Current Status and Future Perspectives of Preoperative and Intraoperative Marking in Thoracic Surgery
Abstract
:Simple Summary
Abstract
1. Introduction
2. Preoperative Marking
2.1. Clinical Overview and Three-Dimensional Reconstruction
2.2. Percutaneous Marking
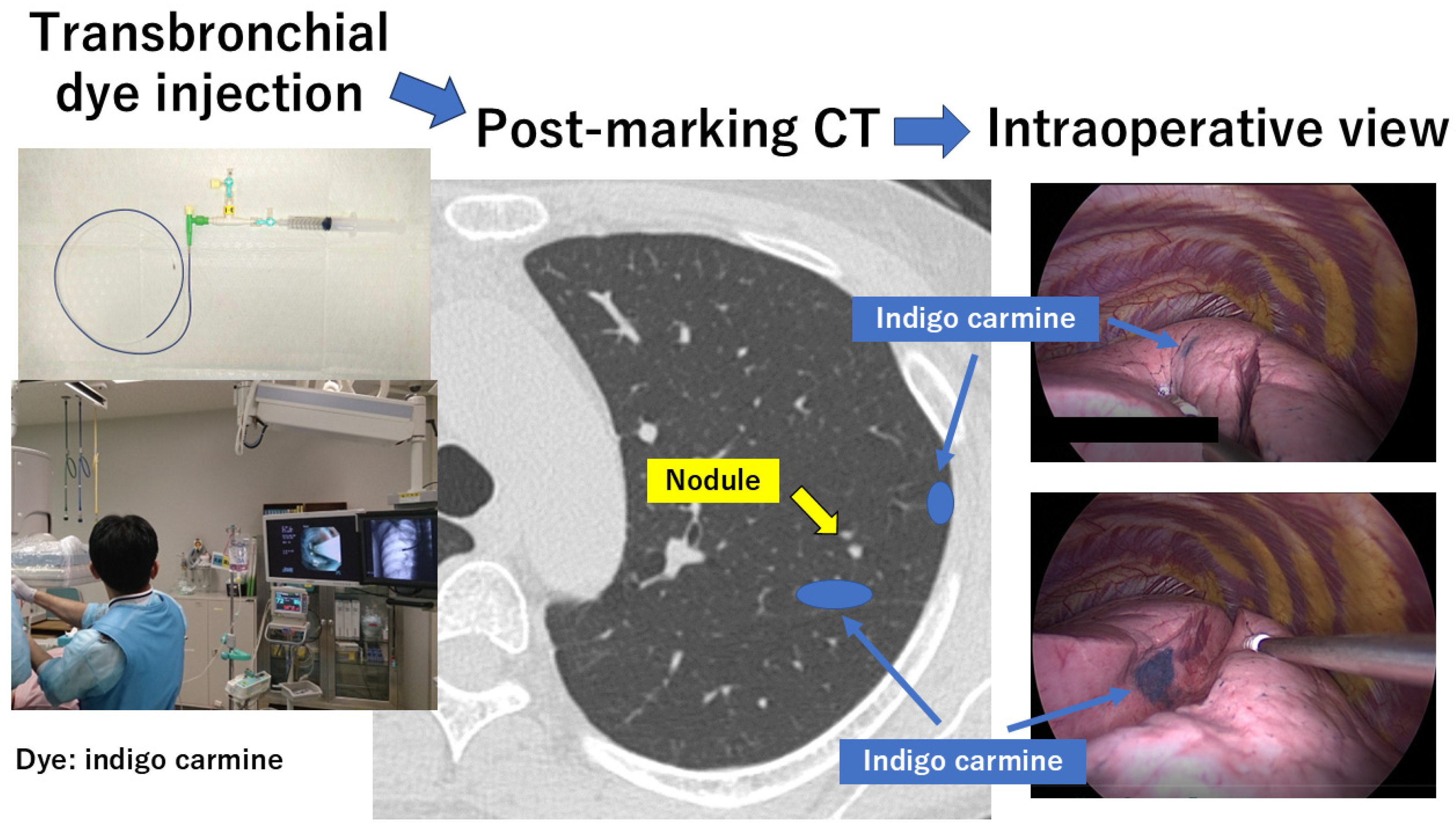
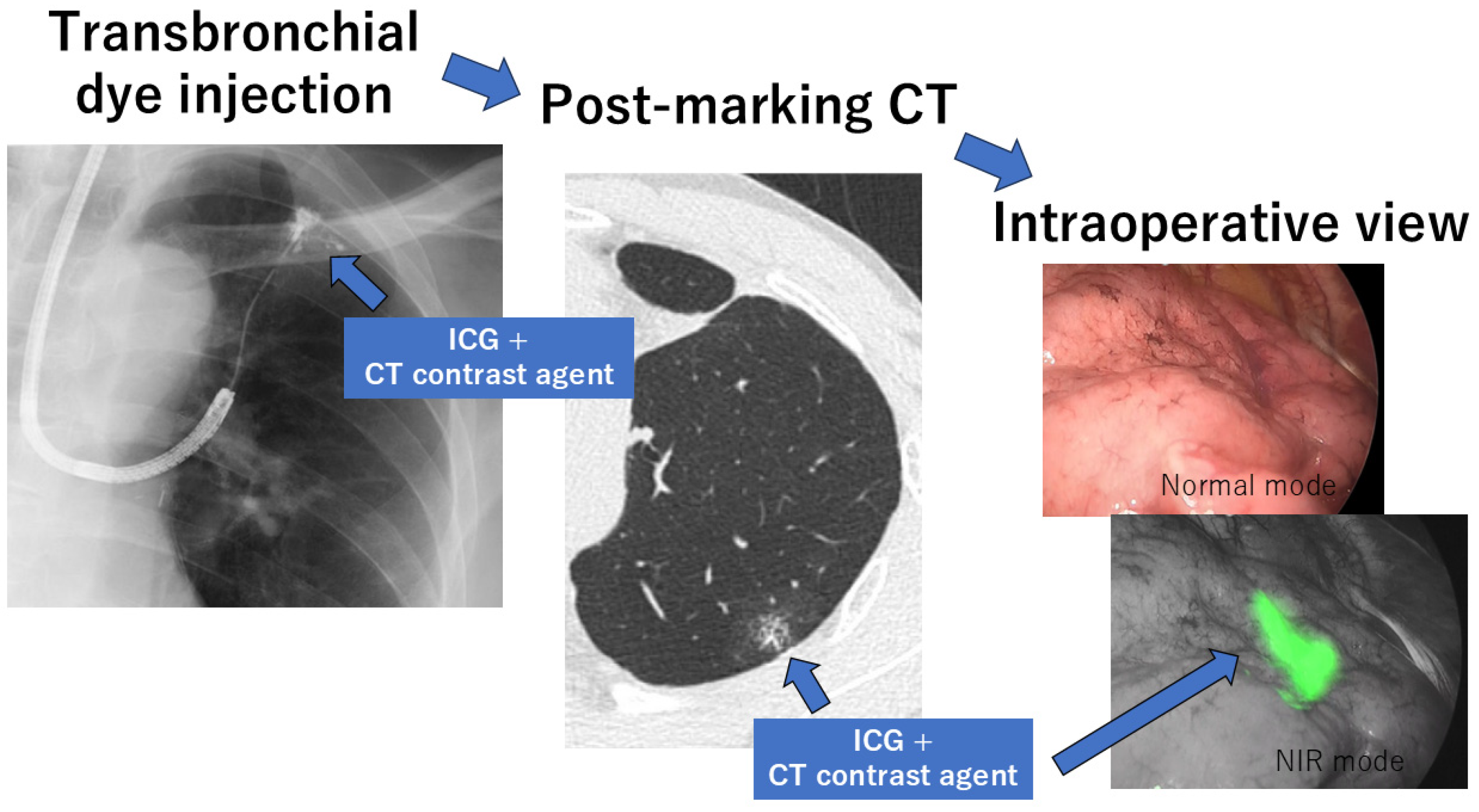
2.3. Transbronchial Marking
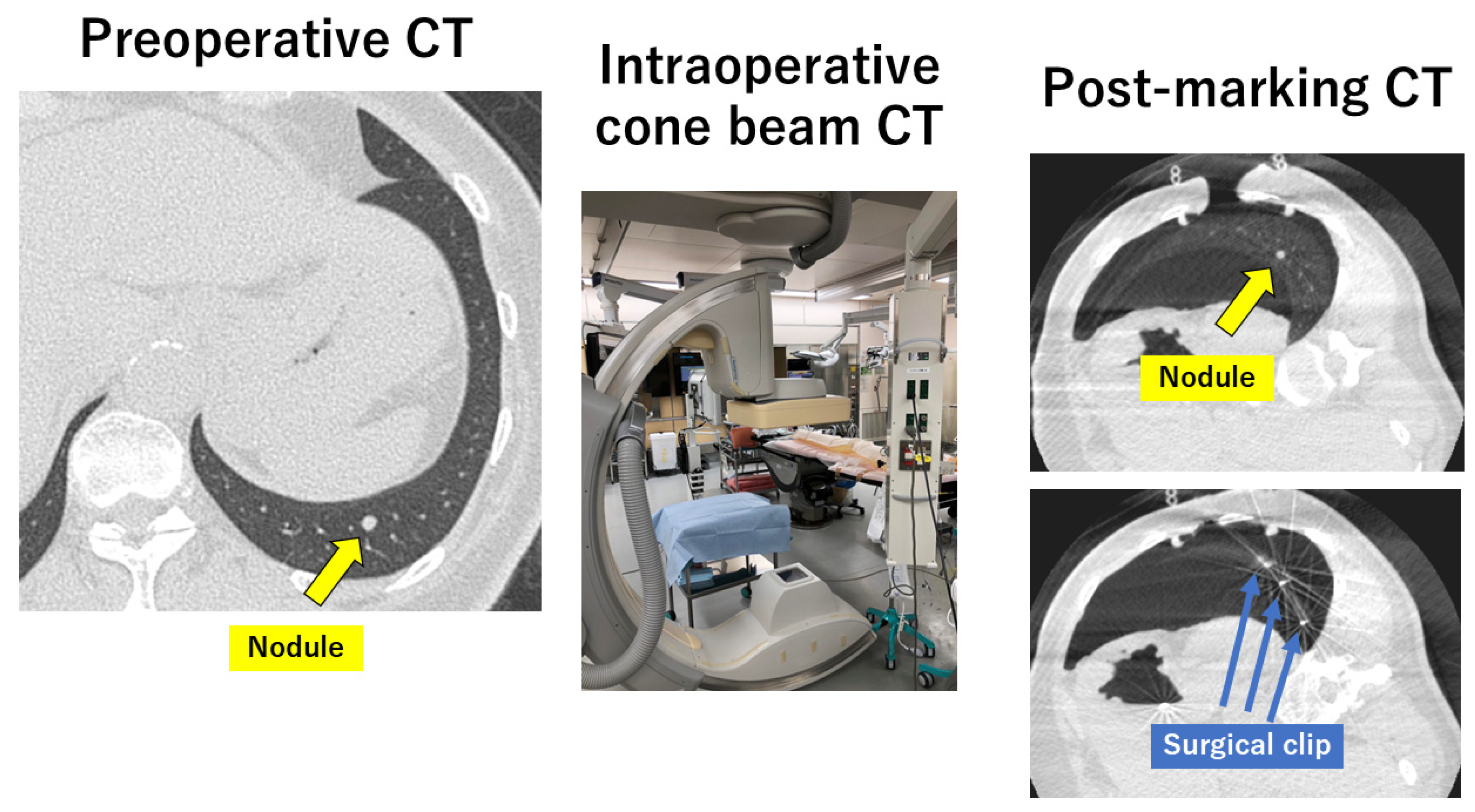
3. Intraoperative Marking
4. Molecular Imaging
5. Real-World Data in a Single Institution
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duma, N.; Santana-Davila, R.; Molima, J.R. Non-small cell lung cancer. Epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.A.; Singer, J.J. Successful removal of an entire lung for carcinoma of the bronchus. CA A Cancer J. Clin. 1974, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Cahan, W.G. Radical lobectomy. J. Thorac. Cardiovasc. Surg. 1960, 39, 555–572. [Google Scholar] [CrossRef]
- Ginsberg, R.J.; Rubinstein, L.V.; Lung Cancer Study Group. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann. Thorac. Surg. 1995, 60, 615–622. [Google Scholar] [CrossRef]
- Okami, J.; Shintani, Y.; Okumura, M.; Ito, H.; Ohtsuka, T.; Toyooka, S.; Mori, T.; Watanabe, S.I.; Date, H.; Yokoi, K.; et al. Demographics, safety and quality, and prognostic information in both the seventh and eighth editions of the TNM classifications in 18,973 surgical cases of the Japanese joint committee of lung cancer registry database in 2010. J. Thorac. Oncol. 2019, 14, 212–222. [Google Scholar] [CrossRef]
- Committee for Scientific Affairs; The Japanese Association for Thoracic Surgery; Yoshimura, N.; Sato, Y.; Takeuchi, H.; Abe, T.; Endo, S.; Hirata, Y.; Ishida, M.; Iwata, H.; et al. Thoracic and cardiovascular surgeries in Japan during 2021: Annual report by the Japanese association for thoracic surgery. Gen. Thorac. Cardiovasc. Surg. 2024, 72, 254–291. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Chen-Yoshikawa, T.F.; Fukui, T.; Nakamura, S.; Ito, T.; Kadomatsu, Y.; Tsubouchi, H.; Ueno, H.; Sugiyama, T.; Goto, M.; Mori, S.; et al. Current trends in thoracic surgery. Nagoya J. Med. Sci. 2020, 82, 161–174. [Google Scholar]
- Chen-Yoshikawa, T.F.; Date, H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J. Thorac. Dis. 2016, 8 (Suppl. S3), S295–S301. [Google Scholar]
- Chen-Yoshikawa, T.F. Evolution of three-dimensional computed tomography imaging in thoracic surgery. Cancers 2024, 16, 2161. [Google Scholar] [CrossRef]
- Vanstraelen, S.; Rocco, G.; Park, B.J.; Jones, D.R. The necessity of preoperative planning and nodule localization in the modern era of thoracic surgery. JTCVS Open 2024, 18, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectony versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomized, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jpnes, D.R.; Conti, M.; Ashrafi, H.; et al. Lobar or sublobar resection peripheral stage IA non-small cell lung cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.; Louie, B.E.; Farivar, A.S.; Onaitis, M.; Park, B.J. Consensus statement on definitions and nomenclature for robotic thoracic surgery. J. Thorac. Cardiovasc. Surg. 2017, 154, 1065–1069. [Google Scholar] [CrossRef]
- Bertolaccini, L.; Batirel, H.; Brunelli, A.; Gonzalez-Rivas, D.; Ismail, M.; Ucar, A.M.; Ng, C.S.H.; Scarci, M.; Shihoe, A.D.L.; Ugalde, P.A.; et al. Uniportal video-assisted thoracic surgery lobectomy: A consensus report from the Uniportal VATS Interest Group (UVIG) of the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardiothorac. Surg. 2019, 56, 224–229. [Google Scholar] [CrossRef]
- Sortini, D.; Feo, C.; Maravegias, K.; Carcoforo, P.; Pozza, E.; Liboni, A.; Sortini, A. Intrathoracoscopic localization techniques. Review of literature. Surg. Endosc. 2006, 30, 1341. [Google Scholar] [CrossRef]
- Ichinose, J.; Kohno, T.; Fujimori, S.; Harano, T.; Suzuki, S. Efficacy and complications of computed tomography-guided hook wire localization. Ann. Thorac. Surg. 2013, 96, 1203–1208. [Google Scholar] [CrossRef]
- Yi, J.H.; Choi, P.J.; Bang, J.H.; Jeong, S.S.; Cho, J.H. Systemic air embolism after computed tomography-guided hook wire localization: Two cases reports and literature review. J. Thorac. Dis. 2018, 10, E59–E64. [Google Scholar] [CrossRef]
- Chang, S.S.; Okamoto, T.; Tokunaga, Y.; Nakano, T. Intraoperative computed tomography navigation during thoracoscopic segmentectomy for small-sized lung tumors. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 96–101. [Google Scholar] [CrossRef]
- Sato, M.; Omasa, M.; Chen, F.; Sato, T.; Sonobe, M.; Bando, T.; Date, H. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J. Thorac. Cardiovasc. Surg. 2014, 147, 1813–1819. [Google Scholar] [CrossRef]
- Keating, J.; Singhal, S. Novel methods of intraoperative localization and margin assessment of pulmonary nodules. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Bakhuis, W.; Sadeghi, A.H.; Moes, I.; Maat, A.P.W.M.; Siregar, S.; Bogers, A.J.J.C.; Mahtab, E.A.F. Essential surgical plan modifications after virtual reality planning in 50 consecutive segmentectomies. Ann. Thorac. Surg. 2023, 115, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, T.; Zhang, C.; Wu, G.; Xiong, R.; Xu, M.; Su, D.; Xie, M. Comparison of perioperative outcomes between precise and routine segmentectomy for patients with early-stage lung cancer presenting as ground-glass opacities: A propensity score-matched study. Front. Oncol. 2021, 11, 661821. [Google Scholar] [CrossRef] [PubMed]
- Anayama, T.; Qiu, J.; Chan, H.; Nakajima, T.; Weersink, R.; Daly, M.; McConnell, J.; Waddell, T.; Keshavjee, S.; Jaffray, D.; et al. Localization of pulmonary nodules using navigation bronchoscope and a near-infrared fluorescence thoracoscope. Ann. Thorac. Surg. 2015, 99, 224–230. [Google Scholar] [CrossRef]
- Ito, K.; Shimada, J.; Shimomura, M.; Terauchi, K.; Nishimura, M.; Yanada, M.; Iwasaki, Y.; Ueshima, Y.; Kato, D.; Suzuki, H.; et al. Safety and reliability of computed tomography-guided lipiodol marking for undetectable pulmonary lesions. Interdiscip. CardioVascular Thorac. Surg. 2020, 30, 546–551. [Google Scholar] [CrossRef]
- Yoshida, R.; Yoshizako, T.; Tanaka, S.; Ando, S.; Nakamura, M.; Kishimoto, K.; Kitagaki, H. CT-guided color marking of impalpable pulmonary nodules prior to video-assisted thoracoscopic surgery. Clin. Imaging 2021, 74, 84–88. [Google Scholar] [CrossRef]
- Doncic, N.; Zech, C.J.; Wild, D.; Bachmann, H.; Mallaev, M.; Tsvetkov, N.; Hojski, A.; Takes, M.T.L.; Lardinois, D. CT-guided percutaneous marking of small pulmonary nodules with [(99m)Tc] Tc-Macrosalb is very accurate and allows minimally invasive lung-sparing resection: A single-centre quality control. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2980–2987. [Google Scholar] [CrossRef]
- Voulaz, E.; Giudici, V.M.; Lanza, E.; Bottoni, E.; Cariboni, Y.; Crepaldi, A.; Ferrillo, G.; Marulli, G.; Alloisio, M.; Mangiameli, G.; et al. Percutaneous CT-guided localization with indocyanine green for the thoracoscopic resection of small pulmonary nodules. J. Clin. Med. 2023, 12, 6149. [Google Scholar] [CrossRef]
- Miyoshi, K.; Toyooka, S.; Gobara, H.; Oto, T.; Mimura, H.; Sano, Y.; Kanazawa, S.; Date, H. Clinical outcomes of short hook wire and suture marking system in thoracoscopic resection for pulmonary nodules. Eur. J. Cardiothorac. Surg. 2009, 36, 378–382. [Google Scholar] [CrossRef]
- Dendo, S.; Kanazawa, S.; Ando, A.; Hyodo, T.; Kouno, Y.; Yasui, K.; Mimura, H.; Akaki, S.; Kuroda, M.; Shimizu, N.; et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: Experience with 168 procedures. Radiology 2002, 225, 511–518. [Google Scholar] [CrossRef]
- Seo, J.M.; Lee, H.Y.; Kim, H.K.; Choi, Y.S.; Kim, J.; Shim, Y.M.; Lee, K.S. Factors determining successful computed tomography-guided localization of lung nodules. J. Thorac. Cardiovasc. Surg. 2012, 143, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Ciriaco, P.; Negri, G.; Puglisi, A.; Nicoletti, R.; Del Maschio, A.; Zannini, P. Video-assisted thoracoscopic surgery for pulmonary nodules. Rationale for preoperative computed tomography-guided hookwire localization. Eur. J. Cardiothorac. Surg. 2009, 36, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama, S.; Kondo, K.; Matsuoka, H.; Yoshida, M.; Miyoshi, T.; Yoshida, S.; Monden, Y. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-yupe marker. J. Thorac. Cardiovasc. Surg. 2003, 126, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Sarsam, M.; Baste, J.M.; Thiberville, L.; Salaun, M.; Lachkar, S. How bronchoscopic dye marking can help minimally invasive lung surgery. J. Clin. Med. 2022, 11, 3246. [Google Scholar] [CrossRef]
- Sato, M.; Yamada, T.; Menju, T.; Aoyama, A.; Sato, T.; Chen, F.; Sonobe, M.; Omasa, M.; Date, H. Virtual-assisted lung mapping: Outcome of 100 consecutive cases in a single institute. Eur. J. Cardiothorac. Surg. 2015, 47, e131–e139. [Google Scholar] [CrossRef]
- Sato, M.; Kuwata, T.; Yamanashi, K.; Kitamura, A.; Misawa, K.; Imashimizu, K.; Kobayashi, M.; Ikeda, M.; Koike, T.; Kosaka, S.; et al. Safety and reproducibility of virtual-assisted lung mapping: A multicentre study in Japan. Eur. J. Cardiothorac. Surg. 2017, 51, 861–868. [Google Scholar] [CrossRef]
- Nagano, M.; Sato, M. Ten-year outcome and development of virtual-assisted lung mapping in thoracic surgery. Cancers 2023, 15, 1971. [Google Scholar] [CrossRef]
- Krimsky, W.S.; Minnich, D.J.; Catanneo, S.M.; Sarkar, S.A.; Harley, D.P.; Finley, D.J.; Browning, R.F.; Parrish, S.C. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 23084. [Google Scholar] [CrossRef]
- Vandori, R.E.; Cuttat, J.F.; Wicky, S.; Suter, M. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur. J. Cardiothorac. Surg. 1998, 14, 265–270. [Google Scholar] [CrossRef]
- Yanagiya, M.; Hiyama, N.; Matsumoto, J. Hybrid technique of virtual-assisted lung mapping and systemic indocyanine green injection for extended segmentectomy. Surg. Case Rep. 2020, 6, 273. [Google Scholar] [CrossRef]
- Tokuno, J.; Chen-Yoshikawa, T.F.; Nakajima, D.; Aoyama, A.; Motoyama, H.; Sato, M.; Date, H. Improved visualization of virtual-assisted lung mapping by indocyanine green. JTCVS Tech. 2021, 10, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Yanagiya, M.; Sato, M.; Ijiri, N.; Kobayashi, K.; Nagano, M.; Konoeda, C.; Kitano, K.; Nakajima, J. Virtual-assisted lung mapping using dual staining with indocyanine green and indigo carmine enhanced marking detectability. J. Thorac. Dis. 2022, 14, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Sato, M.; Yanagiya, M.; Nakao, K.; Konoeda, C.; Kitano, K.; Nakajima, J. Number of dye marks required in virtual-assisted lung mapping. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Yanagiya, M.; Amano, Y.; Hiyama, N.; Matsumoto, J. Initial experience of virtual-assisted lung mapping utilizing both indocyanine green and indigo carmine. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1035. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kobayashi, M.; Sakamoto, J.; Fukai, R.; Takizawa, H.; Shinohara, S.; Kojima, F.; Sakurada, A.; Nakajima, J. The role of virtual-assisted lung mapping 2.0 combining microcoils and dye marks in deep lung resection. J. Thorac. Cardiovasc. Surg. 2022, 164, 243–251. [Google Scholar] [CrossRef]
- Yutaka, Y.; Sato, T.; Hidaka, Y.; Kato, T.; Kayawake, H.; Tanaka, S.; Yamada, Y.; Ohsumi, A.; Nakajima, D.; Hamaji, M.; et al. Electromagnetic navigation bronchoscopy-guided radiofrequency identification marking in wedge resection for fluoroscopically invisible small lung lesions. Eur. J. Cardiothorac. Surg. 2023, 63, ezad006. [Google Scholar] [CrossRef]
- Yutaka, Y.; Sato, T.; Matsushita, K.; Aiba, H.; Muranishi, Y.; Sakaguchi, Y.; Sugiura, T.; Okada, M.; Nakamura, T.; Date, H. Three-dimensional navigation for thoracoscopic sublobar resection using a novel wireless marking system. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 230–237. [Google Scholar] [CrossRef]
- Miyahara, S.; Waseda, R.; Ueda, Y.; Yutaka, Y.; Date, H.; Suzuki, J.; Oizumi, H.; Goto, M.; Nakagawa, T.; Kojima, F.; et al. Evaluation of the radiofrequency identification lung marking system: A multicenmter study in Japan. Surg. Endosc. 2023, 37, 3619–3626. [Google Scholar] [CrossRef]
- Sato, M.; Shinohara, Y.; Yanagiya, M.; Karasaki, T.; Kitano, K.; Nagayama, K.; Nakajima, J. Use of electromagnetic navigation bronchoscopy in virtual-assisted lung mapping: The effect of on-site adjustment. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 1062–1069. [Google Scholar] [CrossRef]
- Shahoud, J.; Weksler, B.; Ghosh, S.; Ganesh, A.; Fernando, H. Robot-assisted bronchoscopy for identification of lung nodules during minimally invasive pulmonary resection. Innovations 2024, 19, 15569845241247549. [Google Scholar] [CrossRef]
- Bawaadam, H.; Benn, B.S.; Colwell, E.M.; Oka, T.; Krishna, G. Lung nodule marking with ICG dye-soaked coil gacilitates localization and delayed surgical resection. Ann. Thorac. Surg. Short Rep. 2023, 1, 221–225. [Google Scholar] [CrossRef]
- Chang, S.S.; Yokomise, H.; Yokota, N.; Yoshida, C.; Katoh, A.; Misaki, N.; Go, T. Dual image navigation to secure surgical margins in thoracoscopic segmentectomy. Ann. Surg. Oncol. 2023, 30, 843–849. [Google Scholar] [CrossRef]
- Cang, C.J.; Lu, C.H.; Fang, H.Y.; Chao, Y.K. Safety and efficacy of cone-beam computed tomography-guided lung tumor localization with a near-infrared marker: A retrospective study of 175 patients. Life 2022, 12, 494. [Google Scholar] [CrossRef]
- Gilberto, G.M.; Falsarella, P.M.; de Andrade, J.R.; Schmid, B.P.; Mariotti, G.C.; Terra, R.M.; de Campos, J.R.M.; Succi, J.E.; Garcia, R.G. Lung nodule localization in hybrid room before minimally invasive thoracic surgery: Series of 20 cases and literature review. Einstein 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Lyberis, P.; Della Beffa, E.; Calandri, M.; Rosboch, G.L.; Femia, F.; Garrone, P.M.; Neitzer, L.; Gazzera, C.; Buttiglieri, A.; Carmelo, A.; et al. All-in-one diagnostic and therapeutic precision thoracic surgery using a hybrid operating theatre: The triple marking technique. Minerva Surg. 2023, 78, 644–650. [Google Scholar] [CrossRef]
- Fujiwara-Kuroda, A.; Aragaki, M.; Hida, Y.; Ujiie, H.; Ohtaka, K.; Shiiya, H.; Kaga, K.; Kato, T. A simple and safe surgical technique for nonpalpable lung tumors: One-stop solution for a nonpalpable lung tumor, marking, resection, and confirmation of the surgical margin in a hybrid operating room (OS-MRCH). Transl. Lung Cancer Res. 2024, 13, 603–611. [Google Scholar] [CrossRef]
- Kaiho, T.; Suzuki, H.; Hata, A.; Ito, T.; Tanaka, K.; Sakairi, Y.; Kato, H.; Shiko, Y.; Kawasaki, Y.; Yoshino, I. Efficacy and safety of intraoperative cone-beam CT-guided localization of small pulmonary nodules. Interdiscip. CardioVascular Thorac. Surg. 2022, 35, ivac236. [Google Scholar] [CrossRef]
- Song, J.W.; Park, I.K.; Bae, S.Y.; Na, K.J.; Park, S.; Kang, C.H.; Kim, Y.T. Electromagnetic navigation bronchoscopy-guided dye marking for localization of pulmonary nodules. Ann. Thorac. Surg. 2022, 113, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Park, H.; Choi, C.M.; Oh, J.H.; Lee, G.D.; Kim, D.K.; Hwang, H.S.; Jang, S.J.; Oh, S.Y.; Kim, M.Y.; et al. Preoperative electromagnetic navigation bronchoscopy-guided one-stage multiple-dye localization for resection of subsolid nodules: A single-center pilot study. Thorac. Cancer 2022, 13, 466–473. [Google Scholar] [CrossRef]
- Taton, O.; Sokolow, Y.; Bondue, B.; Vandermeeren, C.; Kuylen, M.V.; Gevenois, P.A.; Remmelink, M.; Ngono, Z.M.; Berghmans, T.; Leduc, D. Cryobiopsy and dye marking guided by electromagnetic navigation bronchoscopy before resection of pulmonary nodule. Respir. Med. Res. 2022, 81, 108911. [Google Scholar] [CrossRef]
- Chan, J.W.Y.; Chang, A.T.C.; Yu, P.S.Y.; Lau, R.W.H.; Ng, C.S.H. Robotic assisted-bronchoscopy with cone-beam CT ICG dye marking for lung nodule localization: Experience beyond USA. Front. Surg. 2022, 9, 943531. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Bove, M.; Natale, G.; Noro, A.; Martone, M.; Opromolla, G.; Di Filippo, V.; Leonardi, B.; Fasano, M.; Polito, R.; et al. Ultrasound location of ground-glass opacity during thoracoscopic surgery. Interdiscip. Cardiovasc. Thorac. Surg. 2022, 35, ivac234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, Z.; Zheng, Z.; Cao, J.; Zhang, C.; He, Z.; Lv, W.; Hu, J. An “alterative finger” in robotic-assisted thoracic surgery: Intraoperative ultrasound localization of pulmonary nodules. Med. Ultrason. 2017, 19, 374–379. [Google Scholar] [CrossRef]
- Okusanya, O.T.; Holt, D.; Heitjan, D.; Deshpande, C.; Venegas, O.; Jiang, J.; Judy, R.; DeJesus, E.; Madajewski, B.; Oh, K.; et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann. Thorac. Surg. 2014, 98, 1223–1230. [Google Scholar] [CrossRef]
- Jeon, O.H.; Choi, B.H.; Rho, J.; Kim, K.; Lee, J.H.; Lee, J.; Kim, B.M.; Kim, H.K. Optimization of indocyanine green for intraoperative fluorescent image-guided localization of lung cancer; analysis based on solid component of lung nodule. Cancers 2023, 15, 3643. [Google Scholar] [CrossRef]
- Kennedy, G.T.; Okusanya, O.T.; Keating, J.J.; Heitjan, D.; Deshpande, C.; Litzky, L.A.; Albelda, S.M.; Drebin, J.A.; Nie, S.; Low, P.S.; et al. The optical biopsy: A novel technique for rapid intraoperative diagnosis of primary pulmonary adenocarcinomas. Ann. Surg. 2015, 262, 602–609. [Google Scholar] [CrossRef]
- Okusanya, O.T.; DeJesus, E.M.; Jiang, J.X.; Judy, R.P.; Venegas, O.G.; Deshpande, C.G.; Heitjan, D.F.; Nie, S.; Low, P.S.; Singhal, S. Intraoperative molecular imaging can identify lung adenocarcinomas during pulmonary resection. J. Thorac. Cardiovasc. Surg. 2015, 150, 28–35. [Google Scholar] [CrossRef]
- Keating, J.J.; Kennedy, G.T.; Singhal, S. Identification of a subcentimeter pulmonary adenocarcinoma using intraoperative near-infrared imaging during video-assisted thoracoscopic surgery. J. Thorac. Cardiovasc. Surg. 2015, 149, e51–e53. [Google Scholar] [CrossRef]
- Sarkaria, I.S.; Martin, L.W.; Rice, D.C.; Blackmon, S.H.; Slade, H.B.; Singhal, S.; Elucidate Study Group. Pafolacianine for intraoperative molecular imaging of cancer in the lung: The ELUCIDATE trial. J. Thorac. Cardiovasc. Surg. 2023, 166, e468–e478. [Google Scholar] [CrossRef]
- Azari, F.; Kennedy, G.; Zhang, K.; Bernstein, E.; Chang, A.; Nadeem, B.; Segil, A.; Desphande, C.; Delikatny, J.; Kucharczuk, J.; et al. Effects of light-absorbing carbons in intaroperative molecular imaging-guided lung cancer resections. Mol. Imaging Biol. 2023, 25, 156–167. [Google Scholar] [CrossRef]
- Kennedy, G.T.; Azari, F.S.; Chang, A.; Nadeem, B.; Bernstein, E.; Segil, A.; Din, A.; Desphande, C.; Okusanya, O.; Keating, J.; et al. Single-institution experience of 500 pulmonary resections guided by intraoperative molecular imaging. J. Thorac. Cardiovasc. Surg. 2023, 165, 1928–1938. [Google Scholar] [CrossRef]
- Cannone, G.; Verzeletti, V.; Busetto, A.; Lione, L.; Bonis, A.; Nicotra, S.; Rebusso, A.; Mammana, M.; Schiavon, M.; Dell’Amore, A.; et al. Three-dimensional imaging-guided lung anatomic segmentectomy: A single-center preliminary experiment. Medicina 2023, 59, 2079. [Google Scholar] [CrossRef] [PubMed]
- Moal, J.L.; Peillon, C.; Dacher, J.N.; Baste, J.M. Three-dimensional computed tomography reconstruction for operative planning in robotic segmentectomy: A pilot study. J. Thorac. Dis. 2018, 10, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Tokuno, J.; Chen-Yoshikawa, T.F.; Nakao, M.; Matsuda, T.; Date, H. Resection Process Map: A novel dynamic simulation system for pulmonary resection. J. Thorac. Cardiovasc. Surg. 2020, 159, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Tokuno, J.; Chen-Yoshikawa, T.F.; Nakao, M.; Iwakura, M.; Motoki, T.; Matsuda, T.; Date, H. Creation of a video library for education and virtual simulation of anatomical lung resection. Interdiscip. Cardiovasc. Thorac. Surg. 2022, 34, 808–813. [Google Scholar] [CrossRef]
- Kadomatsu, Y.; Nakao, M.; Ueno, H.; Nakamura, S.; Fukumoto, K.; Chen-Yoshikawa, T.F. Clinical application of resection process map as a novel surgical guide in thoracic surgery. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad059. [Google Scholar] [CrossRef]
- Okado, S.; Kadomatsu, Y.; Nakao, M.; Ueno, H.; Fukumoto, K.; Nakamura, S.; Chen-Yoshikawa, T.F. New method for delineation of the intersegmental line in a deflated lung. J. Thorac. Dis. 2023, 15, 4736–4744. [Google Scholar] [CrossRef]


| Marking Method | Before 2019 | 2020 | 2021 | 2022 | 2023 | Sum |
|---|---|---|---|---|---|---|
| Hook wire | All | 15 | 8 | 18 | 21 | 62 |
| VAL-MAP | 2 | 11 | 12 | 12 | 37 | |
| CBCT | 5 | 20 | 13 | 1 | 39 | |
| (Multiple: VAL-MAP + CBCT) | (2) | (4) | (7) | (1) | (14) | |
| Patients undergoing preoperativeor intraoperative marking | 20 | 35 | 36 | 33 | 124 | |
| Surgery for malignant tumors | 305 | 325 | 342 | 370 | 1342 | |
| (Rate of marking in surgery for malignant tumors) | (7%) | (11%) | (11%) | (9%) | (9%) |
| Marking Method | Place | Performer | Condition | Timing |
|---|---|---|---|---|
| Hook wire | CT suite | Radiologist | With thoracic surgeon | Almost every day (One day before or on the day of surgery) |
| VAL-MAP | Bronchoscopy suite | Respirologist | With thoracic surgeon | Once a week (Thursday) (One day before surgery) |
| CBCT | Hybrid OR | Thoracic surgeon | When not used by other departments with priority rights | Once a week (Friday) (On the day of surgery) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen-Yoshikawa, T.F.; Nakamura, S.; Ueno, H.; Kadomatsu, Y.; Kato, T.; Mizuno, T. Current Status and Future Perspectives of Preoperative and Intraoperative Marking in Thoracic Surgery. Cancers 2024, 16, 3284. https://doi.org/10.3390/cancers16193284
Chen-Yoshikawa TF, Nakamura S, Ueno H, Kadomatsu Y, Kato T, Mizuno T. Current Status and Future Perspectives of Preoperative and Intraoperative Marking in Thoracic Surgery. Cancers. 2024; 16(19):3284. https://doi.org/10.3390/cancers16193284
Chicago/Turabian StyleChen-Yoshikawa, Toyofumi Fengshi, Shota Nakamura, Harushi Ueno, Yuka Kadomatsu, Taketo Kato, and Tetsuya Mizuno. 2024. "Current Status and Future Perspectives of Preoperative and Intraoperative Marking in Thoracic Surgery" Cancers 16, no. 19: 3284. https://doi.org/10.3390/cancers16193284
APA StyleChen-Yoshikawa, T. F., Nakamura, S., Ueno, H., Kadomatsu, Y., Kato, T., & Mizuno, T. (2024). Current Status and Future Perspectives of Preoperative and Intraoperative Marking in Thoracic Surgery. Cancers, 16(19), 3284. https://doi.org/10.3390/cancers16193284







