] [CrossRef]
- Luo, J.; Deng, L.; Zou, H.; Guo, Y.; Tong, T.; Huang, M.; Ling, G.; Li, P. New insights into the ambivalent role of YAP/TAZ in human cancers. J. Exp. Clin. Cancer Res. 2023, 42, 130. [Google Scholar] [CrossRef]
- Tocci, P.; Cianfrocca, R.; Sestito, R.; Rosano, L.; Di Castro, V.; Blandino, G.; Bagnato, A. Endothelin-1 axis fosters YAP-induced chemotherapy escape in ovarian cancer. Cancer Lett. 2020, 492, 84–95. [Google Scholar] [CrossRef]
- Zeng, R.; Dong, J. The Hippo Signaling Pathway in Drug Resistance in Cancer. Cancers 2021, 13, 318. [Google Scholar] [CrossRef]
- Hall, C.A.; Wang, R.; Miao, J.; Oliva, E.; Shen, X.; Wheeler, T.; Hilsenbeck, S.G.; Orsulic, S.; Goode, S. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010, 70, 8517–8525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; George, J.; Deb, S.; Degoutin, J.L.; Takano, E.A.; Fox, S.B.; AOCS Study group; Bowtell, D.D.; Harvey, K.F. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 2011, 30, 2810–2822. [Google Scholar] [CrossRef]
- Mouillet-Richard, S.; Laurent-Puig, P. YAP/TAZ Signalling in Colorectal Cancer: Lessons from Consensus Molecular Subtypes. Cancers 2020, 12, 3160. [Google Scholar] [CrossRef]
- Yuen, H.F.; McCrudden, C.M.; Huang, Y.H.; Tham, J.M.; Zhang, X.; Zeng, Q.; Zhang, S.D.; Hong, W. TAZ expression as a prognostic indicator in colorectal cancer. PLoS ONE 2013, 8, e54211. [Google Scholar] [CrossRef]
- Li, N.; Lu, N.; Xie, C. The Hippo and Wnt signalling pathways: Crosstalk during neoplastic progression in gastrointestinal tissue. FEBS J. 2019, 286, 3745–3756. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lee, S.S.; Kim, S.B.; Sohn, B.H.; Lee, H.S.; Jang, H.J.; Park, Y.Y.; Kopetz, S.; Kim, S.S.; Oh, S.C.; et al. Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients. Clin. Cancer Res. 2015, 21, 357–364. [Google Scholar] [CrossRef]
- Ou, C.; Sun, Z.; Li, S.; Li, G.; Li, X.; Ma, J. Dual roles of yes-associated protein (YAP) in colorectal cancer. Oncotarget 2017, 8, 75727–75741. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, D. Role of the transcriptional coactivators YAP/TAZ in liver cancer. Curr. Opin. Cell Biol. 2019, 61, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Y.; Liao, Z.; Mo, J.; Zhang, Q.; Zhang, B.; Zhang, L. The role of YAP1 in liver cancer stem cells: Proven and potential mechanisms. Biomark. Res. 2022, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Cai, Q.; Xu, Y. Hypoxic conditions differentially regulate TAZ and YAP in cancer cells. Arch. Biochem. Biophys. 2014, 562, 31–36. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.Y.; Wang, J.; Wang, Y.; Zhang, P.; Ma, N.; Mo, S.J. Phosphorylation of 14-3-3zeta links YAP transcriptional activation to hypoxic glycolysis for tumorigenesis. Oncogenesis 2019, 8, 31. [Google Scholar] [CrossRef]
- Zhong, Z.; Jiao, Z.; Yu, F.X. The Hippo signaling pathway in development and regeneration. Cell Rep. 2024, 43, 113926. [Google Scholar] [CrossRef]
- Furth, N.; Aylon, Y. The LATS1 and LATS2 tumor suppressors: Beyond the Hippo pathway. Cell Death Differ. 2017, 24, 1488–1501. [Google Scholar] [CrossRef]
- Segrelles, C.; Paramio, J.M.; Lorz, C. The transcriptional co-activator YAP: A new player in head and neck cancer. Oral. Oncol. 2018, 86, 25–32. [Google Scholar] [CrossRef]
- Kim, W.; Jho, E.H. The history and regulatory mechanism of the Hippo pathway. BMB Rep. 2018, 51, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, M.; Yangzhong, X.; Zhang, X.; Zu, A.; Hou, Y.; Li, L.; Sun, S. Hippo signaling pathway and respiratory diseases. Cell Death Discov. 2022, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, P.; Maroni, P.; Matteucci, E.; Luzzati, A.; Perrucchini, G.; Desiderio, M.A. Hypoxia inducible factor-1 is activated by transcriptional co-activator with PDZ-binding motif (TAZ) versus WWdomain-containing oxidoreductase (WWOX) in hypoxic microenvironment of bone metastasis from breast cancer. Eur. J. Cancer 2013, 49, 2608–2618. [Google Scholar] [CrossRef]
- Lopez-Hernandez, A.; Sberna, S.; Campaner, S. Emerging Principles in the Transcriptional Control by YAP and TAZ. Cancers 2021, 13, 4242. [Google Scholar] [CrossRef] [PubMed]
- Strepkos, D.; Markouli, M.; Papavassiliou, K.A.; Papavassiliou, A.G.; Piperi, C. Emerging roles for the YAP/TAZ transcriptional regulators in brain tumour pathology and targeting options. Neuropathol. Appl. Neurobiol. 2022, 48, e12762. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Taouk, G.M. A Potential Role of YAP/TAZ in the Interplay Between Metastasis and Metabolic Alterations. Front. Oncol. 2020, 10, 928. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, M.; Cai, M.; Zhang, C.; Qiu, Y.; Wang, X.; Zhang, T.; Zhou, H.; Wang, J.; Zhao, W.; et al. Transcriptional co-activators YAP/TAZ: Potential therapeutic targets for metastatic breast cancer. Biomed. Pharmacother. 2021, 133, 110956. [Google Scholar] [CrossRef]
- Hansen, C.G.; Moroishi, T.; Guan, K.L. YAP and TAZ: A nexus for Hippo signaling and beyond. Trends Cell Biol. 2015, 25, 499–513. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Lai, Y.S.; Chen, Y.C.; Lin, T.C.; Nguyen, N.T.; Chiu, W.T. Hypoxia-induced YAP activation and focal adhesion turnover to promote cell migration in mesenchymal TNBC cells. Cancer Med. 2023, 12, 9723–9737. [Google Scholar] [CrossRef]
- Liang, H.; Xu, Y.; Zhao, J.; Chen, M.; Wang, M. Hippo pathway in non-small cell lung cancer: Mechanisms, potential targets, and biomarkers. Cancer Gene Ther. 2024, 31, 652–666. [Google Scholar] [CrossRef]

| Function | Gene | Major Pathway | References |
|---|---|---|---|
| Anti-apoptosis | Survivin | YAP promotes sorafenib resistance through upregulation of Survivin expression. | [29] |
| CTGF | CTGF acts as a direct target gene for YAP, promoting cell proliferation and anchorage for independent growth. It also functions as a transcriptional co-repressor to promote cell survival by repressing DNA damage. | [30] | |
| AXL | AXL is a tyrosine kinase receptor that acts as the main downstream effector responsible for sustaining YAP-driven resistance. In addition, YAP and its downstream target AXL play a crucial role in resistance to EGFR TKIs. | [31] | |
| Bcl | Overexpression of TAZ may upregulate its target genes, including connective tissue growth factor (CTGF) and B-cell lymphoma-2 (Bcl-2) and decrease expression of Bcl-2 associated X protein (Bax). | [32] | |
| Proliferation (Cell cycle and growth) | Cyclin and CDK | YAP, as well as mutant p53 and the transcription factor NF-Y, bind onto the regulatory regions of mutant p53 pro-proliferative transcriptional activity genes, such as cyclin A, cyclin B, and CDK1. | [33] |
| MCMs | Hyperactivated YAP in gastric cancer (GC) induces MCM transcription via binding to its promoter. The YAP–MCM axis facilitates GC progression by inducing PI3K/Akt signaling. | [34] | |
| CDC25 | CDC25 is a member of the phosphatase family and is a protein phosphatase that plays an important role in the regulation of the cell cycle. Activation of YAP/TAZ/YKI may lead to the upregulation of CDC25/string. | [35] | |
| SMAD | SMADs activated by TGF-β translocate into the nucleus and bind to YAP, thus promoting the expression of the target gene and cell proliferation. | [13] | |
| TERT | The Hippo pathway effector Yes-associated protein (YAP) promotes the expression of human telomerase reverse transcriptase (hTERT). YAP transcriptionally activates the hTERT promoter through interaction with TEAD. | [36] | |
| Angiogenesis | VEGF | YAP/TAZ activity is controlled by VEGF during angiogenesis. VEGF induces a YAP/TAZ-dependent transcriptome linked to cytoskeleton remodeling. | [37] |
| Axl | YAP/TAZ promote angiogenesis by fueling nutrient-dependent mTORC1 signaling. The upregulated genes observed were prototypical YAP/TAZ targets, such as CTGF, AXL, CYR61, as well as numerous genes linked to mechanistic targeting of mTORC1 signaling. | [38] | |
| CTGF | YAP/TAZ are activated by blood circulation in the endothelial cells. This leads to induction of CTGF and actin polymerization. | [39] | |
| Ang2 | Overexpression of an active form of YAP promotes hypersprouting via angiogenic growth factor angiopoietin-2 (Ang2) signaling. Hypoxia stabilizes hypoxia inducible transcription factor 1α (HIF1α) in tumor cells, initiating the transcription and secretion of pro-angiogenic factors such as VEGF and Ang2. | [23,37] | |
| MMPs | YAP/TAZ-mediated tumor angiogenesis occurs through MMP-mediated ECM remodeling. | [23] | |
| MCMs | YAP/TAZ promote EC proliferation in a MCM-dependent manner. | [40] | |
| Immune Suppression | CXCL5 | YAP, in complex with TEAD in cancer cells, stimulates the recruitment of MDSCs within the TME by transcriptionally inducing the production of cytokines, including CSF and CXCL5. | [41] |
| CCL2 | YAP and TAZ bind to the Ccl2 promoter. Increased TAZ expression was correlated with increased expression of the inflammatory cytokine CCL2. | [41,42] | |
| TGF-beta | The expression of YAP is increased in Tregs, and signaling through YAP increases SMAD/TGFβ signaling and promotes Treg differentiation. | [43] | |
| IL-10 | Activation of the YAP/TAZ–TEAD pathway increases the proportion of MDSCs and enhances the expression of the immunosuppressive cytokines IL-10 and INF-r, which promote Treg cell proliferation. | [44] | |
| PD-L1 | TAZ promotes immune evasion in human cancer through PD-L1; TAZ/YAP/TEAD increase PD-L1 promoter activity. TAZ-induced PD-L1 upregulation in human cancer cells is sufficient to inhibit T-cell function | [45,46] | |
| IL-35 | Tregs in YAP-induced TME secrete the cytokines TGF-β, interleukin-10 (IL-10), and interleukin-35 (IL-35) to maintain their immunosuppressive effects. | [47] | |
| ICOS | When YAP-induced, high expression of TEAD4 regulates immune checkpoint genes (PDCD1, IDO1, ICOS), cytokines (IL-10, CXCL11), cytokine receptors (CCR2, CXCR3, CXCR6, IL2RA), and some other mediators of immune function. | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.H.; Kim, D.Y. Regulation of Tumor Microenvironment through YAP/TAZ under Tumor Hypoxia. Cancers 2024, 16, 3030. https://doi.org/10.3390/cancers16173030
Choi SH, Kim DY. Regulation of Tumor Microenvironment through YAP/TAZ under Tumor Hypoxia. Cancers. 2024; 16(17):3030. https://doi.org/10.3390/cancers16173030
Chicago/Turabian StyleChoi, Sung Hoon, and Do Young Kim. 2024. "Regulation of Tumor Microenvironment through YAP/TAZ under Tumor Hypoxia" Cancers 16, no. 17: 3030. https://doi.org/10.3390/cancers16173030
APA StyleChoi, S. H., & Kim, D. Y. (2024). Regulation of Tumor Microenvironment through YAP/TAZ under Tumor Hypoxia. Cancers, 16(17), 3030. https://doi.org/10.3390/cancers16173030







