Effects of High-Linear-Energy-Transfer Heavy Ion Radiation on Intestinal Stem Cells: Implications for Gut Health and Tumorigenesis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Irradiations
2.2. Isolation of Lgr5+ ISCs
2.3. Intracellular and Mitochondrial Reactive Oxygen Species (ROS) Detection
2.4. β-Galactosidase Staining
2.5. In Situ Immunostaining
2.6. mRNA Expression Analysis Using Real-Time PCR Assays
2.7. Imaging, Quantification, and Statistical Analysis
2.8. List of Antibodies
3. Results
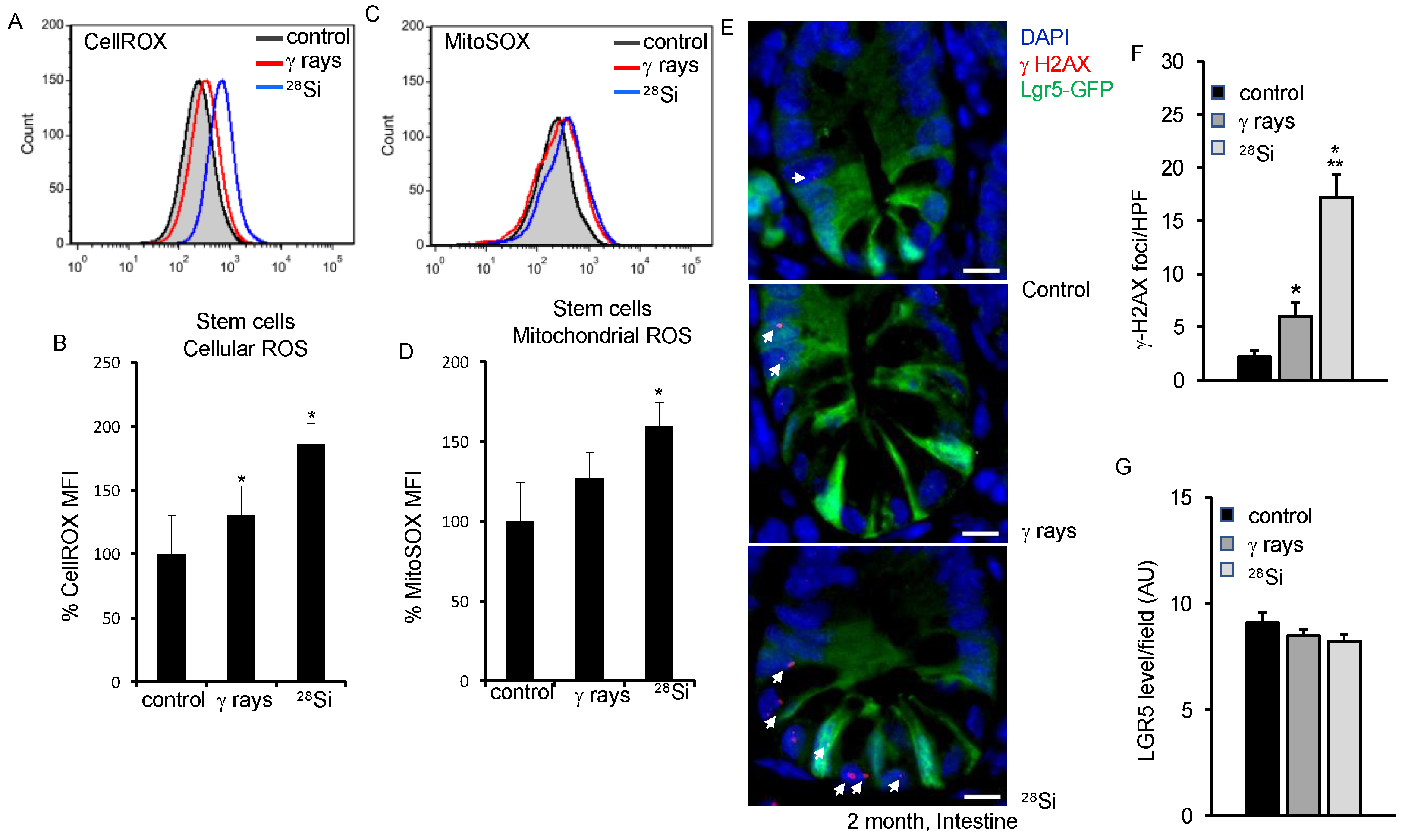
3.1. Assessment of ROS in Lgr5 Expressing ISCs after 28Si Irradiation in Mice
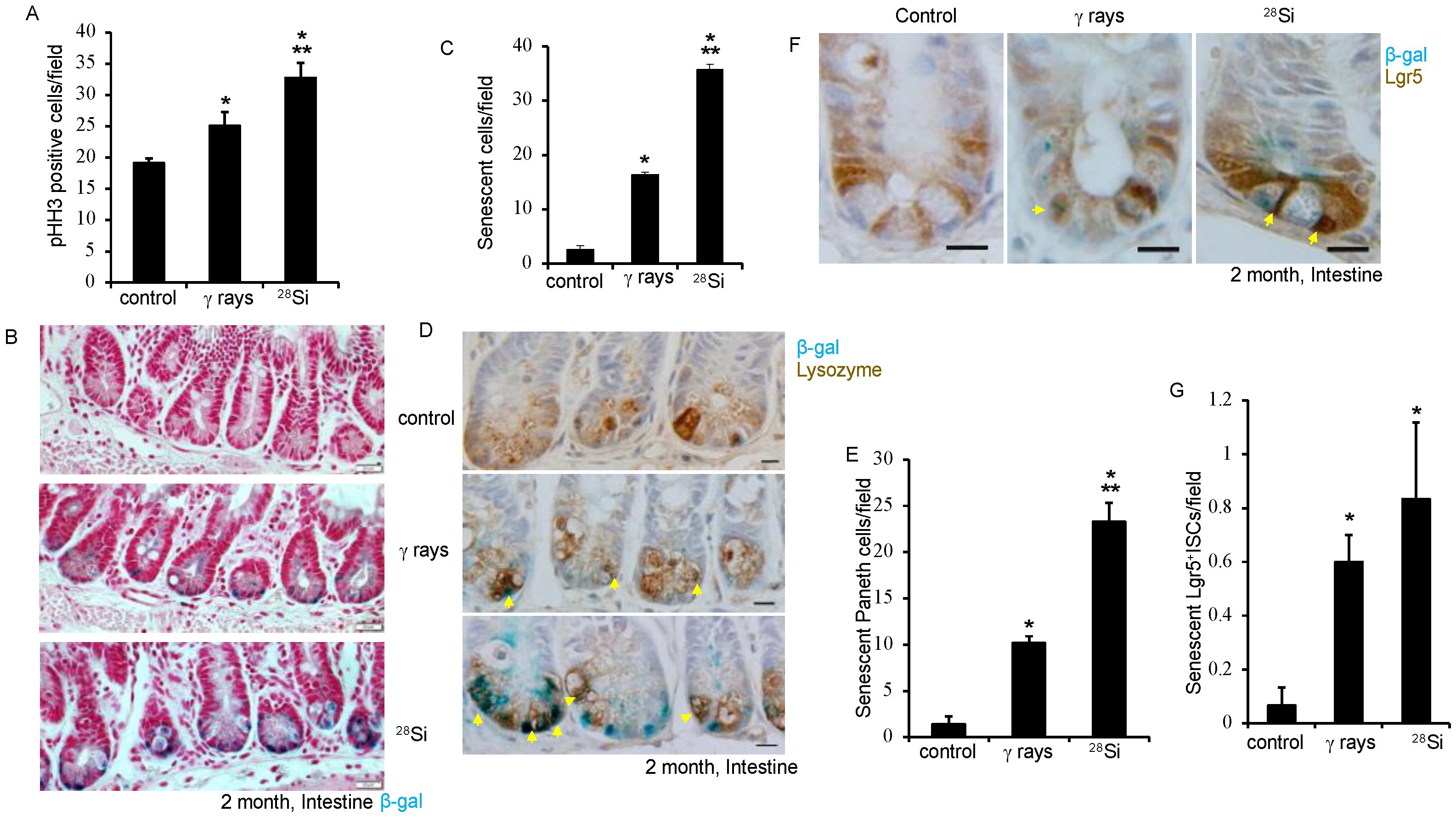
3.2. 28Si Ion Radiation-Induced DNA Damage and Cell Proliferation
3.3. Lgr5+ ISCs and Paneth Cell Senescence after Heavy-Ion 28Si Radiation
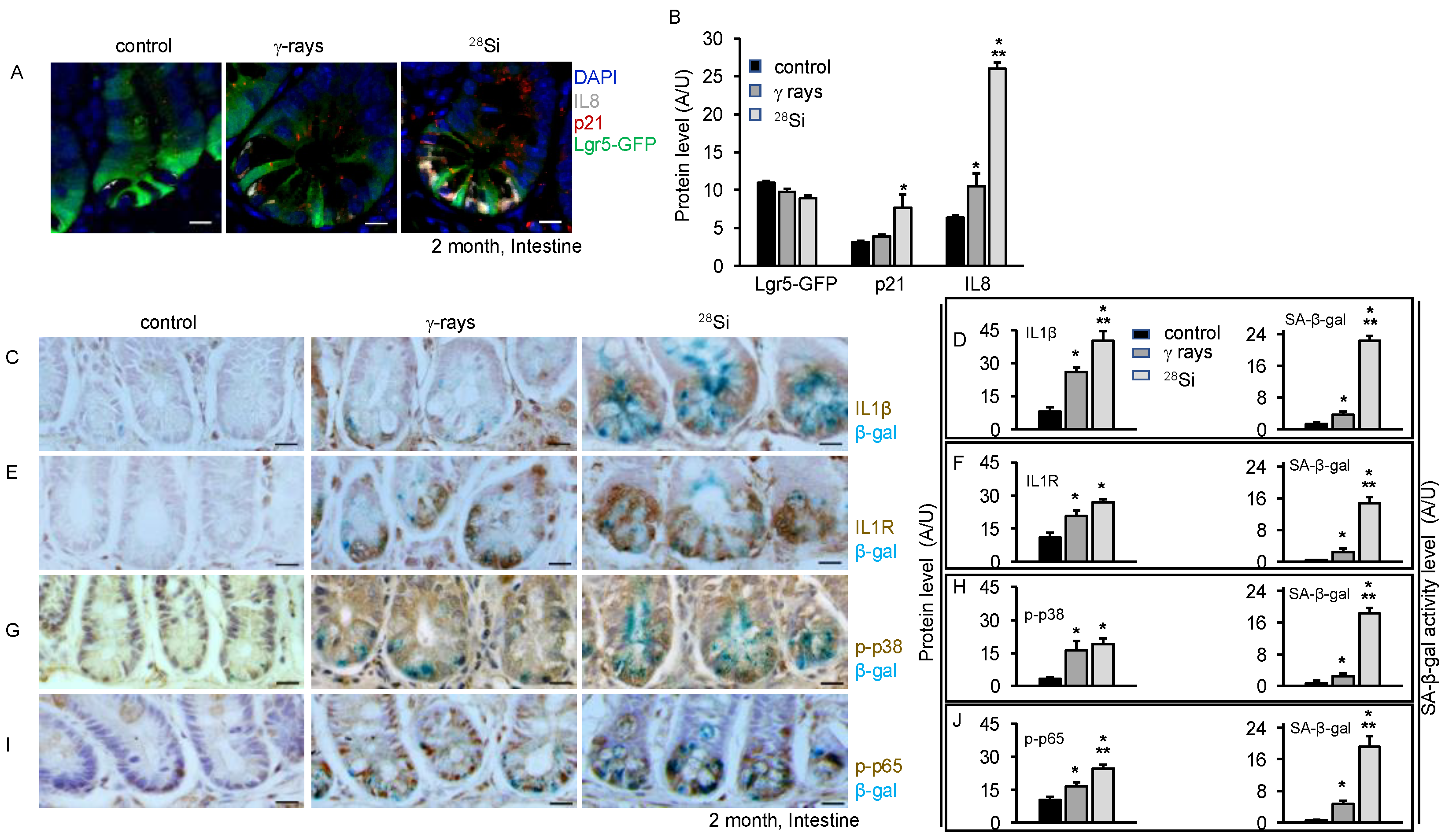
3.4. Increased SASP Markers and Pro-Inflammatory MAPK/NFκB Signaling after 28Si Radiation
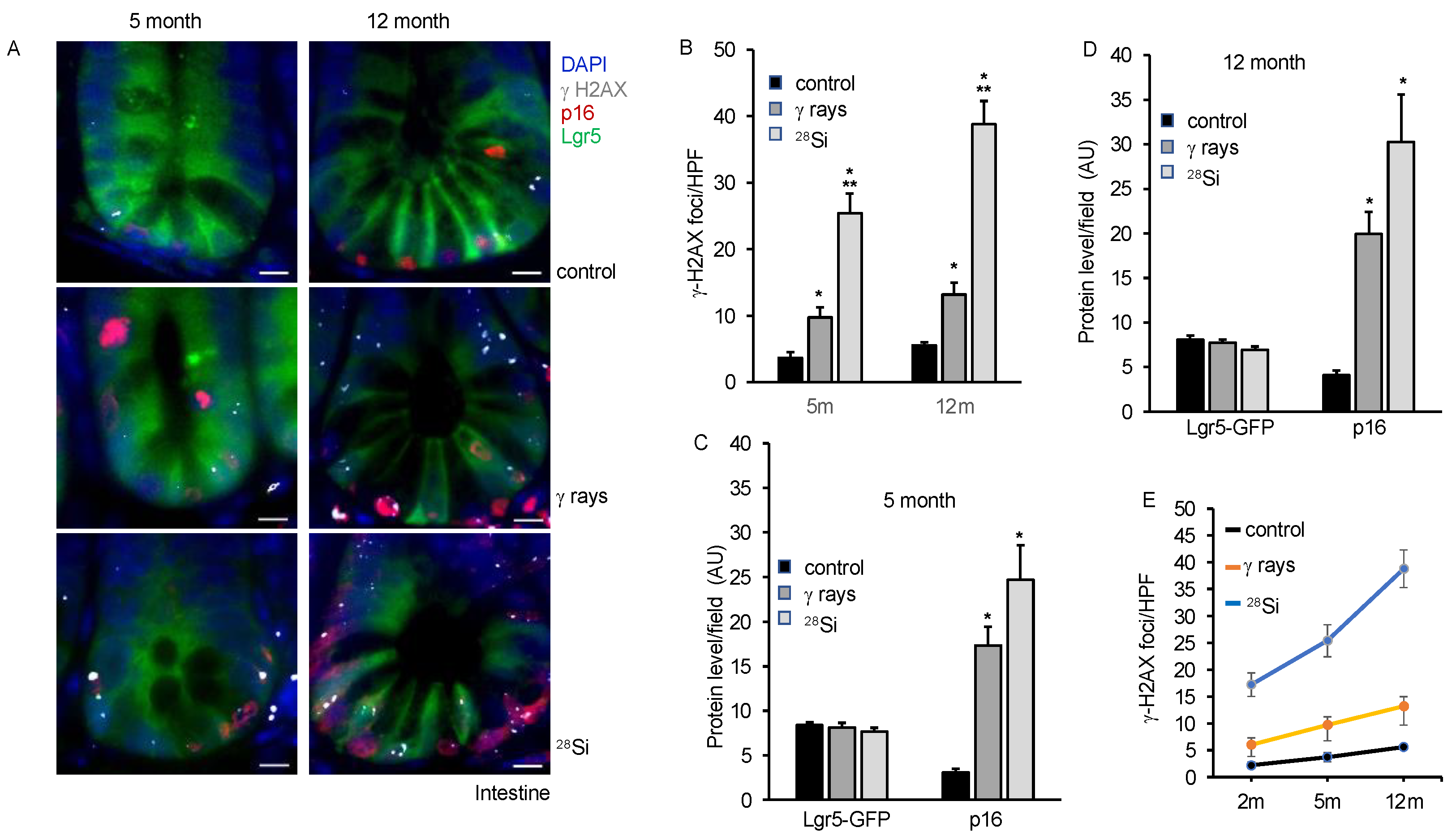
3.5. Persistent DNA Damage, Senescence and SASP Marker in Intestine Long-Term after Heavy-Ion Radiation
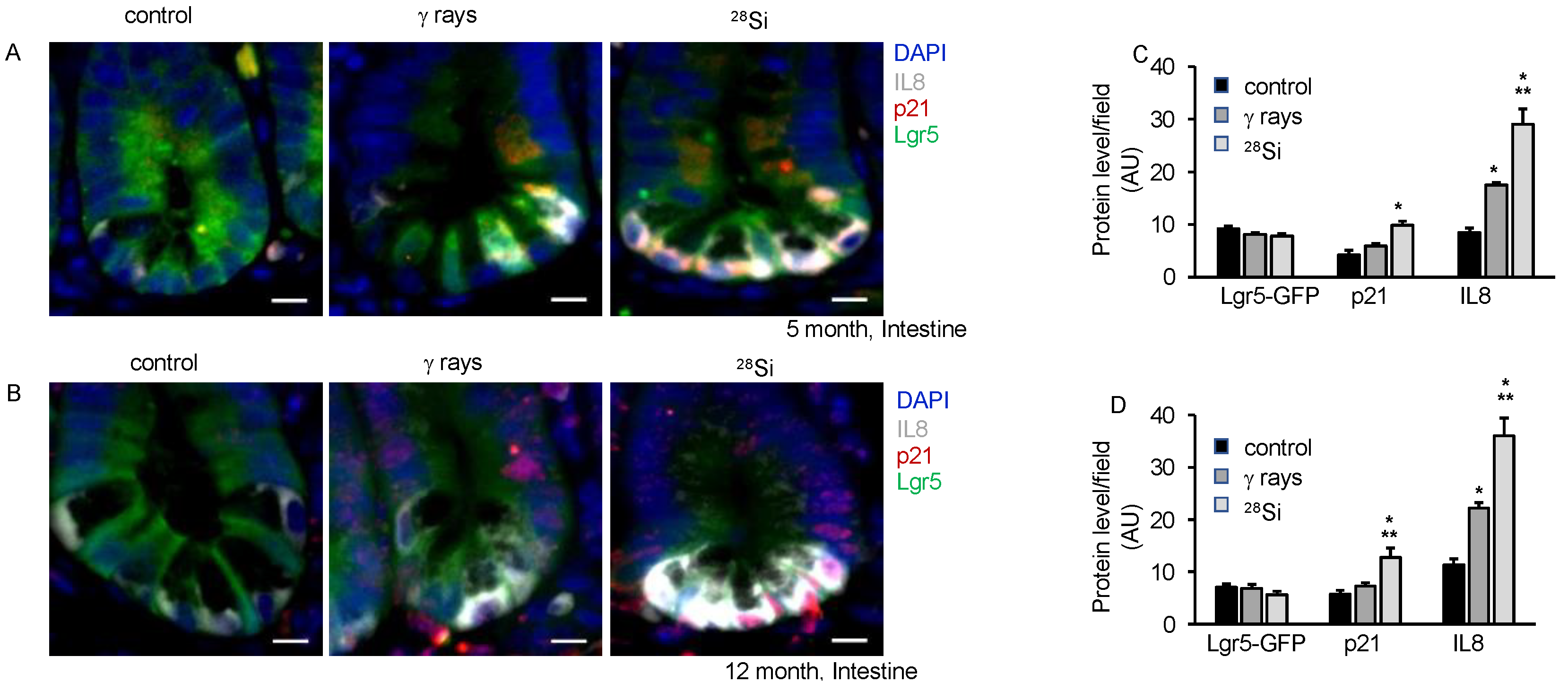
3.6. IR-Induced Accelerated Aging Is Associated with Altered Nutrient Absorption and Barrier Function
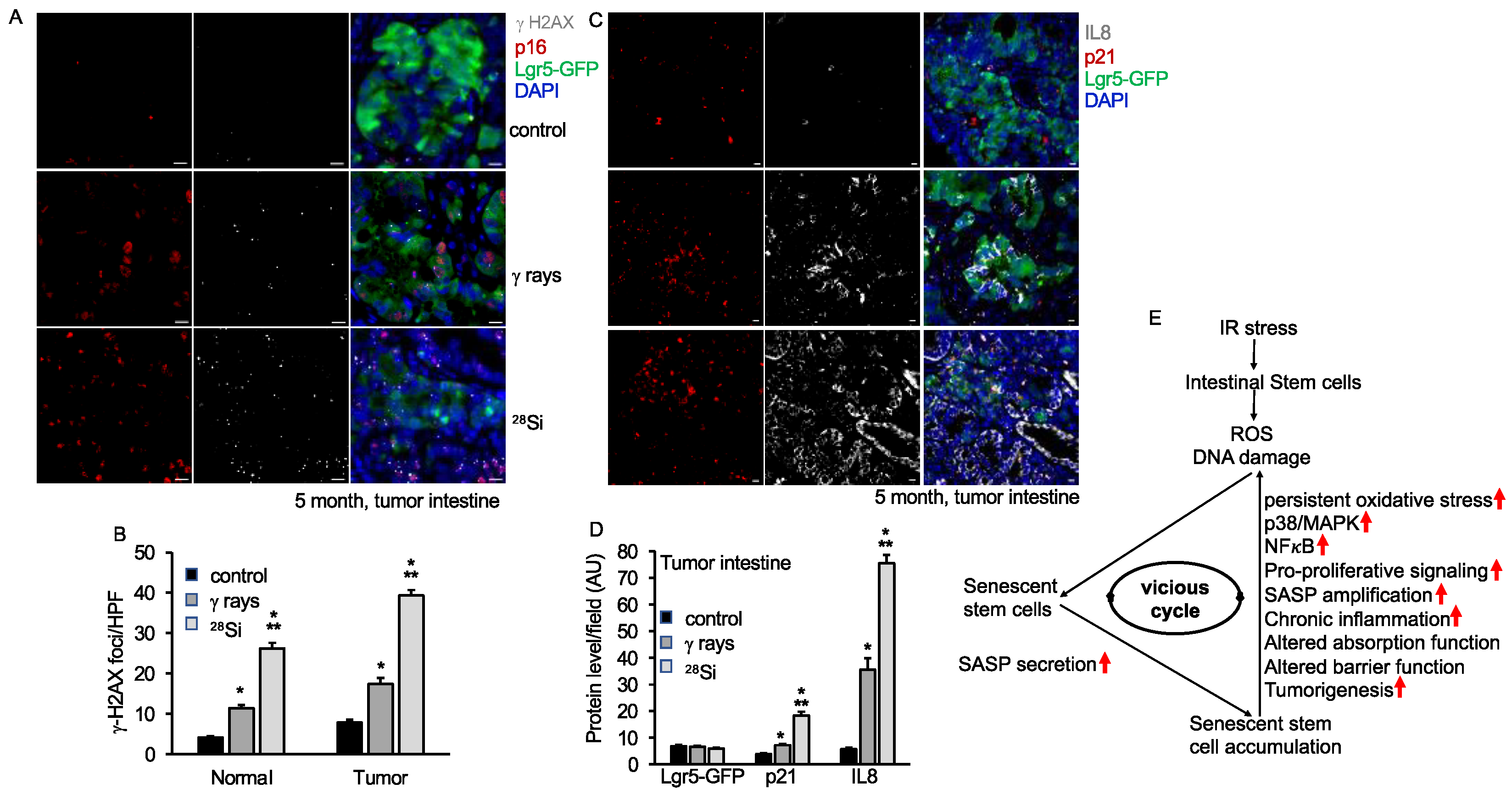
3.7. Increased Senescence and SASP Factors in Intestinal Tumor in Lgr5+Apc1638N/+ Mice after 28Si Radiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barcellos-Hoff, M.H.; Blakely, E.A.; Burma, S.; Fornace, A.J.; Gerson, S.; Hlatky, L.; Kirsch, D.G.; Luderer, U.; Shay, J.; Wang, Y.; et al. Concepts and challenges in cancer risk prediction for the space radiation environment. Life Sci. Space Res. 2015, 6, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Schimmerling, W.; Wilson, J.W.; Peterson, L.E.; Badhwar, G.D.; Saganti, P.B.; Dicello, J.F. Space radiation cancer risks and uncertainties for Mars missions. Radiat. Res. 2001, 156, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A. Review of NASA approach to space radiation risk assessments for Mars exploration. Health Phys. 2015, 108, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Toprani, S.M.; Scheibler, C.; Mordukhovich, I.; McNeely, E.; Nagel, Z.D. Cosmic Ionizing Radiation: A DNA Damaging Agent That May Underly Excess Cancer in Flight Crews. Int. J. Mol. Sci. 2024, 25, 7670. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Failla, G.; Finklea, L.R.; Charp, P.; Ansari, A.J. Radiation Exposure of Workers and Volunteers in Shelters and Community Reception Centers in the Aftermath of a Nuclear Detonation. Health Phys. 2019, 116, 619–624. [Google Scholar] [CrossRef]
- Briggs, B.; Kalra, S.; Panacek, E. Risk of Radiation Exposure to Emergency Department Personnel from Portable Radiographs. J. Emerg. Med. 2022, 63, 645–650. [Google Scholar] [CrossRef]
- Daryoush, J.R.; Lancaster, A.J.; Frandsen, J.J.; Gililland, J.M. Occupational Hazards to the Joint Replacement Surgeon: Radiation Exposure. J. Arthroplasty 2022, 37, 1464–1469. [Google Scholar] [CrossRef]
- Miller, D.L.; Klein, L.W.; Balter, S.; Norbash, A.; Haines, D.; Fairobent, L.; Goldstein, J.A.; Multispecialty, O.H.G. Occupational health hazards in the interventional laboratory: Progress report of the Multispecialty Occupational Health Group. J. Vasc. Interv. Radiol. 2010, 21, 1338–1341. [Google Scholar] [CrossRef]
- Naithani, M.; Khapre, M.; Kathrotia, R.; Gupta, P.K.; Dhingra, V.K.; Rao, S. Evaluation of Sensitization Program on Occupational Health Hazards for Nursing and Allied Health Care Workers in a Tertiary Health Care Setting. Front. Public Health 2021, 9, 669179. [Google Scholar] [CrossRef]
- Port, M.; Majewski, M.; Abend, M. Radiation dose is of Limited Clinical Usefulness in Persons with Acute Radiation Syndrome. Radiat. Prot. Dosimetry 2019, 186, 126–129. [Google Scholar] [CrossRef]
- Sanusi, M.S.M.; Ramli, A.T.; Hashim, S.; Lee, M.H. Radiological hazard associated with amang processing industry in Peninsular Malaysia and its environmental impacts. Ecotoxicol. Environ. Saf. 2021, 208, 111727. [Google Scholar] [CrossRef] [PubMed]
- Shuryak, I.; Fornace, A.J.; Datta, K.; Suman, S.; Kumar, S.; Sachs, R.K.; Brenner, D.J. Scaling Human Cancer Risks from Low LET to High LET when Dose-Effect Relationships are Complex. Radiat. Res. 2017, 187, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Suman, S.; Kallakury, B.V.; Fornace, A.J. Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PLoS ONE 2012, 7, e42224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Kumar, S.; Datta, K.; Fornace, A.J.; Suman, S. High-LET-Radiation-Induced Persistent DNA Damage Response Signaling and Gastrointestinal Cancer Development. Curr. Oncol. 2023, 30, 5497–5514. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Suman, S.; Fornace, A.J.; Datta, K. Space radiation triggers persistent stress response, increases senescent signaling, and decreases cell migration in mouse intestine. Proc. Natl. Acad. Sci. USA 2018, 115, E9832–E9841. [Google Scholar] [CrossRef]
- Kumar, S.; Suman, S.; Fornace, A.J.; Datta, K. Intestinal stem cells acquire premature senescence and senescence associated secretory phenotype concurrent with persistent DNA damage after heavy ion radiation in mice. Aging 2019, 11, 4145–4158. [Google Scholar] [CrossRef]
- Adhikary, A.; Becker, D.; Palmer, B.J.; Heizer, A.N.; Sevilla, M.D. Direct formation of the C5′-radical in the sugar-phosphate backbone of DNA by high-energy radiation. J. Phys. Chem. B 2012, 116, 5900–5906. [Google Scholar] [CrossRef]
- Cucinotta, F.A. A new approach to reduce uncertainties in space radiation cancer risk predictions. PLoS ONE 2015, 10, e0120717. [Google Scholar] [CrossRef]
- Huff, J.L.; Poignant, F.; Rahmanian, S.; Khan, N.; Blakely, E.A.; Britten, R.A.; Chang, P.; Fornace, A.J.; Hada, M.; Kronenberg, A.; et al. Galactic cosmic ray simulation at the NASA space radiation laboratory—Progress, challenges and recommendations on mixed-field effects. Life Sci. Space Res. 2023, 36, 90–104. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Luvhengo, T.; Khan, U.; Marumo, T.K. Paneth Cells and Lgr5+ Intestinal Stem Cells in Radiation Enteritis. Appl. Sci. 2023, 13, 2758. [Google Scholar] [CrossRef]
- Sato, T.; van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; van de Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Urbauer, E.; Rath, E.; Haller, D. Mitochondrial Metabolism in the Intestinal Stem Cell Niche-Sensing and Signaling in Health and Disease. Front. Cell Dev. Biol. 2020, 8, 602814. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, F.; Belli, M.; Campa, A.; Chatterjee, A.; Dini, V.; Esposito, G.; Rydberg, B.; Simone, G.; Tabocchini, M.A. DNA fragmentation induced by Fe ions in human cells: Shielding influence on spatially correlated damage. Adv. Space Res. 2004, 34, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, V.; Mladenov, E.; Stuschke, M.; Iliakis, G. DNA Damage Clustering after Ionizing Radiation and Consequences in the Processing of Chromatin Breaks. Molecules 2022, 27, 1540. [Google Scholar] [CrossRef] [PubMed]
- Nikitaki, Z.; Nikolov, V.; Mavragani, I.V.; Mladenov, E.; Mangelis, A.; Laskaratou, D.A.; Fragkoulis, G.I.; Hellweg, C.E.; Martin, O.A.; Emfietzoglou, D.; et al. Measurement of complex DNA damage induction and repair in human cellular systems after exposure to ionizing radiations of varying linear energy transfer (LET). Free Radic. Res. 2016, 50, S64–S78. [Google Scholar] [CrossRef]
- Rydberg, B. Clusters of DNA Damage Induced by Ionizing Radiation: Formation of Short DNA Fragments. II. Experimental Detection. Radiat. Res. 1996, 145, 200–209. Available online: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8606930 (accessed on 15 August 2024). [CrossRef]
- Broustas, C.G.; Mukherjee, S.; Pannkuk, E.L.; Laiakis, E.C.; Fornace, A.J.; Amundson, S.A. Effect of the p38 Mitogen-Activated Protein Kinase Signaling Cascade on Radiation Biodosimetry. Radiat. Res. 2022, 198, 18–27. [Google Scholar] [CrossRef]
- Chou, C.H.; Chen, S.U.; Cheng, J.C. Radiation-induced interleukin-6 expression through MAPK/p38/NF-kappaB signaling pathway and the resultant antiapoptotic effect on endothelial cells through Mcl-1 expression with sIL6-Ralpha. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1553–1561. [Google Scholar] [CrossRef]
- Chowdhury, P.; Dey, P.; De, D.; Ghosh, U. Gamma ray-induced in vitro cell migration via EGFR/ERK/Akt/p38 activation is prevented by olaparib pretreatment. Int. J. Radiat. Biol. 2020, 96, 651–660. [Google Scholar] [CrossRef]
- Mihaescu, A.; Santen, S.; Jeppsson, B.; Thorlacius, H. p38 Mitogen-activated protein kinase signalling regulates vascular inflammation and epithelial barrier dysfunction in an experimental model of radiation-induced colitis. Br. J. Surg. 2010, 97, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Trani, D.; Datta, K.; Doiron, K.; Kallakury, B.; Fornace, A.J. Enhanced intestinal tumor multiplicity and grade in vivo after HZE exposure: Mouse models for space radiation risk estimates. Radiat. Environ. Biophys. 2010, 49, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Suman, S.; Kallakury, B.V.; Fornace, A.J. Heavy ion radiation exposure triggered higher intestinal tumor frequency and greater β-catenin activation than γ radiation in APC(Min/+) mice. PLoS ONE 2013, 8, e59295. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Kumar, S.; Moon, B.H.; Fornace, A.J.; Datta, K. Low and high dose rate heavy ion radiation-induced intestinal and colonic tumorigenesis in APC1638N/+ mice. Life Sci. Space Res. 2017, 13, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Kumar, S.; Moon, B.H.; Strawn, S.J.; Thakor, H.; Fan, Z.; Shay, J.W.; Fornace, A.J.; Datta, K. Relative Biological Effectiveness of Energetic Heavy Ions for Intestinal Tumorigenesis Shows Male Preponderance and Radiation Type and Energy Dependence in APC(1638N/+) Mice. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 131–138. [Google Scholar] [CrossRef]
- Kwiatkowski, E.; Suman, S.; Kallakury, B.V.S.; Datta, K.; Fornace, A.J.; Kumar, S. Expression of Stem Cell Markers in High-LET Space Radiation-Induced Intestinal Tumors in Apc1638N/+ Mouse Intestine. Cancers 2023, 15, 4240. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, X.; Chen, Y.G. Dedifferentiation: The return road to repair the intestinal epithelium. Cell Regen. 2020, 9, 2. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.G. Intestinal epithelial plasticity and regeneration via cell dedifferentiation. Cell Regen. 2020, 9, 14. [Google Scholar] [CrossRef]
- Guo, R.M.; Xu, W.M.; Lin, J.C.; Mo, L.Q.; Hua, X.X.; Chen, P.X.; Wu, K.; Zheng, D.D.; Feng, J.Q. Activation of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol. Med. Rep. 2013, 8, 603–608. [Google Scholar] [CrossRef]
- Novoselova, E.G.; Glushkova, O.V.; Khrenov, M.O.; Parfenyuk, S.B.; Lunin, S.M.; Vinogradova, E.V.; Novoselova, T.V.; Fesenko, E.E. Involvement of the p38 MAPK signaling cascade in stress response of RAW 264.7 macrophages. Dokl. Biol. Sci. 2017, 476, 203–205. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell. Signal. 2012, 24, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Adhikary, A. The Effects of Particle LET and Fluence on the Complexity and Frequency of Clustered DNA Damage. DNA 2024, 4, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, H.; Dobo, C.; Filippi, R.Z.; Mendes do Nascimento, H.; Palmieri da Silva E Sousa, L.; Meola, J.; Piccinato, C.A.; Podgaec, S. Altered p16Ink4a, IL-1β, and Lamin b1 Protein Expression Suggest Cellular Senescence in Deep Endometriotic Lesions. Int. J. Mol. Sci. 2022, 23, 2476. [Google Scholar] [CrossRef]
- Cinat, D.; Coppes, R.P.; Barazzuol, L. DNA Damage-Induced Inflammatory Microenvironment and Adult Stem Cell Response. Front. Cell Dev. Biol. 2021, 9, 729136. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Podlutsky, A.; Sosnowska, D.; Tucsek, Z.; Toth, P.; Deak, F.; Gautam, T.; Csiszar, A.; Sonntag, W.E. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: Role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ye, C.; Sun, F.; Wei, W.; Hu, B.; Wang, J. Both Complexity and Location of DNA Damage Contribute to Cellular Senescence Induced by Ionizing Radiation. PLoS ONE 2016, 11, e0155725. [Google Scholar] [CrossRef]
- Haines, R.J.; Beard, R.S.; Chen, L.; Eitnier, R.A.; Wu, M.H. Interleukin-1β Mediates β-Catenin-Driven Downregulation of Claudin-3 and Barrier Dysfunction in Caco2 Cells. Dig. Dis. Sci. 2016, 61, 2252–2261. [Google Scholar] [CrossRef]
- Kaminsky, L.W.; Al-Sadi, R.; Ma, T.Y. IL-1β and the Intestinal Epithelial Tight Junction Barrier. Front. Immunol. 2021, 12, 767456. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.L.; Chen, H.L.; Wu, D.; Chen, J.X.; Wang, X.X.; Li, R.L.; He, J.H.; Mo, L.; Cen, X.; et al. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem. Pharmacol. 2014, 88, 334–350. [Google Scholar] [CrossRef]
- He, D.; Wu, H.; Xiang, J.; Ruan, X.; Peng, P.; Ruan, Y.; Chen, Y.G.; Wang, Y.; Yu, Q.; Zhang, H.; et al. Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nat. Commun. 2020, 11, 37. [Google Scholar] [CrossRef]
- Saffrey, M.J. Aging of the mammalian gastrointestinal tract: A complex organ system. Age 2014, 36, 9603. [Google Scholar] [CrossRef] [PubMed]
- Sisakht, M.; Darabian, M.; Mahmoodzadeh, A.; Bazi, A.; Shafiee, S.M.; Mokarram, P.; Khoshdel, Z. The role of radiation induced oxidative stress as a regulator of radio-adaptive responses. Int. J. Radiat. Biol. 2020, 96, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Kendong, S.M.; Raja Ali, R.A.; Nawawi, K.N.M.; Ahmad, H.F.; Mokhtar, N.M. Gut Dysbiosis and Intestinal Barrier Dysfunction: Potential Explanation for Early-Onset Colorectal Cancer. Front. Cell Infect. Microbiol. 2021, 11, 744606. [Google Scholar] [CrossRef] [PubMed]
- Isermann, A.; Mann, C.; Rübe, C.E. Histone Variant H2A.J Marks Persistent DNA Damage and Triggers the Secretory Phenotype in Radiation-Induced Senescence. Int. J. Mol. Sci. 2020, 21, 9130. [Google Scholar] [CrossRef]
- Hafner, L.; Walsh, L.; Schneider, U. Cancer incidence risks above and below 1 Gy for radiation protection in space. Life Sci. Space Res. 2021, 28, 41–56. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Suman, S.; Angdisen, J.; Moon, B.-H.; Kallakury, B.V.S.; Datta, K.; Fornace, A.J., Jr. Effects of High-Linear-Energy-Transfer Heavy Ion Radiation on Intestinal Stem Cells: Implications for Gut Health and Tumorigenesis. Cancers 2024, 16, 3392. https://doi.org/10.3390/cancers16193392
Kumar S, Suman S, Angdisen J, Moon B-H, Kallakury BVS, Datta K, Fornace AJ Jr. Effects of High-Linear-Energy-Transfer Heavy Ion Radiation on Intestinal Stem Cells: Implications for Gut Health and Tumorigenesis. Cancers. 2024; 16(19):3392. https://doi.org/10.3390/cancers16193392
Chicago/Turabian StyleKumar, Santosh, Shubhankar Suman, Jerry Angdisen, Bo-Hyun Moon, Bhaskar V. S. Kallakury, Kamal Datta, and Albert J. Fornace, Jr. 2024. "Effects of High-Linear-Energy-Transfer Heavy Ion Radiation on Intestinal Stem Cells: Implications for Gut Health and Tumorigenesis" Cancers 16, no. 19: 3392. https://doi.org/10.3390/cancers16193392





