Cancer Prevention and Treatment with Polyphenols: Type IV Collagenase-Mediated Mechanisms
Abstract
Simple Summary
Abstract
1. Introduction
2. Search Strategy
3. Polyphenols as Modulators of the Activity and Expression of MMP-2 and MMP-9
3.1. Curcumin
3.1.1. In Vitro Studies
3.1.2. In Vivo Studies
3.2. Epigallocatechin-3-Gallate
3.2.1. In Vitro Studies
3.2.2. In Vivo Studies
3.3. Genistein
3.3.1. In Vitro Studies
3.3.2. In Vivo Studies
3.4. Quercetin
3.4.1. In Vitro Studies
3.4.2. In Vivo Studies
3.5. Resveratrol
3.5.1. In Vitro Studies
3.5.2. In Vivo Studies
3.6. Human Studies
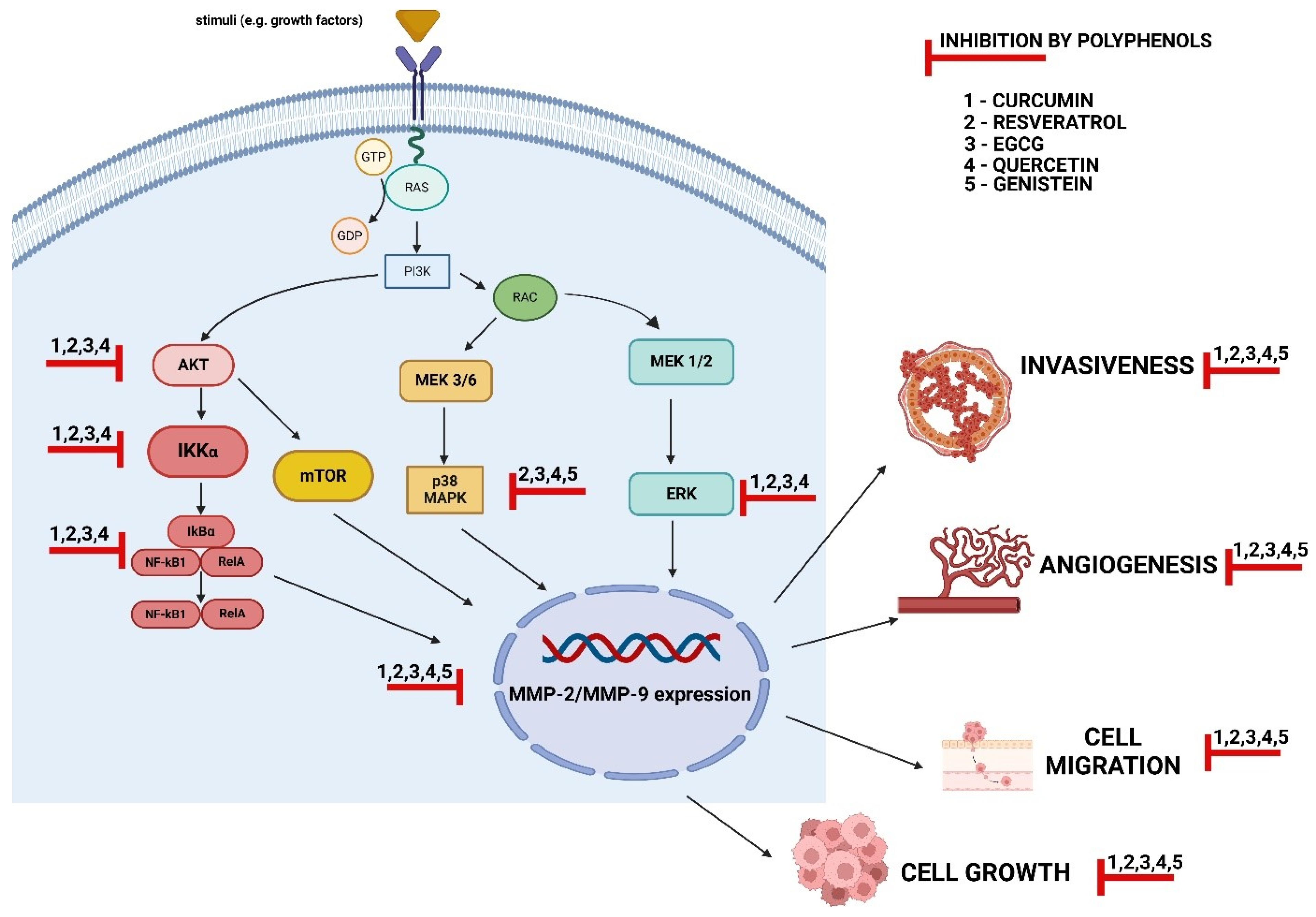
4. The Mechanisms of MMP-2 and MMP-9 Regulation via Polyphenols
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Hanna, N. Advances in systemic therapy for non-small cell lung cancer. BMJ 2021, 375, n2363. [Google Scholar] [CrossRef]
- Chojnacka, K.; Owczarek, K.; Caban, M.; Sosnowska, D.; Kajszczak, D.; Lewandowska, U. Chemopreventive effects of Japanese quince (Chaenomeles japonica L.) phenol leaf extract on colon cancer cells through the modulation of extracellular signal-regulated kinases/Akt signaling pathway. J. Physiol. Pharmacol. 2022, 73, 41–52. [Google Scholar]
- Liang, D.; Liu, L.; Zhao, Y.; Luo, Z.; He, Y.; Li, Y.; Tang, S.; Tang, J.; Chen, N. Targeting extracellular matrix through phytochemicals: A promising approach of multi-step actions on the treatment and prevention of cancer. Front. Pharmacol. 2023, 14, 1186712. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol. Lett. 2017, 14, 5865–5870. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, X.; Sun, S.; Zhang, X.; Yang, W.; Zhang, J.; Zhang, X.; Jiang, Z. Increased expression of MMP-2 and MMP-9 indicates poor prognosis in glioma recurrence. Biomed. Pharmacother. 2019, 118, 109369. [Google Scholar] [CrossRef]
- Chojnacka, K.; Lewandowska, U. Chemopreventive effects of polyphenol-rich extracts against cancer invasiveness and metastasis by inhibition of type IV collagenases expression and activity. J. Funct. Foods 2018, 46, 295–311. [Google Scholar] [CrossRef]
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin. Cancer Biol. 2019, 56, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Owczarek, K.; Lewandowska, U. The Role of Metalloproteinases and Their Tissue Inhibitors on Ocular Diseases: Focusing on Potential Mechanisms. Int. J. Mol. Sci. 2022, 23, 4256. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.N.M.; Zulkafali, N.I.N.; Ugusman, A. Modulation of Matrix Metalloproteinases by Plant-derived Products. Curr. Cancer Drug Targets 2021, 21, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Ang, H.L.; Mohan, C.D.; Shanmugam, M.K.; Leong, H.C.; Makvandi, P.; Rangappa, K.S.; Bishayee, A.; Kumar, A.P.; Sethi, G. Mechanism of epithelial-mesenchymal transition in cancer and its regulation by natural compounds. Med. Res. Rev. 2023, 43, 1141–1200. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Sarkar, N.; Bishayee, A.; Sinha, D. Dietary phytochemicals in the regulation of epithelial to mesenchymal transition and associated enzymes: A promising anticancer therapeutic approach. Semin. Cancer Biol. 2019, 56, 196–218. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Freitas, M.; Ribeiro, D.; Rufino, A.T.; Fernandes, E.; Ferreira de Oliveira, J.M.P. The role of flavonoids in the regulation of epithelial-mesenchymal transition in cancer: A review on targeting signaling pathways and metastasis. Med. Res. Rev. 2023, 43, 1878–1945. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Z.; Granato, D. Polyphenols in foods: Classification, methods of identification, and nutritional aspects in human health. Adv. Food Nutr. Res. 2021, 98, 1–33. [Google Scholar]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Burcher, J.T.; DeLiberto, L.K.; Allen, A.M.; Kilpatrick, K.L.; Bishayee, A. Bioactive phytocompounds for oral cancer prevention and treatment: A comprehensive and critical evaluation. Med. Res. Rev. 2023, 43, 2025–2085. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Lewandowska, U. Polyphenols and the potential mechanisms of their therapeutic benefits against inflammatory bowel diseases. J. Funct. Foods 2022, 95, 105181. [Google Scholar] [CrossRef]
- Caban, M.; Owczarek, K.; Chojnacka, K.; Podsędek, A.; Sosnowska, D.; Lewandowska, U. Chemopreventive properties of spent hops (Humulus lupulus L.) extract against angiogenesis, invasion and migration of colorectal cancer cells. J. Physiol. Pharmacol. 2022, 73, 431–442. [Google Scholar]
- Caban, M.; Owczarek, K.; Podsędek, A.; Sosnowska, D.; Lewandowska, U. Spent hops extract (Humulus lupulus L.) attenuates inflammation and angiogenesis of the retina via the nuclear factor-kappaB and protein kinase B/extracellular signal-regulated kinase pathways. J. Physiol. Pharmacol. 2023, 74, 537–549. [Google Scholar]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Bhatia, M.; Bhalerao, M.; Cruz-Martins, N.; Kumar, D. Curcumin and cancer biology: Focusing regulatory effects in different signalling pathways. Phytother. Res. 2021, 35, 4913–4929. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Daghestani, M.H. Curcumin effect on MMPs and TIMPs genes in a breast cancer cell line. Asian Pac. J. Cancer Prev. 2012, 13, 3259–3264. [Google Scholar] [CrossRef]
- Sun, J. Matrix metalloproteinases and tissue inhibitor of metalloproteinases are essential for the inflammatory response in cancer cells. J. Signal Transduct. 2010, 2010, 985132. [Google Scholar] [CrossRef]
- Lin, H.J.; Su, C.C.; Lu, H.F.; Yang, J.S.; Hsu, S.C.; Ip, S.W.; Wu, J.J.; Li, Y.C.; Ho, C.C.; Wu, C.C.; et al. Curcumin blocks migration and invasion of mouse-rat hybrid retina ganglion cells (N18) through the inhibition of MMP-2, -9, FAK, Rho A and Rock-1 gene expression. Oncol. Rep. 2010, 23, 665–670. [Google Scholar] [PubMed]
- Sirohi, V.K.; Popli, P.; Sankhwar, P.; Kaushal, J.B.; Gupta, K.; Manohar, M.; Dwivedi, A. Curcumin exhibits anti-tumor effect and attenuates cellular migration via Slit-2 mediated down-regulation of SDF-1 and CXCR4 in endometrial adenocarcinoma cells. J. Nutr. Biochem. 2017, 44, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Su, J.; Zhao, J.; Chen, J.; Cui, X.; Sun, M.; Zhang, X. Curcumin inhibits invasion and metastasis of human hepatoma cells through Bclaf1-mediated Wnt/β-catenin signalling. Food Agric. Immunol. 2022, 33, 664–676. [Google Scholar] [CrossRef]
- Jia, W.; Deng, F.; Fu, W.; Hu, J.; Chen, G.; Gao, X.; Tan, X.; Li, G.; Liu, G.; Zhu, S. Curcumin suppresses wilms’ tumor metastasis by inhibiting RECK methylation. Biomed. Pharmacother. 2019, 111, 1204–1212. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zhang, Y.; Cheng, Y.N.; Gong, F.L.; Cao, Z.Q.; Yu, L.G.; Guo, X.L. Metformin incombination with curcumin inhibits the growth, metastasis, and angiogenesis of hepatocellular carcinoma in vitro and in vivo. Mol. Carcinog. 2018, 57, 44–56. [Google Scholar] [CrossRef]
- Wang, P.; Hao, X.; Li, X.; Yan, Y.; Tian, W.; Xiao, L.; Wang, Z.; Dong, J. Curcumin inhibits adverse psychological stress-induced proliferation and invasion of glioma cells via down-regulating the ERK/MAPK pathway. J. Cell. Mol. Med. 2021, 25, 7190–7203. [Google Scholar] [CrossRef]
- Zhu, G.; Shen, Q.; Jiang, H.; Ji, O.; Zhu, L.; Zhang, L. Curcumin inhibited the growth and invasion of human monocytic leukaemia SHI-1 cells in vivo by altering MAPK and MMP signalling. Pharm. Biol. 2020, 58, 25–34. [Google Scholar] [CrossRef]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Karthika, C.; et al. Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef]
- Tavakoli, F.; Jahanban-Esfahlan, R.; Seidi, K.; Jabbari, M.; Behzadi, R.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Effects of nano-encapsulated curcumin-chrysin on telomerase, MMPs and TIMPs gene expression in mouse B16F10 melanoma tumour model. Artif. Cells Nanomed. Biotechnol. 2018, 46, 75–86. [Google Scholar] [CrossRef]
- Sesarman, A.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Patras, L.; Porav, S.; Banciu, M.; Porfire, A. Co-delivery of curcumin and doxorubicin in PEGylated liposomes favored the antineoplastic C26 murine colon carcinoma microenvironment. Drug Deliv. Transl. Res. 2019, 9, 260–272. [Google Scholar] [CrossRef]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, C.; Yan, P.; Zhang, M.; Wang, Y.; Hu, Y.; Wu, X.; Wang, X.; Sheng, J. EGCG Reduces Obesity and White Adipose Tissue Gain Partly Through AMPK Activation in Mice. Front. Pharmacol. 2018, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Benelli, R.; Venè, R.; Bisacchi, D.; Garbisa, S.; Albini, A. Anti-invasive effects of green tea polyphenol epigallocatechin-3-gallate (EGCG), a natural inhibitor of metallo and serine proteases. Biol Chem. 2002, 383, 101–105. [Google Scholar] [CrossRef]
- Vayalil, P.K.; Katiyar, S.K. Treatment of epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and -9 via inhibition of activation of mitogen-activated protein kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145 cells. Prostate 2004, 59, 33–42. [Google Scholar] [CrossRef]
- Huang, Y.J.; Wang, K.L.; Chen, H.Y.; Chiang, Y.F.; Hsia, S.M. Protective Effects of Epigallocatechin Gallate (EGCG) on Endometrial, Breast, and Ovarian Cancers. Biomolecules 2020, 10, 1481. [Google Scholar] [CrossRef]
- Roomi, M.W.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Modulation of MMP-2 and -9 secretion by cytokines, inducers and inhibitors in human melanoma A-2058 cells. Oncol. Rep. 2017, 37, 3681–3687. [Google Scholar] [CrossRef]
- Roomi, M.W.; Bhanap, B.; Niedzwiecki, A.; Rath, M. In vitro modulation of MMP-2 and MMP-9 secretion by cytokines, inducers, and inhibitors in head and neck squamous carcinoma cells (FaDu) and tongue carcinoma cells (SCC-25). J. Otolaryngol. Rhinol. 2017, 3, 29. [Google Scholar] [CrossRef]
- Roomi, M.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. In vitro modulation of MMP-2 and MMP-9 in pediatric human sarcoma cell lines by cytokines, inducers and inhibitors. Int. J. Oncol. 2014, 44, 27–34. [Google Scholar] [CrossRef]
- Roomi, M.W.; Kalinovsky, T.; Monterrey, J.; Rath, M.; Niedzwiecki, A. In vitro modulation of MMP-2 and MMP-9 in adult human sarcoma cell lines by cytokines, inducers and inhibitors. Int. J. Oncol. 2013, 43, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. In vitro modulation of MMP-2 and MMP-9 in human cervical and ovarian cancer cell lines by cytokines, inducers and inhibitors. Oncol. Rep. 2010, 23, 605–614. [Google Scholar] [PubMed]
- Roomi, M.W.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Modulation of human renal cell carcinoma 786-0 MMP-2 and MMP-9 activity by inhibitors and inducers in vitro. Med. Oncol. 2006, 23, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.C.; Huang, C.C.; Lu, Y.T.; Yeh, C.M.; Ho, Y.T.; Yang, S.F.; Hsin, C.H.; Lin, C.W. Epigallocatechin-3-gallate inhibits migration of human nasopharyngeal carcinoma cells by repressing MMP-2 expression. J. Cell. Physiol. 2019, 234, 20915–20924. [Google Scholar] [CrossRef]
- Chen, S.J.; Yao, X.D.; Peng, B.O.; Xu, Y.F.; Wang, G.C.; Huang, J.; Liu, M.; Zheng, J.H. Epigallocatechin-3-gallate inhibits migration and invasion of human renal carcinoma cells by downregulating matrix metalloproteinase-2 and matrix metalloproteinase-9. Exp. Ther. Med. 2016, 11, 1243–1248. [Google Scholar] [CrossRef]
- Kwak, T.W.; Park, S.B.; Kim, H.J.; Jeong, Y.I.; Kang, D.H. Anticancer activities of epigallocatechin-3-gallate against cholangiocarcinoma cells. Onco Targets Ther. 2016, 10, 137–144. [Google Scholar] [CrossRef]
- Luo, K.W.; Chen, W.; Lung, W.Y.; Wei, X.Y.; Cheng, B.H.; Cai, Z.M.; Huang, W.R. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Tu, G.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Enhanced Chemotherapeutic Efficacy of PLGA-Encapsulated Epigallocatechin Gallate (EGCG) Against Human Lung Cancer. Int. J. Nanomed. 2020, 15, 4417–4429. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, J.; Gan, R.; Wu, Z.; Luo, H.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Synergistic inhibition of lung cancer cells by EGCG and NF-κB inhibitor BAY11-7082. J. Cancer 2019, 10, 6543–6556. [Google Scholar] [CrossRef]
- Deng, Y.T.; Lin, J.K. EGCG inhibits the invasion of highly invasive CL1-5 lung cancer cells through suppressing MMP-2 expression via JNK signaling and induces G2/M arrest. J. Agric. Food Chem. 2011, 59, 13318–13327. [Google Scholar] [CrossRef]
- Fang, C.Y.; Wu, C.C.; Hsu, H.Y.; Chuang, H.Y.; Huang, S.Y.; Tsai, C.H.; Chang, Y.; Tsao, G.S.; Chen, C.L.; Chen, J.Y. EGCG inhibits proliferation, invasiveness and tumor growth by up-regulation of adhesion molecules, suppression of gelatinases activity, and induction of apoptosis in nasopharyngeal carcinoma cells. Int. J. Mol. Sci. 2015, 16, 2530–2558. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.N.; Chu, S.C.; Kuo, W.H.; Chou, M.Y.; Lin, J.K.; Hsieh, Y.S. Epigallocatechin-3 gallate inhibits invasion, epithelial-mesenchymal transition, and tumor growth in oral cancer cells. J. Agric. Food Chem. 2011, 59, 3836–3844. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Marsh, L.; Srivastava, R.K. EGCG inhibits growth of human pancreatic tumors orthotopically implanted in Balb C nude mice through modulation of FKHRL1/FOXO3a and neuropilin. Mol. Cell. Biochem. 2013, 372, 83–94. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Russo, G.L.; Daglia, M.; Kasi, P.D.; Ravi, S.; Nabavi, S.F.; Nabavi, S.M. Understanding genistein in cancer: The “good” and the “bad” effects: A review. Food Chem. 2016, 196, 589–600. [Google Scholar] [CrossRef]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef]
- Shafiee, G.; Saidijam, M.; Tayebinia, H.; Khodadadi, I. Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch. Physiol. Biochem. 2022, 128, 694–702. [Google Scholar] [CrossRef]
- Shafiee, G.; Saidijam, M.; Tavilani, H.; Ghasemkhani, N.; Khodadadi, I. Genistein Induces Apoptosis and Inhibits Proliferation of HT29 Colon Cancer Cells. Int. J. Mol. Cell. Med. 2016, 5, 178–191. [Google Scholar]
- Zhu, J.; Ren, J.; Tang, L. Genistein inhibits invasion and migration of colon cancer cells by recovering WIF1 expression. Mol. Med. Rep. 2018, 17, 7265–7273. [Google Scholar] [CrossRef]
- Hussain, A.; Harish, G.; Prabhu, S.A.; Mohsin, J.; Khan, M.A.; Rizvi, T.A.; Sharma, C. Inhibitory effect of genistein on the invasive potential of human cervical cancer cells via modulation of matrix metalloproteinase-9 and tissue inhibitors of matrix metalloproteinase-1 expression. Cancer Epidemiol. 2012, 36, e387–e393. [Google Scholar] [CrossRef]
- Bi, Y.L.; Min, M.; Shen, W.; Liu, Y. Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of STAT3 signalling pathway. Phytomedicine 2018, 39, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Spoerlein, C.; Mahal, K.; Schmidt, H.; Schobert, R. Effects of chrysin, apigenin, genistein and their homoleptic copper(II) complexes on the growth and metastatic potential of cancer cells. J. Inorg. Biochem. 2013, 127, 107–115. [Google Scholar] [CrossRef]
- Noori, D.M.; Saffari, M.; Saydi, D.O.; Rahmani, B.; Noori, D.A.; Salehi, A.; Ghazarian, A. Study of antimetastatic effect of genistein through inhibition of expression of matrix metalloproteinase in A-549 cell line. J. Sci. Islam. Repub. Iran 2012, 23, 115–122. [Google Scholar]
- Han, L.; Zhang, H.W.; Zhou, W.P.; Chen, G.M.; Guo, K.J. The effects of genistein on transforming growth factor-β1-induced invasion and metastasis in human pancreatic cancer cell line Panc-1 in vitro. Chin. Med. J. 2012, 125, 2032–2040. [Google Scholar]
- Xiao, X.; Liu, Z.; Wang, R.; Wang, J.; Zhang, S.; Cai, X.; Wu, K.; Bergan, R.C.; Xu, L.; Fan, D. Genistein suppresses FLT4 and inhibits human colorectal cancer metastasis. Oncotarget 2015, 6, 3225–3239. [Google Scholar] [CrossRef]
- Chen, P.; Hu, M.D.; Deng, X.F.; Li, B. Genistein reinforces the inhibitory effect of Cisplatin on liver cancer recurrence and metastasis after curative hepatectomy. Asian Pac. J. Cancer Prev. 2013, 14, 759–764. [Google Scholar] [CrossRef]
- Kidani, T.; Nakamura, A.; Kamei, S.; Norimatsu, Y.; Miura, H.; Masuno, H. Overexpression of cytoplasmic β-catenin inhibits the metastasis of the murine osteosarcoma cell line LM8. Cancer Cell Int. 2014, 14, 31. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Quercetin and its role in biological functions: An updated review. EXCLI J. 2018, 17, 856–863. [Google Scholar]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Ezhilarasan, D.; Raghunandhakumar, S. Quercetin Inhibits the Epithelial to Mesenchymal Transition through Suppressing Akt Mediated Nuclear Translocation of β-Catenin in Lung Cancer Cell Line. Nutr Cancer 2022, 74, 1894–1906. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Chan, S.T.; Chang, C.N.; Yu, P.S.; Chuang, C.H.; Yeh, S.L. Quercetin and chrysin inhibit nickel-induced invasion and migration by downregulation of TLR4/NF-κB signaling in A549 cells. Chem. Biol. Interact. 2018, 292, 101–109. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z.G.; Lin, Y.; Qu, X.G.; Lv, W.; Wang, G.B.; Li, C.L. Effects of quercetin on proliferation and migration of human glioblastoma U251 cells. Biomed. Pharmacother. 2017, 92, 33–38. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z.G.; Yang, J.Q.; Zhou, Y.; Meng, L.H.; Wang, H.; Li, C.L. Low concentration of quercetin antagonizes the invasion and angiogenesis of human glioblastoma U251 cells. Onco. Targets Ther. 2017, 10, 4023–4028. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; Azevedo, J.; De Freitas, V.; Mateus, N.; Calhau, C. Flavonoid transport across RBE4 cells: A blood-brain barrier model. Cell Mol Biol Lett. 2010, 15, 234–241. [Google Scholar] [CrossRef]
- Zhao, J.; Fang, Z.; Zha, Z.; Sun, Q.; Wang, H.; Sun, M.; Qiao, B. Quercetin inhibits cell viability, migration and invasion by regulating miR-16/HOXA10 axis in oral cancer. Eur. J. Pharmacol. 2019, 847, 11–18. [Google Scholar] [CrossRef]
- Li, S.; Pei, Y.; Wang, W.; Liu, F.; Zheng, K.; Zhang, X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Biomed. Pharmacother. 2019, 114, 108839. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.L.; Xu, Q.Q.; Cheng, D.; Liu, K.D.; Sun, Z.Q. Quercetin inhibits invasion and angiogenesis of esophageal cancer cells. Pathol. Res. Pract. 2021, 222, 153455. [Google Scholar] [CrossRef]
- Li, H.; Chen, C. Quercetin Has Antimetastatic Effects on Gastric Cancer Cells via the Interruption of uPA/uPAR Function by Modulating NF-κb, PKC-δ, ERK1/2, and AMPKα. Integr. Cancer Ther. 2018, 17, 511–523. [Google Scholar] [CrossRef]
- Yu, D.; Ye, T.; Xiang, Y.; Shi, Z.; Zhang, J.; Lou, B.; Zhang, F.; Chen, B.; Zhou, M. Quercetin inhibits epithelial-mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial-mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells. Onco Targets Ther. 2017, 10, 4719–4729. [Google Scholar] [CrossRef] [PubMed]
- Dhanaraj, T.; Mohan, M.; Arunakaran, J. Quercetin attenuates metastatic ability of human metastatic ovarian cancer cells via modulating multiple signaling molecules involved in cell survival, proliferation, migration and adhesion. Arch. Biochem. Biophys. 2021, 701, 108795. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, D.; Zheng, X.; Huang, B.; Xia, X.; Pan, X. Quercetin exerts bidirectional regulation effects on the efficacy of tamoxifen in estrogen receptor-positive breast cancer therapy: An in vitro study. Environ. Toxicol. 2020, 35, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Iaffaioli, R.V.; Armenia, E.; Clemente, O.; Barbarisi, M.; Nasti, G.; Berretta, M.; Ottaiano, A.; Barbarisi, A. Hyaluronic Acid Nanohydrogel Loaded With Quercetin Alone or in Combination to a Macrolide Derivative of Rapamycin RAD001 (Everolimus) as a New Treatment for Hormone-Responsive Human Breast Cancer. J. Cell. Physiol. 2017, 232, 2063–2074. [Google Scholar] [CrossRef]
- Roshanazadeh, M.; Babaahmadi Rezaei, H.; Rashidi, M. Quercetin Enhances the Suppressive Effects of Doxorubicin on the Migration of MDA-MB-231 Breast Cancer Cell Line. Int. J. Cancer Manag. 2021, 14, e119049. [Google Scholar] [CrossRef]
- Ozkan, E.; Bakar-Ates, F. Potentiation of the Effect of Lonidamine by Quercetin in MCF-7 human breast cancer cells through downregulation of MMP-2/9 mRNA Expression. An. Acad. Bras. Cienc. 2020, 92, e20200548. [Google Scholar] [CrossRef]
- Tang, H.; Kuang, Y.; Wu, W.; Peng, B.; Fu, Q. Quercetin inhibits the metabolism of arachidonic acid by inhibiting the activity of CYP3A4, thereby inhibiting the progression of breast cancer. Mol Med. 2023, 29, 127. [Google Scholar] [CrossRef]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Bhat, F.A.; Raja Singh, P.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016, 49, 678–697. [Google Scholar] [CrossRef]
- Chang, J.H.; Lai, S.L.; Chen, W.S.; Hung, W.Y.; Chow, J.M.; Hsiao, M.; Lee, W.J.; Chien, M.H. Quercetin suppresses the metastatic ability of lung cancer through inhibiting Snail-dependent Akt activation and Snail-independent ADAM9 expression pathways. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1746–1758. [Google Scholar] [CrossRef] [PubMed]
- Kee, J.Y.; Han, Y.H.; Kim, D.S.; Mun, J.G.; Park, J.; Jeong, M.Y.; Um, J.Y.; Hong, S.H. Inhibitory effect of quercetin on colorectal lung metastasis through inducing apoptosis, and suppression of metastatic ability. Phytomedicine 2016, 23, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhou, T.; Xiong, J.; Zhang, Z.; Tian, S.; Wang, Y.; Chen, J.; Tian, X. Quercetin, the Ingredient of Xihuang Pills, Inhibits Hepatocellular Carcinoma by Regulating Autophagy and Macrophage Polarization. Front. Biosci. (Landmark Ed.) 2022, 27, 323. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Hong, S.C.; Chang, C.M.; Chen, Y.H.; Liao, P.C.; Huang, C.Y. Oral Squamous Cell Carcinoma Cells with Acquired Resistance to Erlotinib Are Sensitive to Anti-Cancer Effect of Quercetin via Pyruvate Kinase M2 (PKM2). Cells 2023, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.H.; Tse, A.K.; Kwan, H.Y.; Yu, H.; Cheng, C.Y.; Su, T.; Fong, W.F.; Yu, Z.L. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem. Pharmacol. 2014, 87, 424–434. [Google Scholar] [CrossRef]
- Lan, H.; Hong, W.; Fan, P.; Qian, D.; Zhu, J.; Bai, B. Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma Cells. Cell. Physiol. Biochem. 2017, 43, 553–567. [Google Scholar] [CrossRef]
- Pradhan, S.J.; Mishra, R.; Sharma, P.; Kundu, G.C. Quercetin and sulforaphane in combination suppress the progression of melanoma through the down-regulation of matrix metalloproteinase-9. Exp. Ther. Med. 2010, 1, 915–920. [Google Scholar] [CrossRef]
- Vervandier-Fasseur, D.; Latruffe, N. The Potential Use of Resveratrol for Cancer Prevention. Molecules 2019, 24, 4506. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Najafiyan, B.; Bokaii Hosseini, Z.; Esmaelian, S.; Firuzpour, F.; Rahimipour Anaraki, S.; Kalantari, L.; Hheidari, A.; Mesgari, H.; Nabi-Afjadi, M. Unveiling the potential effects of resveratrol in lung cancer treatment: Mechanisms and nanoparticle-based drug delivery strategies. Biomed. Pharmacother. 2024, 172, 116207. [Google Scholar] [CrossRef]
- Xiong, W.; Yin, A.; Mao, X.; Zhang, W.; Huang, H.; Zhang, X. Resveratrol suppresses human glioblastoma cell migration and invasion via activation of RhoA/ROCK signaling pathway. Oncol. Lett. 2016, 11, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.Y.; Hsieh, Y.H.; Yang, S.F.; Chen, C.T.; Tang, C.H.; Chou, M.Y.; Chuang, Y.T.; Lin, C.W.; Chen, M.K. Resveratrol suppresses TPA-induced matrix metalloproteinase-9 expression through the inhibition of MAPK pathways in oral cancer cells. J. Oral Pathol. Med. 2015, 44, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Tsai, C.W.; Yang, J.S.; Hsu, Y.M.; Shih, L.C.; Chiu, H.Y.; Bau, D.T.; Tsai, F.J. Resveratrol inhibited the metastatic behaviors of cisplatin-resistant human oral cancer cells via phosphorylation of ERK/p-38 and suppression of MMP-2/9. J. Food Biochem. 2021, 45, e13666. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, G.; Di Prima, G.; Belfiore, E.; Campisi, G.; De Caro, V. Chemopreventive and Anticancer Role of Resveratrol against Oral Squamous Cell Carcinoma. Pharmaceutics 2023, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sull, J.W.; Sung, H.J. Suppressing effect of resveratrol on the migration and invasion of human metastatic lung and cervical cancer cells. Mol. Biol. Rep. 2012, 39, 8709–8716. [Google Scholar] [CrossRef]
- Park, S.Y.; Chae, S.Y.; Park, J.O.; Lee, K.J.; Park, G. Gold-conjugated resveratrol nanoparticles attenuate the invasion and MMP-9 and COX-2 expression in breast cancer cells. Oncol. Rep. 2016, 35, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Q.M.; Lu, Y.Y.; Zhang, H.; Chen, Q.L.; Zhao, M.; Su, S.B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-β1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef]
- Suh, J.; Kim, D.H.; Surh, Y.J. Resveratrol suppresses migration, invasion and stemness of human breast cancer cells by interfering with tumor-stromal cross-talk. Arch. Biochem. Biophys. 2018, 643, 62–71. [Google Scholar] [CrossRef]
- Shin, H.J.; Han, J.M.; Choi, Y.S.; Jung, H.J. Pterostilbene Suppresses both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules 2020, 25, 228. [Google Scholar] [CrossRef]
- Buhrmann, C.; Shayan, P.; Goel, A.; Shakibaei, M. Resveratrol Regulates Colorectal Cancer Cell Invasion by Modulation of Focal Adhesion Molecules. Nutrients 2017, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Resveratrol Chemosensitizes TNF-β-Induced Survival of 5-FU-Treated Colorectal Cancer Cells. Nutrients 2018, 10, 888. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, J.; Ma, Q.; Li, B.; Han, L.; Liu, J.; Xu, Q.; Duan, W.; Yu, S.; Wang, F.; et al. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-κB pathway. Curr. Med. Chem. 2013, 20, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, L.; Chen, X.; Lei, J.; Ma, Q. Resveratrol inhibits hypoxia-driven ROS-induced invasive and migratory ability of pancreatic cancer cells via suppression of the Hedgehog signaling pathway. Oncol. Rep. 2016, 35, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yang, M.; Qu, Z.; Zhou, J.; Zhang, Q. Resveratrol enhances anticancer effects of paclitaxel in HepG2 human liver cancer cells. BMC Complement. Altern. Med. 2017, 17, 477. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Kunnumakkara, A.B.; Sethi, G.; Diagaradjane, P.; Anand, P.; Pandey, M.K.; Gelovani, J.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer 2010, 127, 257–268. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, H.; Zeng, X.; Ye, D.; Liu, J. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed. Pharmacother. 2018, 98, 36–44. [Google Scholar] [CrossRef]
- Dai, L.; Chen, L.; Wang, W.; Lin, P. Resveratrol inhibits ACHN cells via regulation of histone acetylation. Pharm. Biol. 2020, 58, 231–238. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, H.; Zhang, G.; Hu, L.; Lei, Y.; Qin, Y.; Yang, Y.; Wang, Q.; Li, R.; Mao, Q. Inhibitory effects of resveratrol on the adhesion, migration and invasion of human bladder cancer cells. Mol. Med. Rep. 2017, 15, 885–889. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Shi, J.; Feng, Y.; Yu, M.; Sun, Y.; Zhuang, Q.; Liang, B.; Luo, G.; Xu, X.; et al. Resveratrol inhibits the tumor migration and invasion by upregulating TET1 and reducing TIMP2/3 methylation in prostate carcinoma cells. Prostate 2020, 80, 977–985. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, M.; Luo, T.; Huang, H. Resveratrol raises in vitro anticancer effects of lentinan in SW579 human thyroid squamous cell carcinoma. Int. J. Clin. Exp. Med. 2016, 9, 20777–20789. [Google Scholar]
- Xie, D.; Zheng, G.Z.; Xie, P.; Zhang, Q.H.; Lin, F.X.; Chang, B.; Hu, Q.X.; Du, S.X.; Li, X.D. Antitumor activity of resveratrol against human osteosarcoma cells: A key role of Cx43 and Wnt/β-catenin signaling pathway. Oncotarget 2017, 8, 111419–111432. [Google Scholar] [CrossRef] [PubMed]
- Gweon, E.J.; Kim, S.J. Resveratrol attenuates matrix metalloproteinase-9 and -2-regulated differentiation of HTB94 chondrosarcoma cells through the p38 kinase and JNK pathways. Oncol. Rep. 2014, 32, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gweon, E.J.; Kim, S.J. Resveratrol induces MMP-9 and cell migration via the p38 kinase and PI-3K pathways in HT1080 human fibrosarcoma cells. Oncol Rep. 2013, 29, 826–834. [Google Scholar] [CrossRef]
- Lee, H.S.; Ha, A.W.; Kim, W.K. Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutr. Res. Pract. 2012, 6, 294–300. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Xu, Y.; Peng, W.; Zhang, S.; Li, R.; Zhang, H.; Zhang, H.; Cheng, S.; Wang, Y.; et al. Resveratrol-Loaded TPGS-Resveratrol-Solid Lipid Nanoparticles for Multidrug-Resistant Therapy of Breast Cancer: In Vivo and In Vitro Study. Front. Bioeng. Biotechnol. 2021, 9, 762489. [Google Scholar] [CrossRef]
- Pradhan, R.; Chatterjee, S.; Hembram, K.C.; Sethy, C.; Mandal, M.; Kundu, C.N. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J. Nutr. Biochem. 2021, 92, 108624. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, J.; Zhou, J.; Zhu, M.; Wang, L.; Yan, L. Resveratrol inhibits Interleukin-6 induced invasion of human gastric cancer cells. Biomed. Pharmacother. 2018, 99, 766–773. [Google Scholar] [CrossRef]
- Ji, Q.; Liu, X.; Han, Z.; Zhou, L.; Sui, H.; Yan, L.; Jiang, H.; Ren, J.; Cai, J.; Li, Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/S expression. BMC Cancer 2015, 15, 97. [Google Scholar] [CrossRef]
- Wang, G.; Dai, F.; Yu, K.; Jia, Z.; Zhang, A.; Huang, Q.; Kang, C.; Jiang, H.; Pu, P. Resveratrol inhibits glioma cell growth via targeting oncogenic microRNAs and multiple signaling pathways. Int. J. Oncol. 2015, 46, 1739–1747. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, H.; Liu, Y.; Guo, A.; Xu, X.; Qu, X.; Wang, S.; Zhao, J.; Li, Y.; Cao, Y. Resveratrol Inhibits the Invasion of Glioblastoma-Initiating Cells via Down-Regulation of the PI3K/Akt/NF-κB Signaling Pathway. Nutrients 2015, 7, 4383–4402. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Lee, W.J.; Tan, P.; Tang, C.H.; Hsiao, M.; Hsieh, F.K.; Chien, M.H. Upregulation of miR-328 and inhibition of CREB-DNA-binding activity are critical for resveratrol-mediated suppression of matrix metalloproteinase-2 and subsequent metastatic ability in human osteosarcomas. Oncotarget 2015, 6, 2736–2753. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, A.H.; Fyia, A.A.; Ali, M.M.; Soliman, S.M. Anticancer properties of resveratrol on chemically induced hepatocellular carcinoma in rats: Inhibition of metastasis and angiogenesis. J. Chem. Pharm. Res. 2015, 7, 913–921. [Google Scholar]
- Ganapathy, S.; Chen, Q.; Singh, K.P.; Shankar, S.; Srivastava, R.K. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS ONE 2010, 5, 15627. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.W.; Lung, W.Y.; Luo, X.L.; Huang, W.R. EGCG inhibited bladder cancer T24 and 5637 cell proliferation and migration via PI3K/AKT pathway. Oncotarget 2018, 9, 12261–12272. [Google Scholar] [CrossRef]
- He, Q.; Liu, C.; Wang, X.; Rong, K.; Zhu, M.; Duan, L.; Zheng, P.; Mi, Y. Exploring the mechanism of curcumin in the treatment of colon cancer based on network pharmacology and molecular docking. Front. Pharmacol. 2023, 14, 1102581. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Zhao, S.; Wang, H. IL-17A/IL-17RA promotes invasion and activates MMP-2 and MMP-9 expression via p38 MAPK signaling pathway in non-small cell lung cancer. Mol. Cell. Biochem. 2019, 455, 195–206. [Google Scholar] [CrossRef]
- Lee, G.H.; Jin, S.W.; Kim, S.J.; Pham, T.H.; Choi, J.H.; Jeong, H.G. Tetrabromobisphenol A Induces MMP-9 Expression via NADPH Oxidase and the activation of ROS, MAPK, and Akt Pathways in Human Breast Cancer MCF-7 Cells. Toxicol. Res. 2019, 35, 93–101. [Google Scholar] [CrossRef]
- Rasheduzzaman, M.; Jeong, J.K.; Park, S.Y. Resveratrol sensitizes lung cancer cell to TRAIL by p53 independent and suppression of Akt/NF-κB signaling. Life Sci. 2018, 208, 208–220. [Google Scholar] [CrossRef]
- Biswas, P.; Dey, D.; Biswas, P.K.; Rahaman, T.I.; Saha, S.; Parvez, A.; Khan, D.A.; Lily, N.J.; Saha, K.; Sohel, M.; et al. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int. J. Mol. Sci. 2022, 23, 11746. [Google Scholar] [CrossRef]
- Neamtu, A.A.; Maghiar, T.A.; Alaya, A.; Olah, N.K.; Turcus, V.; Pelea, D.; Totolici, B.D.; Neamtu, C.; Maghiar, A.M.; Mathe, E. A Comprehensive View on the Quercetin Impact on Colorectal Cancer. Molecules 2022, 27, 1873. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Lewandowska, U. Encapsulation of Polyphenolic Compounds Based on Hemicelluloses to Enhance Treatment of Inflammatory Bowel Diseases and Colorectal Cancer. Molecules 2023, 28, 4189. [Google Scholar] [CrossRef] [PubMed]
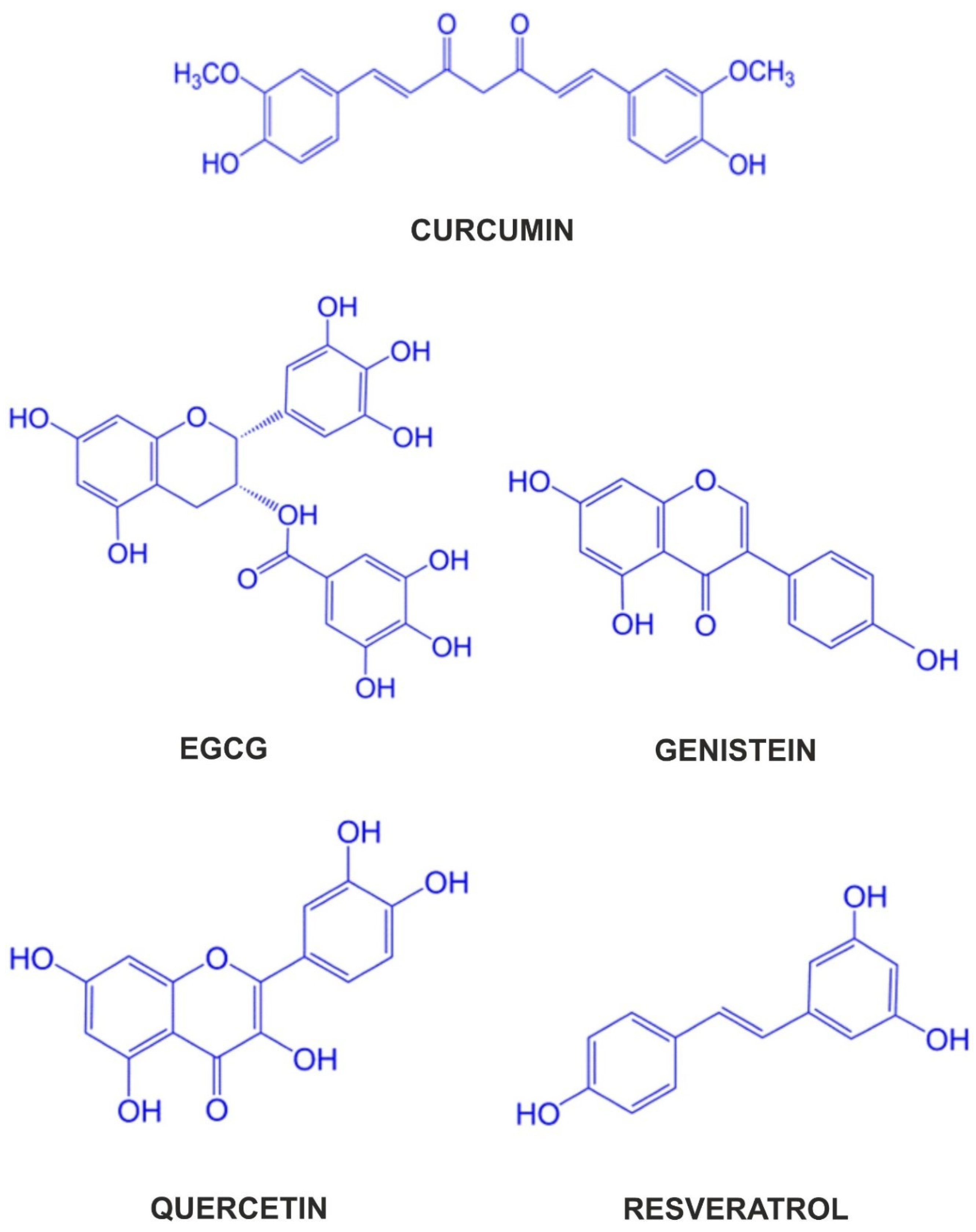
| Polyphenol | Class of Compounds | Main Pro-Health Activities | Main Food Sources of Polyphenol |
|---|---|---|---|
| Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) | Curcuminoids | Anti-cancer, anti-inflammatory, anti-migratory, anti-metastatic, anti-aging | The rhizome of Curcuma longa |
| EGCG (2 R,3 R)-3′,4′,5,5′,7-pentahydroxyflavan-3-yl 3,4,5-trihydroxybenzoate) | Flavonoids: flavan-3-ols | Anti-oxidative, anti-cancer, anti-inflammatory | Green tea |
| Genistein (4′,5,7-trihydroxyisoflavone) | Flavonoids: isoflavones | Anti-cancer, anti-oxidant, anti-inflammatory, anti-angiogenic, proapoptotic, neuroprotective | Fermented soya (miso and natto), soy nuts, soy powder, soy milk, tofu |
| Quercetin (3,3′,4′,5,7-pentahydroxyflavone) | Flavonoids: flavonols | Anti-proliferative, anti-inflammatory, anti-carcinogenic, anti-oxidant, anti-bacterial, anti-viral | Onions, capers, green tea, apples, broccoli, red leaf lettuce, cherries, ginkgo, American elderberry, hypericum |
| Resveratrol (3,5,4′-trihydroxystilbene) | Stilbenes | Cardioprotective, anti-oxidative, anti-inflammatory, neuroprotective, anti-diabetic, anti-cancer | Grapes, red wine, chocolate, berries, mulberries, peanuts |
| Polyphenol | Cell Line | Concentration/ Duration | Anti-Cancer Effects | Type IV Collagenase-Mediated Mechanisms | Reference |
|---|---|---|---|---|---|
| Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] | N18 | 7,5, 15 μM for 6, 12, 24, 48 h | ↓cell migration ↓cell invasion | ↓MMP-2 mRNA, protein ↓MMP-9 protein ↓NF-κB p65 ↓ERK-1/-2 | [31] |
| MDA-MB-231 | 10, 20, 40 μM for 24, 48, 72 h | not specified | ↓MMP-2, -9 mRNA ↑TIMP-1, -2, -3, -4 mRNA | [29] | |
| Ishikawa Hec-1 B Primary endometrial adenocarcinoma cells | 6 μM for 24, 30, 36, 42, 48 h | ↓proliferation ↓cell migration | ↓MMP-2 protein ↓MMP-9 protein | [32] | |
| HepG2 PLC/PRF/5 | 2,5, 5, 10 μM for 12, 24, 48 h with or without 2,5, 5, 10 mM metformin | ↓angiogenesis ↓cell migration ↓cell invasion ↓proliferation ↑apoptosis | ↓MMP-2, -9 protein, activity ↓PI3 K protein ↓pAkt protein ↓pmTOR protein | [35] | |
| WT Primary cells WT-1, WT-2, WT-3 | 10, 20 mM for 6, 12, 18, 24 h | ↓cell migration ↓cell invasion ↓proliferation ↑apoptosis | ↓MMP-2, -9 mRNA, protein | [34] | |
| LN229 U87 MG | 8, 16, 24 μM for 24, 48, 72 h with or without 0,1, 1, 10 μM of norepinephrine | ↓cell migration ↓cell invasion ↓proliferation ↑apoptosis | ↓MMP-2, -9 mRNA, protein, activity ↓pERK-1/-2 protein | [36] | |
| HepG2 SK-Hep-1 | 20, 40, 60 μM for 24 h | ↓cell migration ↓cell invasion ↓proliferation | ↓MMP-2, -9 protein | [33] |
| Polyphenol | Animal Model | Dose/ Duration | Anti-Tumor Effects | Type IV Collagenase-Mediated Mechanisms | Reference |
|---|---|---|---|---|---|
| Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] | NOD-SCID mice injected with Ishikawa | 50 mg/kg daily intraperitoneally for 31 days | ↓tumor volume | ↓MMP-2 protein ↓MMP-9 protein | [32] |
| Balb/c mice injected with C26 | 5 mg/kg curcumin with or without 2.5 mg/kg doxorubicin (in free form or long-circulating liposomes, LCLs) intravenously at days 7 and 10 after inoculation | ↓tumor volume | ↔MMP-2 activity ↔MMP-9 activity (free curcumin) ↓MMP-9 activity (LCL–curcumin, curcumin with doxorubicin) ↓pNF-κB p65 protein | [40] | |
| C57 B16 mice injected with B16 F10 | 15 mg/kg of free curcumin or 30 mg/kg of nano-encapsulated curcumin daily intraperitoneally for 12 days (with or without identical doses of chrysin) | ↓tumor volume | ↓MMP-2, -9 mRNA ↑TIMP-1, -2 mRNA | [39] | |
| Balb/c-nu mice injected with HepG2 | 60 mg/kg daily intraperitoneally for 21 days with or without 150 mg/kg metformin orally | ↓tumor volume | ↓MMP-2, -9 protein ↓PI3 K protein ↓pAkt protein ↓pmTOR protein | [35] | |
| Nude mice injected with primary WT-1 and WT-3 | 20 mg/mL of curcumin in corn oil for 21 days | ↓tumor weight ↓tumor volume ↑apoptosis | not specified | [34] | |
| SCID mice injected with SHI-1 | 15, 30 mg/kg daily intraperitoneally for 15 days | ↓tumor weight ↓tumor volume ↑apoptosis | ↓MMP-2, -9 mRNA, protein ↑pp38 protein ↓pERK-1/-2 protein ↓pNF-κB p65 protein | [37] | |
| Balb/c nu/nu mice injected with LN229 | 60 mg/kg daily intraperitoneally for 4 weeks, accompanied or not by restraint stress for 8 h per day | ↓tumor volume | ↓MMP-2, -9 protein | [36] | |
| Balb/c-nu mice injected with HepG2 | 0.2 mL of 20, 40, 60 μM curcumin daily intraperitoneally for 10 days | ↓tumor volume ↓metastatic incidence in lungs | ↓MMP-2, -9 protein | [33] |
| Polyphenol | Cell Line | Concentration/ Duration | Anti-Cancer Effects | Type IV Collagenase-Mediated Mechanisms | Reference |
|---|---|---|---|---|---|
| EGCG [(2 R,3 R)-3′,4′,5,5′,7-pentahydroxyflavan-3-yl 3,4,5-trihydroxybenzoate] | DU-145 | 2, 5, 10, 20, 40 μg/mL for 24 h | not specified | ↓MMP-2, -9 secretion, activity ↓NF-κB ↓ERK-1/-2 ↓p38 | [46] |
| 786-O | 10, 50, 100, 200 μg/mL for 24 h with or without PMA treatment | not specified | ↓MMP-2, -9 activity | [53] | |
| HeLa DoTc2-4510 SK-OV-3 | 10, 25, 50, 100 μM for 24 h with or without PMA treatment | not specified | ↓MMP-2 activity (HeLa, SK-OV-3) ↓MMP-9 activity (HeLa, DoTc2-4510) | [52] | |
| SCC-9 | 5, 10, 15, 20 μM for 24 h with or without PMA treatment | ↓cell migration ↓cell invasion | ↓MMP-2 mRNA, protein, activity ↓MMP-9 activity ↑TIMP-2 mRNA, protein ↓NF-κB protein | [62] | |
| CL1-5 | 5, 10, 20, 30, 40, 50 μM for 9, 12, 16, 24 h | ↓cell migration ↓cell invasion | ↓MMP-2 mRNA, protein, activity, promoter activity ↓MMP-9 mRNA, protein, activity ↓NF-κB nuclear translocation | [60] | |
| SW-1353, HT-1080, SW-872, SW-982 | 10, 25, 50, 100 μM for 24 h with or without PMA treatment | not specified | ↓MMP-2, -9 activity | [51] | |
| U2 OS RD | 10, 25, 50, 100 μM for 24 h with or without PMA treatment | not specified | ↓MMP-2 activity ↓MMP-9 activity | [50] | |
| TW01 (EBV-negative) NA (EBV-positive, reinfected TW01) | 10, 20, 30, 50 μM for 9, 12, 24, 48 h (cells) 1, 2.5, 5, 10, 25 μM for 7 days (spheroid) | ↓spheroid formation ↓cell migration ↓cell invasion ↓proliferation | ↓MMP-2 mRNA, activity ↓MMP-9 activity ↓pERK-1/-2 protein | [61] | |
| 786-O ACHN | 10, 20, 40 μg/mL for 24 h | ↓cell migration ↓cell invasion | ↓MMP-2, -9 activity, protein | [55] | |
| HuCC-T1 | 0.1, 0.5, 1, 5, 10, 20, 50 μg/mL for 24 h | ↓cell migration ↓cell invasion ↑apoptosis | ↓MMP-2 activity ↓MMP-9 activity | [56] | |
| SW780 | 12.5, 25, 50, 100 μM for 24 h | ↓cell invasion ↓cell migration ↓proliferation ↑apoptosis | ↓MMP-9 mRNA, protein ↓pNF-κB p65 protein ↓NF-κB mRNA | [57] | |
| A-2058 | 10, 25, 50, 100 μM for 24 h | not specified | ↓MMP-2, -9 activity | [48] | |
| FaDu SCC-25 | 10, 25, 50, 100 μM for 24 h with or without PMA treatment | not specified | ↓MMP-2, -9 activity | [49] | |
| NPC-39 HONE-1 NPC-BM | 6, 12, 25, 50 μM for 12, 24 h | ↓cell migration ↓cell invasion | ↓MMP-2 activity, protein | [54] | |
| A549 H1299 | 20, 80, 160 μM for 24, 48 h or 20 μM with 0,625, 1.25, 2.5, 5 μM of BAY11-7082 for 24, 48 h | ↓proliferation ↑apoptosis ↓cell invasion ↓cell migration | ↓MMP-2 mRNA ↓NF-κB, pNF-κB mRNA, protein | [59] | |
| A549 H1299 | 12.5, 25 μM for 48 h (free EGCG or PLGA-encapsulated EGCG) | ↓proliferation ↑apoptosis | ↓MMP-2 mRNA ↓NF-κB, pNF-κB protein | [58] |
| Polyphenol | Animal Model | Dose/ Duration | Anti-Tumor Effects | Type IV Collagenase-Mediated Mechanisms | Reference |
|---|---|---|---|---|---|
| EGCG [(2 R,3 R)-3′,4′,5,5′,7-pentahydroxyflavan-3-yl 3,4,5-trihydroxybenzoate] | Balb/c nu/nu mice injected with SCC-9 | 10, 20 mg/kg daily through oral gavage for 45 days | ↓tumor weight ↓tumor volume | not specified | [62] |
| Balb/c nude mice injected with CL1-5 | 50 mg/kg intraperitoneally twice a week for 6 weeks | ↓metastatic incidence in lungs | not specified | [60] | |
| Balb/c nu/nu mice injected with PANC-1 | 60, 80, 100 mg/kg daily by gavage, 5 days a week for 28 days | ↓angiogenesis ↑apoptosis ratio ↓pancreas weight | ↓MMP-2 mRNA ↓pAkt protein ↓pPI3 K protein ↓pERK protein | [63] | |
| SCID mice injected with NA (EBV-positive) | 30 mg/kg every day or 50 mg/kg every two days, by oral gavage for 8 weeks | ↓tumor volume | not specified | [61] | |
| Balb/c mice injected with HuCC-T1 | 20 mg/kg once subcutaneously beside tumor when it reached 4–5 mm in diameter | ↓tumor volume | ↓MMP-2, -9 protein | [56] | |
| Balb/c mice injected with SW780 | 25, 50, 100 mg/kg daily intraperitoneally for 3 weeks | ↓tumor weight ↓tumor volume | ↓MMP-9 mRNA, protein ↓pNF-κB p65 protein ↓NF-κB mRNA | [57] | |
| Balb/c athymic nude mice injected with A549 | 20 mg/kg with or without 10 mg/kg BAY11-7082, intraperitoneally for 21 days | ↓tumor weight ↓tumor volume | ↓pNF-κB protein | [59] | |
| Balb/c athymic nude mice injected with patient-derived lung cancer samples | 10 mg/kg (free EGCG) or 5 mg/kg (PLGA-encapsulated EGCG) daily intraperitoneally for a month | ↓tumor weight ↓tumor volume | ↓pNF-κB protein | [58] |
| Polyphenol | Cell Line | Concentration/ Duration | Anti-Cancer Effects | Type IV Collagenase-Mediated Mechanisms | Reference |
|---|---|---|---|---|---|
| Genistein (4′,5,7-trihydroxyisoflavone) | TGF-β1-induced Panc-1 | 1, 25, 50 μM for 24, 48 h | ↓cell migration ↓cell invasion | ↓MMP-2 mRNA, activity ↔MMP-9 mRNA, activity ↓uPA mRNA, protein ↓p-p38 MAPK | [75] |
| HeLa | 5, 25, 100 μM for 6, 24, 48 h | ↓cell migration ↑apoptosis | ↓MMP-9 mRNA ↑TIMP-1 mRNA | [70] | |
| A549 | 25, 50, 75, 100 μM for 24, 48 h | ↓proliferation | ↓MMP-2 mRNA, activity ↔MMP-9 mRNA, activity | [74] | |
| HCCLM3 | 40 μM for 24, 48 h with or without 20 μM of cisplatin | ↓proliferation | ↓MMP-2 protein | [77] | |
| 518 A2 | 25, 50 μM for 6, 24, 48 h Free genistein and Cu(II)–genistein complex | ↓cell migration ↓cell invasion ↓proliferation | ↓MMP-2, -9 secretion, activity | [73] | |
| HCT116 SW620 HT29 | 10, 50 μM for 24, 48 h | ↓cell migration ↓cell invasion ↓proliferation | ↓MMP-2 mRNA, protein | [76] | |
| HT29 | 10, 30, 50, 70 μM for 12, 24, 48 h | ↓cell migration ↓proliferation ↑apoptosis | ↓MMP-2 activity ↓p38 MAPK mRNA ↓p-p38 MAPK | [68] | |
| Mia-PaCa2 | 5, 10, 20, 40 μM for 5, 10, 12, 20, 24, 48 h | ↓cell migration ↑apoptosis | ↓MMP-2, -9 protein | [71] | |
| HT29 | 10, 20, 60 μM for 24, 48, 72 h | ↓cell migration ↓cell invasion | ↓MMP-2, -9 mRNA, protein ↑TIMP-1 mRNA, protein | [69] | |
| PC3 | 10, 30, 50, 70 μM for 6, 12, 24 h | ↓cell migration ↓proliferation ↑apoptosis | ↓MMP-2 activity ↓p38 MAPK mRNA ↓p-p38 MAPK | [67] |
| Polyphenol | Animal Model | Dose/ Duration | Anti-Tumor Effects | Type IV Collagenase-Mediated Mechanisms | Reference |
|---|---|---|---|---|---|
| Genistein (4′,5,7-trihydroxyisoflavone) | Balb/c nu/nu mice injected with HCCLM3; partial hepatectomy 10 days after inoculation | 2 mg/kg daily for 4 weeks or 2 mg/kg cisplatin for 7 days or combined therapy of above, intraperitoneally, beginning 3 days after liver lobe resection | ↓recurrent tumor volume ↓metastatic incidence in lungs | ↓MMP-2 mRNA, protein | [77] |
| Balb/cA Jcl-nu and C3 H mice injected with LM8; LM8 previously treated with 50 μM genistein for 3 days | Laboratory chow and water for 25 days (nude) or 36 days (C3 H), no genistein administration | ↓tumor weight ↓engraftment rate ↓metastatic incidence in liver ↓metastatic incidence in lungs (by 100%) | ↓MMP-2 protein | [78] | |
| Balb/c athymic mice injected with HCT116-LUC | 25, 75 mg/kg daily orally 5 days a week, for 5 weeks | ↓tumor weight ↓tumor volume ↓metastatic incidence in liver ↓metastatic incidence in lungs | ↓MMP-2 protein | [76] |
| Polyphenol | Cell Line | Concentration/ Duration | Anti-Cancer Effects | Type IV Collagenase-Mediated Mechanisms | Reference |
|---|---|---|---|---|---|
| Quercetin (3,3′,4′,5,7-pentahydroxyflavone) | A375 A2058 B16 F10 | 10, 20, 40, 60 µM for 16, 24 h or 60 µM for 3, 6, 12, 24 h or 20, 40, 60, 80 µM for 24 h | ↓cell migration ↓cell invasion ↓proliferation ↑apoptosis | ↓MMP-2, -9 mRNA, activity ↑pERK, ↑pAkt protein | [105] |
| MCF-7 MDA-MB-231 | 50, 100 µM for 24 h (quercetin or gold-nanoparticle-conjugated quercetin) | ↓cell migration ↓cell invasion ↓proliferation ↓angiogenesis | ↓MMP-2, -9 protein ↓pPI3 K, ↓Akt, ↓pAkt | [100] | |
| CT26 MC38 CCD-18 Co | 10, 25, 50, 100 µM for 24 h or 50 µM for 3, 6, 9, 12 h or 50 µM for 15, 30, 60 min or 0.1, 1, 10 µM for 24 h | ↓cell migration ↓cell invasion ↓proliferation ↑apoptosis | ↓MMP-2, -9 mRNA, activity ↑TIMP-1, -2 mRNA ↑pERK, pp38 protein | [102] | |
| A549 H1975 HCC827 | 10, 25, 50 µM for 24 h or 50 µM for 10, 30 min, 6, 24 h or 50 µM for 1, 2, 4, 8, 24 h | ↓cell migration ↓cell invasion | ↓MMP-2 protein ↓pAkt | [101] | |
| HOS MG63 | 25, 50, 100 µM for 6, 12, 24 h | ↓cell migration ↓cell invasion | ↓MMP-2, -9 mRNA, protein | [106] | |
| U251 | 10, 20, 30, 40 μg/mL for 24, 48 h | ↓cell migration ↓cell invasion ↓proliferation ↑apoptosis | ↓MMP-2, -9 protein | [85] | |
| U251 | 10 μg/mL for 24, 48 h | ↓cell migration ↓cell invasion | ↓MMP-2, -9 protein | [86] | |
| MCF-7 | 25, 50, 100, 200 µM for 4, 5, 24, 72 h (quercetin) 0.7, 1.4, 2.8, 5.6 mg/mL for 4, 5, 24, 72 h (hyaluronic acid nanohydrogel of quercetin) with or without 1, 10 nM of Everolimus | ↓proliferation ↑apoptosis | ↓MMP-2, -9 protein | [95] | |
| IL-6-induced PATU-8988 | 20, 40, 80 µM for 24, 48 h | ↓cell migration ↓cell invasion | ↓MMP-2 mRNA, protein | [92] | |
| MCF-7 MDA-MB-231 | 20, 30, 40, 60, 80, 100 µM for 6, 24 h | ↓cell migration ↓cell invasion | ↓MMP-2, -9 protein ↓pAkt protein ↓pmTOR protein | [99] | |
| BGC823 AGS | 10 µM for 72 h | ↓cell migration ↓cell invasion ↓proliferation | ↓MMP-2, -9 activity ↓pNF-κB p65 protein ↓pERK-1/-2 protein | [91] | |
| Nickel-induced A549 | 2, 5 µM for 24 h 2, 5 µM for 4 h before Nickel (1 mM) for 5–10 min or 1, 1.5, 12 h | ↓cell migration ↓cell invasion | ↓MMP-2 activity ↓MMP-9 activity, protein ↓NF-κB p65 protein ↓IKKβ protein ↓I-κBα protein | [84] | |
| U2 OS Saos-2 | 20, 40, 60, 80, 100 µM for 48 h | ↓cell migration ↓cell invasion ↓proliferation ↓cell adhesion ↑apoptosis | ↓MMP-2, -9 mRNA ↑TIMP-1, -2 mRNA | [89] | |
| HSC-6 SCC-9 | 50 µM for 24, 48 h | ↓cell migration ↓cell invasion ↓proliferation | ↓MMP-2, -9 protein | [88] | |
| MCF-7 | 80 µM for 24 h with or without 5 µM of lonidamine | ↓proliferation ↑apoptosis | ↓MMP-2, -9 mRNA | [97] | |
| MCF-7 | 5, 100 µM for 12, 24, 48 h with or without 5 µM of tamoxifen | ↑proliferation (5 µM) ↓proliferation (100 µM) ↑cell migration (5 µM) ↓cell migration (100 µM) ↑cell invasion (5 µM) ↓cell invasion (100 µM) ↓apoptosis (5 µM) ↑apoptosis (100 µM) | ↑MMP-2 mRNA (5 µM) ↓MMP-2 mRNA (100 µM) ↑MMP-9 mRNA (5 µM) ↓MMP-9 mRNA (100 µM) | [94] | |
| PA-1 | 50, 75 µM for 24 h | ↓cell migration ↓cell adhesion ↓proliferation | ↓MMP-2 mRNA, protein, activity ↓MMP-9 mRNA, protein, activity ↓PI3 K/pPI3 K mRNA, protein ↓Akt/pAkt mRNA, protein ↓mTOR/pmTOR mRNA, protein ↓ERK-1/-2 protein | [93] | |
| Eca109 | 5, 10 μg/mL for 8, 12, 24 h | ↓cell migration ↓cell invasion ↓proliferation | ↓MMP-2, -9 protein | [90] | |
| MDA-MB-231 | 50 µM for 24, 48 h with or without 32 nM of doxorubicin | ↓cell migration ↓proliferation | ↓MMP-2, -9 mRNA | [96] | |
| A549 | 20, 40 µM for 24 h | ↓proliferation ↓cell migration ↓cell invasion | ↓MMP-2, -9 protein ↑TIMP-2 protein ↓pAkt ↓NF-κB | [83] | |
| H22 HepG2 | 25, 50, 100 µM for 24, 48, 72 h | ↓proliferation ↓cell migration ↓cell invasion ↑autophagy | ↓MMP-2, -9 protein ↓pNF-κB p65, ↓pIκBα protein | [103] | |
| HSC-3 Erlotinib-resistant ERL-R5, ERL-R10 | 5 µM for 24 h or 5, 10 µM with 5 µM of erlotinib | ↓proliferation ↓cell migration ↓cell invasion ↓spheroid formation ↑apoptosis | ↓MMP-2, -9 protein | [104] | |
| ZR-75-1 MCF-7 T47 D MDA-MB-231 | 2, 5 µM for 2, 10, 12, 24 h | ↓proliferation ↓cell migration ↓cell invasion ↓cell adhesion ↑apoptosis | ↓MMP-2, -9 protein, activity ↑TIMP-1, -2 protein | [98] |
| Polyphenol | Animal Model | Dose/ Duration | Anti-Tumor Effects | Type IV Collagenase-Mediated Mechanisms | Reference |
|---|---|---|---|---|---|
| Quercetin (3,3′,4′,5,7-pentahydroxyflavone) | C57 BL6 mice injected with B16 F10 | 15 mg/kg daily thrice a week into the peripheral sites of tumors for 3 weeks or 7.5 mg/kg quercetin with 1.75 mg/kg sulforaphane or 15 mg/kg quercetin with 3.5 mg/kg sulforaphane, the same way | ↓tumor weight ↓tumor volume | ↓MMP-9 protein, activity | [107] |
| Balb/c nu/nu mice injected with A375 (xenograft) C57 BL/6 mice injected with B16 F10 (metastasis model) | 100 mg/kg daily intragastrically for 21 days (xenograft) 100 mg/kg daily intragastrically, 1 day before cell injection and for next 24 days (metastatic model) | ↓tumor weight ↓tumor volume ↓metastatic incidence in lungs | not specified | [105] | |
| Sprague-Dawley rats stimulated by DMBA | 25 mg/kg free quercetin or 25 mg/kg gold-nanoparticle-conjugated quercetin by daily intratumoral injections for 8 days | ↓tumor weight ↓tumor volume ↑normal breast tissue architecture | not specified | [100] | |
| Balb/c mice injected with CT26 | 10, 50 mg/kg intraperitoneally 2 h prior to cell injection and then once every 2 days for 14 days | ↓lung weight ↓metastatic incidence in lungs | not specified | [102] | |
| SCID mice injected with A549 pretreated with quercetin for 24 h (metastatic model) or not (xenograft) | 500 mg/kg daily intraperitoneally for 5 weeks (xenograft model) | ↑survival time ↓metastatic incidence in distant organs (xenograft) ↓metastatic incidence in bones ↓metastatic incidence in lungs (metastatic model) | not specified | [101] | |
| Balb/c nu/nu mice injected with stably transfected HOS | 25, 50, 100 mg/kg intraperitoneally, twice daily for a month | ↓metastatic incidence in lungs | not specified | [106] | |
| Balb/c nude mice injected with MCF-7 | 50 mg/kg, intraperitoneally, twice daily for 25 days | ↓tumor volume | ↓pAkt protein | [99] | |
| Balb/c mice injected with H22 | 25, 50, 100 mg/kg, by gavage, once a day for 21 days | ↓tumor weight ↓tumor volume | not specified | [103] | |
| Nude mice injected with erlotinib-resistant HSC-3, ERL-R5 | 2, 10 mg/kg, intraperitoneally, daily for 18 days | ↓tumor weight ↓tumor volume | not specified | [104] | |
| Balb/c nude mice injected with ZR-75-1 and MCF-7 | 20, 40 mg/kg daily, intraperitoneally for 28 days | ↓tumor weight ↓tumor volume | not specified | [98] |
| Polyphenol | Cell Line | Concentration/ Duration | Anti-Cancer Effects | Type IV Collagenase-Mediated Mechanisms | Reference |
|---|---|---|---|---|---|
| Resveratrol (3,5,4′-trihydroxystilbene) | PMA-induced A549 HeLa | 10, 30 µM for 24 h | ↓proliferation ↓cell migration ↓cell invasion | ↔MMP-2 mRNA, activity ↓MMP-9 mRNA, activity ↓NF-κB activity | [116] |
| 4 T1 | 10, 20, 30 μM for 12, 16, 24, 48 h | ↓cell adhesion ↓cell migration ↓cell invasion | ↓MMP-9 mRNA, activity | [135] | |
| HT1080 | 50 μM for 10, 30 min, 1, 3, 6, 12, 24 h or 10, 20, 30, 40, 50 μM for 24 h or 20, 30, 50 μM for 24, 48 h | ↓proliferation ↑cell migration | ↑MMP-9 protein, activity ↓pp38 ↓pAkt | [134] | |
| BxPC-3 Panc-1 | 12.5, 25, 50 μM for 24, 48 h | ↓proliferation ↓cell migration ↓cell invasion | ↓MMP-2, -9 mRNA, protein ↓pNF-κB protein ↓pAkt protein | [123] | |
| HTB94 | 50 μM for 10, 30 min, 1, 3, 6, 12, 24 h or 10, 20, 30, 40, 50 μM for 24 h | ↓proliferation | ↓MMP-2, -9 protein, activity ↑pp38 | [133] | |
| TGF-β1-induced LoVo | 6, 12 μM for 24, 48 h | ↓proliferation ↓cell migration ↓cell invasion | ↓MMP-2, -9 protein | [139] | |
| Glioblastoma-initiating cells (GICs) derived from patients | 5, 10, 20 μM for 24, 48 h | ↓proliferation ↓cell adhesion ↓cell migration ↓cell invasion | ↓MMP-2 protein, activity ↓NF-κB p65 nuclear translocation ↑I-κBα, ↓pI-κBα protein ↑IKKα/β, ↓pIKKα/β protein ↓pAkt, ↓pmTOR protein | [141] | |
| TPA-induced SCC-9 | 25, 50, 75, 100 μM for 24 h | ↓proliferation ↓cell migration ↓cell invasion | ↔MMP-2 activity ↓MMP-9 mRNA, protein, activity ↓pERK protein | [113] | |
| C6 | 50, 100, 150 μM for 24, 48 h | ↓proliferation ↑apoptosis | ↓MMP-9 protein ↓PI3 K, pAkt, mTOR, STAT3 protein ↓NF-κB protein | [140] | |
| HOS MNNG/HOS 143 B | 25, 50, 75, 100 μM for 12, 24, 48 h or 100 μM for 2, 4 h | ↓proliferation ↓cell adhesion ↓cell migration ↓cell invasion | ↓MMP-2 mRNA, protein, activity, promoter activity ↔TIMP-2 protein ↓pp38, pAkt protein ↑pERK protein | [142] | |
| SW579 | 10 μg/mL for 24 h with 100, 200 μg/mL of lentinan | ↓proliferation ↑apoptosis | ↓MMP-2, -9 mRNA ↑TIMP-1, -2 mRNA ↓NF-κB mRNA ↑I-κBα mRNA | [131] | |
| Hypoxia-induced BxPC-3 Panc-1 | 12.5, 25, 50 μM for 24, 48 h | ↓proliferation ↓cell migration ↓cell invasion | ↓MMP-2 mRNA, protein | [124] | |
| TPA-stimulated MCF-7 | 10 μM for 1, 24 h (resveratrol) 1, 5, 10 μM for 1, 4, 12, 24 h (gold-conjugated resveratrol nanoparticles) | ↓proliferation ↓cell migration ↓cell invasion | ↓MMP-2 protein ↓MMP-9 mRNA, protein, activity, secretion ↑TIMP-1, -2 protein ↓NF-κB p65/pNF-κB p65 protein ↓pAkt, pERK protein | [117] | |
| U87 MG T98 G U251 | 40 μM for 24, 48 h | ↓proliferation ↓cell migration ↓cell invasion | ↓MMP-2 protein, activity | [112] | |
| T24 | 10, 25, 50 μM for 6, 12, 24 h | ↓cell migration ↓cell invasion | ↓MMP-2, -9 protein ↓pERK-1/-2 protein | [129] | |
| SW480 HCT116 | 5 μM for 12, 24 h or 10, 28 days | ↓proliferation ↓cell invasion (in alginate culture) ↑apoptosis | ↓MMP-9 protein ↓pNF-κB p65 protein ↓pNF-κB p50 protein | [121] | |
| HepG2 | 10 μg/mL for 24 h with 5, 10 μg/mL of paclitaxel | ↑apoptosis | ↓MMP-2, -9 mRNA, protein ↑TIMP-1, -2 mRNA, protein ↓NF-κB mRNA, protein ↑I-κBα mRNA, protein | [125] | |
| U2 OS | 6, 12 μg/mL for 24, 48 h | ↓proliferation ↓cell migration ↓cell invasion ↑apoptosis | ↓MMP-2, -9 protein | [132] | |
| HCT116 5-Fluorouracil-resistant HCT116 R | 5 μM for 24, 72 h or 10 days | ↓proliferation ↓cell invasion (in alginate culture) ↑apoptosis | ↓MMP-9 protein ↓pNF-κB p65 protein | [122] | |
| Cancer-associated fibroblasts (CAF)-induced MCF-7 MDA-MB-231 | 50 μM for 24, 36, 48 h | ↓proliferation ↓cell migration ↓cell invasion | ↓MMP-2, -9 mRNA ↓pAkt | [119] | |
| IL-6-induced SGC-7901 HSC-39 | 10, 20 μM for 48 h | ↓proliferation ↓cell invasion | ↓MMP-2, -9 activity ↓pERK protein | [138] | |
| ACHN A498 | 25, 50, 100 μM for 24, 48 h | ↓proliferation ↓cell migration ↓cell invasion | ↓MMP-2, -9 protein ↑TIMP-1 protein ↓pAkt, ERK-1/-2, pERK-1/-2 | [127] | |
| TGF-1 β-induced MDA-MB-231 MDA-MB-436 MDA-MB-453 BT549 | 12.5, 25, 50 μM for 24 h | ↓proliferation ↓cell migration ↓cell invasion | ↓MMP-2 protein, secretion (MDA-MB-231) ↓MMP-9 protein, secretion (MDA-MB-231) ↓pAkt, pPI3 K | [118] | |
| HeLa Cancer Stem Cells (CSCs) | 10, 20, 40 μM for 24, 48 h | ↓proliferation ↑cell migration (slightly, after 24 h) ↓cell migration (after 48 h) ↓cell invasion ↑apoptosis | ↓MMP-2, -9 protein | [120] | |
| PC3 DU145 LNCap | 150 μM for 12, 24, 48 h | ↓cell migration ↓cell invasion | ↓MMP-2, -9 protein ↑TIMP-2, -3 protein ↓TIMP-2, -3 methylation level | [130] | |
| Cisplatin-resistant CAL27 | 25, 50, 75 μM for 6, 12, 18, 24 h | ↓proliferation ↓cell migration ↓cell invasion | ↓MMP-2, -9 protein ↓pERK, pp38 protein | [114] | |
| H-357 | 1, 3.5, 5 μM for 48 h (resveratrol nanoparticles) | ↓angiogenesis ↓proliferation ↓cell invasion | ↓MMP-2, -9 activity ↓NF-κB, IKKα protein ↓Akt, MAPK protein | [137] | |
| Paclitaxel-resistant SKBR3/PR | 30 μM for 24, 48 h (free resveratrol) or equivalent dose (resveratrol–solid lipid nanoparticles with or without TPGS) | ↓proliferation ↓cell migration ↓cell invasion ↑apoptosis | ↓MMP-2, -9 protein | [136] |
| Polyphenol | Animal Model | Dose/ Duration | Anti-Tumor Effects | Type IV Collagenase-Mediated Mechanisms | Reference |
|---|---|---|---|---|---|
| Resveratrol (3,5,4′-trihydroxystilbene) | Balb c nu/nu mice injected with PC-3 | 30 mg/kg through gavage thrice a week, with or without 15 mg/kg TRAIL intravenously on days 1, 7, 14, 21 | ↓angiogenesis ↓tumor volume | ↓MMP-2, -9 protein | [144] |
| Balb/c mice injected with 4 T1 | 100, 200 mg/kg orally, daily, for 21 days | ↓metastatic incidence in lungs | ↓MMP-9 activity (in plasma) | [135] | |
| Sprague-Dawley rats stimulated by DENA and CCl4 | diet containing resveratrol 300, 450 mg/kg for 9 months; 300, 450 mg/kg for 4 weeks as pretreatment and for 9 months as post-treatment | ↑normal hepatic tissue architecture (post-treatment group) | ↓MMP-2, -9 serum concentration | [143] | |
| Balb/c nude mice injected with LoVo | 50, 100, 150 mg/kg via intragastric administration daily for 3 weeks | ↓metastatic incidence in lungs (metastatic model and xenograft) ↓metastatic incidence in liver (xenograft) ↓tumor weight (xenograft) | not specified | [139] | |
| NOD-SCID mice injected with glioblastoma-initiating cells (GICs) derived from patients | 10 mg/kg daily intraperitoneally for 28 days | ↓mean depth of tumor invasion | not specified | [141] | |
| Wistar rats injected with C6 | 8 mg/kg daily orally for different lengths of time, depending on the animals’ lifespan, minimum 13 days, maximum 90 days | ↑survival time ↑apoptosis ↓tumor growth | ↓MMP-9 protein ↓NF-κB protein | [140] | |
| SCID mice injected with MNNG/HOS | 40, 100 mg/kg five times a week by oral gavage for 24 days | ↓tumor volume ↓metastatic incidence in lungs | ↓MMP-2 protein | [142] | |
| NOD-SCID mice injected with HSC-39 | 10, 20 μM for 3 weeks | ↓metastatic incidence in lungs | not specified | [138] | |
| Balb/c mice injected with H-357 | 40 mg/kg daily orally for 55 days (resveratrol–nanoparticles) | ↓tumor volume | not specified | [137] | |
| Balb/c nude mice injected with SKBR3/PR | 20 mg/kg (free resveratrol) or equivalent dose (resveratrol–solid lipid nanoparticles with or without TPGS), intraperitoneally, 5 times a day for 21 days | ↓tumor weight ↓tumor volume | not specified | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowski, W.; Caban, M.; Lewandowska, U. Cancer Prevention and Treatment with Polyphenols: Type IV Collagenase-Mediated Mechanisms. Cancers 2024, 16, 3193. https://doi.org/10.3390/cancers16183193
Pawłowski W, Caban M, Lewandowska U. Cancer Prevention and Treatment with Polyphenols: Type IV Collagenase-Mediated Mechanisms. Cancers. 2024; 16(18):3193. https://doi.org/10.3390/cancers16183193
Chicago/Turabian StylePawłowski, Wojciech, Miłosz Caban, and Urszula Lewandowska. 2024. "Cancer Prevention and Treatment with Polyphenols: Type IV Collagenase-Mediated Mechanisms" Cancers 16, no. 18: 3193. https://doi.org/10.3390/cancers16183193
APA StylePawłowski, W., Caban, M., & Lewandowska, U. (2024). Cancer Prevention and Treatment with Polyphenols: Type IV Collagenase-Mediated Mechanisms. Cancers, 16(18), 3193. https://doi.org/10.3390/cancers16183193








