Plaque Radiotherapy for Ocular Melanoma
Simple Summary
Abstract
1. Introduction
2. Indications for Treatment
3. Comparison with Other Treatment Options
4. Types of Radioisotopes and Comparison
5. Plaque Size, Materials, and Dosimetry Innovations
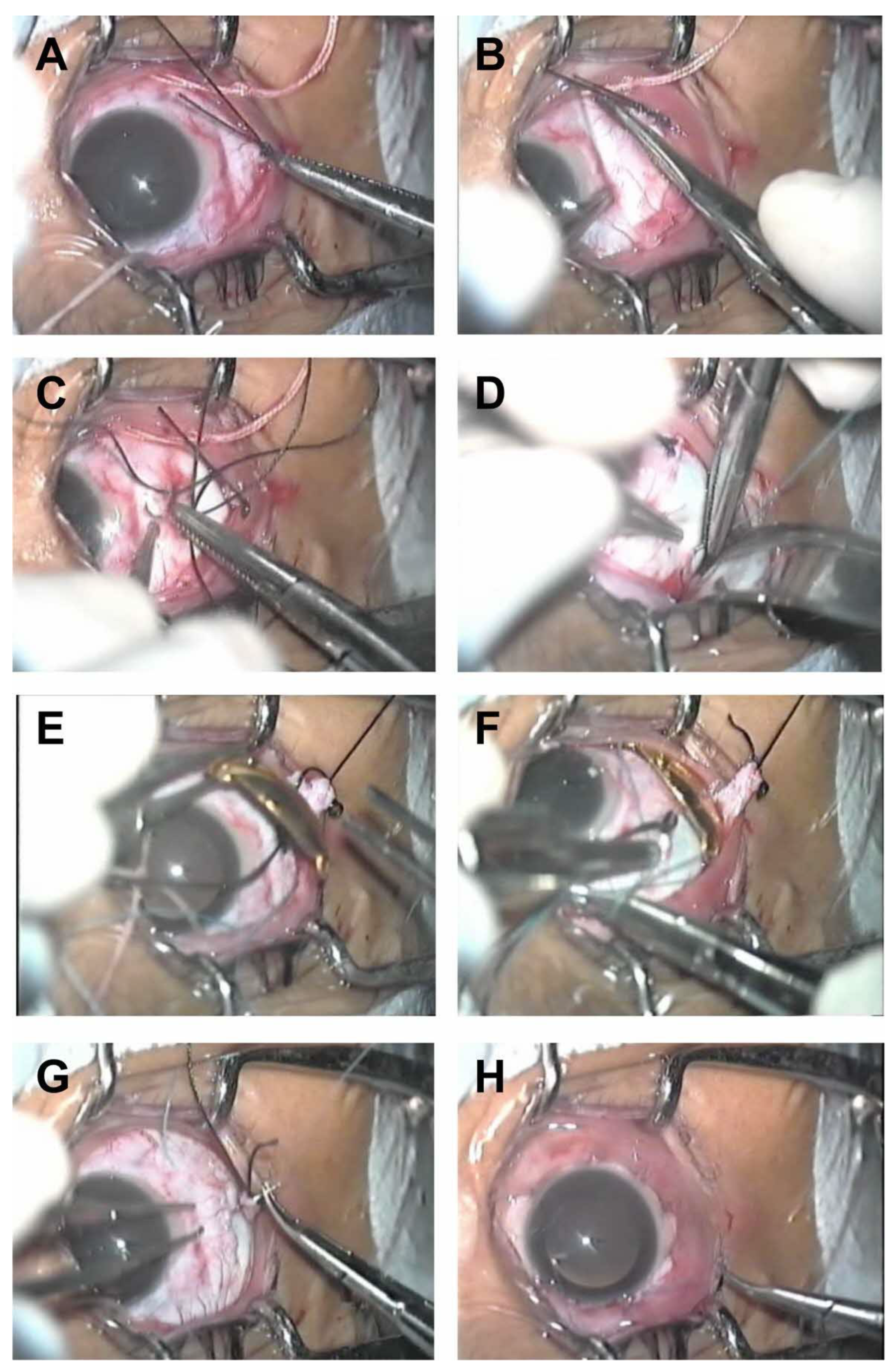
6. Surgical Technique
7. High Dose Rate (HDR) Brachytherapy
8. Adjunctive Procedures: Cytology and Cytogenetics
9. Efficacy of Treatment
10. Complications of Plaque Radiotherapy and Its Treatment
10.1. Scleral and Corneal Complications
10.2. Neovascular Glaucoma
10.3. Radiation-Induced Cataract
10.4. Radiation-Induced Retinopathy and Maculopathy
10.5. Radiation-Induced Optic Neuropathy
10.6. Visual Acuity
10.7. Extraocular Muscle Dysfunction and Diplopia
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, R. Choroidal sarcoma treated by the intraocular insertion of radon seeds. Br. J. Ophthalmol. 1930, 14, 145–156. [Google Scholar] [CrossRef]
- Stallard, H.B. Radiotherapy for malignant melanoma of the choroid. Br. J. Ophthalmol. 1966, 50, 147–155. [Google Scholar] [CrossRef]
- Lommatzsch, P.K. Results after beta-irradiation (106Ru/106Rh) of choroidal melanomas. Twenty years’ experience. Am. J. Clin. Oncol. 1987, 10, 146–151. [Google Scholar] [CrossRef]
- Packer, S.; Rotman, M. Radiotherapy of choroidal melanoma with iodine-125. Ophthalmology 1980, 87, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Sealy, R.; le Roux, P.L.; Rapley, F. The treatment of ophthalmic tumours with low-energy sources. Br. J. Radiol. 1976, 49, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T.; Chin, K.J.; Duvall, G. Palladium-103 ophthalmic plaque radiation therapy for choroidal melanoma: 400 treated patients. Ophthalmology 2009, 116, 790–796. [Google Scholar] [CrossRef]
- Rivard, M.J.; Melhus, C.S.; Sioshansi, S. The impact of prescription depth, dose rate, plaque size, and source loading on the central axis using 103Pd, 125I, and 131Cs. Brachytherapy 2008, 7, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T. Radiation therapy for choroidal melanoma. Surv. Ophthalmol. 1997, 42, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Vakulenko, M.P.; Dedenkov, A.N.; Brovkina, A.F.; Zarubeĭ, G.D. Results of beta-therapy of choroidal melanoma. Med. Radiol. 1980, 25, 73–74. [Google Scholar]
- Brovkina, A.F.; Zarubei, G.D.; Val’skii, V.V. Criteria for assessing the efficacy of brachytherapy of uveal melanomas, complications of therapy and their prevention. Vestn. Oftalmol. 1997, 113, 14–16. [Google Scholar]
- Murakami, N.; Suzuki, S.; Ito, Y.; Yoshimura, R.; Inaba, K.; Kuroda, Y.; Morota, M.; Mayahara, H.; Sakudo, M.; Wakita, A.; et al. 106Ruthenium plaque therapy (RPT) for retinoblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Ocular Melanoma Study Group. Chapter 12: Radiation therapy. In COMS Manual of Procedures; National Technical Information Service (NTIS): Springfield, VA, USA, 1995. [Google Scholar]
- Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar] [CrossRef]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10, 1758834018757175. [Google Scholar] [CrossRef]
- Khoja, L.; Atenafu, E.G.; Suciu, S.; Leyvraz, S.; Sato, T.; Marshall, E.; Keilholz, U.; Zimmer, L.; Patel, S.; Piperno-Neumann, S.; et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: An international rare cancers initiative (IRCI) ocular melanoma study. Ann. Oncol. 2019, 30, 1370–1380. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; EUROCARE Working Group. Incidence of uveal melanoma in Europe. Ophthalmology 2007, 114, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- American Brachytherapy Society—Ophthalmic Oncology Task Force. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy 2014, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rouberol, F.; Roy, P.; Kodjikian, L.; Gerard, J.P.; Jean-Louis, B.; Grange, J.D. Survival, anatomic, and functional long-term results in choroidal and ciliary body melanoma after ruthenium brachytherapy (15 years’ experience with beta-rays). Am. J. Ophthalmol. 2004, 137, 893–900. [Google Scholar] [CrossRef]
- Karimi, S.; Arabi, A.; Shahraki, T. Plaque brachytherapy in iris and iridociliary melanoma: A systematic review of efficacy and complications. J. Contemp. Brachytherapy 2021, 13, 46–50. [Google Scholar] [CrossRef]
- Pagliara, M.M.; Kakkassery, V.; Fionda, B.; Lepore, D.; Kovács, G.; Tagliaferri, L.; Blasi, M.A. Interventional radio-therapy (brachytherapy) in eyelid and ocular surface tumors: A review for treatment of naïve and recurrent malignancies. Neurosignals 2022, 30, 1–10. [Google Scholar] [PubMed]
- Lewis, G.D.; Li, H.K.; Quan, E.M.; Scarboro, S.B.; Teh, B.S. The role of eye plaque brachytherapy and MR imaging in the management of diffuse choroidal hemangioma: An illustrative case report and literature review. Pract. Radiat. Oncol. 2019, 9, e452–e456. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, N.J.; Marinkovic, M.; Peters, F.P.; Hulshof, M.C.; Pieters, B.R.; de Keizer, R.J.; Horeweg, N.; Laman, M.S.; Bleeker, J.C.; van Duinen, S.G.; et al. Management of conjunctival melanoma with local excision and adjuvant brachytherapy. Eye 2021, 35, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Astrahan, M.A.; Luxton, G.; Jozsef, G.; Kampp, T.D.; Liggett, P.E.; Sapozink, M.D.; Petrovich, Z. An interactive treatment planning system for ophthalmic plaque radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 679–687. [Google Scholar] [CrossRef]
- Margo, C.E. The Collaborative Ocular Melanoma Study: An Overview. Cancer Control 2004, 11, 304–309. [Google Scholar] [CrossRef]
- Collaborative Ocular Melanoma Study Group. Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma I. visual acuity after 3 years COMS report no. 16. Ophthalmology 2001, 108, 348–366. [Google Scholar] [CrossRef]
- Miguel, D.; de Frutos, J.M.; Alonso, P.; García-Álvarez, C.; Saornil, M.A.; Diezhandino, P.; Garavis, M.I.; Valencia, P. A study comparing baseline and best-corrected visual acuity after iodine-125 episcleral brachytherapy in uveal melanoma. J. Contemp. Brachytherapy 2023, 15, 350–356. [Google Scholar] [CrossRef]
- Furdova, A.; Babal, P.; Kobzova, D.; Zahorjanova, P.; Kapitanova, K.; Sramka, M.; Kralik, G.; Furda, R.; Krasnik, V. Uveal melanoma survival rates after single dose stereotactic radiosurgery. Neoplasma 2018, 65, 965–971. [Google Scholar] [CrossRef]
- Furdova, A.; Slezak, P.; Chorvath, M.; Waczulikova, I.; Sramka, M.; Kralik, G. No differences in outcome between radical surgical treatment (enucleation) and stereotactic radiosurgery in patients with posterior uveal melanoma. Neoplasma 2010, 57, 377–381. [Google Scholar]
- Miguel, D.; Frutos-Baraja, J.; López-Lara, F.; Saornil, M.; García-Álvarez, C.; Alonso, P.; Diezhandino, P. Radiobiological doses, tumor, and treatment features influence on local control, enucleation rates, and survival after episcleral brachytherapy. A 20-year retrospective analysis from a single institution: Part I. J. Contemp. Brachytherapy 2018, 10, 337–346. [Google Scholar] [CrossRef]
- Abrams, M.J.; Gagne, N.L.; Melhus, C.S.; Mignano, J.E. Brachytherapy vs. external beam radiotherapy for choroidal melanoma: Sur-vival and patterns-of-care analyses. Brachytherapy 2016, 15, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.T.; Sari, S.Y.; Zorlu, F.; Yazici, G. External Beam Radiotherapy in the Management of Uveal Melanoma. Curr. Treat. Options Oncol. 2024, 25, 932–951. [Google Scholar] [CrossRef]
- Thomson, R.M.; Furutani, K.M.; Kaulich, T.W.; Mourtada, F.; Rivard, M.J.; Soares, C.G.; Vanneste, F.M.; Melhus, C.S. AAPM recommendations on medical physics practices for ocular plaque brachytherapy: Report of Task Group 221. Med. Phys. 2020, 47, e92–e124. [Google Scholar] [CrossRef] [PubMed]
- Leonard, K.L.; Gagne, N.L.; Mignano, J.E.; Duker, J.S.; Bannon, E.A.; Rivard, M.J. A 17-year retrospective study of institutional results for eye plaque brachytherapy of uveal melanoma using 125I, 103Pd, and 131Cs and historical perspective. Brachytherapy 2011, 10, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Pellizzon, A.C.A.; Salvajoli, J.V.; Novaes, P.E.; Fogaroli, R.; Ferrigno, R.; Maia, M.A.C.; Chojniak, M.M.; Erwene, C.M. Single institutional retrospective analysis: Treatment of choroidal melanomas with cobalt-60 brachytherapy. Arq. Bras. Oftalmol. 2004, 67, 451–454. [Google Scholar] [CrossRef][Green Version]
- Zografos, L.; Bercher, L.; Chamot, L.; Gailloud, C.; Raimondi, S.; Egger, E. Cobalt-60 treatment of choroidal hemangiomas. Am. J. Ophthalmol. 1996, 121, 190–199. [Google Scholar] [CrossRef]
- Fass, D.; McCormick, B.; Abramson, D.; Ellsworth, R. Cobalt-60 plaques in recurrent retinoblastoma. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 625–627. [Google Scholar] [CrossRef]
- Laskar, S.; Gurram, L.; Laskar, S.G.; Chaudhari, S.; Khanna, N.; Upreti, R. Superficial ocular malignancies treated with strontium-90 brachytherapy: Long-term outcomes. J. Contemp. Brachytherapy 2015, 7, 369–373. [Google Scholar] [CrossRef]
- Van Ginderdeuren, R.; Van Limbergen, E.; Spileers, W. 18 years’ experience with high dose rate strontium-90 brachytherapy of small to medium sized posterior uveal melanoma. Br. J. Ophthalmol. 2005, 89, 1306–1310. [Google Scholar] [CrossRef]
- Fukushima, S.; Inoue, T.; Inoue, T.; Ozeki, S. Postoperative irradiation of pterygium with 90Sr eye applicator. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 597–600. [Google Scholar] [CrossRef]
- Missotten, L.; Dirven, W.; Van der Schueren, A.; Leys, A.; De Meester, G.; Van Limbergen, E. Results of treatment of choroidal malignant melanoma with high-dose-rate strontium-90 brachytherapy: A retrospective study of 46 patients treated between 1983 and 1995. Graefe’s Arch Clin. Exp. Ophthalmol. 1998, 236, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Dupere, J.M.; Munro, J.J.; Medich, D.C. Shielded high dose rate ocular brachytherapy using Yb-169. Phys. Med. Biol. 2021, 66, 125003. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, F.; Valverde, S.; Cárdenes, H.; Cajigal, C.; de la Torre, A.; Magallón, R.; Regueiro, C.; Encinas, J.L.; Aragón, G. Episcleral iridium-192 wire therapy for choroidal melanomas. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 1091–1097. [Google Scholar] [CrossRef]
- Reichstein, D.; Karan, K. Plaque brachytherapy for posterior uveal melanoma in 2018: Improved techniques and expanded indications. Curr. Opin. Ophthalmol. 2018, 29, 191–198. [Google Scholar] [CrossRef]
- National Nuclear Data Center. Chart of Nuclides. Available online: http://www.nndc.bnl.gov/chart/ (accessed on 16 March 2024).
- Ghassemi, F.; Sheibani, S.; Arjmand, M.; Poorbaygi, H.; Kouhestani, E.; Sabour, S.; Samiei, F.; Beiki-Ardakani, A.; Jabarvand, M.; Sadeghi Tari, A. Comparison of iodide-125 and ruthenium-106 brachytherapy in the treatment of choroidal melanomas. Clin. Ophthalmol. 2020, 14, 339–346. [Google Scholar] [CrossRef]
- Filì, M.; Trocme, E.; Bergman, L.; See, T.R.O.; André, H.; Bartuma, K.; Girnita, L.; All-Eriksson, C.; Seregard, S.; Stålhammar, G. Ruthenium-106 versus iodine-125 plaque brachytherapy of 571 choroidal melanomas with a thickness of ≥5.5 mm. Br. J. Ophthalmol. 2020, 104, 26–32. [Google Scholar] [CrossRef]
- Takiar, V.; Gombos, D.S.; Mourtada, F.; Rechner, L.A.; Lawyer, A.A.; Morrison, W.H.; Garden, A.S.; Beadle, B.M. Disease control and toxicity outcomes using ruthenium eye plaque brachytherapy in the treatment of uveal melanoma. Pract. Radiat. Oncol. 2014, 4, e189–e194. [Google Scholar] [CrossRef] [PubMed]
- Danish, H.; Ferris, M.J.; Balagamwala, E.; Switchenko, J.M.; Patel, K.R.; Choudhary, M.; Craven, C.; Mendoza, P.; Suh, J.; Bergstrom, C.; et al. Comparative outcomes and toxicities for ruthenium-106 versus palladium-103 in the treatment of choroidal melanoma. Melanoma Res. 2018, 28, 120–125. [Google Scholar] [CrossRef]
- Powell, B.E.; Finger, P.T. Anti–VEGF therapy immediately after plaque radiation therapy prevents or delays radiation maculopathy. Ophthalmol. Retina 2020, 4, 547–550. [Google Scholar] [CrossRef]
- Finger, P.T.; Tomar, A.S.; Chin, K.J. Palladium-103 plaque therapy for multifocal iris melanoma: Radiation of the entire anterior segment of the eye. Eur. J. Ophthalmol. 2021, 31, 1375–1383. [Google Scholar] [CrossRef]
- Maheshwari, A.; Finger, P.T. Palladium-103 plaque brachytherapy for retinoblastoma: Long-term follow up. Am. J. Ophthalmol. Case Rep. 2022, 27, 101636. [Google Scholar] [CrossRef] [PubMed]
- Chiu-Tsao, S.T.; Astrahan, M.A.; Finger, P.T.; Followill, D.S.; Meigooni, A.S.; Melhus, C.S.; Mourtada, F.; Napolitano, M.E.; Nath, R.; Rivard, M.J.; et al. Dosimetry of 125I and 103Pd COMS eye plaques for intraocular tumors: Report of Task Group 129 by the AAPM and ABS. Med. Phys. 2012, 39, 6161–6184. [Google Scholar] [CrossRef] [PubMed]
- Chiu-Tsao, S.T.; Anderson, L.L.; O’Brien, K.; Stabile, L.; Liu, J.C. Dosimetry for 125I seed (model 6711) in eye plaques. Med. Phys. 1993, 20, 383–389. [Google Scholar] [CrossRef]
- Thomson, R.M.; Taylor, R.E.P.; Rogers, D.W.O. Monte Carlo dosimetry for an eye plaque brachytherapy. Med. Phys. 2008, 35, 5530–5543. [Google Scholar] [CrossRef] [PubMed]
- Hegde, J.V.; McCannel, T.A.; McCannel, C.A.; Lamb, J.; Wang, P.C.; Veruttipong, D.; Almanzor, R.; Demanes, D.J.; Kamrava, M. Juxtapapillary and circumpapillary choroidal melanoma: Globe-sparing treatment outcomes with iodine-125 notched plaque brachytherapy. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1843–1850. [Google Scholar] [CrossRef]
- Lesperance, M.; Inglis-Whalen, M.; Thomson, R.M. Model-based dose calculations for COMS eye plaque brachytherapy using an anatomically realistic eye phantom. Med. Phys. 2014, 41, 021717. [Google Scholar] [CrossRef]
- Lesperance, M.; Martinov, M.; Thomson, R.M. Monte Carlo dosimetry for 103Pd,125I, and131Cs ocular brachytherapy with various plaque models using an eye phantom. Med. Phys. 2014, 41, 031706. [Google Scholar] [CrossRef]
- Studenski, M.T.; Patel, N.V.; Markoe, A.; Harbour, J.W.; Samuels, S.E. Influence of tumor shape and location in eye plaque brachytherapy dosimetry. Brachytherapy 2020, 19, 249–254. [Google Scholar] [CrossRef]
- Karvat, A.; Duzenli, C.; Ma, R.; Paton, K.; Pickles, T. The treatment of choroidal melanoma with 198Au plaque brachytherapy. Radiother. Oncol. 2001, 59, 153–156. [Google Scholar] [CrossRef]
- Ballester, F.; Granero, D.; Perez-Calatayud, J.; Casal, E.; Puchades, V.; Cases, R. Dosimetric study of the 15 mm ropes eye plaque. Radiother. Oncol. 2004, 71, S84. [Google Scholar]
- Astrahan, M.A.; Szechter, A.; Finger, P.T. Design and dosimetric considerations of a modified COMS plaque: The reusable “seed-guide” insert. Med. Phys. 2005, 32, 2706–2716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- EyePhysics. Plaque Simulator. Available online: https://www.eyephysics.com/PS/Index.html (accessed on 5 April 2024).
- Chang, M.Y.; Kamrava, M.; Demanes, D.J.; Leu, M.; Agazaryan, N.; Lamb, J.; Moral, J.N.; Almanzor, R.; McCannel, T.A. Intraoperative ultrasonography-guided positioning of iodine 125 plaque brachytherapy in the treatment of choroidal melanoma. Ophthalmology 2012, 119, 1073–1077. [Google Scholar] [CrossRef]
- Aziz, H.A.; Al Zahrani, Y.A.; Bena, J.; Lorek, B.; Wilkinson, A.; Suh, J.; Singh, A.D. Episcleral brachytherapy of uveal melanoma: Role of intraoperative echographic confirmation. Br. J. Ophthalmol. 2017, 101, 747–751. [Google Scholar] [CrossRef]
- Schefler, A.C.; Yu, H.J. Surgical considerations of radiation plaques and enucleation. In Uveal Melanoma: Biology and Management; Springer International Publishing: Cham, Switzerland, 2021; pp. 69–88. [Google Scholar]
- Finger, P.T. Yttrium-90 episcleral plaque brachytherapy for choroidal melanoma. J. Vitreoretinal Dis. 2024, 8, 210–214. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Yoshida, K.; Yamazaki, H.; Nonomura, N.; Ogawa, K. The emerging role of high-dose-rate (HDR) brachytherapy as monotherapy for prostate cancer. J. Radiat. Res. 2013, 54, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T.; Stewart, R.; Rivard, M.J.; Beers, R.J.; Kamen, J.; Lama, S.; Chin, K.J.; Mohney, K.; Welles, T.S.; Sauerwein, W.A.; et al. First clinical implementation of yttrium-90 disc brachytherapy after FDA clearance. Brachytherapy 2023, 22, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Rivard, M.J.; Mohney, K.; Welles, T.; Finger, P. Dosimetry of beta-emitting brachytherapy sources for age-related macular degeneration. Med. Phys. 2018, 45, e172. [Google Scholar]
- Semeniuk, O.; Yu, E.; Rivard, M.J. Current and emerging radiotherapy options for uveal melanoma. Cancers 2024, 16, 1074. [Google Scholar] [CrossRef]
- Kirwan, J.F.; Constable, P.H.; Murdoch, I.E.; Khaw, P.T. Beta irradiation: New uses for an old treatment: A review. Eye 2003, 17, 207–215. [Google Scholar] [CrossRef]
- Diener-West, M.; Earle, J.D.; Fine, S.L.; Hawkins, B.S.; Moy, C.S.; Reynolds, S.M.; Schachat, A.P.; Straatsma, B.R.; Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine-125 brachytherapy for choroidal melanoma, III: Initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001, 119, 969–982. [Google Scholar]
- Kujala, E.; Makitie, T.; Kivela, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.D.; Gozzo, L.; Tracia, L.; Cicciù, M.; Drago, F.; Bucolo, C.; Avitabile, T.; Rejdak, R.; Nowomiejska, K.; Zweifel, S.; et al. New therapeutic perspectives in the treatment of uveal melanoma: A systematic review. Biomedicines 2021, 9, 1311. [Google Scholar] [CrossRef] [PubMed]
- Tosi, A.; Cappellesso, R.; Dei Tos, A.P.; Rossi, V.; Aliberti, C.; Pigozzo, J.; Fabozzi, A.; Sbaraglia, M.; Blandamura, S.; Del Bianco, P.; et al. The immune cell landscape of metastatic uveal melanoma correlates with overall survival. J. Exp. Clin. Cancer Res. 2021, 40, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Terai, M.; Londin, E.; Sato, T. The role of HGF/MET signaling in metastatic uveal melanoma. Cancers 2021, 13, 5457. [Google Scholar] [CrossRef] [PubMed]
- Triozzi, P.L.; Elson, P.; Aldrich, W.; Achberger, S.; Tubbs, R.; Biscotti, C.V.; Singh, A.D. Elevated blood β-2 microglobulin is associated with tumor monosomy-3 in patients with primary uveal melanoma. Melanoma Res. 2013, 23, 1–7. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Hardy, P. Emerging roles of microRNAs and their implications in uveal melanoma. Cell. Mol. Life Sci. 2021, 78, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.C.; Madic, J.; Mariani, P.; Piperno-Neumann, S.; Rampanou, A.; Servois, V.; Cassoux, N.; Desjardins, L.; Milder, M.; Vaucher, I.; et al. Detection rate and prognostic value of circulating tumor cells and circulating tumor DNA in metastatic uveal melanoma. Int. J. Cancer 2014, 134, 1207–1213. [Google Scholar] [CrossRef]
- Damato, B.; Duke, C.; Coupland, S.E.; Hiscott, P.; Smith, P.A.; Campbell, I.; Douglas, A.; Howard, P. Cytogenetics of uveal melanoma: A 7-year clinical experience. Ophthalmology 2007, 114, 1925–1931. [Google Scholar] [CrossRef]
- Finger, P.T. Intraocular melanoma. In Cancer: Principles and Practice of Oncology, 10th ed.; DeVita, V.T., Lawrence, T.S., Rosenberg, S.A., Eds.; Wolters Kluwer; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2014; pp. 1770–1779. [Google Scholar]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef]
- Ragusa, M.; Barbagallo, C.; Statello, L.; Caltabiano, R.; Russo, A.; Puzzo, L.; Avitabile, T.; Longo, A.; Toro, M.D.; Barbagallo, D.; et al. miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: Pathological and diagnostic implications. Cancer Biol. Ther. 2015, 16, 1387–1396. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Piperno-Neumann, S.; Kapiteijn, E.; Chapman, P.B.; Frank, S.; Joshua, A.M.; Piulats, J.M.; Wolter, P.; Cocquyt, V.; Chmielowski, B.; et al. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: A phase III, multicenter, randomized trial (SUMIT). J. Clin. Oncol. 2018, 36, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, F.; Grosso, M.; Picasso, V.; Tornari, E.; Pesce, M.; Queirolo, P. Treatment of metastatic uveal melanoma with intravenous fotemustine. Melanoma Res. 2013, 23, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Piperno-Neumann, S.; Diallo, A.; Etienne-Grimaldi, M.C.; Bidard, F.C.; Rodrigues, M.; Plancher, C.; Servois, V. Phase II trial of bevacizumab in combination with temozolomide as first-line treatment in patients with metastatic uveal melanoma. Oncol. 2016, 21, 281–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Homsi, J.; Bedikian, A.Y.; Papadopoulos, N.E.; Kim, K.B.; Hwu, W.J.; Mahoney, S.L.; Hwu, P. Phase 2 open-label study of weekly docosahexaenoic acid–paclitaxel in patients with metastatic uveal melanoma. Melanoma Res. 2010, 20, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Bedikian, A.Y.; Papadopoulos, N.E.; Kim, K.B.; Vardeleon, A.; Smith, T.; Lu, B.; Deitcher, S.R. A pilot study with vincristine sulfate liposome infusion in patients with metastatic melanoma. Melanoma Res. 2008, 18, 400–404. [Google Scholar] [CrossRef]
- Peters, S.; Voelter, V.; Zografos, L.; Pampallona, S.; Popescu, R.; Gillet, M.; Leyvraz, S. Intra-arterial hepatic fotemustine for the treatment of liver metastases from uveal melanoma: Experience in 101 patients. Ann. Oncol. 2006, 17, 578–583. [Google Scholar] [CrossRef]
- Huppert, P.E.; Fierlbeck, G.; Pereira, P.; Schanz, S.; Duda, S.H.; Wietholtz, H.; Claussen, C.D. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur. J. Radiol. 2010, 74, e38–e44. [Google Scholar] [CrossRef]
- Bethlehem, M.S.; Katsarelias, D.; Olofsson Bagge, R. Meta-analysis of isolated hepatic perfusion and percutaneous hepatic perfusion as a treatment for uveal melanoma liver metastases. Cancers 2021, 13, 4726. [Google Scholar] [CrossRef]
- Pyrhönen, S.; Hahka-Kemppinen, M.; Muhonen, T.; Nikkanen, V.; Eskelin, S.; Summanen, P.; Kivelä, T. Chemo-immunotherapy with bleomycin, vincristine, lomustine, dacarbazine (BOLD), and human leukocyte interferon for metastatic uveal melanoma. Cancer 2002, 95, 2366–2372. [Google Scholar] [CrossRef]
- Komatsubara, K.M.; Carvajal, R.D. Immunotherapy for the treatment of uveal melanoma: Current status and emerging therapies. Curr. Oncol. Rep. 2017, 19, 1–11. [Google Scholar] [CrossRef]
- Triozzi, P.L.; Eng, C.; Singh, A.D. Targeted therapy for uveal melanoma. Cancer Treat Rev. 2008, 34, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Summanen, P.; Immonen, I.; Kivela, T. Radiation-related complications after ruthenium plaque radiotherapy of uveal melanoma. Br. J. Ophthalmol. 1996, 80, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, M.; Zehetmayer, M.; Ruhswurm, I. Tumour regression of uveal melanoma after ruthenium-106 brachytherapy or stereotactic radiotherapy with gamma knife or linear accelerator. Ophthalmologica 2003, 217, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Damato, B.; Patel, I.; Campbell, I.R. Local tumor control after 106Ru brachytherapy of choroidal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 385–391. [Google Scholar] [CrossRef]
- Karimi, S.; Arabi, A.; Siavashpour, Z.; Shahraki, T.; Ansari, I. Efficacy and complications of ruthenium-106 brachytherapy for uveal melanoma: A systematic review and meta-analysis. J. Contemp. Brachytherapy 2021, 13, 358–364. [Google Scholar] [CrossRef]
- Shields, C.L.; Naseripour, M.; Cater, J.; Shields, J.A.; Demirci, H.; Youseff, A.; Freire, J. Plaque radiotherapy for large posterior uveal melanomas (≥8-mm thick) in 354 consecutive patients. Ophthalmology 2002, 109, 1838–1849. [Google Scholar] [CrossRef]
- Gündüz, K.; Shields, C.L.; Shields, J.A.; Cater, J.; Freire, J.E.; Brady, L.W. Radiation complications and tumor control after plaque radiotherapy of choroidal melanoma with macular involvement. Am. J. Ophthalmol. 1999, 127, 579–589. [Google Scholar] [CrossRef]
- Barker, C.A.; Francis, J.H.; Cohen, G.N.; Marr, B.P.; Wolden, S.L.; McCormick, B.; Abramson, D.H. (106)Ru plaque brachytherapy for uveal melanoma: Factors associated with local tumor recurrence. Brachytherapy 2014, 13, 584–590. [Google Scholar] [CrossRef]
- Oittinen, H.A.L.; O’Shaughnessy, M.; Cullinane, A.B.; Keohane, C. Malignant melanoma of the ciliary body presenting as extraocular metastasis in the temporalis muscle. J. Clin. Pathol. 2007, 60, 834–835. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A.; Cater, J.; Othmane, I.; Singh, A.D.; Micaily, B. Plaque radiotherapy for retinoblastoma: Long-term tumor control and treatment complications in 208 tumors. Ophthalmology 2001, 108, 2116–2121. [Google Scholar] [CrossRef]
- Quivey, J.M.; Augsburger, J.; Snelling, L.; Brady, L.W. 125I plaque therapy for uveal melanoma: Analysis of the impact of time and dose factors on local control. Cancer 1996, 77, 2356–2362. [Google Scholar] [CrossRef]
- Bol, K.F.; Mensink, H.W.; Aarntzen, E.H.; Schreibelt, G.; Keunen, J.E.; Coulie, P.G.; de Klein, A.; Punt, C.J.; Paridaens, D.; Figdor, C.G.; et al. Long Overall Survival After Dendritic Cell Vaccination in Metastatic Uveal Melanoma Patients. Arch. Ophthalmol. 2014, 158, 939–947.e5. [Google Scholar] [CrossRef] [PubMed]
- Heimann, H.; Coupland, S.E.; Gochman, R.; Hellmich, M.; Foerster, M.H. Alterations in expression of mucin, tenascin-C, and syndecan-1 in the conjunctiva following retinal surgery and plaque radiotherapy. Graefes Arch Clin. Exp. Ophthalmol. 2001, 239, 488–495. [Google Scholar] [CrossRef]
- Peddada, K.V.; Sangani, R.; Menon, H.; Verma, V. Complications and adverse events of plaque brachytherapy for ocular melanoma. J. Contemp. Brachyther. 2019, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T. Tumour location affects the incidence of cataract and retinopathy after ophthalmic plaque radiation therapy. Br. J. Ophthalmol. 2000, 84, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Stack, R.; Elder, M.; Abdelaal, A.; Hidajat, R.; Clemett, R. New Zealand experience of I-125 brachytherapy for choroidal melanoma. Clin. Exp. Ophthalmol. 2005, 33, 490–494. [Google Scholar] [CrossRef]
- Quivey, J.M.; Char, D.H.; Phillips, T.L.; Weaver, K.A.; Castro, J.R.; Kroll, S.M. High intensity 125-iodine (125I) plaque treatment of uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 613–618. [Google Scholar] [CrossRef]
- Detorakis, E.T.; Engstrom, R.E.; Wallace, R.; Straatsma, B.R. Iris and anterior chamber angle neovascularization after iodine 125 brachytherapy for uveal melanoma. Ophthalmology 2005, 112, 505–510. [Google Scholar] [CrossRef]
- Easom, H.A. Sympathetic Ophthalmia Associated with Malignant Melanoma. Arch Ophthalmol. 1963, 70, 786–790. [Google Scholar] [CrossRef]
- Wen, J.C.; Oliver, S.C.; McCannel, T.A. Ocular complications following I-125 brachytherapy for choroidal melanoma. Eye 2009, 23, 1254–1268. [Google Scholar] [CrossRef]
- Kim, E.A.; Salazar, D.; McCannel, C.A.; Kamrava, M.; Demanes, D.J.; Lamb, J.; Caprioli, J.; McCannel, T.A. Glaucoma after iodine-125 brachytherapy for uveal melanoma: Incidence and risk factors. J. Glaucoma 2020, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Ocular Melanoma Study Group. Incidence of cataract and outcomes after cataract surgery in the first 5 years after iodine-125 brachytherapy in the Collaborative Ocular Melanoma Study. COMS Report No. 27. Ophthalmology 2007, 114, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, J.; Meyer, D.; Xu, S.; Tai, D. Treatment of choroidal melanoma with I-125 plaque. Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 619–626. [Google Scholar] [CrossRef]
- Archer, D.B.; Amoaku, W.M.; Gardiner, T.A. Radiation retinopathy: Clinical, histopathological, ultrastructural, and experimental correlations. Eye 1991, 5, 239–251. [Google Scholar] [CrossRef]
- Finger, P.T.; Kurli, M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br. J. Ophthalmol. 2005, 89, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Dalvin, L.A.; Chang, M.; Mazloumi, M.; Fortin, P.; McGarrey, M.; Martin, A.; Yaghy, A.; Yang, X.; Vichitvejpaisal, P.; et al. Visual outcome at 4 years following plaque radiotherapy and prophylactic intravitreal bevacizumab (every 4 months for 2 years) for uveal melanoma. JAMA Ophthalmol. 2020, 138, 136–146. [Google Scholar] [CrossRef]
- Puusaari, I.; Heikkonen, J.; Kivela, T. Ocular complications after iodine brachytherapy for large uveal melanomas. Ophthalmology 2004, 111, 1768–1777. [Google Scholar] [CrossRef]
- Shields, C.L.; Demirci, H.; Marr, B.P.; Mashayekhi, A.; Dai, V.V.; Materin, M.A.; Shields, J.A. Intravitreal triamcinolone acetonide for acute radiation papillopathy. Retina 2006, 26, 537–544. [Google Scholar] [CrossRef]
- Levy, R.L.; Miller, N.R. Hyperbaric oxygen therapy for radiation-induced optic neuropathy. Ann. Acad. Med. Singap. 2006, 35, 151–157. [Google Scholar] [CrossRef]
- Lumbroso-Le Rouic, L.; Charif Chefchaouni, M.; Levy, C.; Plancher, C.; Dendale, R.; Asselain, B.; Solignac, S.; Mazal, A.; Desjardins, L. 125I plaque brachytherapy for anterior uveal melanomas. Eye 2004, 18, 911–916. [Google Scholar] [CrossRef]
- Bechrakis, N.E.; Bornfeld, N.; Zoller, I.; Foerster, M.H. Iodine-125 plaque brachytherapy vs transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology 2002, 109, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, J.L.; Packer, S.; Rotman, M.; Ho, T.; Finger, P.T. Choroidal melanoma: I-125 plaque therapy. Radiology 1988, 169, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Garretson, B.R.; Robertson, D.M.; Earle, J.D. Choroidal melanoma treatment with iodine-125 brachytherapy. Arch Ophthalmol. 1987, 105, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Gore, E.; Mieler, W.; Murray, K.; Gillin, M.; Albano, K.; Erickson, B. Posttreatment visual acuity in patients treated with episcleral plaque therapy for choroidal melanomas: Dose and dose rate effects. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Sener, E.C.; Kiratli, H.; Gedik, S.; Sanac, A.S. Ocular motility disturbances after episcleral plaque brachytherapy for uveal melanoma. J. AAPOS 2004, 8, 38–45. [Google Scholar] [CrossRef]
- Dawson, E.; Sagoo, M.S.; Mehta, J.S.; Comer, R.; Hungerford, J.; Lee, J. Strabismus in adults with uveal melanoma following episcleral plaque brachytherapy. J. AAPOS 2007, 11, 584–588. [Google Scholar] [CrossRef]
| General information |
|
| Ruthenium-106 [34] |
|
| Iodine-125 [31] | |
| Palladium-103 [18] |
| Complication | Mechanism | Management |
|---|---|---|
| Keratitis and dry eye syndrome | Radiation exposure during plaque radiotherapy may lead to goblet cell and limbal stem cell injury [101]. | Topical lubricants and gels |
| Neovascular glaucoma | Upregulation of vascular endothelial growth factor (VEGF) and iris vasculature damage can cause rubeosis iridis, potentially progressing to neovascular glaucoma (NVG), which carries a poor prognosis and may require enucleation if untreated [107]. | Topical eye-pressure-lowering medications, oral acetazolamide, laser cyclophotocoagulation, glaucoma filtration surgery [107] |
| Radiation-induced cataract | Ionizing radiation can damage lens fibers, leading to posterior subcapsular, cortical, or nuclear sclerotic cataracts, with posterior subcapsular cataracts being most common [108]. | Cataract surgery, treated similarly to age-related cataracts [108] |
| Radiation-induced retinopathy and maculopathy | Ionizing radiation can lead to closure of the retinal vascular bed, causing intraretinal hemorrhage, cotton-wool spots, non-perfusion of vessels, neovascularization, cystoid macular edema, and vitreous hemorrhage [109]. | Macular edema: intravitreal anti-VEGF and steroid injections Ischemic radiation retinopathy: laser photocoagulation, anti-VEGF injections [109] |
| Radiation-induced optic neuropathy | Characterized by optic disk hyperemia, edema, hemorrhage, and cotton-wool spots [110]. | Intravitreal triamcinolone acetate injections, hyperbaric oxygen therapy [110] |
| Visual acuity loss | Various; linked to higher radiation doses, larger tumor size, and proximity of the tumor to critical structures [111]. | Treatment of the specific cause |
| Extraocular muscle dysfunction and diplopia | Intraoperative muscle disinsertion, radiation-induced extraocular muscle dysfunction [112]. | May recover spontaneously; prisms and botulinum toxin injections for symptoms persisting <6 months; strabismus surgery if symptoms persist >6 months [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, G.N.; Chou, I.-L.; Gopal, L. Plaque Radiotherapy for Ocular Melanoma. Cancers 2024, 16, 3386. https://doi.org/10.3390/cancers16193386
Thomas GN, Chou I-L, Gopal L. Plaque Radiotherapy for Ocular Melanoma. Cancers. 2024; 16(19):3386. https://doi.org/10.3390/cancers16193386
Chicago/Turabian StyleThomas, George Naveen, I-Ling Chou, and Lingam Gopal. 2024. "Plaque Radiotherapy for Ocular Melanoma" Cancers 16, no. 19: 3386. https://doi.org/10.3390/cancers16193386
APA StyleThomas, G. N., Chou, I.-L., & Gopal, L. (2024). Plaque Radiotherapy for Ocular Melanoma. Cancers, 16(19), 3386. https://doi.org/10.3390/cancers16193386






