Modulation of PRC1 Promotes Anticancer Effects in Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. UALCAN Database Analysis
2.2. GEPIA Database Analysis
2.3. TIMER Database Analysis
2.4. Immunohistochemistry (IHC) Staining Analysis
2.5. Cell Culture and siRNA Transfection
2.6. Cell Viability Assay
2.7. Western Blot
2.8. Wound Healing Assay
2.9. Annexin V Staining Assay
2.10. Statistical Analysis
3. Results
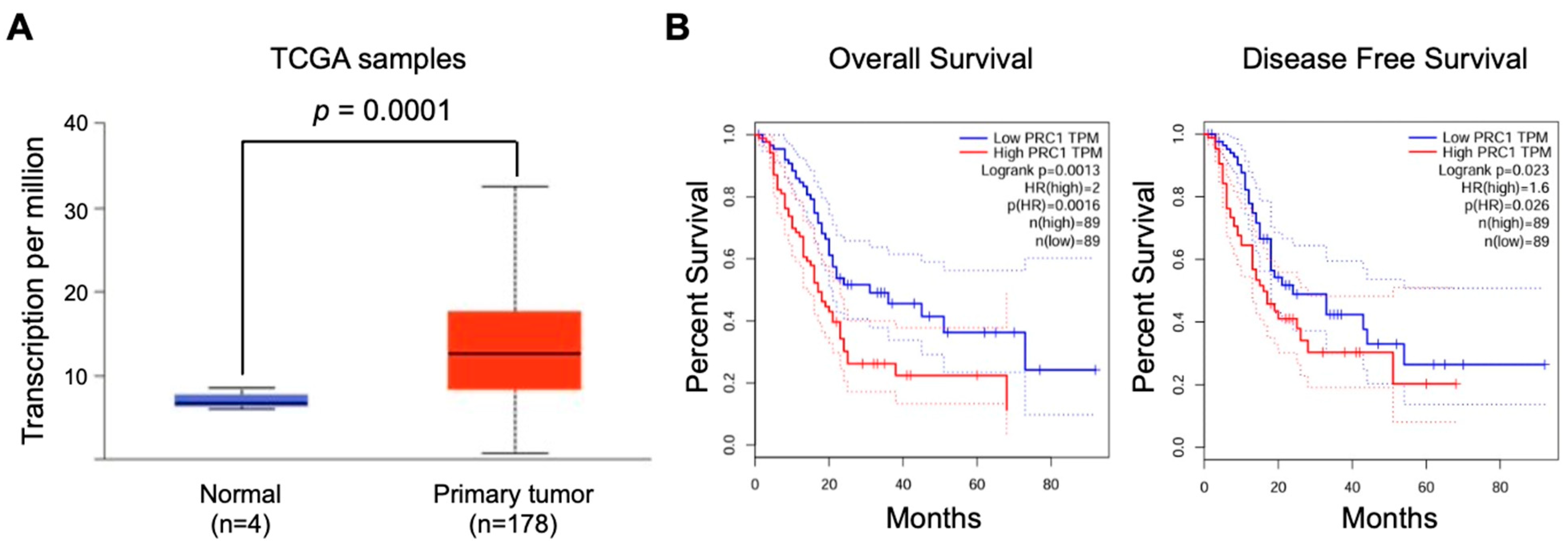
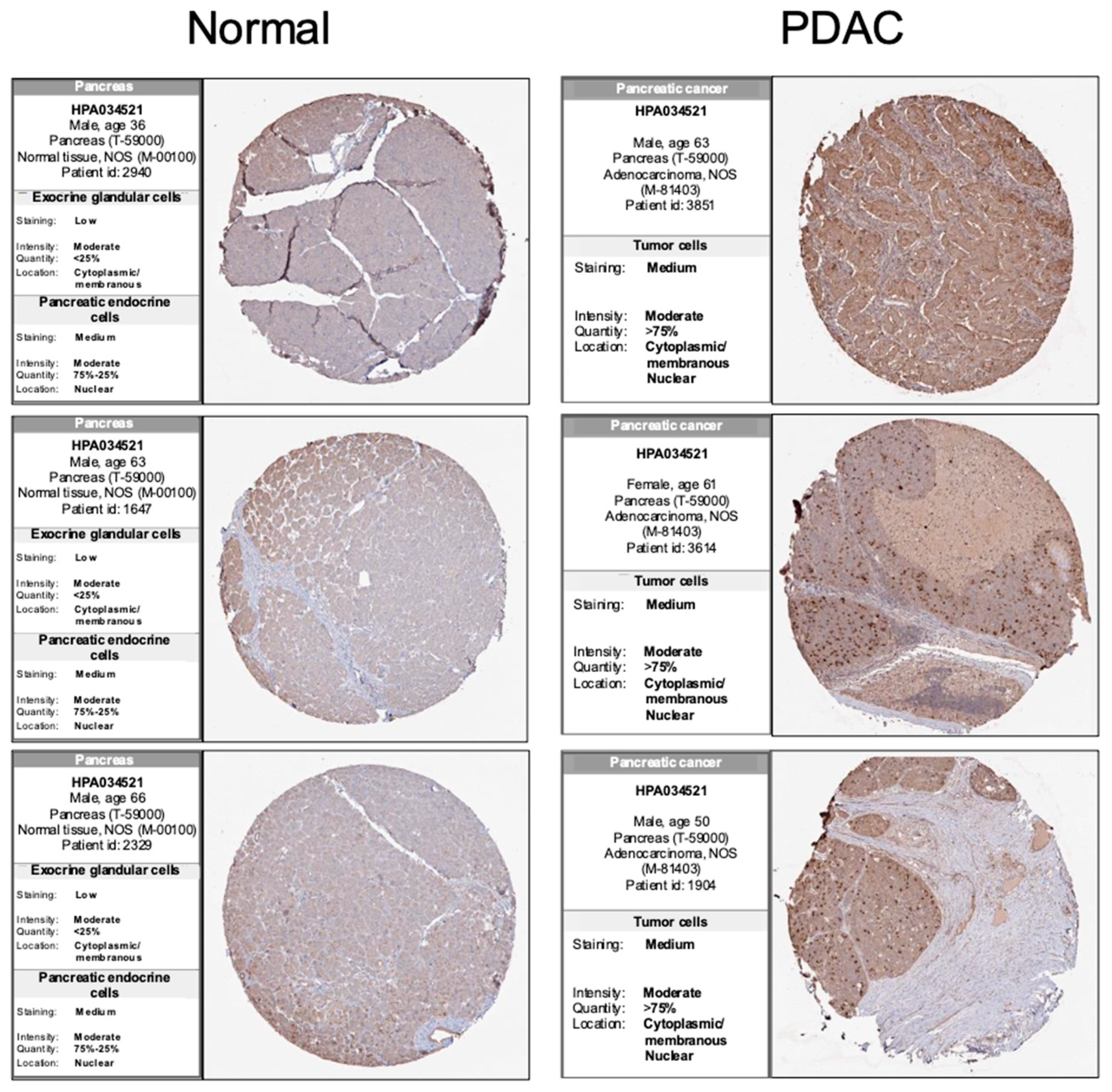
3.1. PRC1 Is Significantly Overexpressed in Pancreatic Cancer Patients and Has Potential Clinical Significance
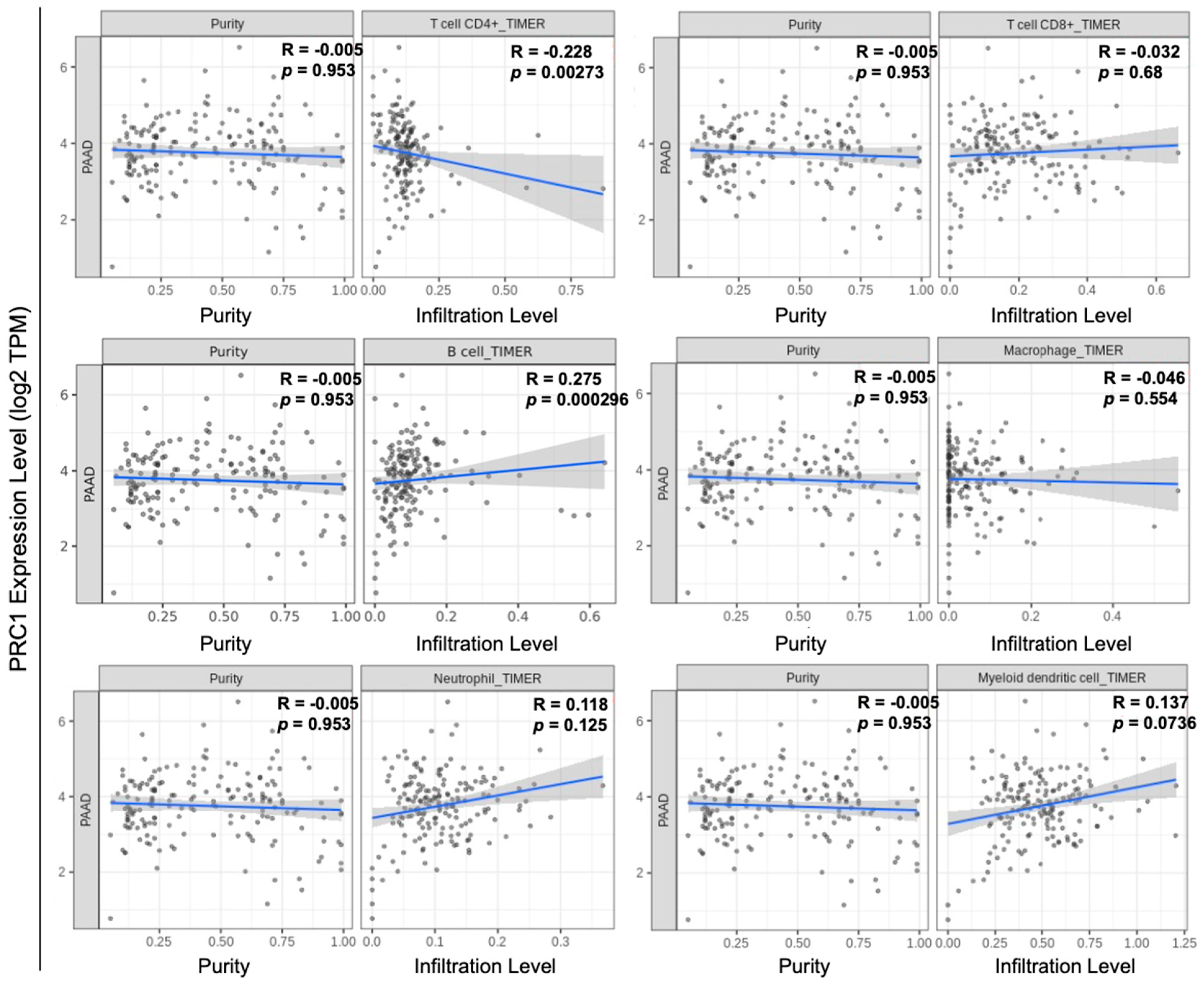
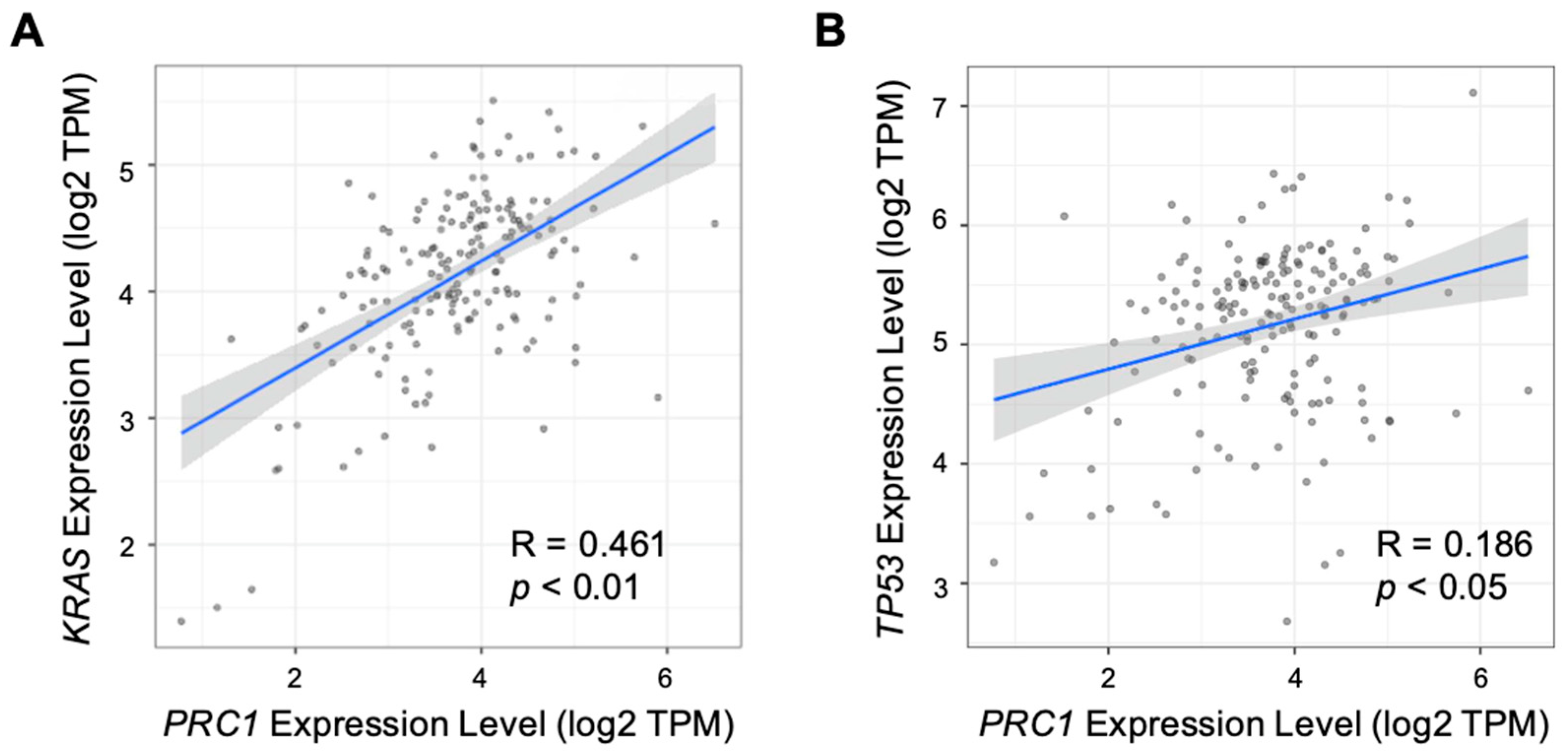
3.2. PRC1 May Be Related to Immune Cell Infiltration and Other PDAC Markers
3.3. Reduced PRC1 Expression Inhibits the Proliferation of Pancreatic Cancer Cells
3.4. Blocking of PRC1 Increases Sensitivity to a Chemotherapeutic Drug in Pancreatic Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic cancer. A review. J. Am. Med. Assoc. 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Halbrook, C.J.; Lyssiotis, C.A.; di Magliano, M.P.; Maitra, A. Pancreatic cancer: Advances and challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef]
- Bengtsson, A.; Andersson, R.; Ansari, D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci. Rep. 2020, 10, 16425. [Google Scholar] [CrossRef] [PubMed]
- Stoop, T.F.; Theijse, R.T.; Seelen, L.W.F.; Koerkamp, B.G.; van Eijck, C.H.J.; Wolfgang, C.L.; van Tienhoven, G.; van Santvoort, H.C.; Molenaar, I.Q.; Wilmink, J.W.; et al. Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 101–124. [Google Scholar] [CrossRef]
- Garajová, I.; Peroni, M.; Gelsomino, F.; Leonardi, F. A simple overview of pancreatic cancer treatment for clinical oncologists. Curr. Oncol. 2023, 30, 9587–9601. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.Y.; Kitahara, K.; Sasaki, N.; Nakao, N.; Sato, K.; Narita, H.; Shimoda, H.; Matsusaki, M.; Nishihara, H.; Masamune, A.; et al. Pancreatic stellate cells derived from human pancreatic cancer demonstrate aberrant SPARC-dependent ECM remodeling in 3D engineered fibrotic tissue of clinically relevant thickness. Biomaterials 2019, 192, 355–367. [Google Scholar] [CrossRef]
- Hosein, A.N.; Dougan, S.K.; Aguirre, A.J.; Maitra, A. Translational advances in pancreatic ductal adenocarcinoma therapy. Nat. Cancer 2022, 3, 272–286. [Google Scholar] [CrossRef]
- Min, K.K.M.; Ffrench, C.B.; Jessup, C.F.; Shepherdson, M.; Barreto, S.G.; Bonder, C.S. Overcoming the fibrotic fortress in pancreatic ductal adenocarcinoma: Challenges and opportunities. Cancers 2023, 15, 2354. [Google Scholar] [CrossRef]
- Hude, I.; Sasse, S.; Engert, A.; Bröckelmann, P.J. The emerging role of immune checkpoint inhibition in malignant lymphoma. Haematologica 2017, 102, 30–42. [Google Scholar] [CrossRef]
- Yeo, D.; Giardina, C.; Saxena, P.; Rasko, J.E. The next wave of cellular immunotherapies in pancreatic cancer. Mol. Ther. Oncolytics 2022, 24, 561–576. [Google Scholar] [CrossRef]
- Laface, C.; Memeo, R.; Maselli, F.M.; Santoro, A.N.; Iaia, M.L.; Ambrogio, F.; Laterza, M.; Cazzato, G.; Guarini, C.; De Santis, P.; et al. Immunotherapy and Pancreatic Cancer: A Lost Challenge? Life 2023, 13, 1482. [Google Scholar] [CrossRef]
- Li, S.; Motiño, O.; Lambertucci, F.; Martins, I.; Sun, L.; Kroemer, G. Protein regulator of cytokinesis 1: A potential oncogenic driver. Mol. Cancer 2023, 22, 128. [Google Scholar] [CrossRef]
- Jiang, W.; Jimenez, G.; Wells, N.J.; Hope, T.J.; Wahl, G.M.; Hunter, T.; Fukunaga, R. Prc1: A human mitotic spindle-associated cdk substrate protein required for cytokinesis. Mol. Cell 1998, 2, 877–885. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, D.; Kong, X.; Han, Z.; Hao, X.; Wang, X.; Wen, X.; Liang, C. Protein regulator of cytokinesis 1 regulates chromosome dynamics and cytoplasmic division during mouse oocyte meiotic maturation and early embryonic development. FEBS J. 2020, 287, 5130–5147. [Google Scholar] [CrossRef]
- Xu, T.; Wang, X.; Jia, X.; Gao, W.; Li, J.; Gao, F.; Zhan, P.; Ji, W. Overexpression of protein regulator of cytokinesis 1 facilitates tumor growth and indicates unfavorable prognosis of patients with colon cancer. Cancer Cell Int. 2020, 20, 528. [Google Scholar] [CrossRef]
- Hu, C.-K.; Özlü, N.; Coughlin, M.; Steen, J.J.; Mitchison, T.J. Plk1 negatively regulates PRC1 to prevent premature midzone formation before cytokinesis. Mol. Biol. Cell 2012, 23, 2702–2711. [Google Scholar] [CrossRef]
- Gluszek-Kustusz, A.; Craske, B.; Legal, T.; McHugh, T.; Welburn, J.P. Phosphorylation controls spatial and temporal activities of motor-PRC1 complexes to complete mitosis. EMBO J. 2023, 42, e113647. [Google Scholar] [CrossRef]
- Hanselmann, S.; Wolter, P.; Malkmus, J.; Gaubatz, S. The microtubule-associated protein PRC1 is a potential therapeutic target for lung cancer. Oncotarget 2018, 9, 4985–4997. [Google Scholar] [CrossRef]
- Liang, Z.; Li, X.; Chen, J.; Cai, H.; Zhang, L.; Li, C.; Tong, J.; Hu, W. PRC1 promotes cell proliferation and cell cycle progression by regulating p21/p27-pRB family molecules and FAK-paxillin pathway in non-small cell lung cancer. Transl. Cancer Res. 2019, 8, 2059–2072. [Google Scholar] [CrossRef]
- Bu, H.; Li, Y.; Jin, C.; Yu, H.; Wang, X.; Chen, J.; Wang, Y.; Ma, Y.; Zhang, Y.; Kong, B. Overexpression of PRC1 indicates a poor prognosis in ovarian cancer. Int. J. Oncol. 2020, 56, 685–696. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Bear, A.S.; Vonderheide, R.H.; O’Hara, M.H. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell 2020, 38, 788–802. [Google Scholar] [CrossRef]
- Ullman, N.A.; Burchard, P.R.; Dunne, R.F.; Linehan, D.C. Immunologic strategies in pancreatic cancer: Making cold tumors Hot. J. Clin. Oncol. 2022, 40, 2789–2805. [Google Scholar] [CrossRef]
- Hartupee, C.; Nagalo, B.M.; Chabu, C.Y.; Tesfay, M.Z.; Coleman-Barnett, J.; West, J.T.; Moaven, O. Pancreatic cancer tumor microenvironment is a major therapeutic barrier and target. Front. Immunol. 2024, 15, 1287459. [Google Scholar] [CrossRef]
- Gu, A.; Li, J.; Qiu, S.; Hao, S.; Yue, Z.-Y.; Zhai, S.; Li, M.-Y.; Liu, Y. Pancreatic cancer environment: From patient-derived models to single-cell omics. Mol. Omics 2024, 20, 220–233. [Google Scholar] [CrossRef]
- Morton, J.P.; Timpson, P.; Karim, S.A.; Ridgway, R.A.; Athineos, D.; Doyle, B.; Jamieson, N.B.; Oien, K.A.; Lowy, A.M.; Brunton, V.G.; et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 246–251. [Google Scholar] [CrossRef]
- Collins, M.A.; Bednar, F.; Zhang, Y.; Brisset, J.-C.; Galbán, S.; Galbán, C.J.; Rakshit, S.; Flannagan, K.S.; Adsay, N.V.; di Magliano, M.P. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J. Clin. Investig. 2012, 122, 639–653. [Google Scholar] [CrossRef]
- Collins, M.A.; Brisset, J.-C.; Zhang, Y.; Bednar, F.; Pierre, J.; Heist, K.A.; Galbán, C.J.; Galbán, S.; di Magliano, M.P. Metastatic Pancreatic Cancer Is Dependent on Oncogenic Kras in Mice. PLoS ONE 2012, 7, e49707. [Google Scholar] [CrossRef]
- Gujral, T.S.; Kirschner, M.W. Hippo pathway mediates resistance to cytotoxic drugs. Proc. Natl. Acad. Sci. USA 2017, 114, E3729–E3738. [Google Scholar] [CrossRef]
- Chen, S.; Hsu, Y.; Wang, S.; Chen, Y.; Hong, C.; Yen, G. Lucidone inhibits autophagy and MDR1 via HMGB1/RAGE/PI3K/Akt signaling pathway in pancreatic cancer cells. Phytother. Res. 2022, 36, 1664–1677. [Google Scholar] [CrossRef]
- Lyu, P.-W.; Xu, X.-D.; Zong, K.; Qiu, X.-G. Overexpression of DUOX2 mediates doxorubicin resistance and predicts prognosis of pancreatic cancer. Gland. Surg. 2022, 11, 115–124. [Google Scholar] [CrossRef]
- Waters, A.M.; Der, C.J. KRAS: The critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef]
- Luo, J. KRAS mutation in pancreatic cancer. Semin. Oncol. 2021, 48, 10–18. [Google Scholar] [CrossRef]
- Luo, J.; Ostrem, J.; Pellini, B.; Imbody, D.; Stern, Y.; Solanki, H.S.; Haura, E.B.; Villaruz, L.C. Overcoming KRAS-mutant lung cancer. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 700–710. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Yang, F.; Zhu, L.; Zhu, X.-Q.; Wang, Z.-F.; Wu, X.-L.; Zhou, C.-H.; Yan, J.-Y.; Hu, B.-Y.; et al. The molecular biology of pancreatic adenocarcinoma: Translational challenges and clinical perspectives. Signal Transduct. Target. Ther. 2021, 6, 249. [Google Scholar] [CrossRef]
- Dai, M.; Chen, S.; Teng, X.; Chen, K.; Cheng, W. KRAS as a key oncogene in the clinical precision diagnosis and treatment of pancreatic cancer. J. Cancer 2022, 13, 3209–3220. [Google Scholar] [CrossRef]
- Shen, R.; Wang, Q.; Cheng, S.; Liu, T.; Jiang, H.; Zhu, J.; Wu, Y.; Wang, L. The biological features of PanIN initiated from oncogenic Kras mutation in genetically engineered mouse models. Cancer Lett. 2013, 339, 135–143. [Google Scholar] [CrossRef]
- Chen, J.; Rajasekaran, M.; Xia, H.; Zhang, X.; Kong, S.N.; Sekar, K.; Seshachalam, V.P.; Deivasigamani, A.; Goh, B.K.P.; Ooi, L.L.; et al. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/β-catenin signalling pathway. Gut 2016, 65, 1522–1534. [Google Scholar] [CrossRef]
- Li, J.; Ohmura, S.; Marchetto, A.; Orth, M.F.; Imle, R.; Dallmayer, M.; Musa, J.; Knott, M.M.L.; Hölting, T.L.B.; Stein, S.; et al. Therapeutic targeting of the PLK1-PRC1-axis triggers cell death in genomically silent childhood cancer. Nat. Commun. 2021, 12, 5356. [Google Scholar] [CrossRef]
- Hung, K.; Hayashi, R.; Lafond-Walker, A.; Lowenstein, C.; Pardoll, D.; Levitsky, H. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 1998, 188, 2357–2368. [Google Scholar] [CrossRef]
- Ostroumov, D.; Fekete-Drimusz, N.; Saborowski, M.; Kühnel, F.; Woller, N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell. Mol. Life Sci. 2018, 75, 689–713. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy—New insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef]
- Taniuchi, I. CD4 Helper and CD8 Cytotoxic T Cell Differentiation. Annu. Rev. Immunol. 2018, 36, 579–601. [Google Scholar] [CrossRef]
- Wakil, A.E.; Wang, Z.-E.; Ryan, J.C.; Fowell, D.J.; Locksley, R.M. Interferon γ derived from CD4+ T cells is sufficient to mediate t helper cell type 1 development. J. Exp. Med. 1998, 188, 1651–1656. [Google Scholar] [CrossRef]
- Fischer, J.; Dirks, J.; Haase, G.; Holl-Wieden, A.; Hofmann, C.; Girschick, H.; Morbach, H. IL-21+ CD4+ T helper cells co-expressing IFN-γ and TNF-α accumulate in the joints of antinuclear antibody positive patients with juvenile idiopathic arthritis. Clin. Immunol. 2020, 217, 108484. [Google Scholar] [CrossRef]
- Jeong, S.; Jang, N.; Kim, M.; Choi, I.-K. CD4+ cytotoxic T cells: An emerging effector arm of anti-tumor immunity. BMB Rep. 2023, 56, 140–144. [Google Scholar] [CrossRef]
- Hasegawa, T.; Oka, T.; Son, H.G.; Oliver-García, V.S.; Azin, M.; Eisenhaure, T.M.; Lieb, D.J.; Hacohen, N.; Demehri, S. Cytotoxic CD4+ T cells eliminate senescent cells by targeting cytomegalovirus antigen. Cell 2023, 186, 1417–1431.e20. [Google Scholar] [CrossRef] [PubMed]
- Muranski, P.; Restifo, N.P. Adoptive immunotherapy of cancer using CD4+ T cells. Curr. Opin. Immunol. 2009, 21, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kravtsov, D.S.; Erbe, A.K.; Sondel, P.M.; Rakhmilevich, A.L. Roles of CD4+ T cells as mediators of antitumor immunity. Front. Immunol. 2022, 13, 972021. [Google Scholar] [CrossRef]
- Bove, C.; Arcangeli, S.; Falcone, L.; Camisa, B.; El Khoury, R.; Greco, B.; De Lucia, A.; Bergamini, A.; Bondanza, A.; Ciceri, F.; et al. CD4 CAR-T cells targeting CD19 play a key role in exacerbating cytokine release syndrome, while maintaining long-term responses. J. Immunother. Cancer 2023, 11, e005878. [Google Scholar] [CrossRef] [PubMed]
- Chinni, S.S.; Taylor, M.F.; Borger, J.G.; Quinn, K.M. Highlight of 2023: Virtues and vices of CD4 CAR T cells. Immunol. Cell Biol. 2024, 102, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Quiñonero, F.; Mesas, C.; Doello, K.; Cabeza, L.; Perazzoli, G.; Jimenez-Luna, C.; Rama, A.R.; Melguizo, C.; Prados, J. The challenge of drug resistance in pancreatic ductal adenocarcinoma: A current overview. Cancer Biol. Med. 2019, 16, 688–699. [Google Scholar] [CrossRef]
- Hsu, S.-K.; Jadhao, M.; Liao, W.-T.; Chang, W.-T.; Hung, C.-T.; Chiu, C.-C. Culprits of PDAC resistance to gemcitabine and immune checkpoint inhibitor: Tumour microenvironment components. Front. Mol. Biosci. 2022, 9, 1020888. [Google Scholar] [CrossRef]
- Hanicinec, V.; Brynychova, V.; Rosendorf, J.; Palek, R.; Liska, V.; Oliverius, M.; Kala, Z.; Mohelnikova-Duchonova, B.; Krus, I.; Soucek, P. Gene expression of cytokinesis regulators prc1, kif14 and cit has no prognostic role in colorectal and pancreatic cancer. Oncol. Lett. 2021, 22, 598. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Bae, A.-N.; Yang, H.; Lee, J.-H.; Park, J.H. Modulation of PRC1 Promotes Anticancer Effects in Pancreatic Cancer. Cancers 2024, 16, 3310. https://doi.org/10.3390/cancers16193310
Lee H, Bae A-N, Yang H, Lee J-H, Park JH. Modulation of PRC1 Promotes Anticancer Effects in Pancreatic Cancer. Cancers. 2024; 16(19):3310. https://doi.org/10.3390/cancers16193310
Chicago/Turabian StyleLee, Hajin, An-Na Bae, Huiseong Yang, Jae-Ho Lee, and Jong Ho Park. 2024. "Modulation of PRC1 Promotes Anticancer Effects in Pancreatic Cancer" Cancers 16, no. 19: 3310. https://doi.org/10.3390/cancers16193310
APA StyleLee, H., Bae, A.-N., Yang, H., Lee, J.-H., & Park, J. H. (2024). Modulation of PRC1 Promotes Anticancer Effects in Pancreatic Cancer. Cancers, 16(19), 3310. https://doi.org/10.3390/cancers16193310




