Role of Receptor for Advanced Glycation End-Products in Endometrial Cancer: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Molecular Dialogue between the RAGE Signaling Pathway and Endometrial Pathology
3. RAGE and Its Ligands in Endometrial Cancer (EC)
3.1. HMGB1
3.2. S100B
3.3. Diaph1
4. Cancer and Diabetes
5. DNA Methylation in Endometrial Cancer (EC)
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fazeli, A.; Holt, W.V. Cross talk during the periconception period. Theriogenology 2016, 86, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, M.; Lee, S.K. Role of endometrial immune cells in implantation. Clin. Exp. Reprod. Med. 2011, 38, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.L.; Martin, A.R.; Kosasih, M.; Caruana, B.T.; Farrell, R. The Role of Hyperglycemia in Endometrial Cancer Pathogenesis. Cancers 2020, 12, 1191. [Google Scholar] [CrossRef]
- Sidorkiewicz, I.; Jóźwik, M.; Buczyńska, A.; Erol, A.; Jóźwik, M.; Moniuszko, M.; Jarząbek, K.; Niemira, M.; Krętowski, A. Identification and subsequent validation of transcriptomic signature associated with metabolic status in endometrial cancer. Sci. Rep. 2023, 13, 13763. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, D.; Zhou, Y.M.; Yang, H.; Cheng, D.; Ma, X.X. Effects of receptor for advanced glycation endproducts on microvessel formation in endometrial cancer. BMC Cancer 2016, 16, 93. [Google Scholar] [CrossRef]
- Zglejc-Waszak, K.; Korytko, A.; Pomianowski, A.; Wojtkiewicz, J.; Wąsowicz, K.; Juranek, J.K. A mouse model of uterine exposure to long-term hyperglycemia and a high-fat diet. Anim. Sci. Pap. Rep. 2024, 42, 203–216. [Google Scholar] [CrossRef]
- Pintican, R.; Bura, V.; Zerunian, M.; Smith, J.; Addley, H.; Freeman, S.; Caruso, D.; Laghi, A.; Sala, E.; Jimenez-Linan, M. MRI of the endometrium—From normal appearances to rare pathology. Br. J. Radiol. 2021, 94, 20201347. [Google Scholar] [CrossRef]
- Zglejc-Waszak, K.; Mukherjee, K.; Juranek, J.K. The cross-talk between RAGE and DIAPH1 in neurological complications of diabetes: A review. Eur. J. Neurosci. 2021, 54, 5982–5999. [Google Scholar] [CrossRef]
- Zglejc-Waszak, K.; Mukherjee, K.; Korytko, A.; Lewczuk, B.; Pomianowski, A.; Wojtkiewicz, J.; Banach, M.; Załęcki, M.; Nowicka, N.; Jarosławska, J.; et al. Novel insights into the nervous system affected by prolonged hyperglycemia. J. Mol. Med. 2023, 101, 1015–1028. [Google Scholar] [CrossRef]
- Nowicka, N.; Zglejc-Waszak, K.; Juranek, J.; Korytko, A.; Wąsowicz, K.; Chmielewska-Krzesińska, M.; Wojtkiewicz, J. Novel insights into RAGE signaling pathways during the progression of amyotrophic lateral sclerosis in RAGE-deficient SOD1 G93A mice. PLoS ONE 2024, 19, e0299567. [Google Scholar] [CrossRef]
- Nowicka, N.; Szymańska, K.; Juranek, J.; Zglejc-Waszak, K.; Korytko, A.; Załęcki, M.; Chmielewska-Krzesińska, M.; Wąsowicz, K.; Wojtkiewicz, J. The Involvement of RAGE and Its Ligands during Progression of ALS in SOD1 G93A Transgenic Mice. Int. J. Mol. Sci. 2022, 23, 2184. [Google Scholar] [CrossRef] [PubMed]
- Juranek, J.; Mukherjee, K.; Kordas, B.; Załęcki, M.; Korytko, A.; Zglejc-Waszak, K.; Szuszkiewicz, J.; Banach, M. Role of RAGE in the Pathogenesis of Neurological Disorders. Neurosci. Bull. 2022, 38, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- Zglejc-Waszak, K.; Schmidt, A.M.; Juranek, J.K. The receptor for advanced glycation end products and its ligands’ expression in OVE26 diabetic sciatic nerve during the development of length-dependent neuropathy. Neuropathology 2023, 43, 84–94. [Google Scholar] [CrossRef]
- Zglejc-Waszak, K.; Pomianowski, A.; Wojtkiewicz, J.; Banach, M.; Juranek, J.K. New insights into RAGE/Diaph1 interaction as a modulator of actin cytoskeleton dynamics in peripheral nervous system in long-term hyperglycaemia. Eur. J. Neurosci. 2023, 57, 1642–1656. [Google Scholar] [CrossRef] [PubMed]
- Jaroslawska, J.; Korytko, A.; Zglejc-Waszak, K.; Antonowski, T.; Pomianowski, A.S.; Wasowicz, K.; Wojtkiewicz, J.; Juranek, J.K. Peripheral Neuropathy Presents Similar Symptoms and Pathological Changes in Both High-Fat Diet and Pharmacologically Induced Pre- and Diabetic Mouse Models. Life 2021, 11, 126. [Google Scholar] [CrossRef]
- Bao, J.M.; He, M.Y.; Liu, Y.W.; Lu, Y.J.; Hong, Y.Q.; Luo, H.H.; Ren, Z.L.; Zhao, S.C.; Jiang, Y. AGE/RAGE/Akt pathway contributes to prostate cancer cell proliferation by promoting Rb phosphorylation and degradation. Am. J. Cancer Res. 2015, 5, 1741–1750. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, H.; Wang, T.; Sun, Y.; Ni, P.; Liu, Y.; Tian, S.; Amoah Barnie, P.; Shen, H.; Xu, W.; et al. Exogenous high-mobility group box 1 inhibits apoptosis and promotes the proliferation of lewis cells via RAGE/TLR4-dependent signal pathways. Scand. J. Immunol. 2014, 79, 386–394. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Metformin inhibits advanced glycation end products (AGEs)-induced growth and VEGF expression in MCF-7 breast cancer cells by suppressing AGEs receptor expression via AMP-activated protein kinase. Horm. Metab. Res. 2013, 45, 387–390. [Google Scholar] [CrossRef]
- Nowicka, N.; Juranek, J.; Juranek, J.K.; Wojtkiewicz, J. Risk Factors and Emerging Therapies in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2019, 20, 2616. [Google Scholar] [CrossRef]
- Manigrasso, M.B.; Juranek, J.; Ramasamy, R.; Schmidt, A.M. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol. Metab. 2014, 25, 15–22. [Google Scholar] [CrossRef]
- Daffu, G.; del Pozo, C.H.; O’Shea, K.M.; Ananthakrishnan, R.; Ramasamy, R.; Schmidt, A.M. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int. J. Mol. Sci. 2013, 14, 19891–19910. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. World Cancer Report 2014; World Health Organization: Geneva, Switzerland, 2014; Chapter 5.12; ISBN 978-92-832-0429-9. [Google Scholar]
- International Agency for Research on Cancer. World Cancer Report: Cancer Research for Cancer Prevention 2020; World Cancer Reports; International Agency for Research on Cancer: Lyon, France, 2020; ISBN 24978-92-832-0447-3. [Google Scholar]
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, W1, W60–W64. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, A.; Malvi, P.; Wajapeyee, N. Oncogene-directed alterations in cancer cell metabolism. Trends Cancer 2016, 2, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg efect: How does it beneft cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Li, W.; Wang, J. Uncovering the underlying mechanisms of cancer metabolism through the landscapes and probability fux quantifcations. IScience 2020, 23, 101002. [Google Scholar] [CrossRef]
- Byrne, F.L.; Poon, I.K.; Modesitt, S.C.; Tomsig, J.L.; Chow, J.D.; Healy, M.E.; Baker, W.D.; Atkins, K.A.; Lancaster, J.M.; Marchion, D.C.; et al. Metabolic vulnerabilities in endometrial cancer. Cancer Res. 2014, 74, 5832–5845. [Google Scholar] [CrossRef]
- Roncolato, F.; Lindemann, K.; Willson, M.L.; Martyn, J.; Mileshkin, L. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database Syst. Rev. 2019, 10, CD012160. [Google Scholar] [CrossRef]
- Ramasubbu, K.; Devi Rajeswari, V. Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases: A perspective review. Mol. Cell Biochem. 2023, 478, 1307–1324. [Google Scholar] [CrossRef]
- Jarosławska, J.; Kordas, B.; Miłowski, T.; Juranek, J.K. Mammalian Diaphanous1 signalling in neurovascular complications of diabetes. Eur. J. Neurosci. 2024, 59, 2628–2645. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xia, P.; Zheng, J.J.; Sun, X.B.; Pan, X.D.; Zhang, X.; Wu, C.Z. Receptors for advanced glycation end products (RAGE) is associated with microvessel density and is a prognostic biomarker for clear cell renal cell carcinoma. Biomed. Pharmacother. 2015, 73, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhong, Y.; Zhou, S.; Peng, L. Knockdown of RAGE expression inhibits colorectal cancer cell invasion and suppresses angiogenesis in vitro and in vivo. Cancer Lett. 2011, 313, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Healey, G.D.; Pan-Castillo, B.; Garcia-Parra, J.; Davies, J.; Roberts, S.; Jones, E.; Dhar, K.; Nandanan, S.; Tofazzal, N.; Piggott, L.; et al. Antibody drug conjugates against the receptor for advanced glycation end products (RAGE), a novel therapeutic target in endometrial cancer. J. Immunother. Cancer 2019, 7, 280. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; Al-Abed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920. [Google Scholar] [CrossRef]
- Senatus, L.; Egaña-Gorroño, L.; López-Díez, R.; Bergaya, S.; Aranda, J.F.; Amengual, J.; Arivazhagan, L.; Manigrasso, M.B.; Yepuri, G.; Nimma, R.; et al. DIAPH1 mediates progression of atherosclerosis and regulates hepatic lipid metabolism in mice. Commun. Biol. 2023, 6, 280. [Google Scholar] [CrossRef] [PubMed]
- Wendt, T.; Tanji, N.; Guo, J.; Hudson, B.I.; Bierhaus, A.; Ramasamy, R.; Arnold, B.; Nawroth, P.P.; Yan, S.F.; D’Agati, V.; et al. Glucose, glycation, and RAGE: Implications for amplification of cellular dysfunction in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, 1383–1395. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Investig. 2001, 108, 949–955. [Google Scholar] [CrossRef]
- Palanissami, G.; Paul, S.F.D. RAGE and Its Ligands: Molecular Interplay between Glycation, Inflammation, and Hallmarks of Cancer-a Review. Horm. Cancer 2018, 9, 295–325. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in inflammatory diseases. Front. Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef] [PubMed]
- Juranek, J.K.; Aleshin, A.; Rattigan, E.M.; Johnson, L.; Qu, W.; Song, F.; Ananthakrishnan, R.; Quadri, N.; Yan, S.D.; Ramasamy, R.; et al. Morphological Changes and Immunohistochemical Expression of RAGE and its Ligands in the Sciatic Nerve of Hyperglycemic Pig (Sus Scrofa). Biochem. Insights 2010, 2010, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Banerjee, S.; Palmer, A.; Zheng, G.; Chen, A.; Bosland, M.C.; Kajdacsy-Balla, A.; Kalyanasundaram, R.; Munirathinam, G. HMGB1 in hormone-related cancer: A potential therapeutic target. Horm. Cancer 2014, 5, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.C.; Huang, Y.C.; Kan, J.Y.; Kuo, P.L.; Hou, M.F.; Hsu, Y.L. S100B expression in breast cancer as a predictive marker for cancer metastasis. Int. J. Oncol. 2018, 52, 433–440. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Piperi, C.; Papavassiliou, A.G. High-mobility group box 1 in Parkinson’s disease: From pathogenesis to therapeutic approaches. J. Neurochem. 2018, 146, 211–218. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Emerging role of S100B protein implication in Parkinson’s disease pathogenesis. Cell Mol. Life Sci. 2021, 78, 1445–1453. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s Disease (AD): From Risk Factors to Therapeutic Targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195, Erratum in Nature 2010, 467, 622. [Google Scholar] [CrossRef] [PubMed]
- Todorova, J.; Pasheva, E. High mobility group B1 protein interacts with its receptor RAGE in tumor cells but not in normal tissues. Oncol. Lett. 2012, 3, 214–218. [Google Scholar] [CrossRef]
- Luan, X.; Ma, C.; Wang, P.; Lou, F. HMGB1 is negatively correlated with the development of endometrial carcinoma and prevents cancer cell invasion and metastasis by inhibiting the process of epithelial-to-mesenchymal transition. Onco Targets Ther. 2017, 10, 1389–1402. [Google Scholar] [CrossRef]
- Hori, O.; Brett, J.; Slattery, T.; Cao, R.; Zhang, J.; Chen, J.X.; Nagashima, M.; Lundh, E.R.; Vijay, S.; Nitecki, D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 1995, 270, 25752–25761. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Tang, D.; Schapiro, N.E.; Livesey, K.M.; Farkas, A.; Loughran, P.; Bierhaus, A.; Lotze, M.T.; Zeh, H.J. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010, 17, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 proteins in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118677. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Vetter, S.W. The role of S100 proteins and their receptor RAGE in pancreatic cancer. BBA-Mol. Basis Dis. 2015, 852, 2706–2711. [Google Scholar] [CrossRef]
- Bresnick, A.R.; Weber, D.J.; Zimmer, D.B. S100 proteins in cancer. Nat. Rev. Cancer. 2015, 15, 96–109. [Google Scholar] [CrossRef]
- Yin, C.; Li, H.; Zhang, B.; Liu, Y.; Lu, G.; Lu, S.; Sun, L.; Qi, Y.; Li, X.; Chen, W. RAGE-binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial–mesenchymal transition. Breast Cancer Res. Treat. 2013, 142, 297–309. [Google Scholar] [CrossRef]
- Bonfrer, J.M.; Korse, C.M.; Nieweg, O.E.; Rankin, E.M. The luminescence immunoassay S-100: A sensitive test to measure circulating S-100B: Its prognostic value in malignant melanoma. Br. J. Cancer 1998, 77, 2210–2214. [Google Scholar] [CrossRef]
- Choi, H.; Puvenna, V.; Brennan, C.; Mahmoud, S.; Wang, X.F.; Phillips, M.; Janigro, D.; Mazzone, P. S100B and S100B auto-antibody as biomarkers for early detection of brain metastases in lung cancer. Transl. Lung Cancer Res. 2016, 5, 413–419. [Google Scholar] [CrossRef]
- Heizmann, C.W. S100B protein in clinical diagnostics: Assay specificity. Clin. Chem. 2004, 50, 249–251. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Q.; Wilder, P.T.; Carrier, F.; Weber, D.J. The calcium-binding protein S100B down-regulates p53 and apoptosis in malignant melanoma. J. Biol. Chem. 2010, 285, 27487–27498. [Google Scholar] [CrossRef]
- Yoshimura, C.; Miyafusa, T.; Tsumoto, K. Identification of small-molecule inhibitors of the human S100B-p53 interaction and evaluation of their activity in human melanoma cells. Bioorg Med. Chem. 2013, 21, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Surget, S.; Khoury, M.P.; Bourdon, J.C. Uncovering the role of p53 splice variants in human malignancy: A clinical perspective. Onco Targets Ther. 2013, 7, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Shekhtman, A.; Ramasamy, R.; Schmidt, A.M. Glycation & the RAGE axis: Targeting signal trans-duction through DIAPH1. Expert. Rev. Proteom. 2017, 14, 147–156. [Google Scholar] [CrossRef]
- Arivazhagan, L.; Popp, C.J.; Ruiz, H.H.; Wilson, R.A.; Manigrasso, M.B.; Shekhtman, A.; Ramasamy, R.; Sevick, M.A.; Schmidt, A.M. The RAGE/DIAPH1 axis: Mediator of obesity and proposed biomarker of human cardiometabolic disease. Cardiovasc. Res. 2024, 119, 2813–2824. [Google Scholar] [CrossRef]
- Labat-de-Hoz, L.; Alonso, M.A. Formins in Human Disease. Cells 2021, 10, 2554. [Google Scholar] [CrossRef]
- Manigrasso, M.B.; Pan, J.; Rai, V.; Zhang, J.; Reverdatto, S.; Quadri, N.; DeVita, R.J.; Ramasamy, R.; Shekhtman, A.; Schmidt, A.M. Small Molecule Inhibition of Ligand-Stimulated RAGE-DIAPH1 Signal Transduction. Sci. Rep. 2016, 6, 22450. [Google Scholar] [CrossRef] [PubMed]
- Manigrasso, M.B.; Friedman, R.A.; Ramasamy, R.; D’Agati, V.; Schmidt, A.M. Deletion of the formin Diaph1 protects from structural and functional abnormalities in the murine diabetic kidney. Am. J. Physiol. Renal Physiol. 2018, 315, F1601–F1612. [Google Scholar] [CrossRef]
- Manigrasso, M.B.; Rabbani, P.; Egaña-Gorroño, L.; Quadri, N.; Frye, L.; Zhou, B.; Reverdatto, S.; Ramirez, L.S.; Dansereau, S.; Pan, J.; et al. Small-molecule antagonism of the interaction of the RAGE cytoplasmic domain with DIAPH1 reduces diabetic complications in mice. Sci. Transl. Med. 2021, 13, eabf7084. [Google Scholar] [CrossRef]
- Reis, A.H.O.; Figalo, L.B.; Orsini, M.; Lemos, B. The implications of DNA methylation for amyotrophic lateral sclerosis. An. Acad. Bras. Cienc. 2023, 95 (Suppl. S2), e20230277. [Google Scholar] [CrossRef]
- Flat, W.; Borowski, S.; Paraschiakos, T.; Blechner, C.; Windhorst, S. DIAPH1 facilitates paclitaxel-mediated cytotoxicity of ovarian cancer cells. Biochem Pharmacol. 2022, 197, 114898. [Google Scholar] [CrossRef]
- Wu, C.H.; Fallini, C.; Ticozzi, N.; Keagle, P.; Sapp, P.C.; Piotrowska, K.; Lowe, P.; Koppers, M.; McKenna-Yasek, D.; Baron, D.M.; et al. Mutations in the profilin 1 gene cause familial amyotrophic lateral sclerosis. Nature 2012, 488, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Lu, L.; Chen, W.; Chen, H.; Xu, X.; Zheng, Z. RhoA/mDia-1/profilin-1 signaling targets microvascular endothelial dysfunction in diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.; Nyström, L.E.; Sundkvist, I.; Markey, F.; Lindberg, U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J. Mol. Biol. 1977, 115, 465–483. [Google Scholar] [CrossRef]
- Da Silva, J.S.; Medina, M.; Zuliani, C.; Di Nardo, A.; Witke, W.; Dotti, C.G. RhoA/ROCK regulation of neuritogenesis via profilin IIa-mediated control of actin stability. J. Cell Biol. 2003, 162, 1267–1279. [Google Scholar] [CrossRef]
- Hirose, A.; Tanikawa, T.; Mori, H.; Okada, Y.; Tanaka, Y. Advanced glycation end products increase endothelial permeability through the RAGE/Rho signaling pathway. FEBS Lett. 2010, 584, 61–66. [Google Scholar] [CrossRef]
- Bros, M.; Haas, K.; Moll, L.; Grabbe, S. RhoA as a Key Regulator of Innate and Adaptive Immunity. Cells 2019, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Wang, B.; Shirvaikar, A.; Khan, S.; Kamat, S.; Schelling, J.R.; Konieczkowski, M.; Sedor, J.R. The IL-1 receptor and Rho directly associate to drive cell activation in inflammation. J. Clin. Investig. 1999, 103, 1561–1570. [Google Scholar] [CrossRef]
- Xu, J.; Huang, Y.; Zhao, J.; Wu, L.; Qi, Q.; Liu, Y.; Li, G.; Li, J.; Liu, H.; Wu, H. Cofilin: A Promising Protein Implicated in Cancer Metastasis and Apoptosis. Front. Cell Dev. Biol. 2021, 9, 599065. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Wan, R.; Wang, Y.; Zhang, C.; Li, M.; Deng, P.; Cao, L.; Hu, C. Profilin 1 Induces Tumor Metastasis by Promoting Microvesicle Secretion Through the ROCK 1/p-MLC Pathway in Non-Small Cell Lung Cancer. Front. Pharmacol. 2022, 13, 890891. [Google Scholar] [CrossRef]
- Santos, J.C.; Profitós-Pelejà, N.; Sánchez-Vinces, S.; Roué, G. RHOA Therapeutic Targeting in Hema-tological Cancers. Cells 2023, 12, 433. [Google Scholar] [CrossRef]
- Ghavami, S.; Rashedi, I.; Dattilo, B.M.; Eshraghi, M.; Chazin, W.J.; Hashemi, M.; Wesselborg, S.; Kerkhoff, C.; Los, M. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J. Leucoc. Biol. 2008, 83, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Young, K.G.; Copeland, J.W. Formins in cell signaling. Biochim. Biophys. Acta 2010, 1803, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Arber, S.; Barbayannis, F.A.; Hanser, H.; Schneider, C.; Stanyon, C.A.; Bernard, O.; Caroni, P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998, 393, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Qu, S. The Relationship between Diabetes Mellitus and Cancers and Its Underlying Mechanisms. Front. Endocrinol. 2022, 13, 800995. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Gui, D.Y. Vander Heiden MG. Altered metabolite levels in cancer: Implications for tumour biology and cancer therapy. Nat. Rev. Cancer 2016, 16, 680–693. [Google Scholar] [CrossRef]
- Kellenberger, L.D.; Bruin, J.E.; Greenaway, J.; Campbell, N.E.; Moorehead, R.A.; Holloway, A.C.; Petrik, J. The role of dysregulated glucose metabolism in epithelial ovarian cancer. J. Oncol. 2010, 2010, 514310. [Google Scholar] [CrossRef]
- Naka, Y.; Bucciarelli, L.G.; Wendt, T.; Lee, L.K.; Rong, L.L.; Ramasamy, R.; Yan, S.F.; Schmidt, A.M. RAGE axis: Animal models and novel insights into the vascular complications of diabetes. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1342–1349. [Google Scholar] [CrossRef]
- Juranek, J.; Ray, R.; Banach, M.; Rai, V. Receptor for advanced glycation end-products in neuro-degenerative diseases. Rev. Neurosci. 2015, 26, 691–698. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, X. HMGB1 siRNA can reduce damage to retinal cells induced by high glucose in vitro and in vivo. Drug Des. Devel Ther. 2017, 11, 783–795. [Google Scholar] [CrossRef]
- Juranek, J.K.; Daffu, G.K.; Geddis, M.S.; Li, H.; Rosario, R.; Kaplan, B.J.; Kelly, L.; Schmidt, A.M. Soluble RAGE Treatment Delays Progression of Amyotrophic Lateral Sclerosis in SOD1 Mice. Front. Cell Neurosci. 2016, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Juranek, J.; Osowski, A.; Wojtkiewicz, J.; Banach, M. Plasma levels of soluble RAGE, AGEs and AOPPs at the early stage of amyotrophic lateral sclerosis: A preliminary study. Polim. Med. 2023, 53, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Aglago, E.K.; Rinaldi, S.; Freisling, H.; Jiao, L.; Hughes, D.J.; Fedirko, V.; Schalkwijk, C.G.; Weiderpass, E.; Dahm, C.C.; Overvad, K.; et al. Soluble Receptor for Advanced Glycation End-products (sRAGE) and Colorectal Cancer Risk: A Case-Control Study Nested within a European Prospective Cohort. Cancer Epidemiol. Biomark. Prev. 2021, 30, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Erusalimsky, J.D. The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox Biol. 2021, 42, 101958. [Google Scholar] [CrossRef]
- Ganss, R. Tumour vessel remodelling: New opportunities in cancer treatment. Vasc. Biol. 2020, 2, R35–R43. [Google Scholar] [CrossRef]
- Roda, N.; Blandano, G.; Pelicci, P.G. Blood Vessels and Peripheral Nerves as Key Players in Cancer Progression and Therapy Resistance. Cancers 2021, 13, 4471. [Google Scholar] [CrossRef] [PubMed]
- Juranek, J.K.; Geddis, M.S.; Song, F.; Zhang, J.; Garcia, J.; Rosario, R.; Yan, S.F.; Brannagan, T.H.; Schmidt, A.M. RAGE deficiency improves postinjury sciatic nerve regeneration in type 1 diabetic mice. Diabetes 2013, 62, 931–943. [Google Scholar] [CrossRef]
- Inoue, F.; Sone, K.; Toyohara, Y.; Takahashi, Y.; Kukita, A.; Hara, A.; Taguchi, A.; Tanikawa, M.; Tsuruga, T.; Osuga, Y. Targeting Epigenetic Regulators for Endometrial Cancer Therapy: Its Molecular Biology and Potential Clinical Applications. Int. J. Mol. Sci. 2021, 22, 2305. [Google Scholar] [CrossRef]
- Qian, Y.; Shi, L.; Luo, Z. Long Non-coding RNAs in Cancer: Implications for Diagnosis, Prognosis, and Therapy. Front. Med. 2020, 7, 612393. [Google Scholar] [CrossRef]
- Franczak, A.; Zglejc, K.; Waszkiewicz, E.; Wojciechowicz, B.; Martyniak, M.; Sobotka, W.; Okrasa, S.; Kotwica, G. Periconceptional undernutrition affects in utero methyltransferase expression and steroid hormone concentrations in uterine flushings and blood plasma during the peri-implantation period in domestic pigs. Reprod. Fertil. Dev. 2017, 29, 1499–1508. [Google Scholar] [CrossRef]
- Zglejc, K.; Franczak, A. Peri-conceptional under-nutrition alters the expression of TRIM28 and ZFP57 in the endometrium and embryos during peri-implantation period in domestic pigs. Reprod. Domest. Anim. 2017, 52, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Zglejc-Waszak, K.; Waszkiewicz, E.M.; Franczak, A. Periconceptional undernutrition affects the levels of DNA methylation in the peri-implantation pig endometrium and in embryos. Theriogenology 2019, 123, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, E.M.; Kozlowska, W.; Zmijewska, A.; Franczak, A. Nutritional restriction during the peri-conceptional period alters the myometrial transcriptome during the peri-implantation period. Sci. Rep. 2021, 11, 21187. [Google Scholar] [CrossRef] [PubMed]
- Zglejc, K.; Martyniak, M.; Waszkiewicz, E.; Kotwica, G.; Franczak, A. Peri-conceptional under-nutrition alters transcriptomic profile in the endometrium during the peri-implantation period-The study in domestic pigs. Reprod. Domest. Anim. 2018, 53, 74–84. [Google Scholar] [CrossRef]
- Kan, S.; Wu, J.; Sun, C.; Hao, J.; Wu, Z. Correlation between RAGE gene promoter methylation and diabetic retinal inflammation. Exp. Ther. Med. 2018, 15, 242–246. [Google Scholar] [CrossRef]
- Wu, X.; Shi, X.; Chen, X.; Yin, Z. Advanced glycation end products regulate the receptor of AGEs epigenetically. Front. Cell Dev. Biol. 2023, 11, 1062229. [Google Scholar] [CrossRef]
- Li, P.; Wang, T.; Chen, M.; Chen, J.; Shen, Y.; Chen, L. RAGE-mediated functional DNA methylat-ed modification contributes to cigarette smoke-induced airway inflammation in mice. Biosci. Rep. 2021, 41, BSR20210308. [Google Scholar] [CrossRef]
- Wang, J.; Zhen, Y.; Zhou, Y.; Yan, S.; Jiang, L.; Zhang, L. Promoter methylation cooperates with SNPs to modulate RAGE transcription and alter UC risk. Biochem. Biophys. Rep. 2018, 17, 17–22. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]

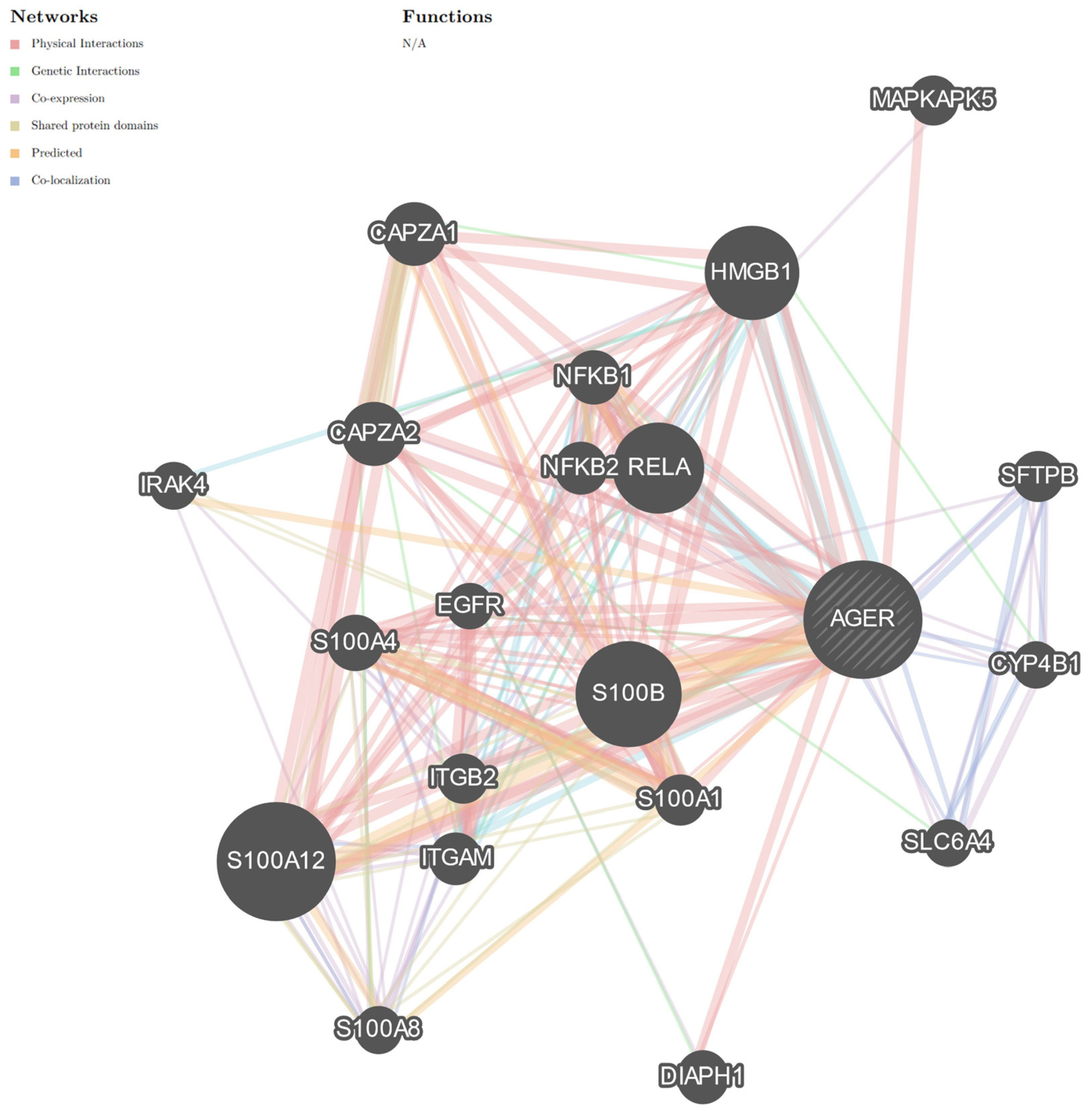

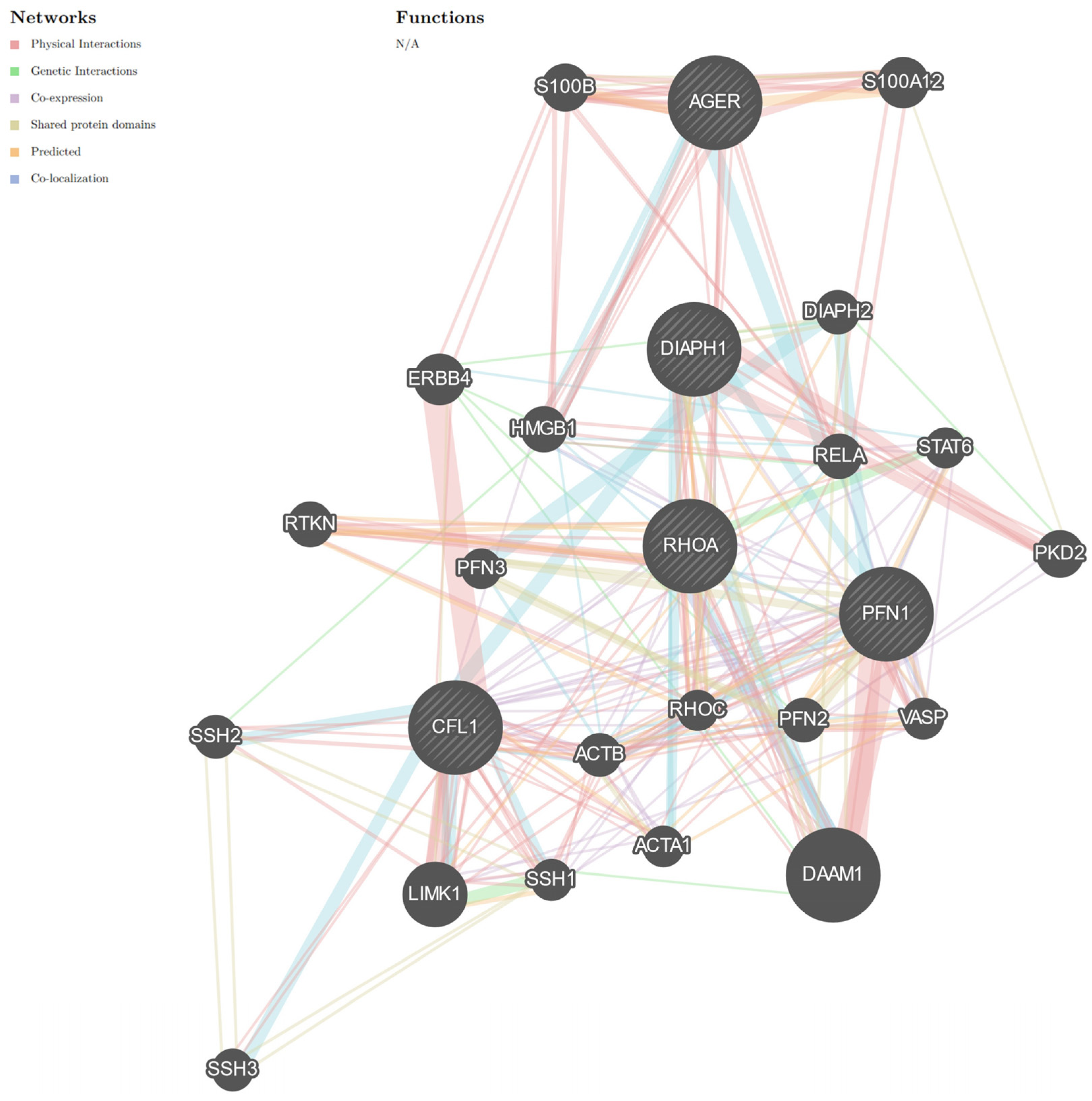

| Function | Official Symbol | Number of Genes |
|---|---|---|
| pyruvate metabolic process | PDK1, PDP1, PDP2, PDPR, LDHA, PKLR, PGAM1, ENO2, ENO1, ENO3 | 13 |
| glucose catabolic process to pyruvate | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2 | 7 |
| glycolytic process through fructose-6-phosphate | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2 | 7 |
| glycolytic process through glucose-6-phosphate | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2 | 7 |
| glucose catabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2 | 7 |
| NADH metabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2 | 7 |
| ADP metabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2, LDHA, ENO4 | 9 |
| ATP generation from ADP | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2, LDHA, ENO4 | 9 |
| NAD metabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2 | 7 |
| purine ribonucleoside diphosphate metabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2, LDHA, ENO4 | 9 |
| carbohydrate catabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2, LDHA, ENO4, ENOSF1 | 10 |
| purine nucleoside diphosphate metabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2, LDHA, ENO4 | 9 |
| ribonucleoside diphosphate metabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2, LDHA, ENO4 | 9 |
| nucleoside diphosphate metabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2, LDHA, ENO4 | 9 |
| glucose metabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2, PDK1, SLC25A1 | 9 |
| hexose catabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2 | 7 |
| monosaccharide catabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2 | 7 |
| hexose metabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2, PDK1, SLC25A1 | 9 |
| glycolytic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2 | 7 |
| ATP metabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2, LDHA, ENO4 | 9 |
| monosaccharide metabolic process | PKLR, PGAM1, ENO2, PKM, ENO1, ENO3, PGAM2, PDK1, SLC25A1 | 9 |
| hexose biosynthetic process | SLC25A1, PGAM1, ENO2, ENO1, ENO3, PGAM2 | 6 |
| regulation of sulfur metabolic process | PDP2, PDPR, PDP1, PDK1 | 4 |
| monosaccharide biosynthetic process | SLC25A1, PGAM1, ENO2, ENO1, ENO3, PGAM2 | 6 |
| regulation of purine nucleotide metabolic process | PGAM1, PDP2, PDPR, PDP1, PDK1, ENO1 | 6 |
| regulation of nucleotide metabolic process | PGAM1, PDP2, PDPR, PDP1, PDK1, ENO1 | 6 |
| thioester biosynthetic process | PDP2, PDPR, PDP1, PDK1, SLC25A1 | 5 |
| acyl-CoA biosynthetic process | PDP2, PDPR, PDP1, PDK1, SLC25A1 | 5 |
| acetyl-CoA metabolic process | PDP2, PDPR, PDP1, PDK1 | 4 |
| acyl-CoA metabolic process | PDP2, PDPR, PDP1, PDK1, SLC25A1 | 5 |
| carbohydrate biosynthetic process | SLC25A1, PGAM1, ENO2, ENO1, ENO3, PGAM2 | 6 |
| regulation of fatty acid metabolic process | PDP2, PDPR, PDP1, PDK1 | 4 |
| cellular ketone metabolic process | PDP2, PDPR, PDP1, PDK1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zglejc-Waszak, K.; Jozwik, M.; Thoene, M.; Wojtkiewicz, J. Role of Receptor for Advanced Glycation End-Products in Endometrial Cancer: A Review. Cancers 2024, 16, 3192. https://doi.org/10.3390/cancers16183192
Zglejc-Waszak K, Jozwik M, Thoene M, Wojtkiewicz J. Role of Receptor for Advanced Glycation End-Products in Endometrial Cancer: A Review. Cancers. 2024; 16(18):3192. https://doi.org/10.3390/cancers16183192
Chicago/Turabian StyleZglejc-Waszak, Kamila, Marcin Jozwik, Michael Thoene, and Joanna Wojtkiewicz. 2024. "Role of Receptor for Advanced Glycation End-Products in Endometrial Cancer: A Review" Cancers 16, no. 18: 3192. https://doi.org/10.3390/cancers16183192





