PLK1 Inhibitor Onvansertib Enhances the Efficacy of Alpelisib in PIK3CA-Mutated HR-Positive Breast Cancer Resistant to Palbociclib and Endocrine Therapy: Preclinical Insights
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Cell Viability and Synergy Analysis
2.3. Colony Formation Assay
2.4. Flow Cytometric Analysis of Cell Cycle Distribution and Apoptosis
2.5. Protein Analysis Using Simple Western
2.6. In Vivo PDX Studies
2.7. Pharmacodynamic Studies
2.8. Statistical Analysis
3. Results
3.1. Onvansertib and Alpelisib Are Synergistic in ER+ Breast Cancer Cell Lines
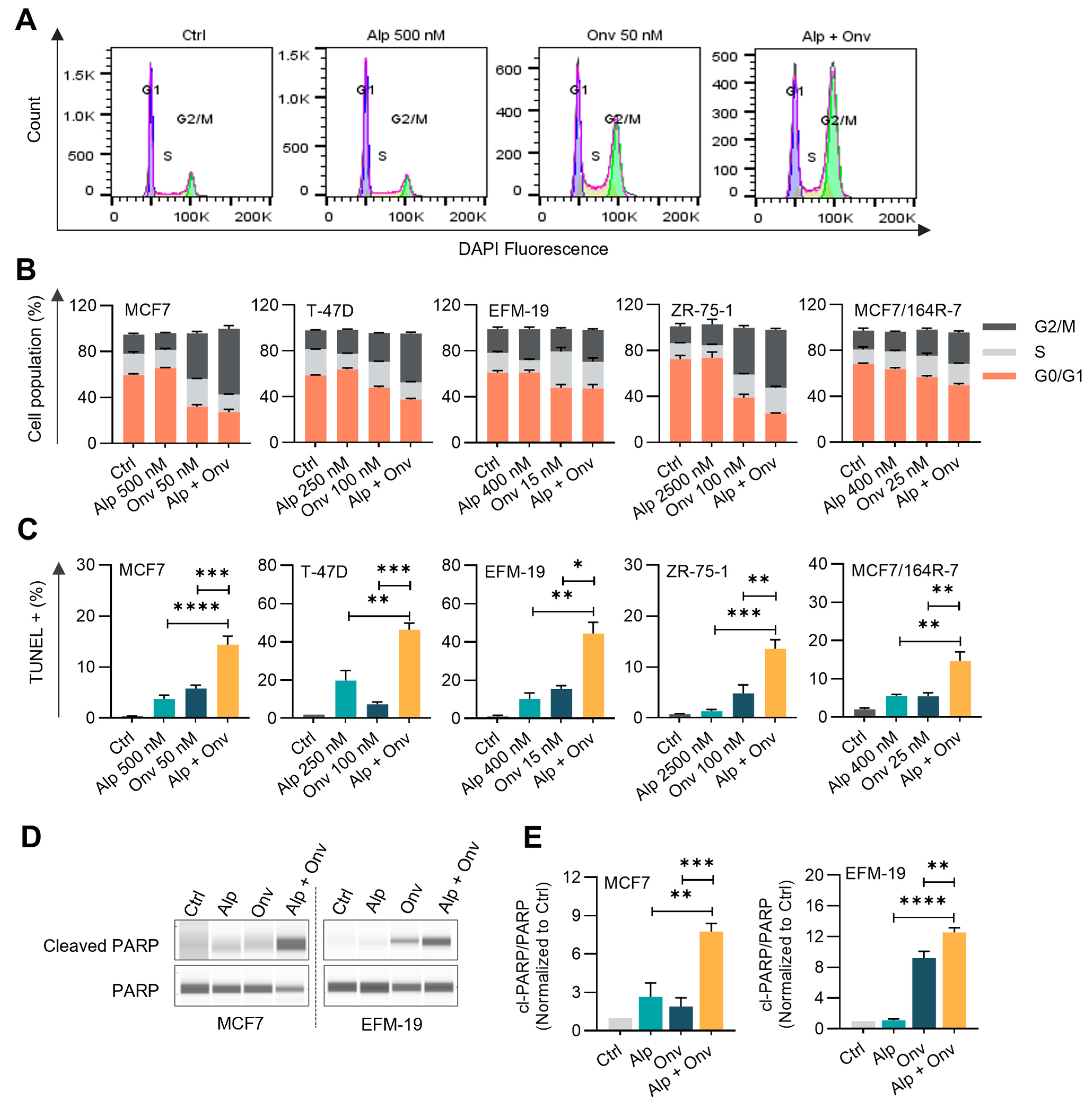
3.2. The Combination of Onvansertib and Alpelisib Suppresses PI3K-AKT Signaling, Induces G2/M Arrest, and Apoptosis
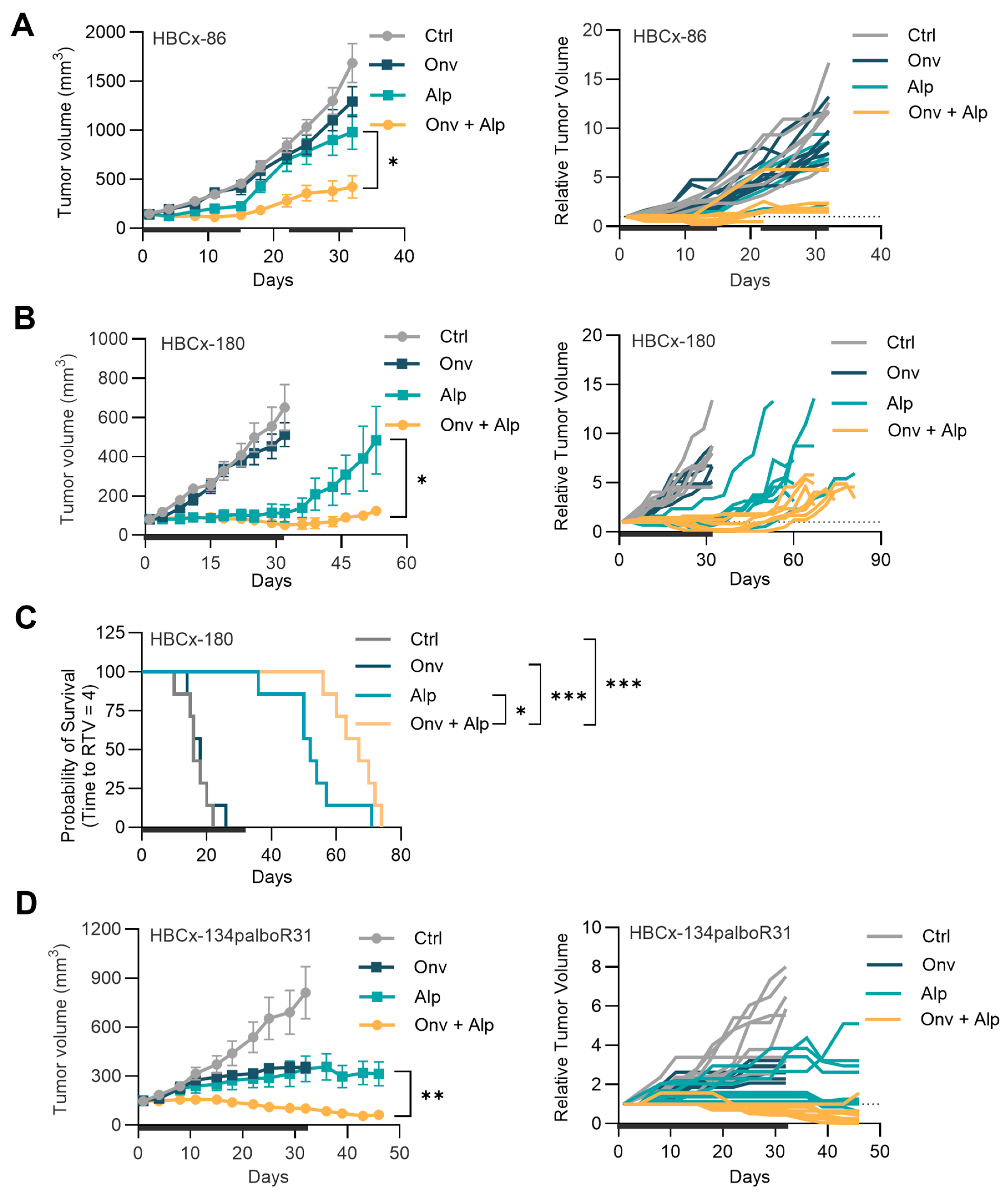
3.3. Co-Administration of Onvansertib and Alpelisib Inhibits the Growth of PIK3CA-Mutated, ET, and Palbociclib-Resistant HR+ Breast Cancer PDXs
3.4. Onvansertib and Alpelisib Combination Inhibits PLK1 and PI3K Activity and Induces Pronounced Apoptosis in the Combination-Treated Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.H.; Downton, T.; Freelander, A.; Hurwitz, J.; Caldon, C.E.; Lim, E. CDK4/6 Inhibitor Resistance in Estrogen Receptor Positive Breast Cancer, a 2023 Perspective. Front. Cell Dev. Biol. 2023, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.L.; Schillaci, R. Emerging Targeted Therapies for HER2-Positive Breast Cancer. Cancers 2023, 15, 1987. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Molto Valiente, C.; Tamimi, F.; Schlam, I.; Sammons, S.; Tolaney, S.M.; Tarantino, P. Filling the Gap after CDK4/6 Inhibitors: Novel Endocrine and Biologic Treatment Options for Metastatic Hormone Receptor Positive Breast Cancer. Cancers 2023, 15, 2015. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Gomez Moreno, H.L.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib plus Fulvestrant in PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer after a CDK4/6 Inhibitor (BYLieve): One Cohort of a Phase 2, Multicentre, Open-Label, Non-Comparative Study. Lancet Oncol. 2021, 22, 489–498. [Google Scholar] [CrossRef]
- Piccart, M.; Hortobagyi, G.N.; Campone, M.; Pritchard, K.I.; Lebrun, F.; Ito, Y.; Noguchi, S.; Perez, A.; Rugo, H.S.; Deleu, I.; et al. Everolimus plus Exemestane for Hormone-Receptor-Positive, Human Epidermal Growth Factor Receptor-2-Negative Advanced Breast Cancer: Overall Survival Results from BOLERO-2†. Ann. Oncol. 2014, 25, 2357–2362. [Google Scholar] [CrossRef]
- Verret, B.; Cortes, J.; Bachelot, T.; Andre, F.; Arnedos, M. Efficacy of PI3K Inhibitors in Advanced Breast Cancer. Ann. Oncol. 2019, 30 (Suppl. S1), x12–x20. [Google Scholar] [CrossRef]
- Pal, I.; Mandal, M. PI3K and Akt as Molecular Targets for Cancer Therapy: Current Clinical Outcomes. Acta Pharmacol. Sin. 2012, 33, 1441–1458. [Google Scholar] [CrossRef]
- Alves, C.L.; Ditzel, H.J. Drugging the PI3K/AKT/MTOR Pathway in ER+ Breast Cancer. Int. J. Mol. Sci. 2023, 24, 4522. [Google Scholar] [CrossRef]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; Neve, R.M.; Kuo, W.-L.; Davies, M.; Carey, M.; Hu, Z.; Guan, Y.; Sahin, A.; et al. An Integrative Genomic and Proteomic Analysis of PIK3CA, PTEN, and AKT Mutations in Breast Cancer. Cancer Res. 2008, 68, 6084–6091. [Google Scholar] [CrossRef] [PubMed]
- Mollon, L.E.; Anderson, E.J.; Dean, J.L.; Warholak, T.L.; Aizer, A.; Platt, E.A.; Tang, D.H.; Davis, L.E. A Systematic Literature Review of the Prognostic and Predictive Value of PIK3CA Mutations in HR+/HER2- Metastatic Breast Cancer. Clin. Breast Cancer 2020, 20, e232–e243. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, A.; Bertucci, F.; Gonçalves, A. Phosphoinositide 3-Kinase (PI3K) Inhibitors and Breast Cancer: An Overview of Current Achievements. Cancers 2023, 15, 1416. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects. Int. J. Mol. Sci. 2021, 22, 3464. [Google Scholar] [CrossRef] [PubMed]
- Juric, D.; Castel, P.; Griffith, M.; Griffith, O.L.; Won, H.H.; Ellis, H.; Ebbesen, S.H.; Ainscough, B.J.; Ramu, A.; Iyer, G.; et al. Convergent Loss of PTEN Leads to Clinical Resistance to a PI(3)Kα Inhibitor. Nature 2015, 518, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Razavi, P.; Dickler, M.N.; Shah, P.D.; Toy, W.; Brown, D.N.; Won, H.H.; Li, B.T.; Shen, R.; Vasan, N.; Modi, S.; et al. Alterations in PTEN and ESR1 Promote Clinical Resistance to Alpelisib plus Aromatase Inhibitors. Nat. Cancer 2020, 1, 382–393. [Google Scholar] [CrossRef]
- King, S.I.; Purdie, C.A.; Bray, S.E.; Quinlan, P.R.; Jordan, L.B.; Thompson, A.M.; Meek, D.W. Immunohistochemical Detection of Polo-like Kinase-1 (PLK1) in Primary Breast Cancer Is Associated with TP53 Mutation and Poor Clinical Outcom. Breast Cancer Res. 2012, 14, R40. [Google Scholar] [CrossRef]
- Donizy, P.; Halon, A.; Surowiak, P.; Kaczorowski, M.; Kozyra, C.; Matkowski, R. Augmented Expression of Polo-like Kinase 1 Is a Strong Predictor of Shorter Cancer-Specific Overall Survival in Early Stage Breast Cancer at 15-Year Follow-Up. Oncol. Lett. 2016, 12, 1667–1674. [Google Scholar] [CrossRef]
- Zitouni, S.; Nabais, C.; Jana, S.C.; Guerrero, A.; Bettencourt-Dias, M. Polo-like Kinases: Structural Variations Lead to Multiple Functions. Nat. Rev. Mol. Cell Biol. 2014, 15, 433–452. [Google Scholar] [CrossRef]
- Gutteridge, R.E.A.; Ndiaye, M.A.; Liu, X.; Ahmad, N. Plk1 Inhibitors in Cancer Therapy: From Laboratory to Clinics. Mol. Cancer Ther. 2016, 15, 1427–1435. [Google Scholar] [CrossRef]
- Chiappa, M.; Petrella, S.; Damia, G.; Broggini, M.; Guffanti, F.; Ricci, F. Present and Future Perspective on PLK1 Inhibition in Cancer Treatment. Front. Oncol. 2022, 12, 903016. [Google Scholar] [CrossRef] [PubMed]
- Montaudon, E.; Nikitorowicz-Buniak, J.; Sourd, L.; Morisset, L.; El Botty, R.; Huguet, L.; Dahmani, A.; Painsec, P.; Nemati, F.; Vacher, S.; et al. PLK1 Inhibition Exhibits Strong Anti-Tumoral Activity in CCND1-Driven Breast Cancer Metastases with Acquired Palbociclib Resistance. Nat. Commun. 2020, 11, 4053. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Zotano, Á.; Belli, S.; Zielinski, C.; Gil-Gil, M.; Fernandez-Serra, A.; Ruiz-Borrego, M.; Ciruelos Gil, E.M.; Pascual, J.; Muñoz-Mateu, M.; Bermejo, B.; et al. CCNE1 and PLK1 Mediate Resistance to Palbociclib in HR+/HER2- Metastatic Breast Cancer. Clin. Cancer Res. 2023, 29, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hou, X.; Shao, C.; Li, J.; Cheng, J.-X.; Kuang, S.; Ahmad, N.; Ratliff, T.; Liu, X. Plk1 Inhibition Enhances the Efficacy of Androgen Signaling Blockade in Castration-Resistant Prostate Cancer. Cancer Res. 2014, 74, 6635–6647. [Google Scholar] [CrossRef]
- Mao, Y.; Xi, L.; Li, Q.; Wang, S.; Cai, Z.; Zhang, X.; Yu, C. Combination of PI3K/Akt Pathway Inhibition and Plk1 Depletion Can Enhance Chemosensitivity to Gemcitabine in Pancreatic Carcinoma. Transl. Oncol. 2018, 11, 852–863. [Google Scholar] [CrossRef]
- De Martino, D.; Yilmaz, E.; Orlacchio, A.; Ranieri, M.; Zhao, K.; Di Cristofano, A. PI3K Blockage Synergizes with PLK1 Inhibition Preventing Endoreduplication and Enhancing Apoptosis in Anaplastic Thyroid Cancer. Cancer Lett. 2018, 439, 56–65. [Google Scholar] [CrossRef]
- Su, S.; Chhabra, G.; Singh, C.K.; Ndiaye, M.A.; Ahmad, N. PLK1 Inhibition-Based Combination Therapies for Cancer Management. Transl. Oncol. 2022, 16, 101332. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Li, G.; Ye, J.; Wang, R.; Zhang, X.; Li, F.; Gao, C.; Li, J.; Jiang, J.; et al. PI3K/MTOR Inhibitor VS-5584 Combined with PLK1 Inhibitor Exhibits Synergistic Anti-Cancer Effects on Non-Small Cell Lung Cancer. Eur. J. Pharmacol. 2023, 957, 176004. [Google Scholar] [CrossRef]
- Beria, I.; Bossi, R.T.; Brasca, M.G.; Caruso, M.; Ceccarelli, W.; Fachin, G.; Fasolini, M.; Forte, B.; Fiorentini, F.; Pesenti, E.; et al. NMS-P937, a 4,5-Dihydro-1H-Pyrazolo [4,3-h]Quinazoline Derivative as Potent and Selective Polo-like Kinase 1 Inhibitor. Bioorg. Med. Chem. Lett. 2011, 21, 2969–2974. [Google Scholar] [CrossRef]
- Affatato, R.; Carrassa, L.; Chilà, R.; Lupi, M.; Restelli, V.; Damia, G. Identification of PLK1 as a New Therapeutic Target in Mucinous Ovarian Carcinoma. Cancers 2020, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Liu, Y.; Armeson, K.; Park, Y.; Ridinger, M.; Erlander, M.; Reuben, J.; Britten, C.; Kappler, C.; Yeh, E.; et al. Polo-like Kinase 1 (Plk1) Inhibition Synergizes with Taxanes in Triple Negative Breast Cancer. PLoS ONE 2019, 14, e0224420. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Varkaris, A.; Ridinger, M.; Dalrymple, S.L.; Dennehy, C.M.; Isaacs, J.T.; Einstein, D.J.; Brennen, W.N.; Balk, S.P. AKT Inhibition Sensitizes to Polo-Like Kinase 1 Inhibitor Onvansertib in Prostate Cancer. Mol. Cancer Ther. 2024. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, M.; Decio, A.; Guarrera, L.; Mengoli, I.; Karki, A.; Yemane, D.; Ghilardi, C.; Scanziani, E.; Canesi, S.; Barbera, M.C.; et al. Onvansertib Treatment Overcomes Olaparib Resistance in High-Grade Ovarian Carcinomas. Cell Death Dis. 2024, 15, 521. [Google Scholar] [CrossRef]
- Valsasina, B.; Beria, I.; Alli, C.; Alzani, R.; Avanzi, N.; Ballinari, D.; Cappella, P.; Caruso, M.; Casolaro, A.; Ciavolella, A.; et al. NMS-P937, an Orally Available, Specific Small-Molecule Polo-like Kinase 1 Inhibitor with Antitumor Activity in Solid and Hematologic Malignancies. Mol. Cancer Ther. 2012, 11, 1006–1016. [Google Scholar] [CrossRef]
- BLISS, C.I. The Toxicity of Poisons Applied Jointly. Ann. Appl. Biol. 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic Assay of Cells in Vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Guzmán, C.; Bagga, M.; Kaur, A.; Westermarck, J.; Abankwa, D. ColonyArea: An ImageJ Plugin to Automatically Quantify Colony Formation in Clonogenic Assays. PLoS ONE 2014, 9, e92444. [Google Scholar] [CrossRef]
- Li, X.; Traganos, F.; Melamed, M.R.; Darzynkiewicz, Z. Single-Step Procedure for Labeling DNA Strand Breaks with Fluorescein- or BODIPY-Conjugated Deoxynucleotides: Detection of Apoptosis and Bromodeoxyuridine Incorporation. Cytometry 1995, 20, 172–180. [Google Scholar] [CrossRef]
- El-Botty, R.; Morriset, L.; Montaudon, E.; Tariq, Z.; Schnitzler, A.; Bacci, M.; Lorito, N.; Sourd, L.; Huguet, L.; Dahmani, A.; et al. Oxidative Phosphorylation Is a Metabolic Vulnerability of Endocrine Therapy and Palbociclib Resistant Metastatic Breast Cancers. Nat. Commun. 2023, 14, 4221. [Google Scholar] [CrossRef]
- Jacquemetton, J.; Kassem, L.; Poulard, C.; Dahmani, A.; De Plater, L.; Montaudon, E.; Sourd, L.; Morisset, L.; El Botty, R.; Chateau-Joubert, S.; et al. Analysis of Genomic and Non-Genomic Signaling of Estrogen Receptor in PDX Models of Breast Cancer Treated with a Combination of the PI3K Inhibitor Alpelisib (BYL719) and Fulvestrant. Breast Cancer Res. 2021, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Coussy, F.; de Koning, L.; Lavigne, M.; Bernard, V.; Ouine, B.; Boulai, A.; El Botty, R.; Dahmani, A.; Montaudon, E.; Assayag, F.; et al. A Large Collection of Integrated Genomically Characterized Patient-Derived Xenografts Highlighting the Heterogeneity of Triple-Negative Breast Cancer. Int. J. Cancer 2019, 145, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Cottu, P.; Marangoni, E.; Assayag, F.; de Cremoux, P.; Vincent-Salomon, A.; Guyader, C.; de Plater, L.; Elbaz, C.; Karboul, N.; Fontaine, J.J.; et al. Modeling of Response to Endocrine Therapy in a Panel of Human Luminal Breast Cancer Xenografts. Breast Cancer Res. Treat. 2012, 133, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, I.; Paoli, M.; Risi, E.; Biagioni, C.; Biganzoli, L.; Benelli, M.; Malorni, L. PIK3CA Co-Occurring Mutations and Copy-Number Gain in Hormone Receptor Positive and HER2 Negative Breast Cancer. NPJ Breast Cancer 2022, 8, 24. [Google Scholar] [CrossRef]
- Hanan, E.J.; Braun, M.-G.; Heald, R.A.; MacLeod, C.; Chan, C.; Clausen, S.; Edgar, K.A.; Eigenbrot, C.; Elliott, R.; Endres, N.; et al. Discovery of GDC-0077 (Inavolisib), a Highly Selective Inhibitor and Degrader of Mutant PI3Kα. J. Med. Chem. 2022, 65, 16589–16621. [Google Scholar] [CrossRef]
- Gómez Tejeda Zañudo, J.; Mao, P.; Alcon, C.; Kowalski, K.; Johnson, G.N.; Xu, G.; Baselga, J.; Scaltriti, M.; Letai, A.; Montero, J.; et al. Cell Line-Specific Network Models of ER+ Breast Cancer Identify Potential PI3Kα Inhibitor Resistance Mechanisms and Drug Combinations. Cancer Res. 2021, 81, 4603–4617. [Google Scholar] [CrossRef]
- Jones, R.B.; Farhi, J.; Adams, M.; Parwani, K.K.; Cooper, G.W.; Zecevic, M.; Lee, R.S.; Hong, A.L.; Spangle, J.M. Targeting MLL Methyltransferases Enhances the Antitumor Effects of PI3K Inhibition in Hormone Receptor—Positive Breast Cancer. Cancer Res. Commun. 2022, 2, 1569–1578. [Google Scholar] [CrossRef]
- Caswell-Jin, J.L.; Plevritis, S.K.; Tian, L.; Cadham, C.J.; Xu, C.; Stout, N.K.; Sledge, G.W.; Mandelblatt, J.S.; Kurian, A.W. Change in Survival in Metastatic Breast Cancer with Treatment Advances: Meta-Analysis and Systematic Review. JNCI Cancer Spectr. 2018, 2, pky062. [Google Scholar] [CrossRef]
- Kothari, R.; Fong, Y.; Storrie-Lombardi, M.C. Review of Laser Raman Spectroscopy for Surgical Breast Cancer Detection: Stochastic Backpropagation Neural Networks. Sensors 2020, 20, 6260. [Google Scholar] [CrossRef]
- Lopez-Tarruella, S.; Echavarria, I.; Jerez, Y.; Herrero, B.; Gamez, S.; Martin, M. How We Treat HR-Positive, HER2-Negative Early Breast Cancer. Future Oncol. 2022, 18, 1003–1022. [Google Scholar] [CrossRef]
- Fasching, P.A.; Kreipe, H.; Del Mastro, L.; Ciruelos, E.; Freyer, G.; Korfel, A.; Chouaki, N.; Stoffregen, C.; Sapunar, F.; Cameron, D. Identification of Patients with Early HR+ HER2- Breast Cancer at High Risk of Recurrence. Geburtshilfe Frauenheilkd. 2024, 84, 164–184. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.H.; Barzi, A.; Ridinger, M.; Samuëlsz, E.; Subramanian, R.A.; Croucher, P.J.P.; Smeal, T.; Kabbinavar, F.F.; Lenz, H.-J. Onvansertib in Combination with FOLFIRI and Bevacizumab in Second-Line Treatment of KRAS-Mutant Metastatic Colorectal Cancer: A Phase Ib Clinical Study. Clin. Cancer Res. 2024, 30, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wu, J.; Chen, Y.; Nie, J.; Chen, C. Activation of PI3K/AKT/MTOR Pathway Causes Drug Resistance in Breast Cancer. Front. Pharmacol. 2021, 12, 628690. [Google Scholar] [CrossRef]
- Jacobson, A. Alpelisib Plus Fulvestrant or Letrozole Demonstrates Sustained Benefits Across Subgroups of Patients with PIK3CA-Mutated HR+/HER2- Advanced Breast Cancer. Oncologist 2022, 27, S13–S14. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Ridinger, M.; Lin, T.L.; Becker, P.S.; Schiller, G.J.; Patel, P.A.; Spira, A.I.; Tsai, M.L.; Samuëlsz, E.; Silberman, S.L.; et al. A Phase Ib Study of Onvansertib, a Novel Oral PLK1 Inhibitor, in Combination Therapy for Patients with Relapsed or Refractory Acute Myeloid Leukemia. Clin. Cancer Res. 2020, 26, 6132–6140. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.J.; Jameson, G.; Von Hoff, D.D.; Valsasina, B.; Davite, C.; Di Giulio, C.; Fiorentini, F.; Alzani, R.; Carpinelli, P.; Di Sanzo, A.; et al. Phase I Dose Escalation Study of NMS-1286937, an Orally Available Polo-like Kinase 1 Inhibitor, in Patients with Advanced or Metastatic Solid Tumors. Investig. New Drugs 2018, 36, 85–95. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreekumar, S.; Montaudon, E.; Klein, D.; Gonzalez, M.E.; Painsec, P.; Derrien, H.; Sourd, L.; Smeal, T.; Marangoni, E.; Ridinger, M. PLK1 Inhibitor Onvansertib Enhances the Efficacy of Alpelisib in PIK3CA-Mutated HR-Positive Breast Cancer Resistant to Palbociclib and Endocrine Therapy: Preclinical Insights. Cancers 2024, 16, 3259. https://doi.org/10.3390/cancers16193259
Sreekumar S, Montaudon E, Klein D, Gonzalez ME, Painsec P, Derrien H, Sourd L, Smeal T, Marangoni E, Ridinger M. PLK1 Inhibitor Onvansertib Enhances the Efficacy of Alpelisib in PIK3CA-Mutated HR-Positive Breast Cancer Resistant to Palbociclib and Endocrine Therapy: Preclinical Insights. Cancers. 2024; 16(19):3259. https://doi.org/10.3390/cancers16193259
Chicago/Turabian StyleSreekumar, Sreeja, Elodie Montaudon, Davis Klein, Migdalia E. Gonzalez, Pierre Painsec, Héloise Derrien, Laura Sourd, Tod Smeal, Elisabetta Marangoni, and Maya Ridinger. 2024. "PLK1 Inhibitor Onvansertib Enhances the Efficacy of Alpelisib in PIK3CA-Mutated HR-Positive Breast Cancer Resistant to Palbociclib and Endocrine Therapy: Preclinical Insights" Cancers 16, no. 19: 3259. https://doi.org/10.3390/cancers16193259
APA StyleSreekumar, S., Montaudon, E., Klein, D., Gonzalez, M. E., Painsec, P., Derrien, H., Sourd, L., Smeal, T., Marangoni, E., & Ridinger, M. (2024). PLK1 Inhibitor Onvansertib Enhances the Efficacy of Alpelisib in PIK3CA-Mutated HR-Positive Breast Cancer Resistant to Palbociclib and Endocrine Therapy: Preclinical Insights. Cancers, 16(19), 3259. https://doi.org/10.3390/cancers16193259






