The Tumor Microenvironment as a Therapeutic Target in Cutaneous T Cell Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
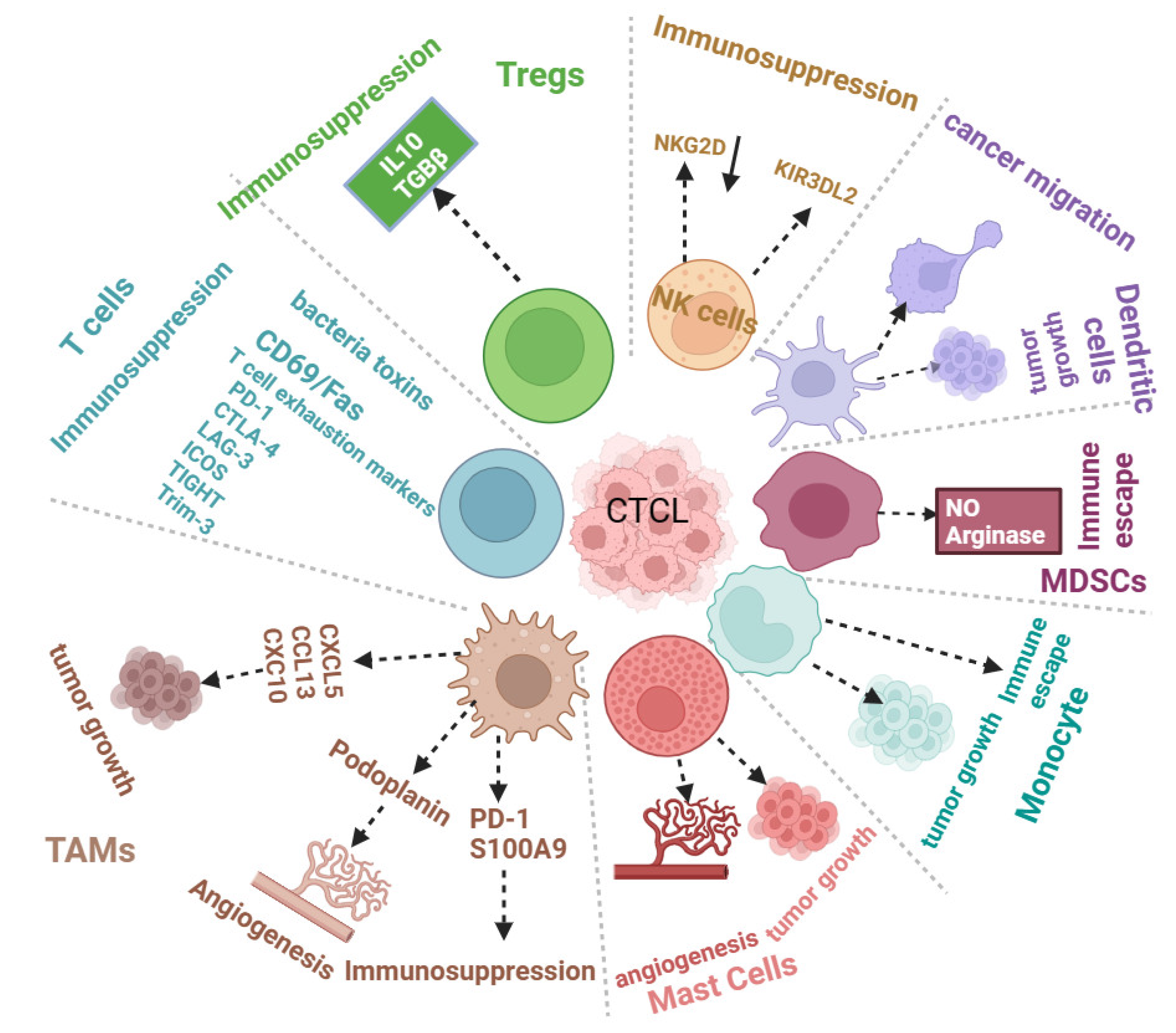
2. Role of CTCL Tumor Microenvironment in CTCL Progression
2.1. The Immune Tumor Microenvironment and CTCL Progression
2.1.1. Macrophages
2.1.2. Mast Cells
2.1.3. Eosinophils and Neutrophils
2.1.4. Dendritic Cells and Natural Killer Cells
2.1.5. Myeloid-Derived Suppressor Cells (MDSCs)
2.1.6. Tumor-Infiltrating Lymphocytes
2.1.7. Regulatory T Cells
2.2. Role of Cancer-Associated Fibroblasts in CTCL
2.3. Role of Vascular or Endothelial Cells in CTCLs
3. Molecular Mechanisms of CTCL Immune Evasion
3.1. Apoptosis Resistance
3.2. Cytokine Dysregulation in CTCLs
3.3. Genetic Alterations in CTCLs
3.4. Immune Checkpoint-Mediated Suppression of T Cells
3.5. Exosomes in CTCLs
4. Advances in Strategies to Target CTCLs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rubio Gonzalez, B.; Zain, J.; Rosen, S.T.; Querfeld, C. Tumor microenvironment in mycosis fungoides and Sézary syndrome. Curr. Opin. Oncol. 2016, 28, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Agar, N.S.; Wedgeworth, E.; Crichton, S.; Mitchell, T.J.; Cox, M.; Ferreira, S.; Robson, A.; Calonje, E.; Stefanato, C.M.; Wain, E.M.; et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: Validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4730–4739. [Google Scholar] [CrossRef] [PubMed]
- Criscione, V.D.; Weinstock, M.A. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch. Dermatol. 2007, 143, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Trautinger, F.; Eder, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Gniadecki, R.; Klemke, C.D.; Ortiz-Romero, P.L.; Papadavid, E.; et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2017. Eur. J. Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef]
- Najidh, S.; Tensen, C.P.; van der Sluijs-Gelling, A.J.; Teodosio, C.; Cats, D.; Mei, H.; Kuipers, T.B.; Out-Luijting, J.J.; Zoutman, W.H.; van Hall, T.; et al. Improved Sézary cell detection and novel insights into immunophenotypic and molecular heterogeneity in Sézary syndrome. Blood 2021, 138, 2539–2554. [Google Scholar] [CrossRef]
- Wilcox, R.A. Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2016, 91, 151–165. [Google Scholar] [CrossRef]
- Lessin, S.R.; Duvic, M.; Guitart, J.; Pandya, A.G.; Strober, B.E.; Olsen, E.A.; Hull, C.M.; Knobler, E.H.; Rook, A.H.; Kim, E.J.; et al. Topical chemotherapy in cutaneous T-cell lymphoma: Positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013, 149, 25–32. [Google Scholar] [CrossRef]
- Quaglino, P.; Maule, M.; Prince, H.M.; Porcu, P.; Horwitz, S.; Duvic, M.; Talpur, R.; Vermeer, M.; Bagot, M.; Guitart, J.; et al. Global patterns of care in advanced stage mycosis fungoides/Sezary syndrome: A multicenter retrospective follow-up study from the Cutaneous Lymphoma International Consortium. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2517–2525. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Prince, H.M.; Kim, Y.H.; Horwitz, S.M.; Dummer, R.; Scarisbrick, J.; Quaglino, P.; Zinzani, P.L.; Wolter, P.; Sanches, J.A.; Ortiz-Romero, P.L.; et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): An international, open-label, randomised, phase 3, multicentre trial. Lancet 2017, 390, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, G.M.; Guan, J.Y.; Chen, Y.J.; Zhao, Y.Z.; Wang, K.; Bai, O. Epigenetic regulation of cutaneous T-cell lymphoma is mediated by dysregulated lncRNA MALAT1 through modulation of tumor microenvironment. Front. Oncol. 2022, 12, 977266. [Google Scholar] [CrossRef] [PubMed]
- Gaydosik, A.M.; Stonesifer, C.J.; Tabib, T.; Lafyatis, R.; Geskin, L.J.; Fuschiotti, P. The mycosis fungoides cutaneous microenvironment shapes dysfunctional cell trafficking, antitumor immunity, matrix interactions, and angiogenesis. JCI Insight 2023, 8, e170015. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.A.; Rivet, J.; Daneshpouy, M.; Briere, J.; Morel, P.; Janin, A. In situ eosinophil activation in 26 primary cutaneous T-cell lymphomas with blood eosinophilia. J. Am. Acad. Dermatol. 2005, 52, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kwantwi, L.B.; Rosen, S.T.; Querfeld, C. The role of signaling lymphocyte activation molecule family receptors in hematologic malignancies. Curr. Opin. Oncol. 2024, 36, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Alcántara-Hernández, M.; Torres-Zárate, C.; Pérez-Montesinos, G.; Jurado-Santacruz, F.; Domínguez-Gómez, M.A.; Peniche-Castellanos, A.; Ferat-Osorio, E.; Neri, N.; Nambo, M.J.; Alvarado-Cabrero, I.; et al. Overexpression of hypoxia-inducible factor 1 alpha impacts FoxP3 levels in mycosis fungoides--cutaneous T-cell lymphoma: Clinical implications. Int. J. Cancer 2014, 134, 2136–2145. [Google Scholar] [CrossRef]
- Lauenborg, B.; Litvinov, I.V.; Zhou, Y.; Willerslev-Olsen, A.; Bonefeld, C.M.; Nastasi, C.; Fredholm, S.; Lindahl, L.M.; Sasseville, D.; Geisler, C.; et al. Malignant T cells activate endothelial cells via IL-17 F. Blood Cancer J. 2017, 7, e586. [Google Scholar] [CrossRef]
- Lauenborg, B.; Christensen, L.; Ralfkiaer, U.; Kopp, K.L.; Jønson, L.; Dabelsteen, S.; Bonefeld, C.M.; Geisler, C.; Gjerdrum, L.M.; Zhang, Q.; et al. Malignant T cells express lymphotoxin α and drive endothelial activation in cutaneous T cell lymphoma. Oncotarget 2015, 6, 15235–15249. [Google Scholar] [CrossRef]
- Du, Y.; Cai, Y.; Lv, Y.; Zhang, L.; Yang, H.; Liu, Q.; Hong, M.; Teng, Y.; Tang, W.; Ma, R.; et al. Single-cell RNA sequencing unveils the communications between malignant T and myeloid cells contributing to tumor growth and immunosuppression in cutaneous T-cell lymphoma. Cancer Lett. 2022, 551, 215972. [Google Scholar] [CrossRef]
- Geskin, L.J.; Akilov, O.E.; Kwon, S.; Schowalter, M.; Watkins, S.; Whiteside, T.L.; Butterfield, L.H.; Falo, L.D. Therapeutic reduction of cell-mediated immunosuppression in mycosis fungoides and Sézary syndrome. Cancer Immunol. Immunother. 2018, 67, 423–434. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Gao, Y.; Sun, J.; Li, Y.; Lin, Y.; Liu, X.; Wen, Y.; Yi, S.; Dang, J.; et al. High Expression of IKZF2 in Malignant T Cells Promotes Disease Progression in Cutaneous T Cell Lymphoma. Acta Derm. Venereol. 2021, 101, adv00613. [Google Scholar] [CrossRef] [PubMed]
- Aronovich, A.; Moyal, L.; Gorovitz, B.; Amitay-Laish, I.; Naveh, H.P.; Forer, Y.; Maron, L.; Knaneh, J.; Ad-El, D.; Yaacobi, D.; et al. Cancer-Associated Fibroblasts in Mycosis Fungoides Promote Tumor Cell Migration and Drug Resistance through CXCL12/CXCR4. J. Investig. Dermatol. 2021, 141, 619–627.e612. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, I.H.; Willerslev-Olsen, A.; Vetter-Kauczok, C.; Krejsgaard, T.; Lauenborg, B.; Kopp, K.L.; Geisler, C.; Bonefeld, C.M.; Zhang, Q.; Wasik, M.A.; et al. Vascular endothelial growth factor receptor-3 expression in mycosis fungoides. Leuk. Lymphoma 2013, 54, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Klemke, C.-D.; Brenner, D.; Weiβ, E.-M.; Schmidt, M.; Leverkus, M.; Gülow, K.; Krammer, P.H. Lack of T-Cell Receptor–Induced Signaling Is Crucial for CD95 Ligand Up-regulation and Protects Cutaneous T-Cell Lymphoma Cells from Activation-Induced Cell Death. Cancer Res. 2009, 69, 4175–4183. [Google Scholar] [CrossRef]
- Wu, J.; Salva, K.A.; Wood, G.S. c-CBL E3 ubiquitin ligase is overexpressed in cutaneous T-cell lymphoma: Its inhibition promotes activation-induced cell death. J. Investig. Dermatol. 2015, 135, 861–868. [Google Scholar] [CrossRef]
- Ni, X.; Zhang, C.; Talpur, R.; Duvic, M. Resistance to activation-induced cell death and bystander cytotoxicity via the Fas/Fas ligand pathway are implicated in the pathogenesis of cutaneous T cell lymphomas. J. Investig. Dermatol. 2005, 124, 741–750. [Google Scholar] [CrossRef]
- Wang, Y.; Su, M.; Zhou, L.L.; Tu, P.; Zhang, X.; Jiang, X.; Zhou, Y. Deficiency of SATB1 expression in Sezary cells causes apoptosis resistance by regulating FasL/CD95L transcription. Blood 2011, 117, 3826–3835. [Google Scholar] [CrossRef]
- Jankowska-Konsur, A.; Kobierzycki, C.; Reich, A.; Piotrowska, A.; Gomulkiewicz, A.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P.; Szepietowski, J.C. Expression of SOX18 in Mycosis Fungoides. Acta Derm. Venereol. 2017, 97, 17–23. [Google Scholar] [CrossRef]
- Narducci, M.G.; Scala, E.; Bresin, A.; Caprini, E.; Picchio, M.C.; Remotti, D.; Ragone, G.; Nasorri, F.; Frontani, M.; Arcelli, D.; et al. Skin homing of Sézary cells involves SDF-1-CXCR4 signaling and down-regulation of CD26/dipeptidylpeptidase IV. Blood 2006, 107, 1108–1115. [Google Scholar] [CrossRef]
- Tuzova, M.; Richmond, J.; Wolpowitz, D.; Curiel-Lewandrowski, C.; Chaney, K.; Kupper, T.; Cruikshank, W. CCR4+ T cell recruitment to the skin in mycosis fungoides: Potential contributions by thymic stromal lymphopoietin and interleukin-16. Leuk. Lymphoma 2015, 56, 440–449. [Google Scholar] [CrossRef]
- Ito, M.; Teshima, K.; Ikeda, S.; Kitadate, A.; Watanabe, A.; Nara, M.; Yamashita, J.; Ohshima, K.; Sawada, K.; Tagawa, H. MicroRNA-150 inhibits tumor invasion and metastasis by targeting the chemokine receptor CCR6, in advanced cutaneous T-cell lymphoma. Blood 2014, 123, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T.; Sugaya, M.; Oka, T.; Takahashi, N.; Kawaguchi, M.; Suga, H.; Fujita, H.; Yoshizaki, A.; Asano, Y.; Sato, S. Placental Growth Factor and Vascular Endothelial Growth Factor Together Regulate Tumour Progression via Increased Vasculature in Cutaneous T-cell Lymphoma. Acta Derm. Venereol. 2017, 97, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Beksaç, B.; Gleason, L.; Baik, S.; Ringe, J.M.; Porcu, P.; Nikbakht, N. Dermal fibroblasts promote cancer cell proliferation and exhibit fibronectin overexpression in early mycosis fungoides. J. Dermatol. Sci. 2022, 106, 53–60. [Google Scholar] [CrossRef] [PubMed]
- McGirt, L.Y.; Jia, P.; Baerenwald, D.A.; Duszynski, R.J.; Dahlman, K.B.; Zic, J.A.; Zwerner, J.P.; Hucks, D.; Dave, U.; Zhao, Z. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood J. Am. Soc. Hematol. 2015, 126, 508–519. [Google Scholar] [CrossRef]
- Gluud, M.; Fredholm, S.; Blümel, E.; Willerslev-Olsen, A.; Buus, T.B.; Nastasi, C.; Krejsgaard, T.; Bonefeld, C.M.; Woetmann, A.; Iversen, L.; et al. MicroRNA-93 Targets p21 and Promotes Proliferation in Mycosis Fungoides T Cells. Dermatology 2021, 237, 277–282. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, X.; Li, W.; Zhang, Q.; Zhang, C. PAK1 overexpression promotes cell proliferation in cutaneous T cell lymphoma via suppression of PUMA and p21. J. Dermatol. Sci. 2018, 90, 60–67. [Google Scholar] [CrossRef]
- Kumari, N.; Choi, S.H. Tumor-associated macrophages in cancer: Recent advancements in cancer nanoimmunotherapies. J. Exp. Clin. Cancer Res. 2022, 41, 68. [Google Scholar] [CrossRef]
- Johanny, L.D.; Sokumbi, O.; Hobbs, M.M.; Jiang, L. Polarization of Macrophages in Granulomatous Cutaneous T Cell Lymphoma Granulomatous Mycosis Fungoides Microenvironment. Dermatopathology 2022, 9, 54–59. [Google Scholar] [CrossRef]
- Di Raimondo, C.; Han, Z.; Su, C.; Wu, X.; Qin, H.; Sanchez, J.F.; Yuan, Y.-C.; Martinez, X.; Abdulla, F.; Zain, J.; et al. Identification of a Distinct miRNA Regulatory Network in the Tumor Microenvironment of Transformed Mycosis Fungoides. Cancers 2021, 13, 5854. [Google Scholar] [CrossRef]
- Huang, S.; Liao, M.; Chen, S.; Zhang, P.; Xu, F.; Zhang, H. Immune signatures of CD4 and CD68 predicts disease progression in cutaneous T cell lymphoma. Am. J. Transl. Res. 2022, 14, 3037–3051. [Google Scholar]
- El-Guindy, D.M.; Elgarhy, L.H.; Elkholy, R.A.; Ali, D.A.; Helal, D.S. Potential role of tumor-associated macrophages and CD163/CD68 ratio in mycosis fungoides and Sézary syndrome in correlation with serum sCD163 and CCL22. J. Cutan. Pathol. 2022, 49, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Furudate, S.; Fujimura, T.; Kakizaki, A.; Kambayashi, Y.; Asano, M.; Watabe, A.; Aiba, S. The possible interaction between periostin expressed by cancer stroma and tumor-associated macrophages in developing mycosis fungoides. Exp. Dermatol. 2016, 25, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T.; Sugaya, M.; Suga, H.; Ohmatsu, H.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. Increased CCL18 expression in patients with cutaneous T-cell lymphoma: Association with disease severity and prognosis. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e60–e67. [Google Scholar] [CrossRef]
- Günther, C.; Zimmermann, N.; Berndt, N.; Grosser, M.; Stein, A.; Koch, A.; Meurer, M. Up-regulation of the chemokine CCL18 by macrophages is a potential immunomodulatory pathway in cutaneous T-cell lymphoma. Am. J. Pathol. 2011, 179, 1434–1442. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Seiwert, T.Y. The role of macrophages in the tumor microenvironment and tumor metabolism. Semin. Immunopathol. 2023, 45, 187–201. [Google Scholar] [CrossRef]
- Han, Z.; Wu, X.; Qin, H.; Yuan, Y.C.; Schmolze, D.; Su, C.; Zain, J.; Moyal, L.; Hodak, E.; Sanchez, J.F.; et al. Reprogramming of PD-1+ M2-like tumor-associated macrophages with anti-PD-L1 and lenalidomide in cutaneous T cell lymphoma. JCI Insight 2023, 8, e163518. [Google Scholar] [CrossRef]
- Wu, X.; Schulte, B.C.; Zhou, Y.; Haribhai, D.; Mackinnon, A.C.; Plaza, J.A.; Williams, C.B.; Hwang, S.T. Depletion of M2-like tumor-associated macrophages delays cutaneous T-cell lymphoma development in vivo. J. Investig. Dermatol. 2014, 134, 2814–2822. [Google Scholar] [CrossRef]
- Wilcox, R.A.; David, W.A.; Feldman, A.L.; Elsawa, S.F.; Grote, D.M.; Ziesmer, S.C.; Novak, A.J.; Pittelkow, M.R.; Witzig, T.E.; Ansell, S.M. Monocytes Promote Survival of Malignant T Cells in Cutaneous T-Cell Lymphoma and Are Recruited to the Tumor Microenvironment by CCL5 (RANTES). Blood 2008, 112, 378. [Google Scholar] [CrossRef]
- Rabenhorst, A.; Schlaak, M.; Heukamp, L.C.; Förster, A.; Theurich, S.; von Bergwelt-Baildon, M.; Büttner, R.; Kurschat, P.; Mauch, C.; Roers, A.; et al. Mast cells play a protumorigenic role in primary cutaneous lymphoma. Blood 2012, 120, 2042–2054. [Google Scholar] [CrossRef]
- Terhorst-Molawi, D.; Lohse, K.; Ginter, K.; Puhl, V.; Metz, M.; Hu, M.; Maurer, M.; Altrichter, S. Mast cells and tryptase are linked to itch and disease severity in mycosis fungoides: Results of a pilot study. Front. Immunol. 2022, 13, 930979. [Google Scholar] [CrossRef]
- Eder, J.; Rogojanu, R.; Jerney, W.; Erhart, F.; Dohnal, A.; Kitzwögerer, M.; Steiner, G.; Moser, J.; Trautinger, F. Mast Cells Are Abundant in Primary Cutaneous T-Cell Lymphomas: Results from a Computer-Aided Quantitative Immunohistological Study. PLoS ONE 2016, 11, e0163661. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Sheng, Y.; Xiao, H.; Ye, Y.; Kwantwi, L.B.; Cheng, L.; Wang, Y.; Xu, J.; Wu, Q. Lung Adenocarcinoma Cells Promote Self-Migration and Self-Invasion by Activating Neutrophils to Upregulate Notch3 Expression of Cancer Cells. Front. Mol. Biosci. 2022, 8, 762729. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, M.; Boafo Kwantwi, L.; Bi, X.; Zhang, C.; Cheng, Z.; Ding, X.; Su, T.; Wang, H.; Wu, Q. Breast cancer cells promote self-migration by secreting interleukin 8 to induce NET formation. Gene 2020, 754, 144902. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Peng, W.; Huang, Y.; Cheng, L.; Meng, Y.; Kwantwi, L.B.; Yang, J.; Xu, J.; Xiao, H.; Kzhyshkowska, J.; et al. Tumor-activated neutrophils promote metastasis in breast cancer via the G-CSF-RLN2-MMP-9 axis. J. Leukoc. Biol. 2023, 113, 383–399. [Google Scholar] [CrossRef]
- Lee, T.-L.; Chen, T.-H.; Kuo, Y.-J.; Lan, H.-Y.; Yang, M.-H.; Chu, P.-Y. Tumor-associated tissue eosinophilia promotes angiogenesis and metastasis in head and neck squamous cell carcinoma. Neoplasia 2023, 35, 100855. [Google Scholar] [CrossRef]
- Diwan, A.H.; Prieto, V.G.; Herling, M.; Duvic, M.; Jone, D. Primary Sézary syndrome commonly shows low-grade cytologic atypia and an absence of epidermotropism. Am. J. Clin. Pathol. 2005, 123, 510–515. [Google Scholar] [CrossRef]
- Suzuki, H.; Sugaya, M.; Nakajima, R.; Oka, T.; Takahashi, N.; Nakao, M.; Miyagaki, T.; Asano, Y.; Sato, S. Serum amyloid A levels in the blood of patients with atopic dermatitis and cutaneous T-cell lymphoma. J. Dermatol. 2018, 45, 1440–1443. [Google Scholar] [CrossRef]
- Fredholm, S.; Gjerdrum, L.M.; Willerslev-Olsen, A.; Petersen, D.L.; Nielsen, I.; Kauczok, C.S.; Wobser, M.; Ralfkiaer, U.; Bonefeld, C.M.; Wasik, M.A.; et al. STAT3 activation and infiltration of eosinophil granulocytes in mycosis fungoides. Anticancer Res. 2014, 34, 5277–5286. [Google Scholar]
- Cirée, A.; Michel, L.; Camilleri-Bröet, S.; Jean Louis, F.; Oster, M.; Flageul, B.; Senet, P.; Fossiez, F.; Fridman, W.H.; Bachelez, H.; et al. Expression and activity of IL-17 in cutaneous T-cell lymphomas (Mycosis fungoides and Sezary syndrome). Int. J. Cancer 2004, 112, 113–120. [Google Scholar] [CrossRef]
- Abreu, M.; Miranda, M.; Castro, M.; Fernandes, I.; Cabral, R.; Santos, A.H.; Fonseca, S.; Rodrigues, J.; Leander, M.; Lau, C.; et al. IL-31 and IL-8 in Cutaneous T-Cell Lymphoma: Looking for Their Role in Itch. Adv. Hematol. 2021, 2021, 5582581. [Google Scholar] [CrossRef]
- Wylie, B.; Macri, C.; Mintern, J.D.; Waithman, J. Dendritic Cells and Cancer: From Biology to Therapeutic Intervention. Cancers 2019, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.S.P.; Anandasabapathy, N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin. Immunopathol. 2017, 39, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.L.; Hanlon, D.; Kanada, D.; Dhodapkar, M.; Lombillo, V.; Wang, N.; Christensen, I.; Howe, G.; Crouch, J.; El-Fishawy, P.; et al. The growth of cutaneous T-cell lymphoma is stimulated by immature dendritic cells. Blood 2002, 99, 2929–2939. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, P.; Obermoser, G.; Nguyen, V.A.; Fritsch, P.; Sepp, N.; Romani, N. Distribution and maturation of skin dendritic cell subsets in two forms of cutaneous T-cell lymphoma: Mycosis fungoides and Sézary syndrome. Acta Derm. Venereol. 2012, 92, 269–275. [Google Scholar] [CrossRef]
- Vieyra-Garcia, P.; Crouch, J.D.; O’Malley, J.T.; Seger, E.W.; Yang, C.H.; Teague, J.E.; Vromans, A.M.; Gehad, A.; Win, T.S.; Yu, Z.; et al. Benign T cells drive clinical skin inflammation in cutaneous T cell lymphoma. JCI Insight 2019, 4, e124233. [Google Scholar] [CrossRef]
- Manfrere, C.K.; Torrealba, M.P.; Miyashiro, D.R.; Pereira, N.Z.; Yoshikawa, F.S.Y.; Oliveira, L.d.M.; Cury-Martins,, J.; Duarte, A.J.S.; Sanches, J.A.; Sato, M.N. Profile of differentially expressed Toll-like receptor signaling genes in the natural killer cells of patients with Sézary syndrome. Oncotarget 2017, 8, 92183–92194. [Google Scholar] [CrossRef]
- Sako, N.; Schiavon, V.; Bounfour, T.; Dessirier, V.; Ortonne, N.; Olive, D.; Ram-Wolff, C.; Michel, L.; Sicard, H.; Marie-Cardine, A.; et al. Membrane expression of NK receptors CD160 and CD158k contributes to delineate a unique CD4+ T-lymphocyte subset in normal and mycosis fungoides skin. Cytom. Part A 2014, 85, 869–882. [Google Scholar] [CrossRef]
- Kwantwi, L.B. SLAM family-mediated crosstalk between tumor and immune cells in the tumor microenvironment: A promising biomarker and a potential therapeutic target for immune checkpoint therapies. Clin. Transl. Oncol. 2024, 1–8. [Google Scholar] [CrossRef]
- Argyropoulos, K.V.; Pulitzer, M.; Perez, S.; Korkolopoulou, P.; Angelopoulou, M.; Baxevanis, C.; Palomba, M.L.; Siakantaris, M. Tumor-infiltrating and circulating granulocytic myeloid-derived suppressor cells correlate with disease activity and adverse clinical outcomes in mycosis fungoides. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2020, 22, 1059–1066. [Google Scholar] [CrossRef]
- Hergott, C.B.; Dudley, G.; Dorfman, D.M. Circulating Myeloid-Derived Suppressor Cells Reflect Mycosis Fungoides/Sezary Syndrome Disease Stage and Response to Treatment. Blood 2018, 132, 4127. [Google Scholar] [CrossRef]
- Maliniemi, P.; Laukkanen, K.; Väkevä, L.; Dettmer, K.; Lipsanen, T.; Jeskanen, L.; Bessede, A.; Oefner, P.J.; Kadin, M.E.; Ranki, A. Biological and clinical significance of tryptophan-catabolizing enzymes in cutaneous T-cell lymphomas. Oncoimmunology 2017, 6, e1273310. [Google Scholar] [CrossRef] [PubMed]
- Kwantwi, L.B.; Wang, S.; Zhang, W.; Peng, W.; Cai, Z.; Sheng, Y.; Xiao, H.; Wang, X.; Wu, Q. Tumor-associated neutrophils activated by tumor-derived CCL20 (C-C motif chemokine ligand 20) promote T cell immunosuppression via programmed death-ligand 1 (PD-L1) in breast cancer. Bioengineered 2021, 12, 6996–7006. [Google Scholar] [CrossRef] [PubMed]
- Querfeld, C.; Leung, S.; Myskowski, P.L.; Curran, S.A.; Goldman, D.A.; Heller, G.; Wu, X.; Kil, S.H.; Sharma, S.; Finn, K.J.; et al. Primary T Cells from Cutaneous T-cell Lymphoma Skin Explants Display an Exhausted Immune Checkpoint Profile. Cancer Immunol. Res. 2018, 6, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.; McMurray, J.L.; Eldershaw, S.; Pearce, H.; Davies, N.; Scarisbrick, J.J.; Moss, P. Progression of mycosis fungoides occurs through divergence of tumor immunophenotype by differential expression of HLA-DR. Blood Adv. 2019, 3, 519–530. [Google Scholar] [CrossRef]
- Blümel, E.; Munir Ahmad, S.; Nastasi, C.; Willerslev-Olsen, A.; Gluud, M.; Fredholm, S.; Hu, T.; Surewaard, B.G.J.; Lindahl, L.M.; Fogh, H.; et al. Staphylococcus aureus alpha-toxin inhibits CD8(+) T cell-mediated killing of cancer cells in cutaneous T-cell lymphoma. Oncoimmunology 2020, 9, 1751561. [Google Scholar] [CrossRef]
- Torrealba, M.P.; Manfrere, K.C.; Miyashiro, D.R.; Lima, J.F.; Oliveira, L.d.M.; Pereira, N.Z.; Cury-Martins, J.; Pereira, J.; Duarte, A.J.S.; Sato, M.N.; et al. Chronic activation profile of circulating CD8+ T cells in Sézary syndrome. Oncotarget 2018, 9, 3497–3506. [Google Scholar] [CrossRef]
- Han, Z.; Estephan, R.J.; Wu, X.; Su, C.; Yuan, Y.C.; Qin, H.; Kil, S.H.; Morales, C.; Schmolze, D.; Sanchez, J.F.; et al. MicroRNA Regulation of T-Cell Exhaustion in Cutaneous T Cell Lymphoma. J. Investig. Dermatol. 2022, 142, 603–612.e607. [Google Scholar] [CrossRef]
- Nielsen, P.R.; Eriksen, J.O.; Sørensen, M.D.; Wehkamp, U.; Lindahl, L.M.; Bzorek, M.; Iversen, L.; Woetman, A.; Ødum, N.; Litman, T.; et al. Role of B-cells in Mycosis Fungoides. Acta Derm. Venereol. 2021, 101, adv00413. [Google Scholar] [CrossRef]
- Shareef, M.M.; Elgarhy, L.H.; Wasfy Rel, S. Expression of Granulysin and FOXP3 in Cutaneous T Cell Lymphoma and Sézary Syndrome. Asian Pac. J. Cancer Cancer Prev. 2015, 16, 5359–5364. [Google Scholar] [CrossRef]
- Fried, I.; Cerroni, L. FOXP3 in Sequential Biopsies of Progressive Mycosis Fungoides. Am. J. Dermatopathol. 2012, 34, 263–265. [Google Scholar] [CrossRef]
- Wada, D.A.; Wilcox, R.A.; Weenig, R.H.; Gibson, L.E. Paucity of intraepidermal FoxP3-positive T cells in cutaneous T-cell lymphoma in contrast with spongiotic and lichenoid dermatitis. J. Cutan. Pathol. 2010, 37, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Vonderheid, E.C.; Hess, A.D.; Eischen, C.M.; McGirt, L.Y. Genetic markers associated with progression in early mycosis fungoides. J. Eur. Acad. Dermatol. Venereol. JEADV 2014, 28, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.L.; Tigelaar, R.; Cohen, J.; Mariwalla, K.; Trinh, J.; Wang, N.; Edelson, R.L. Cutaneous T-cell lymphoma: Malignant proliferation of T-regulatory cells. Blood 2005, 105, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Willerslev-Olsen, A.; Buus, T.B.; Nastasi, C.; Blümel, E.; Gluud, M.; Bonefeld, C.M.; Geisler, C.; Lindahl, L.M.; Vermeer, M.; Wasik, M.A.; et al. Staphylococcus aureus enterotoxins induce FOXP3 in neoplastic T cells in Sézary syndrome. Blood Cancer J. 2020, 10, 57. [Google Scholar] [CrossRef]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Hamberger, F.; Ravichandra, A.; Miller, M.; Nair, A.; Affo, S.; Filliol, A.; Chin, L.; Savage, T.M.; Yin, D.; et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Mehdi, S.J.; Moerman-Herzog, A.; Wong, H.K. Normal and cancer fibroblasts differentially regulate TWIST1, TOX and cytokine gene expression in cutaneous T-cell lymphoma. BMC Cancer 2021, 21, 492. [Google Scholar] [CrossRef]
- Miyagaki, T.; Sugaya, M.; Suga, H.; Morimura, S.; Ohmatsu, H.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. Low Herpesvirus Entry Mediator (HVEM) Expression on Dermal Fibroblasts Contributes to a Th2-Dominant Microenvironment in Advanced Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2012, 132, 1280–1289. [Google Scholar] [CrossRef]
- Miyagaki, T.; Sugaya, M.; Fujita, H.; Ohmatsu, H.; Kakinuma, T.; Kadono, T.; Tamaki, K.; Sato, S. Eotaxins and CCR3 Interaction Regulates the Th2 Environment of Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2010, 130, 2304–2311. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Li, M.; Fang, L.; Kwantwi, L.B.; He, G.; Luo, W.; Yang, L.; Huang, Y.; Yin, S.; Cai, Y.; Ma, W.; et al. N-Myc promotes angiogenesis and therapeutic resistance of prostate cancer by TEM8. Med. Oncol. 2021, 38, 127. [Google Scholar] [CrossRef] [PubMed]
- Kwantwi, L.B. The dual and multifaceted role of relaxin-2 in cancer. Clin. Transl. Oncol. 2023, 25, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Karpova, M.B.; Fujii, K.; Jenni, D.; Dummer, R.; Urosevic-Maiwald, M. Evaluation of lymphangiogenic markers in Sézary syndrome. Leuk. Lymphoma 2011, 52, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Zohdy, M.; Abd El Hafez, A.; Abd Allah, M.Y.Y.; Bessar, H.; Refat, S. Ki67 and CD31 Differential Expression in Cutaneous T-Cell Lymphoma and Its Mimickers: Association with Clinicopathological Criteria and Disease Advancement. Clin. Cosmet. Investig. Dermatol. 2020, 13, 431–442. [Google Scholar] [CrossRef]
- Jankowska-Konsur, A.; Kobierzycki, C.; Grzegrzolka, J.; Piotrowska, A.; Gomulkiewicz, A.; Glatzel-Plucinska, N.; Olbromski, M.; Podhorska-Okolow, M.; Szepietowski, J.C.; Dziegiel, P. Expression of CD31 in Mycosis Fungoides. Anticancer Res. 2016, 36, 4575–4582. [Google Scholar] [CrossRef]
- Jankowska-Konsur, A.; Kobierzycki, C.; Grzegrzółka, J.; Piotrowska, A.; Gomulkiewicz, A.; Glatzel-Plucinska, N.; Reich, A.; Podhorska-Okołów, M.; Dzięgiel, P.; Szepietowski, J.C. Podoplanin Expression Correlates with Disease Progression in Mycosis Fungoides. Acta Derm. Venereol. 2017, 97, 235–241. [Google Scholar] [CrossRef]
- Sakamoto, M.; Miyagaki, T.; Kamijo, H.; Oka, T.; Takahashi, N.; Suga, H.; Yoshizaki, A.; Asano, Y.; Sugaya, M.; Sato, S. Serum vascular endothelial growth factor A levels reflect itch severity in mycosis fungoides and Sézary syndrome. J. Dermatol. 2018, 45, 95–99. [Google Scholar] [CrossRef]
- El-Ashmawy, A.A.; Shamloula, M.M.; Elfar, N.N. Podoplanin as a Predictive Marker for Identification of High-Risk Mycosis Fungoides Patients: An Immunohistochemical Study. Indian J. Dermatol. 2020, 65, 500–505. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K.; Uchida, Y.; Toge, T. The role of Fas ligand and transforming growth factor β in tumor progression. Cancer 2004, 100, 2281–2291. [Google Scholar] [CrossRef]
- Ni, X.; Hazarika, P.; Zhang, C.; Talpur, R.; Duvic, M. Fas ligand expression by neoplastic T lymphocytes mediates elimination of CD8+ cytotoxic T lymphocytes in mycosis fungoides: A potential mechanism of tumor immune escape? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001, 7, 2682–2692. [Google Scholar]
- Ferenczi, K.; Ohtola, J.; Aubert, P.; Kessler, M.; Sugiyama, H.; Somani, A.K.; Gilliam, A.C.; Chen, J.Z.; Yeh, I.; Matsuyama, S.; et al. Malignant T cells in cutaneous T-cell lymphoma lesions contain decreased levels of the antiapoptotic protein Ku70. Br. J. Dermatol. 2010, 163, 564–571. [Google Scholar] [CrossRef] [PubMed]
- da Silva Almeida, A.C.; Abate, F.; Khiabanian, H.; Martinez-Escala, E.; Guitart, J.; Tensen, C.P.; Vermeer, M.H.; Rabadan, R.; Ferrando, A.; Palomero, T. The mutational landscape of cutaneous T cell lymphoma and Sezary syndrome. Nat. Genet 2015, 47, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Vieyra-Garcia, P.A.; Wei, T.; Naym, D.G.; Fredholm, S.; Fink-Puches, R.; Cerroni, L.; Odum, N.; O’Malley, J.T.; Gniadecki, R.; Wolf, P. STAT3/5-Dependent IL9 Overexpression Contributes to Neoplastic Cell Survival in Mycosis Fungoides. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 3328–3339. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dhamija, B.; Marathe, S.; Ghosh, S.; Dwivedi, A.; Karulkar, A.; Sharma, N.; Sengar, M.; Sridhar, E.; Bonda, A.; et al. The Th9 Axis Reduces the Oxidative Stress and Promotes the Survival of Malignant T Cells in Cutaneous T-Cell Lymphoma Patients. Mol. Cancer Res. 2020, 18, 657–668. [Google Scholar] [CrossRef]
- Kwantwi, L.B.; Wang, S.; Sheng, Y.; Wu, Q. Multifaceted roles of CCL20 (C-C motif chemokine ligand 20): Mechanisms and communication networks in breast cancer progression. Bioengineered 2021, 12, 6923–6934. [Google Scholar] [CrossRef]
- Kwantwi, L.B. The dual role of autophagy in the regulation of cancer treatment. Amino Acids 2024, 56, 7. [Google Scholar] [CrossRef]
- Kwantwi, L.B.; Tandoh, T. Focal adhesion kinase-mediated interaction between tumor and immune cells in the tumor microenvironment: Implications for cancer-associated therapies and tumor progression. Clin. Transl. Oncol. 2024, 1–8. [Google Scholar] [CrossRef]
- Krejsgaard, T.; Lindahl, L.M.; Mongan, N.P.; Wasik, M.A.; Litvinov, I.V.; Iversen, L.; Langhoff, E.; Woetmann, A.; Odum, N. Malignant inflammation in cutaneous T-cell lymphoma—A hostile takeover. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 269–282. [Google Scholar]
- Tseng, P.Y.; Hoon, M.A. Oncostatin M can sensitize sensory neurons in inflammatory pruritus. Sci. Transl. Med. 2021, 13, eabe3037. [Google Scholar] [CrossRef]
- Miyagaki, T.; Sugaya, M.; Suga, H.; Kamata, M.; Ohmatsu, H.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. IL-22, but not IL-17, dominant environment in cutaneous T-cell lymphoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 7529–7538. [Google Scholar] [CrossRef]
- Wu, X.; Hsu, D.K.; Wang, K.-H.; Huang, Y.; Mendoza, L.; Zhou, Y.; Hwang, S.T. IL-10 is overexpressed in human cutaneous T-cell lymphoma and is required for maximal tumor growth in a mouse model. Leuk. Lymphoma 2019, 60, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.A.; Wada, D.A.; Ziesmer, S.C.; Elsawa, S.F.; Comfere, N.I.; Dietz, A.B.; Novak, A.J.; Witzig, T.E.; Feldman, A.L.; Pittelkow, M.R. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood J. Am. Soc. Hematol. 2009, 114, 2936–2944. [Google Scholar] [CrossRef] [PubMed]
- Ohmatsu, H.; Humme, D.; Gulati, N.; Gonzalez, J.; Möbs, M.; Suárez-Fariñas, M.; Cardinale, I.; Mitsui, H.; Guttman-Yassky, E.; Sterry, W. IL32 is progressively expressed in mycosis fungoides independent of helper T-cell 2 and helper T-cell 9 polarization. Cancer Immunol. Res. 2014, 2, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.K.; Smith, N.P.; Essien, S.V.; Teague, J.E.; Vieyra-Garcia, P.; Gehad, A.; Zhan, Q.; Crouch, J.D.; Gerard, N.; Larocca, C.; et al. IL-32 Supports the Survival of Malignant T Cells in Cutaneous T-cell Lymphoma. J. Investig. Dermatol. 2022, 142, 2285–2288.e2282. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sugaya, M.; Miyagaki, T.; Kawaguchi, M.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. The role of IL-32 in cutaneous T-cell lymphoma. J. Investig. Dermatol. 2014, 134, 1428–1435. [Google Scholar] [CrossRef]
- Singer, E.M.; Shin, D.B.; Nattkemper, L.A.; Benoit, B.M.; Klein, R.S.; Didigu, C.A.; Loren, A.W.; Dentchev, T.; Wysocka, M.; Yosipovitch, G. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J. Investig. Dermatol. 2013, 133, 2783–2785. [Google Scholar] [CrossRef]
- Santen, S.v.; Out, J.; Zoutman, W.; Quint, K.; Willemze, R.; Vermeer, M.; Tensen, C. Serum and cutaneous transcriptional expression levels of IL31 are minimal in cutaneous T cell lymphoma variants. Biochem. Biophys. Rep. 2021, 26, 101007. [Google Scholar] [CrossRef]
- Mishra, A.; La Perle, K.; Kwiatkowski, S.; Sullivan, L.A.; Sams, G.H.; Johns, J.; Curphey, D.P.; Wen, J.; McConnell, K.; Qi, J.; et al. Mechanism, Consequences, and Therapeutic Targeting of Abnormal IL15 Signaling in Cutaneous T-cell Lymphoma. Cancer Discov. 2016, 6, 986–1005. [Google Scholar] [CrossRef]
- Marzec, M.; Liu, X.; Kasprzycka, M.; Witkiewicz, A.; Raghunath, P.N.; El-Salem, M.; Robertson, E.; Odum, N.; Wasik, M.A. IL-2- and IL-15-induced activation of the rapamycin-sensitive mTORC1 pathway in malignant CD4+ T lymphocytes. Blood 2008, 111, 2181–2189. [Google Scholar] [CrossRef]
- Thode, C.; Woetmann, A.; Wandall, H.H.; Carlsson, M.C.; Qvortrup, K.; Kauczok, C.S.; Wobser, M.; Printzlau, A.; Ødum, N.; Dabelsteen, S. Malignant T cells secrete galectins and induce epidermal hyperproliferation and disorganized stratification in a skin model of cutaneous T-cell lymphoma. J. Investig. Dermatol. 2015, 135, 238–246. [Google Scholar] [CrossRef]
- Willerslev-Olsen, A.; Litvinov, I.V.; Fredholm, S.M.; Petersen, D.L.; Sibbesen, N.A.; Gniadecki, R.; Zhang, Q.; Bonefeld, C.M.; Wasik, M.A.; Geisler, C. IL-15 and IL-17F are differentially regulated and expressed in mycosis fungoides (MF). Cell Cycle 2014, 13, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Senda, N.; Miyagaki, T.; Kamijo, H.; Nakajima, R.; Oka, T.; Takahashi, N.; Suga, H.; Yoshizaki, A.; Asano, Y.; Sugaya, M.; et al. Increased HMGB1 levels in lesional skin and sera in patients with cutaneous T-cell lymphoma. Eur. J. Dermatol. 2018, 28, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Kwantwi, L.B. Genetic alterations shape innate immune cells to foster immunosuppression and cancer immunotherapy resistance. Clin. Exp. Med. 2023, 23, 4289–4296. [Google Scholar] [CrossRef] [PubMed]
- Wooler, G.; Melchior, L.; Ralfkiaer, E.; Rahbek Gjerdrum, L.M.; Gniadecki, R. TP53 gene status affects survival in advanced mycosis fungoides. Front. Med. 2016, 3, 51. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Zhu, M.; Hu, P.; Liu, X.; Qing, Y.; Wang, X.; Wang, H.; Wang, Z.; Xu, J.; et al. Involvement of p53 Acetylation in Growth Suppression of Cutaneous T-Cell Lymphomas Induced by HDAC Inhibition. J. Investig. Dermatol. 2020, 140, 2009–2022.e2004. [Google Scholar] [CrossRef]
- Wei, T.; Biskup, E.; Gjerdrum, L.M.; Niazi, O.; Ødum, N.; Gniadecki, R. Ubiquitin-specific protease 2 decreases p53-dependent apoptosis in cutaneous T-cell lymphoma. Oncotarget 2016, 7, 48391–48400. [Google Scholar] [CrossRef]
- Gu, X.; Wang, Y.; Zhang, C.; Liu, Y. GFI-1 overexpression promotes cell proliferation and apoptosis resistance in mycosis fungoides by repressing Bax and P21. Oncol. Lett. 2021, 22, 521. [Google Scholar] [CrossRef]
- Yang, H.; Ma, P.; Cao, Y.; Zhang, M.; Li, L.; Wei, J.; Tao, L.; Qian, K. ECPIRM, a Potential Therapeutic Agent for Cutaneous T-Cell Lymphoma, Inhibits Cell Proliferation and Promotes Apoptosis via a JAK/STAT Pathway. Anti-Cancer Agents Med. Chem. 2018, 18, 401–411. [Google Scholar] [CrossRef]
- Yanagi, T.; Nishihara, H.; Fujii, K.; Nishimura, M.; Narahira, A.; Takahashi, K.; Iwata, H.; Hata, H.; Kitamura, S.; Imafuku, K.; et al. Comprehensive cancer-related gene analysis reveals that active KRAS mutation is a prognostic mutation in mycosis fungoides. J. Dermatol. Sci. 2017, 88, 367–370. [Google Scholar] [CrossRef]
- Kiessling, M.K.; Oberholzer, P.A.; Mondal, C.; Karpova, M.B.; Zipser, M.C.; Lin, W.M.; Girardi, M.; Macconaill, L.E.; Kehoe, S.M.; Hatton, C.; et al. High-throughput mutation profiling of CTCL samples reveals KRAS and NRAS mutations sensitizing tumors toward inhibition of the RAS/RAF/MEK signaling cascade. Blood 2011, 117, 2433–2440. [Google Scholar] [CrossRef]
- Ungewickell, A.; Bhaduri, A.; Rios, E.; Reuter, J.; Lee, C.S.; Mah, A.; Zehnder, A.; Ohgami, R.; Kulkarni, S.; Armstrong, R.; et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat. Genet. 2015, 47, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Vaqué, J.P.; Gómez-López, G.; Monsálvez, V.; Varela, I.; Martínez, N.; Pérez, C.; Domínguez, O.; Graña, O.; Rodríguez-Peralto, J.L.; Rodríguez-Pinilla, S.M.; et al. PLCG1 mutations in cutaneous T-cell lymphomas. Blood 2014, 123, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Patrone, C.C.; Yang, W.; Rabionet, R.; Gallardo, F.; Espinet, B.; Sharma, M.K.; Girardi, M.; Tensen, C.P.; Vermeer, M.; et al. An Integrated Data Resource for Genomic Analysis of Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2018, 138, 2681–2683. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ni, X.; Covington, K.R.; Yang, B.Y.; Shiu, J.; Zhang, X.; Xi, L.; Meng, Q.; Langridge, T.; Drummond, J.; et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat. Genet. 2015, 47, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Fanok, M.H.; Sun, A.; Fogli, L.K.; Narendran, V.; Eckstein, M.; Kannan, K.; Dolgalev, I.; Lazaris, C.; Heguy, A.; Laird, M.E.; et al. Role of Dysregulated Cytokine Signaling and Bacterial Triggers in the Pathogenesis of Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2018, 138, 1116–1125. [Google Scholar] [CrossRef]
- Willerslev-Olsen, A.; Krejsgaard, T.; Lindahl, L.M.; Litvinov, I.V.; Fredholm, S.; Petersen, D.L.; Nastasi, C.; Gniadecki, R.; Mongan, N.P.; Sasseville, D.; et al. Staphylococcal enterotoxin A (SEA) stimulates STAT3 activation and IL-17 expression in cutaneous T-cell lymphoma. Blood 2016, 127, 1287–1296. [Google Scholar] [CrossRef]
- Pinzaru, A.M.; Hom, R.A.; Beal, A.; Phillips, A.F.; Ni, E.; Cardozo, T.; Nair, N.; Choi, J.; Wuttke, D.S.; Sfeir, A.; et al. Telomere Replication Stress Induced by POT1 Inactivation Accelerates Tumorigenesis. Cell Rep. 2016, 15, 2170–2184. [Google Scholar] [CrossRef]
- Kantekure, K.; Yang, Y.; Raghunath, P.; Schaffer, A.; Woetmann, A.; Zhang, Q.; Odum, N.; Wasik, M. Expression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma/mycosis fungoides. Am. J. Dermatopathol. 2012, 34, 126–128. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, F.; Sun, J.; Wen, Y.; Tu, P.; Kadin, M.E.; Wang, Y. Differential SATB1 Expression Reveals Heterogeneity of Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2021, 141, 607–618.e606. [Google Scholar] [CrossRef]
- Di Raimondo, C.; Rubio-Gonzalez, B.; Palmer, J.; Weisenburger, D.D.; Zain, J.; Wu, X.; Han, Z.; Rosen, S.T.; Song, J.Y.; Querfeld, C. Expression of immune checkpoint molecules programmed death protein 1, programmed death-ligand 1 and inducible T-cell co-stimulator in mycosis fungoides and Sézary syndrome: Association with disease stage and clinical outcome*. Br. J. Dermatol. 2022, 187, 234–243. [Google Scholar] [CrossRef]
- Han, Z.; Wu, X.; Qin, H.; Yuan, Y.C.; Zain, J.; Smith, D.L.; Akilov, O.E.; Rosen, S.T.; Feng, M.; Querfeld, C. Blockade of the Immune Checkpoint CD47 by TTI-621 Potentiates the Response to Anti-PD-L1 in Cutaneous T Cell Lymphoma. J. Investig. Dermatol. 2023, 143, 1569–1578.e5. [Google Scholar] [CrossRef] [PubMed]
- Saulite, I.; Ignatova, D.; Chang, Y.T.; Fassnacht, C.; Dimitriou, F.; Varypataki, E.; Anzengruber, F.; Nägeli, M.; Cozzio, A.; Dummer, R.; et al. Blockade of programmed cell death protein 1 (PD-1) in Sézary syndrome reduces Th2 phenotype of non-tumoral T lymphocytes but may enhance tumor proliferation. Oncoimmunology 2020, 9, 1738797. [Google Scholar] [CrossRef]
- Samimi, S.; Benoit, B.; Evans, K.; Wherry, E.J.; Showe, L.; Wysocka, M.; Rook, A.H. Increased Programmed Death-1 Expression on CD4+ T Cells in Cutaneous T-Cell Lymphoma: Implications for Immune Suppression. Arch. Dermatol. 2010, 146, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Daniels, J.; Wartewig, T.; Ringbloom, K.G.; Martinez-Escala, M.E.; Choi, S.; Thomas, J.J.; Doukas, P.G.; Yang, J.; Snowden, C.; et al. Integrated genomic analyses of cutaneous T-cell lymphomas reveal the molecular bases for disease heterogeneity. Blood 2021, 138, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Kwantwi, L.B. Exosome-mediated crosstalk between tumor cells and innate immune cells: Implications for cancer progression and therapeutic strategies. J. Cancer Res. Clin. Oncol. 2023, 149, 9487–9503. [Google Scholar] [CrossRef]
- Yang, E.; Wang, X.; Gong, Z.; Yu, M.; Wu, H.; Zhang, D. Exosome-mediated metabolic reprogramming: The emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct. Target. Ther. 2020, 5, 242. [Google Scholar] [CrossRef]
- Kwantwi, L.B. Interplay between tumor-derived factors and tumor-associated neutrophils: Opportunities for therapeutic interventions in cancer. Clin. Transl. Oncol. 2023, 25, 1963–1976. [Google Scholar] [CrossRef]
- Moyal, L.; Arkin, C.; Gorovitz-Haris, B.; Querfeld, C.; Rosen, S.; Knaneh, J.; Amitay-Laish, I.; Prag-Naveh, H.; Jacob-Hirsch, J.; Hodak, E. Mycosis fungoides-derived exosomes promote cell motility and are enriched with microRNA-155 and microRNA-1246, and their plasma-cell-free expression may serve as a potential biomarker for disease burden. Br. J. Dermatol. 2021, 185, 999–1012. [Google Scholar] [CrossRef]
- Kwantwi, L.B. Overcoming anti-PD-1/PD-L1 immune checkpoint blockade resistance: The role of macrophage, neutrophils and mast cells in the tumor microenvironment. Clin. Exp. Med. 2023, 23, 3077–3091. [Google Scholar] [CrossRef]
- Cheng, M.; Zain, J.; Rosen, S.T.; Querfeld, C. Emerging drugs for the treatment of cutaneous T-cell lymphoma. Expert Opin. Emerg. Drugs 2022, 27, 45–54. [Google Scholar] [CrossRef]
- Khodadoust, M.; Rook, A.H.; Porcu, P.; Foss, F.M.; Moskowitz, A.J.; Shustov, A.R.; Shanbhag, S.; Sokol, L.; Shine, R.; Fling, S.P. Pembrolizumab for treatment of relapsed/refractory mycosis fungoides and Sezary syndrome: Clinical efficacy in a CITN multicenter phase 2 study. Blood 2016, 128, 181. [Google Scholar] [CrossRef]
- Querfeld, C.; Thompson, J.A.; Taylor, M.H.; DeSimone, J.A.; Zain, J.M.; Shustov, A.R.; Johns, C.; McCann, S.; Lin, G.H.Y.; Petrova, P.S.; et al. Intralesional TTI-621, a novel biologic targeting the innate immune checkpoint CD47, in patients with relapsed or refractory mycosis fungoides or Sezary syndrome: A multicentre, phase 1 study. Lancet Haematol. 2021, 8, e808–e817. [Google Scholar] [CrossRef] [PubMed]
- Querfeld, C.; Chen, L.; Wu, X.; Han, Z.; Su, C.; Banez, M.; Quach, J.; Barnhizer, T.; Crisan, L.; Rosen, S.T.; et al. Preliminary Analysis Demonstrates Durvalumab (Anti-PD-L1) & Lenalidomide Is Superior to Single-Agent Durvalumab (anti-PD-L1) in Refractory/Advanced Cutaneous T Cell Lymphoma in a Randomized Phase 2 Trial. Blood 2023, 142, 3077. [Google Scholar]
- Wu, X.; Singh, R.; Hsu, D.K.; Zhou, Y.; Yu, S.; Han, D.; Shi, Z.; Huynh, M.; Campbell, J.J.; Hwang, S.T. A Small Molecule CCR2 Antagonist Depletes Tumor Macrophages and Synergizes with Anti-PD-1 in a Murine Model of Cutaneous T-Cell Lymphoma (CTCL). J. Investig. Dermatol. 2020, 140, 1390–1400.e1394. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Z.; Liu, J.; Zhang, W.; Zhou, D.; Zhang, Y. Case Report: Outcome and Adverse Events of Anti-PD-1 Antibody Plus Chidamide for Relapsed/Refractory Sézary Syndrome: Case Series and A Literature Review. Front. Oncol. 2022, 12, 842123. [Google Scholar] [CrossRef]
- Querfeld, C.; Zain, J.M.; Wakefield, D.L.; Jovanovic-Talisman, T.; Kil, S.H.; Estephan, R.; Sanchez, J.; Palmer, J.; Rosen, S.T. Phase 1/2 Trial of Durvalumab and Lenalidomide in Patients with Cutaneous T Cell Lymphoma (CTCL): Preliminary Results of Phase I Results and Correlative Studies. Blood 2018, 132, 2931. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Zhang, H.; Ramakrishna, R.; Mintzlaff, D.; Mathes, D.W.; Pomfret, E.A.; Lucia, M.S.; Gao, D.; Haverkos, B.M.; et al. CCR4-IL2 bispecific immunotoxin is more effective than brentuximab for targeted therapy of cutaneous T-cell lymphoma in a mouse CTCL model. FEBS Open Bio. 2023, 13, 1309–1319. [Google Scholar] [CrossRef]
- Querfeld, C.; William, B.M.; Sokol, L.; Akilov, O.; Poligone, B.; Zain, J.; Tagaya, Y.; Azimi, N. Co-Inhibition of il-2, il-9 and il-15 by the novel immunomodulator, bnz-1, provides clinical efficacy in patients with refractory cutaneous T cell lymphoma in a phase 1/2 clinical trial. Blood 2020, 136, 37. [Google Scholar] [CrossRef]
- Ni, X.; Jorgensen, J.L.; Goswami, M.; Challagundla, P.; Decker, W.K.; Kim, Y.H.; Duvic, M.A. Reduction of regulatory T cells by Mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sézary syndrome. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 274–285. [Google Scholar] [CrossRef]
- Bagot, M.; Porcu, P.; Marie-Cardine, A.; Battistella, M.; William, B.M.; Vermeer, M.; Whittaker, S.; Rotolo, F.; Ram-Wolff, C.; Khodadoust, M.S.; et al. IPH4102, a first-in-class anti-KIR3DL2 monoclonal antibody, in patients with relapsed or refractory cutaneous T-cell lymphoma: An international, first-in-human, open-label, phase 1 trial. Lancet Oncol. 2019, 20, 1160–1170. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwantwi, L.B.; Rosen, S.T.; Querfeld, C. The Tumor Microenvironment as a Therapeutic Target in Cutaneous T Cell Lymphoma. Cancers 2024, 16, 3368. https://doi.org/10.3390/cancers16193368
Kwantwi LB, Rosen ST, Querfeld C. The Tumor Microenvironment as a Therapeutic Target in Cutaneous T Cell Lymphoma. Cancers. 2024; 16(19):3368. https://doi.org/10.3390/cancers16193368
Chicago/Turabian StyleKwantwi, Louis Boafo, Steven T. Rosen, and Christiane Querfeld. 2024. "The Tumor Microenvironment as a Therapeutic Target in Cutaneous T Cell Lymphoma" Cancers 16, no. 19: 3368. https://doi.org/10.3390/cancers16193368







