Subependymal Giant Cell Astrocytoma: The Molecular Landscape and Treatment Advances
Abstract
:Simple Summary
Abstract
1. Introduction to Subependymal Giant Cell Astrocytoma Etiology, Clinical Presentation, and Diagnostics
2. Neuropathological Characteristics of SEGA
3. Tuberous Sclerosis Complex
4. Genetic Background of TSC
| Condition | Mutations Reported | Reference |
|---|---|---|
| TSC, familial | Frameshift > splicing, nonsense TSC1 mutations | [67] |
| TSC, familial | Lower proportion of TSC2 mutations in familial cases of TSC than in de novo cases; Dominant in frequency were frameshifts followed by nonsense mutations | [51] |
| TSC, familial | Identification of TSC1 mutations appears to be twice as likely in familial cases as in sporadic cases. Mutations in TSC2 are associated with more severe diseases, including seizures and cognitive dysfunction. | [50] |
| TSC, familial | Germline mutations in TSC1 (9q34.3) encoding hamartin and TSC2 (16p113.3) encoding tuberin | [68] |
| TSC, sporadic | Small TSC2 mutations > small TSC1 mutations > large TSC2 mutations | [46] |
| TSC, sporadic | Nonsense, missense > indels among TSC2 mutations | [48] |
| TSC, sporadic | Sporadic TSC cases more often result from TSC2 than TSC1 mutations | [49] |
| TSC, sporadic | Pathogenic variants in TSC2 > TSC1: 23% nonsense, 22% missense, 19% splice, 18% deletions, 8% large deletions, 2% in-frame deletions | [69] |
| SEGA; TSC-related | TSC2 gene deletions affecting the adjacent PKD1 have the highest risk of early SEGA development. | [70] |
| SEGA; TSC-related | TSC1 nonsense > deletions > insertions; missense or TSC2 nonsence > deletions, splice sites > missense; insertions. Also found were somatic mutations in genes involved in transcriptional and translational regulation, cell cycle regulation, signal transduction, cell adhesion, resistance to anti-cancer drugs, energy metabolism, ubiquitin–proteasome system functioning, immune homeostasis, and cytoskeleton stabilization. | [71] |
| SEGA-like; solitary | NF1 splice mutations | [72] |
| SEGA; solitary | TSC1 or TSC2 mutation limited to the tumor | [73] |
| SEGA; solitary | TSC2 somatic mosaic mutation, including extra-tumor tissues | [74] |
| SEGA; solitary | EGFR amplification, CDKN2A/B homozygous deletion, chromosomal +7/−10 alterations, and TERT promoter mutation, typical molecular abnormalities usually found in GBM, were observed. | [9] |
5. The Mechanisms of Sega Formation in Tuberous Sclerosis
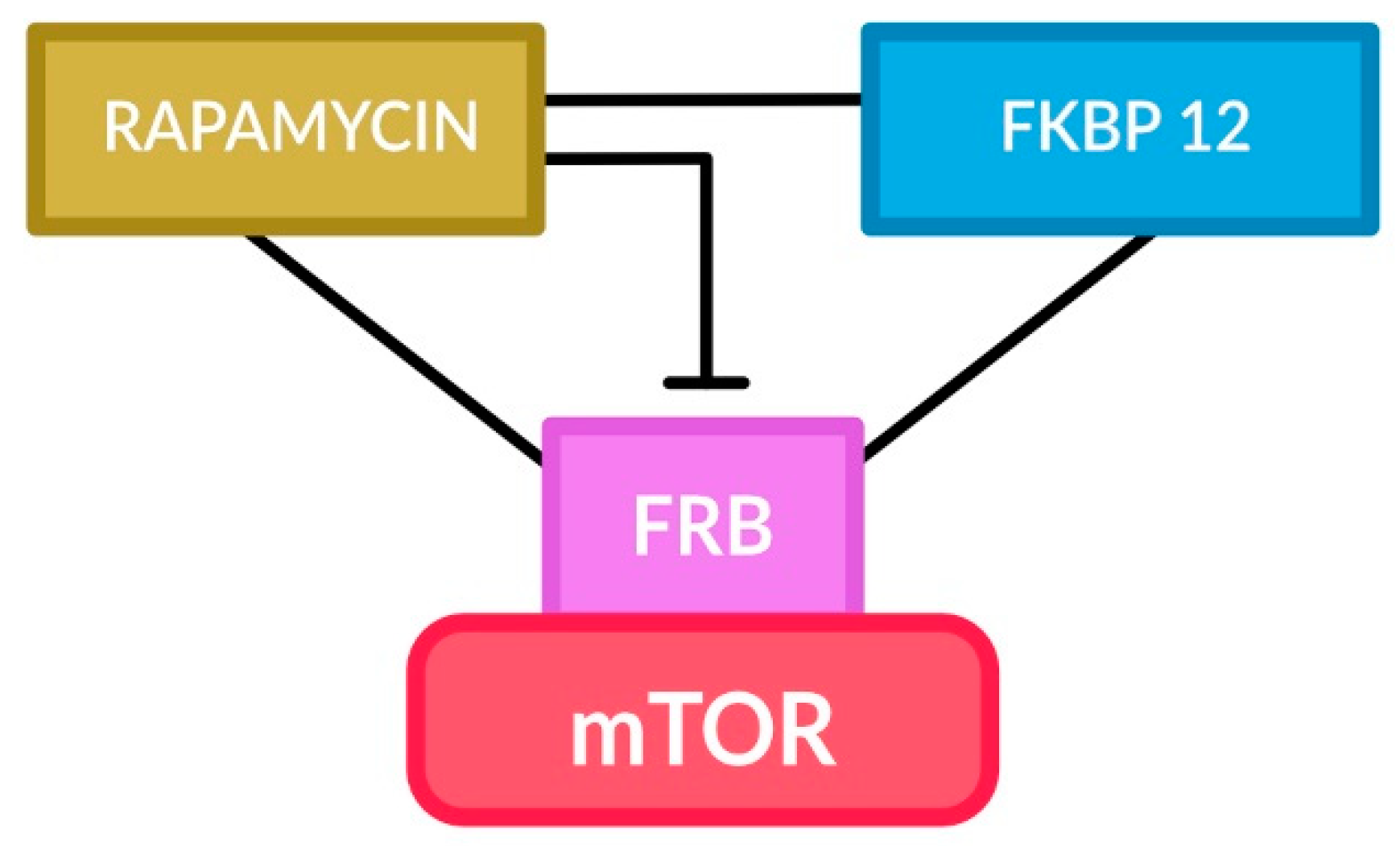
6. Therapeutic Approach for SEGA Tumors
7. Non-Pharmacological Treatment Modalities and Biopharmaceuticals
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buccoliero, A.M.; Franchi, A.; Castiglione, F.; Gheri, C.F.; Mussa, F.; Giordano, F.; Genitori, L.; Taddei, G.L. Subependymal giant cell astrocytoma (SEGA): Is it an astrocytoma? Morphological, immunohistochemical and ultrastructural study. Neuropathology 2009, 29, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Buccoliero, A.M.; Caporalini, C.; Giordano, F.; Mussa, F.; Scagnet, M.; Moscardi, S.; Baroni, G.; Genitori, L.; Taddei, G.L. Subependymal giant cell astrocytoma: A lesion with activated mTOR pathway and constant expression of glutamine synthetase. Clin. Neuropathol. 2016, 35, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, S.; Mandera, M.; Młynarski, W. Natural History and Current Treatment Options for Subependymal Giant Cell Astrocytoma in Tuberous Sclerosis Complex. Semin. Pediatr. Neurol. 2015, 22, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Grajkowska, W.; Kotulska, K.; Jurkiewicz, E.; Matyja, E. Brain lesions in tuberous sclerosis complex. Review. Folia Neuropathol. 2010, 48, 139–149. [Google Scholar] [PubMed]
- Navarro-Ballester, A.; Álvaro-Ballester, R.; Lara-Martínez, M. Beyond Benign: A Case of Subependymal Giant Cell Astrocytomas Provoking Hydrocephalus in Tuberous Sclerosis Complex. Acta Med. Litu. 2024, 31, 61–67. [Google Scholar] [CrossRef]
- Gao, C.; Zabielska, B.; Jiao, F.; Mei, D.; Wang, X.; Kotulska, K.; Jozwiak, S. Subependymal Giant Cell Astrocytomas in Tuberous Sclerosis Complex-Current Views on Their Pathogenesis and Management. J. Clin. Med. 2023, 12, 956. [Google Scholar] [CrossRef]
- Jansen, A.C.; Belousova, E.; Benedik, M.P.; Carter, T.; Cottin, V.; Curatolo, P.; Dahlin, M.; D’Amato, L.; d’Augères, G.B.; de Vries, P.J.; et al. Clinical Characteristics of Subependymal Giant Cell Astrocytoma in Tuberous Sclerosis Complex. Front. Neurol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Almubarak, A.O.; Abdullah, J.; Al Hindi, H.; AlShail, E. Infantile atypical subependymal giant cell astrocytoma. Neurosciences 2020, 25, 61–64. [Google Scholar] [CrossRef]
- Yamada, S.; Tanikawa, M.; Matsushita, Y.; Fujinami, R.; Yamada, H.; Sakomi, K.; Sakata, T.; Inagaki, H.; Yokoo, H.; Ichimura, K.; et al. SEGA-like circumscribed astrocytoma in a non-NF1 patient, harboring molecular profile of GBM. A case report. Neuropathology 2024, 44, 190–199. [Google Scholar] [CrossRef]
- Stawiski, K.; Trelińska, J.; Baranska, D.; Dachowska, I.; Kotulska, K.; Jóźwiak, S.; Fendler, W.; Młynarski, W. What are the true volumes of SEGA tumors? Reliability of planimetric and popular semi-automated image segmentation methods. Magma 2017, 30, 397–405. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, A.L.; Piña-Ballantyne, S.A.; Espinosa-Aguilar, E.J. Subependymal Giant Cell Astrocytoma Non-Associated with Tuberous Sclerosis Complex and Expression of OCT-4 and INI-1: A Case Report. Cureus 2023, 15, e39187. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.; Van den Berge, D. Radiotherapy for subependymal giant cell astrocytoma: Time to challenge a historical ban? A case report and review of the literature. J. Med. Case Rep. 2024, 18, 330. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Pellerino, A.; Rudà, R. Subependymal Giant Cell Astrocytomas (SEGAs): A Model of Targeting Tumor Growth and Epilepsy. Curr. Treat. Options Neurol. 2021, 23, 18. [Google Scholar] [CrossRef]
- Ess, K.C.; Kamp, C.A.; Tu, B.P.; Gutmann, D.H. Developmental origin of subependymal giant cell astrocytoma in tuberous sclerosis complex. Neurology 2005, 64, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Altermatt, H.J.; Scheithauer, B.W. Cytomorphology of subependymal giant cell astrocytoma. Acta Cytol. 1992, 36, 171–175. [Google Scholar]
- Mizuguchi, M.; Takashima, S. Neuropathology of tuberous sclerosis. Brain Dev. 2001, 23, 508–515. [Google Scholar] [CrossRef]
- Bonnin, J.M.; Rubinstein, L.J.; Papasozomenos, S.C.; Marangos, P.J. Subependymal giant cell astrocytoma. Significance and possible cytogenetic implications of an immunohistochemical study. Acta Neuropathol. 1984, 62, 185–193. [Google Scholar] [CrossRef]
- Scheithauer, B.W. The neuropathology of tuberous sclerosis. J. Dermatol. 1992, 19, 897–903. [Google Scholar] [CrossRef]
- Phi, J.H.; Park, S.H.; Chae, J.H.; Hong, K.H.; Park, S.S.; Kang, J.H.; Jun, J.K.; Cho, B.K.; Wang, K.C.; Kim, S.K. Congenital subependymal giant cell astrocytoma: Clinical considerations and expression of radial glial cell markers in giant cells. Childs Nerv. Syst. 2008, 24, 1499–1503. [Google Scholar] [CrossRef]
- Dutta, R.; Sharma, M.C.; Suri, V.; Sarkar, C.; Garg, A.; Suri, A.; Kale, S.S. TTF-1: A Well-Favored Addition to the Immunohistochemistry Armamentarium as a Diagnostic Marker of SEGA. World Neurosurg. 2022, 159, e62–e69. [Google Scholar] [CrossRef]
- Bingle, C.D. Thyroid transcription factor-1. Int. J. Biochem. Cell Biol. 1997, 29, 1471–1473. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, D.; Price, M.; de Felice, M.; Di Lauro, R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 1991, 113, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Cho, G.; Norgren, R.; Junier, M.-P.; Tapia, V.; Costa, M.; Ojeda, S. TTF-1, a Homeodomain Gene Required for Diencephalic Morphogenesis, Is Postnatally Expressed in the Neuroendocrine Brain in a Developmentally Regulated and Cell-Specific Fashion. Mol. Cell. Neurosci. 2001, 17, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Hang, J.F.; Hsu, C.Y.; Lin, S.C.; Wu, C.C.; Lee, H.J.; Ho, D.M. Thyroid transcription factor-1 distinguishes subependymal giant cell astrocytoma from its mimics and supports its cell origin from the progenitor cells in the medial ganglionic eminence. Mod. Pathol. 2017, 30, 318–328. [Google Scholar] [CrossRef]
- Schmid, S.; Solomon, D.A.; Perez, E.; Thieme, A.; Kleinschmidt-DeMasters, B.K.; Giannini, C.; Reinhardt, A.; Asa, S.L.; Mete, O.; Stichel, D.; et al. Genetic and epigenetic characterization of posterior pituitary tumors. Acta Neuropathol. 2021, 142, 1025–1043. [Google Scholar] [CrossRef]
- Feliciano, D.M. The Neurodevelopmental Pathogenesis of Tuberous Sclerosis Complex (TSC). Front. Neuroanat. 2020, 14, 39. [Google Scholar] [CrossRef]
- Bongaarts, A.; Giannikou, K.; Reinten, R.J.; Anink, J.J.; Mills, J.D.; Jansen, F.E.; Spliet, G.M.W.; den Dunnen, W.F.A.; Coras, R.; Blümcke, I.; et al. Subependymal giant cell astrocytomas in Tuberous Sclerosis Complex have consistent TSC1/TSC2 biallelic inactivation, and no BRAF mutations. Oncotarget 2017, 8, 95516–95529. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours: Who Classification of Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, IARC Publications: Lyon, France, 2022; Volume 6, p. 584. [Google Scholar]
- Cuccia, V.; Zuccaro, G.; Sosa, F.; Monges, J.; Lubienieky, F.; Taratuto, A.L. Subependymal giant cell astrocytoma in children with tuberous sclerosis. Childs Nerv. Syst. 2003, 19, 232–243. [Google Scholar] [CrossRef]
- Mei, G.-H.; Liu, X.-X.; Zhou, P.; Shen, M. Clinical and imaging features of subependymal giant cell astrocytoma: Report of 20 cases. Chin. Neurosurg. J. 2017, 3, 14. [Google Scholar] [CrossRef]
- Siedlecka, M.; Szlufik, S.; Grajkowska, W.; Roszkowski, M.; Jóźwiak, J. Erk activation as a possible mechanism of transformation of subependymal nodule into subependymal giant cell astrocytoma. Folia Neuropathol. 2015, 53, 8–14. [Google Scholar] [CrossRef]
- Danassegarane, G.; Tinois, J.; Sahler, Y.; Aouaissia, S.; Riffaud, L. Subependymal giant-cell astrocytoma: A surgical review in the modern era of mTOR inhibitors. Neurochirurgie 2022, 68, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Magri, L.; Galli, R. mTOR signaling in neural stem cells: From basic biology to disease. Cell Mol. Life Sci. 2013, 70, 2887–2898. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, A.; De Waele, L.; Bartalini, G.; Jozwiak, S.; Laforgia, N.; Verhelst, H.; Petrak, B.; Pedespan, J.M.; Witt, O.; Castellana, R.; et al. EFFECTS: An expanded access program of everolimus for patients with subependymal giant cell astrocytoma associated with tuberous sclerosis complex. BMC Neurol. 2016, 16, 126. [Google Scholar] [CrossRef]
- Northrup, H.; Krueger, D.A. Tuberous sclerosis complex diagnostic criteria update: Recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 2013, 49, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Roach, E.S.; Bartels, U.; Jóźwiak, S.; Koenig, M.K.; Weiner, H.L.; Franz, D.N.; Wang, H.Z. Subependymal giant cell astrocytoma: Diagnosis, screening, and treatment. Recommendations from the International Tuberous Sclerosis Complex Consensus Conference 2012. Pediatr. Neurol. 2013, 49, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Weidman, D.R.; Pole, J.D.; Bouffet, E.; Taylor, M.D.; Bartels, U. Dose-level response rates of mTor inhibition in tuberous sclerosis complex (TSC) related subependymal giant cell astrocytoma (SEGA). Pediatr. Blood Cancer 2015, 62, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Appalla, D.; Depalma, A.; Calderwood, S. Mammalian Target of Rapamycin Inhibitor Induced Complete Remission of a Recurrent Subependymal Giant Cell Astrocytoma in a Patient without Features of Tuberous Sclerosis Complex. Pediatr. Blood Cancer 2016, 63, 1276–1278. [Google Scholar] [CrossRef]
- O’Rawe, M.; Chandran, A.S.; Joshi, S.; Simonin, A.; Dyke, J.M.; Lee, S. A case of subependymal giant cell astrocytoma without tuberous sclerosis complex and review of the literature. Childs Nerv. Syst. 2021, 37, 1381–1385. [Google Scholar] [CrossRef]
- Tahiri Elousrouti, L.; Lamchahab, M.; Bougtoub, N.; Elfatemi, H.; Chbani, L.; Harmouch, T.; Maaroufi, M.; Amarti Riffi, A. Subependymal giant cell astrocytoma (SEGA): A case report and review of the literature. J. Med. Case Rep. 2016, 10, 35. [Google Scholar] [CrossRef]
- Cobourn, K.D.; Chesney, K.M.; Mueller, K.; Fayed, I.; Tsering, D.; Keating, R.F. Isolated subependymal giant cell astrocytoma (SEGA) in the absence of clinical tuberous sclerosis: Two case reports and literature review. Childs Nerv. Syst. 2024, 40, 73–78. [Google Scholar] [CrossRef]
- Halmagyi, G.M.; Bignold, L.P.; Allsop, J.L. Recurrent subependymal giant-cell astrocytoma in the absence of tuberous sclerosis. Case report. J. Neurosurg. 1979, 50, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Corlette, L.; Reid, A.; Roberts-Thomson, S.; Christie, M.; Gaillard, F. Solitary subependymal giant cell astrocytoma: Case report and review of the literature. J. Clin. Neurosci. 2020, 82, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kondo, A.; Ogino, I.; Akiyama, O.; Fujita, N.; Shimizu, Y.; Arai, H. A case of solitary subependymal giant cell astrocytoma with histopathological anaplasia and TSC2 gene alteration. Childs Nerv. Syst. 2021, 37, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- De Waele, L.; Lagae, L.; Mekahli, D. Tuberous sclerosis complex: The past and the future. Pediatr. Nephrol. 2015, 30, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Dabora, S.L.; Jozwiak, S.; Franz, D.N.; Roberts, P.S.; Nieto, A.; Chung, J.; Choy, Y.S.; Reeve, M.P.; Thiele, E.; Egelhoff, J.C.; et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am. J. Hum. Genet. 2001, 68, 64–80. [Google Scholar] [CrossRef]
- Man, A.; Di Scipio, M.; Grewal, S.; Suk, Y.; Trinari, E.; Ejaz, R.; Whitney, R. The Genetics of Tuberous Sclerosis Complex and Related mTORopathies: Current Understanding and Future Directions. Genes 2024, 15, 332. [Google Scholar] [CrossRef]
- Avgeris, S.; Fostira, F.; Vagena, A.; Ninios, Y.; Delimitsou, A.; Vodicka, R.; Vrtel, R.; Youroukos, S.; Stravopodis, D.J.; Vlassi, M.; et al. Mutational analysis of TSC1 and TSC2 genes in Tuberous Sclerosis Complex patients from Greece. Sci. Rep. 2017, 7, 16697. [Google Scholar] [CrossRef]
- Caban, C.; Khan, N.; Hasbani, D.M.; Crino, P.B. Genetics of tuberous sclerosis complex: Implications for clinical practice. Appl. Clin. Genet. 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Rosset, C.; Netto, C.B.O.; Ashton-Prolla, P. TSC1 and TSC2 gene mutations and their implications for treatment in Tuberous Sclerosis Complex: A review. Genet. Mol. Biol. 2017, 40, 69–79. [Google Scholar] [CrossRef]
- Sancak, O.; Nellist, M.; Goedbloed, M.; Elfferich, P.; Wouters, C.; Maat-Kievit, A.; Zonnenberg, B.; Verhoef, S.; Halley, D.; van den Ouweland, A. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: Genotype—Phenotype correlations and comparison of diagnostic DNA techniques in Tuberous Sclerosis Complex. Eur. J. Hum. Genet. 2005, 13, 731–741. [Google Scholar] [CrossRef]
- Barron, R.P.; Kainulainen, V.T.; Forrest, C.R.; Krafchik, B.; Mock, D.; Sàndor, G.K. Tuberous sclerosis: Clinicopathologic features and review of the literature. J. Craniomaxillofac. Surg. 2002, 30, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Crino, P.B.; Nathanson, K.L.; Henske, E.P. The tuberous sclerosis complex. N. Engl. J. Med. 2006, 355, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Northrup, H.; Aronow, M.E.; Bebin, E.M.; Bissler, J.; Darling, T.N.; de Vries, P.J.; Frost, M.D.; Fuchs, Z.; Gosnell, E.S.; Gupta, N.; et al. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr. Neurol. 2021, 123, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Patniyot, I.; Qubty, W. Managing Headache Disorders Associated with Tuberous Sclerosis and Neurofibromatosis. Curr. Pain Headache Rep. 2022, 26, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Crainic, N.; Furtner, J.; Pallud, J.; Bielle, F.; Lombardi, G.; Rudà, R.; Idbaih, A. Rare Neuronal, Glial and Glioneuronal Tumours in Adults. Cancers 2023, 15, 1120. [Google Scholar] [CrossRef]
- Gelot, A.B.; Represa, A. Progression of Fetal Brain Lesions in Tuberous Sclerosis Complex. Front. Neurosci. 2020, 14, 899. [Google Scholar] [CrossRef]
- Moavero, R.; Coniglio, A.; Garaci, F.; Curatolo, P. Is mTOR inhibition a systemic treatment for tuberous sclerosis? Ital. J. Pediatr. 2013, 39, 57. [Google Scholar] [CrossRef]
- Leung, A.K.; Robson, W.L. Tuberous sclerosis complex: A review. J. Pediatr. Health Care 2007, 21, 108–114. [Google Scholar] [CrossRef]
- Napolioni, V.; Curatolo, P. Genetics and molecular biology of tuberous sclerosis complex. Curr. Genom. 2008, 9, 475–487. [Google Scholar] [CrossRef]
- Beaumont, T.L.; Godzik, J.; Dahiya, S.; Smyth, M.D. Subependymal giant cell astrocytoma in the absence of tuberous sclerosis complex: Case report. J. Neurosurg. Pediatr. 2015, 16, 134–137. [Google Scholar] [CrossRef]
- Yalon, M.; Ben-Sira, L.; Constantini, S.; Toren, A. Regression of subependymal giant cell astrocytomas with RAD001 (Everolimus) in tuberous sclerosis complex. Child’s Nerv. Syst. 2011, 27, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.L.; Garcia, E.; Pieper, R.O. S6K1 plays a key role in glial transformation. Cancer Res. 2008, 68, 6516–6523. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Cui, D.; Qu, R.; Liu, D.; Xiong, X.; Liang, T.; Zhao, Y. The Cross Talk between p53 and mTOR Pathways in Response to Physiological and Genotoxic Stresses. Front. Cell Dev. Biol. 2021, 9, 775507. [Google Scholar] [CrossRef] [PubMed]
- Carriere, A.; Romeo, Y.; Jaquez, A.; Moreau, J.; Bonneil, E.; Thibault, P.; Fingar, D.; Roux, P. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1). J. Biol. Chem. 2010, 286, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Daniells, C.E.; Snell, R.G.; Tachataki, M.; Idziaszczyk, S.A.; Krawczak, M.; Sampson, J.R.; Cheadle, J.P. Molecular genetic and phenotypic analysis reveals differences between TSC1 and TSC2 associated familial and sporadic tuberous sclerosis. Hum. Mol. Genet. 1997, 6, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Piña-Ballantyne, S.A.; Espinosa-Aguilar, E.J.; Calderón-Garcidueñas, A.L. The Clinicopathological Features of the Solitary Subependymal Giant Cell Astrocytoma: A Systematic Review. Neurol. India 2024, 72, 708–717. [Google Scholar] [CrossRef]
- Ogórek, B.; Hamieh, L.; Hulshof, H.M.; Lasseter, K.; Klonowska, K.; Kuijf, H.; Moavero, R.; Hertzberg, C.; Weschke, B.; Riney, K.; et al. TSC2 pathogenic variants are predictive of severe clinical manifestations in TSC infants: Results of the EPISTOP study. Genet. Med. 2020, 22, 1489–1497. [Google Scholar] [CrossRef]
- Kotulska, K.; Borkowska, J.; Mandera, M.; Roszkowski, M.; Jurkiewicz, E.; Grajkowska, W.; Bilska, M.; Jóźwiak, S. Congenital subependymal giant cell astrocytomas in patients with tuberous sclerosis complex. Child’s Nerv. Syst. 2014, 30, 2037–2042. [Google Scholar] [CrossRef]
- Giannikou, K.; Zhu, Z.; Kim, J.; Winden, K.D.; Tyburczy, M.E.; Marron, D.; Parker, J.S.; Hebert, Z.; Bongaarts, A.; Taing, L.; et al. Subependymal giant cell astrocytomas are characterized by mTORC1 hyperactivation, a very low somatic mutation rate, and a unique gene expression profile. Mod. Pathol. 2021, 34, 264–279. [Google Scholar] [CrossRef]
- Palsgrove, D.N.; Brosnan-Cashman, J.A.; Giannini, C.; Raghunathan, A.; Jentoft, M.; Bettegowda, C.; Gokden, M.; Lin, D.; Yuan, M.; Lin, M.T.; et al. Subependymal giant cell astrocytoma-like astrocytoma: A neoplasm with a distinct phenotype and frequent neurofibromatosis type-1-association. Mod. Pathol. 2018, 31, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Fohlen, M.; Harzallah, I.; Polivka, M.; Giuliano, F.; Pons, L.; Streichenberger, N.; Dorfmüller, G.; Touraine, R. Identification of TSC1 or TSC2 mutation limited to the tumor in three cases of solitary subependymal giant cell astrocytoma using next-generation sequencing technology. Childs Nerv. Syst. 2020, 36, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Uda, T.; Kuki, I.; Kunihiro, N.; Okazaki, S.; Niida, Y.; Goto, T. TSC2 somatic mosaic mutation, including extra-tumor tissue, may be the developmental cause of solitary subependymal giant cell astrocytoma. Childs Nerv. Syst. 2022, 38, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Sharma, M.C.; Kakkar, A.; Malgulwar, P.B.; Pathak, P.; Suri, V.; Sarkar, C.; Chandra, S.P.; Faruq, M.; Gupta, R.K.; et al. Role of mTOR signaling pathway in the pathogenesis of subependymal giant cell astrocytoma—A study of 28 cases. Neurol. India 2016, 64, 988–994. [Google Scholar] [PubMed]
- Popova, N.V.; Jücker, M. The Role of mTOR Signaling as a Therapeutic Target in Cancer. Int. J. Mol. Sci. 2021, 22, 1743. [Google Scholar] [CrossRef] [PubMed]
- Yindeedej, V.; Rojnueangnit, K.; Chotsakulthong, P.; Thamwongskul, C. Bleeding solitary SEGA in non-tuberous sclerosis complex adolescent: A case illustration and review of literature. Childs Nerv. Syst. 2024, 40, 2199–2207. [Google Scholar] [CrossRef]
- Zabielska, B.; Rzewuska, N.; Jóźwiak, S. Subependymal Giant Cell Astrocytoma Tumors in Patients without Clinical Manifestation of Tuberous Sclerosis Complex: A Diagnostic Puzzle. Pediatr. Neurol. 2024, 150, 40–42. [Google Scholar] [CrossRef]
- Hall, A.; Westlake, G.; Short, B.P.; Pearson, M.; Ess, K.C. TSC1 Mosaicism Leading to Subependymal Giant Cell Astrocytoma but Not Tuberous Sclerosis Complex. Pediatr. Neurol. 2021, 123, 38–39. [Google Scholar] [CrossRef]
- Klonowska, K.; Giannikou, K.; Grevelink, J.M.; Boeszoermenyi, B.; Thorner, A.R.; Herbert, Z.T.; Afrin, A.; Treichel, A.M.; Hamieh, L.; Kotulska, K.; et al. Comprehensive genetic and phenotype analysis of 95 individuals with mosaic tuberous sclerosis complex. Am. J. Hum. Genet. 2023, 110, 979–988. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Franz, D.N. Pharmacological treatment strategies for subependymal giant cell astrocytoma (SEGA). Expert Opin. Pharmacother. 2020, 21, 1329–1336. [Google Scholar] [CrossRef]
- Tyburczy, M.E.; Kotulska, K.; Pokarowski, P.; Mieczkowski, J.; Kucharska, J.; Grajkowska, W.; Roszkowski, M.; Jozwiak, S.; Kaminska, B. Novel proteins regulated by mTOR in subependymal giant cell astrocytomas of patients with tuberous sclerosis complex and new therapeutic implications. Am. J. Pathol. 2010, 176, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, S.; Nabbout, R.; Curatolo, P. Management of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC): Clinical recommendations. Eur. J. Paediatr. Neurol. 2013, 17, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.E.; Orlova, K.A.; Heuer, G.G.; Baybis, M.; Aronica, E.; Frost, M.; Wong, M.; Crino, P.B. Enhanced epidermal growth factor, hepatocyte growth factor, and vascular endothelial growth factor expression in tuberous sclerosis complex. Am. J. Pathol. 2011, 178, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Carter, M.; Edwards, R.J.; Pople, I.; Aquilina, K.; Merrifield, J.; Osborne, J.P.; O’Callaghan, F.J. The outcome of surgical management of subependymal giant cell astrocytoma in tuberous sclerosis complex. Eur. J. Paediatr. Neurol. 2013, 17, 36–44. [Google Scholar] [CrossRef]
- Dias, S.F.; Richards, O.; Elliot, M.; Chumas, P. Pediatric-Like Brain Tumors in Adults. Adv. Tech. Stand. Neurosurg. 2024, 50, 147–183. [Google Scholar] [CrossRef]
- Karita, H.; Tsuda, K.; Kono, M.; Yamamoto, T.; Ihara, S. Neoadjuvant Therapy with Everolimus for Subependymal Giant Cell Astrocytoma: A Case Report. NMC Case Rep. J. 2023, 10, 291–297. [Google Scholar] [CrossRef]
- Krueger, D.A.; Care, M.M.; Holland, K.; Agricola, K.; Tudor, C.; Mangeshkar, P.; Wilson, K.A.; Byars, A.; Sahmoud, T.; Franz, D.N. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N. Engl. J. Med. 2010, 363, 1801–1811. [Google Scholar] [CrossRef]
- Capal, J.; Ritter, D.; Franz, D.; Griffith, M.; Currans, K.; Kent, B.; Bebin, M.; Northrup, H.; Koenig, M.; Mizuno, T.; et al. Preventative treatment of tuberous sclerosis complex with sirolimus: Phase I safety and efficacy results. Ann. Child Neurol. Soc. 2024, 2, 106–119. [Google Scholar] [CrossRef]
- Sun, P.; Kohrman, M.; Liu, J.; Guo, A.; Rogerio, J.; Krueger, D. Outcomes of resecting subependymal giant cell astrocytoma (SEGA) among patients with SEGA-related tuberous sclerosis complex: A national claims database analysis. Curr. Med. Res. Opin. 2012, 28, 657–663. [Google Scholar] [CrossRef]
- Sun, P.; Krueger, D.; Liu, J.; Guo, A.; Rogerio, J.; Kohrman, M. Surgical resection of subependymal giant cell astrocytomas (SEGAs) and changes in SEGA-related conditions: A US national claims database study. Curr. Med. Res. Opin. 2012, 28, 651–656. [Google Scholar] [CrossRef]
- Kotulska, K.; Borkowska, J.; Roszkowski, M.; Mandera, M.; Daszkiewicz, P.; Drabik, K.; Jurkiewicz, E.; Larysz-Brysz, M.; Nowak, K.; Grajkowska, W.; et al. Surgical treatment of subependymal giant cell astrocytoma in tuberous sclerosis complex patients. Pediatr. Neurol. 2014, 50, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Yamada, K.; Nakahara, T.; Ishihara, A.; Takaki, S.; Kochi, M.; Ushio, Y. Rapid regrowth of solitary subependymal giant cell astrocytoma—Case report. Neurol. Med.-Chir. 2002, 42, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Campen, C.J.; Porter, B.E. Subependymal Giant Cell Astrocytoma (SEGA) Treatment Update. Curr. Treat. Options Neurol. 2011, 13, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Sasongko, T.H.; Ismail, N.F.; Nik Abdul Malik, N.M.; Zabidi-Hussin, Z.A. Rapamycin and its analogues (rapalogs) for Tuberous Sclerosis Complex-associated tumors: A systematic review on non-randomized studies using meta-analysis. Orphanet J. Rare Dis. 2015, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Śmiałek, D.; Kotulska, K.; Duda, A.; Jóźwiak, S. Effect of mTOR Inhibitors in Epilepsy Treatment in Children with Tuberous Sclerosis Complex Under 2 Years of Age. Neurol. Ther. 2023, 12, 931–946. [Google Scholar] [CrossRef]
- Tomoto, K.; Fujimoto, A.; Inenaga, C.; Okanishi, T.; Imai, S.; Ogai, M.; Fukunaga, A.; Nakamura, H.; Sato, K.; Obana, A.; et al. Experience using mTOR inhibitors for subependymal giant cell astrocytoma in tuberous sclerosis complex at a single facility. BMC Neurol. 2021, 21, 139. [Google Scholar] [CrossRef]
- Yang, G.; Yang, L.; Yang, X.; Shi, X.; Wang, J.; Liu, Y.; Ju, J.; Zou, L. Efficacy and safety of a mammalian target of rapamycin inhibitor in pediatric patients with tuberous sclerosis complex: A systematic review and meta-analysis. Exp. Ther. Med. 2015, 9, 626–630. [Google Scholar] [CrossRef]
- Eng, C.P.; Sehgal, S.N.; Vézina, C. Activity of rapamycin (AY-22,989) against transplanted tumors. J. Antibiot. 1984, 37, 1231–1237. [Google Scholar] [CrossRef]
- Georgakis, G.V.; Younes, A. From Rapa Nui to rapamycin: Targeting PI3K/Akt/mTOR for cancer therapy. Expert Rev. Anticancer. Ther. 2006, 6, 131–140. [Google Scholar] [CrossRef]
- Ballou, L.M.; Lin, R.Z. Rapamycin and mTOR kinase inhibitors. J. Chem. Biol. 2008, 1, 27–36. [Google Scholar] [CrossRef]
- Wagner, A.J.; Ravi, V.; Riedel, R.F.; Ganjoo, K.; Van Tine, B.A.; Chugh, R.; Cranmer, L.; Gordon, E.M.; Hornick, J.L.; Du, H.; et al. nab-Sirolimus for Patients with Malignant Perivascular Epithelioid Cell Tumors. J. Clin. Oncol. 2021, 39, 3660–3670. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.M.; Angel, N.L.; Omelchenko, N.; Chua-Alcala, V.S.; Moradkhani, A.; Quon, D.; Wong, S. A Phase I/II Investigation of Safety and Efficacy of Nivolumab and nab-Sirolimus in Patients with a Variety of Tumors with Genetic Mutations in the mTOR Pathway. Anticancer. Res. 2023, 43, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Schuler, W.; Sedrani, R.; Cottens, S.; Häberlin, B.; Schulz, M.; Schuurman, H.J.; Zenke, G.; Zerwes, H.G.; Schreier, M.H. SDZ RAD, a new rapamycin derivative: Pharmacological properties in vitro and in vivo. Transplantation 1997, 64, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, K.; Kotulska, K.; Jóźwiak, S. Management of side effects of mTOR inhibitors in tuberous sclerosis patients. Pharmacol. Rep. 2016, 68, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Banaszynski, L.A.; Liu, C.W.; Wandless, T.J. Characterization of the FKBP.rapamycin.FRB ternary complex. J. Am. Chem. Soc. 2005, 127, 4715–4721. [Google Scholar] [CrossRef]
- Edwards, S.R.; Wandless, T.J. The Rapamycin-binding Domain of the Protein Kinase Mammalian Target of Rapamycin Is a Destabilizing Domain. J. Biol. Chem. 2007, 282, 13395–13401. [Google Scholar] [CrossRef]
- Ali, E.S.; Mitra, K.; Akter, S.; Ramproshad, S.; Mondal, B.; Khan, I.N.; Islam, M.T.; Sharifi-Rad, J.; Calina, D.; Cho, W.C. Recent advances and limitations of mTOR inhibitors in the treatment of cancer. Cancer Cell Int. 2022, 22, 284. [Google Scholar] [CrossRef]
- Zhao, H.-f.; Wang, J.; Shao, W.; Wu, C.-p.; Chen, Z.-p.; To, S.-s.T.; Li, W.-p. Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: Current preclinical and clinical development. Mol. Cancer 2017, 16, 100. [Google Scholar] [CrossRef]
- Hillmann, P.; Fabbro, D. PI3K/mTOR Pathway Inhibition: Opportunities in Oncology and Rare Genetic Diseases. Int. J. Mol. Sci. 2019, 20, 5792. [Google Scholar] [CrossRef]
- Curran, M.P. Everolimus: In patients with subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Paediatr. Drugs 2012, 14, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Franz, D.N.; Agricola, K.; Mays, M.; Tudor, C.; Care, M.M.; Holland-Bouley, K.; Berkowitz, N.; Miao, S.; Peyrard, S.; Krueger, D.A. Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann. Neurol. 2015, 78, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Franz, D.N.; Belousova, E.; Sparagana, S.; Bebin, E.M.; Frost, M.; Kuperman, R.; Witt, O.; Kohrman, M.H.; Flamini, J.R.; Wu, J.Y.; et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013, 381, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, S.; Watanabe, T.; Takayama, R.; Minagawa, K.; Tsutsumi, H. Everolimus Treatment for an Early Infantile Subependymal Giant Cell Astrocytoma with Tuberous Sclerosis Complex. J. Child Neurol. 2015, 30, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Franz, D.N.; Belousova, E.; Sparagana, S.; Bebin, E.M.; Frost, M.; Kuperman, R.; Witt, O.; Kohrman, M.H.; Flamini, J.R.; Wu, J.Y.; et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol. 2014, 15, 1513–1520. [Google Scholar] [CrossRef]
- Krueger, D.A.; Care, M.M.; Agricola, K.; Tudor, C.; Mays, M.; Franz, D.N. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology 2013, 80, 574–580. [Google Scholar] [CrossRef]
- Tran, L.H.; Zupanc, M.L. Long-Term Everolimus Treatment in Individuals with Tuberous Sclerosis Complex: A Review of the Current Literature. Pediatr. Neurol. 2015, 53, 23–30. [Google Scholar] [CrossRef]
- French, J.A.; Lawson, J.A.; Yapici, Z.; Ikeda, H.; Polster, T.; Nabbout, R.; Curatolo, P.; de Vries, P.J.; Dlugos, D.J.; Berkowitz, N.; et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): A phase 3, randomised, double-blind, placebo-controlled study. Lancet 2016, 388, 2153–2163. [Google Scholar] [CrossRef]
- Trelinska, J.; Dachowska, I.; Kotulska, K.; Baranska, D.; Fendler, W.; Jozwiak, S.; Mlynarski, W. Factors affecting response to everolimus therapy for subependymal giant cell astrocytomas associated with tuberous sclerosis. Pediatr. Blood Cancer 2015, 62, 616–621. [Google Scholar] [CrossRef]
- Vergès, B.; Cariou, B. mTOR inhibitors and diabetes. Diabetes Res. Clin. Pract. 2015, 110, 101–108. [Google Scholar] [CrossRef]
- Jackson, E.R.; Duchatel, R.J.; Staudt, D.E.; Persson, M.L.; Mannan, A.; Yadavilli, S.; Parackal, S.; Game, S.; Chong, W.C.; Jayasekara, W.S.N.; et al. ONC201 in combination with paxalisib for the treatment of H3K27-altered diffuse midline glioma. Cancer Res. 2023, 83, 2421–2437. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.A.; Estranero, J.; Gudka, K.; Grundy, R.G. The therapeutic potential of targeting the PI3K pathway in pediatric brain tumors. Oncotarget 2017, 8, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Szklener, K.; Mazurek, M.; Wieteska, M.; Wacławska, M.; Bilski, M.; Mańdziuk, S. New Directions in the Therapy of Glioblastoma. Cancers 2022, 14, 5377. [Google Scholar] [CrossRef] [PubMed]
- Bongaarts, A.; van Scheppingen, J.; Korotkov, A.; Mijnsbergen, C.; Anink, J.J.; Jansen, F.E.; Spliet, W.G.M.; den Dunnen, W.F.A.; Gruber, V.E.; Scholl, T.; et al. The coding and non-coding transcriptional landscape of subependymal giant cell astrocytomas. Brain 2020, 143, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Angst, G.; Haas, M.; Yang, F.; Wang, C. The Characterization of a Subependymal Giant Astrocytoma-Like Cell Line from Murine Astrocyte with mTORC1 Hyperactivation. Int. J. Mol. Sci. 2021, 22, 4116. [Google Scholar] [CrossRef] [PubMed]
- Pucko, E.; Ostrowski, R.; Matyja, E. Novel small molecule protein kinase CK2 inhibitors exert potent antitumor effects on T98G and SEGA cells in vitro. Folia Neuropathol. 2019, 57, 239–248. [Google Scholar] [CrossRef]
- Aum, D.J.; Reynolds, R.A.; McEvoy, S.D.; Wong, M.; Roland, J.L.; Smyth, M.D. Laser interstitial thermal therapy compared with open resection for treating subependymal giant cell astrocytoma. J. Neurosurg. Pediatr. 2024, 33, 95–104. [Google Scholar] [CrossRef]
- Dadey, D.Y.; Kamath, A.A.; Leuthardt, E.C.; Smyth, M.D. Laser interstitial thermal therapy for subependymal giant cell astrocytoma: Technical case report. Neurosurg. Focus 2016, 41, E9. [Google Scholar] [CrossRef]
- Amin, S.; Mallick, A.A.; Edwards, H.; Cortina-Borja, M.; Laugharne, M.; Likeman, M.; O’Callaghan, F.J.K. The metformin in tuberous sclerosis (MiTS) study: A randomised double-blind placebo-controlled trial. eClinicalMedicine 2021, 32, 100715. [Google Scholar] [CrossRef]
- Bongaarts, A.; de Jong, J.M.; Broekaart, D.W.M.; van Scheppingen, J.; Anink, J.J.; Mijnsbergen, C.; Jansen, F.E.; Spliet, W.G.M.; den Dunnen, W.F.A.; Gruber, V.E.; et al. Dysregulation of the MMP/TIMP Proteolytic System in Subependymal Giant Cell Astrocytomas in Patients with Tuberous Sclerosis Complex: Modulation of MMP by MicroRNA-320d In Vitro. J. Neuropathol. Exp. Neurol. 2020, 79, 777–790. [Google Scholar] [CrossRef]
- Park, K.J.; Kano, H.; Kondziolka, D.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D. Gamma Knife surgery for subependymal giant cell astrocytomas. Clinical article. J. Neurosurg. 2011, 114, 808–813. [Google Scholar] [CrossRef] [PubMed]
- De Roxas, R.C.; Suratos, C.T.R.; Fernandez, M.L.L. Temozolomide as Treatment in Lowgrade Glioma: A Systematic Review. J. Neuro-Oncol. Neurosci. 2016, 1, 7. [Google Scholar] [CrossRef]
- Grajkowska, W.; Kotulska, K.; Jurkiewicz, E.; Roszkowski, M.; Daszkiewicz, P.; Jóźwiak, S.; Matyja, E. Subependymal giant cell astrocytomas with atypical histological features mimicking malignant gliomas. Folia Neuropathol. 2011, 49, 39–46. [Google Scholar] [PubMed]
- Hafazalla, K.; Sahgal, A.; Jaja, B.; Perry, J.R.; Das, S. Procarbazine, CCNU and vincristine (PCV) versus temozolomide chemotherapy for patients with low-grade glioma: A systematic review. Oncotarget 2018, 9, 33623–33633. [Google Scholar] [CrossRef] [PubMed]
- Duchatel, R.J.; Jackson, E.R.; Parackal, S.G.; Kiltschewskij, D.; Findlay, I.J.; Mannan, A.; Staudt, D.E.; Thomas, B.C.; Germon, Z.P.; Laternser, S.; et al. PI3K/mTOR is a therapeutically targetable genetic dependency in diffuse intrinsic pontine glioma. J. Clin. Investig. 2024, 134, e170329. [Google Scholar] [CrossRef]
- Omeljaniuk, W.J.; Krętowski, R.; Ratajczak-Wrona, W.; Jabłońska, E.; Cechowska-Pasko, M. Novel Dual PI3K/mTOR Inhibitor, Apitolisib (GDC-0980), Inhibits Growth and Induces Apoptosis in Human Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 11511. [Google Scholar] [CrossRef]
- Tutak, I.; Ozdil, B.; Uysal, A. Voxtalisib and low intensity pulsed ultrasound combinatorial effect on glioblastoma multiforme cancer stem cells via PI3K/AKT/mTOR. Pathol. Res. Pract. 2022, 239, 154145. [Google Scholar] [CrossRef]
- Wen, P.Y.; Omuro, A.; Ahluwalia, M.S.; Fathallah-Shaykh, H.M.; Mohile, N.; Lager, J.J.; Laird, A.D.; Tang, J.; Jiang, J.; Egile, C.; et al. Phase I dose-escalation study of the PI3K/mTOR inhibitor voxtalisib (SAR245409, XL765) plus temozolomide with or without radiotherapy in patients with high-grade glioma. Neuro Oncol. 2015, 17, 1275–1283. [Google Scholar] [CrossRef]
- Srinivasan, A.; Naga Shashi Kiran, B.; Koduvath, A.; Pal, A.; Suresh, A.; Kannan, A.; Ravichandran, M. Metformin for the management of tuberous sclerosis: What does the evidence tell us? EXCLI J. 2021, 20, 1474–1475. [Google Scholar] [CrossRef]
- Łabuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopień, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Seliger, C.; Genbrugge, E.; Gorlia, T.; Chinot, O.; Stupp, R.; Nabors, B.; Weller, M.; Hau, P. Use of metformin and outcome of patients with newly diagnosed glioblastoma: Pooled analysis. Int. J. Cancer 2020, 146, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.D.; Memon, S.; Qureshi, K.; Farooq, U.; Memon, U.A.; Aparna, F.; Kachhadia, M.P.; Shahzeen, F.; Ali, S.; Varrassi, G.; et al. Seizing the Connection: Exploring the Interplay between Epilepsy and Glycemic Control in Diabetes Management. Cureus 2023, 15, e45606. [Google Scholar] [CrossRef] [PubMed]
- Tornio, A.; Niemi, M.; Neuvonen, P.J.; Backman, J.T. Drug interactions with oral antidiabetic agents: Pharmacokinetic mechanisms and clinical implications. Trends Pharmacol. Sci. 2012, 33, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Pucko, E.; Matyja, E.; Koronkiewicz, M.; Ostrowski, R.P.; Kazimierczuk, Z. Potent Antitumour Effects of Novel Pentabromobenzylisothioureas Studied on Human Glial-derived Tumour Cell Lines. Anticancer Res. 2018, 38, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- Frassanito, P.; Noya, C.; Tamburrini, G. Current trends in the management of subependymal giant cell astrocytomas in tuberous sclerosis. Child’s Nerv. Syst. 2020, 36, 2527–2536. [Google Scholar] [CrossRef]
- Reese, J.C.; Fadel, H.A.; Pawloski, J.A.; Samir, M.; Haider, S.; Komatar, R.J.; Luther, E.; Morell, A.A.; Ivan, M.E.; Robin, A.M.; et al. Laser interstitial thermal therapy for deep-seated perivascular brain tumors is not associated with distal ischemia. J. Neurooncol. 2024, 166, 265–272. [Google Scholar] [CrossRef]
- Boop, S.; Bonda, D.; Randle, S.; Leary, S.; Vitanza, N.; Crotty, E.; Novotny, E.; Friedman, S.; Ellenbogen, R.G.; Durfy, S.; et al. A Comparison of Clinical Outcomes for Subependymal Giant Cell Astrocytomas Treated with Laser Interstitial Thermal Therapy, Open Surgical Resection, and mTOR Inhibitors. Pediatr. Neurosurg. 2023, 58, 150–159. [Google Scholar] [CrossRef]
- Abba, M.; Patil, N.; Allgayer, H. MicroRNAs in the Regulation of MMPs and Metastasis. Cancers 2014, 6, 625–645. [Google Scholar] [CrossRef]
- Beylerli, O.; Gareev, I.; Sufianov, A.; Ilyasova, T.; Zhang, F. The role of microRNA in the pathogenesis of glial brain tumors. Non-Coding RNA Res. 2022, 7, 71–76. [Google Scholar] [CrossRef]
- Dibdiakova, K.; Majercikova, Z.; Galanda, T.; Richterova, R.; Kolarovszki, B.; Racay, P.; Hatok, J. Relationship between the Expression of Matrix Metalloproteinases and Their Tissue Inhibitors in Patients with Brain Tumors. Int. J. Mol. Sci. 2024, 25, 2858. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, X.; Sun, S.; Zhang, X.; Yang, W.; Zhang, J.; Zhang, X.; Jiang, Z. Increased expression of MMP-2 and MMP-9 indicates poor prognosis in glioma recurrence. Biomed. Pharmacother. 2019, 118, 109369. [Google Scholar] [CrossRef] [PubMed]
- Adylova, A.; Kapanova, G.; Datkhayeva, Z.; Raganina, K.; Tanbayeva, G.; Baigonova, K. Nanotechnology-based cancer chemoprevention in glioblastoma. Folia Neuropathol. 2023, 61, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Mondal, I.; Janjua, T.; Popat, A.; Kulshreshtha, R. Engineered smart materials for RNA based molecular therapy to treat Glioblastoma. Bioact. Mater. 2024, 33, 396–423. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhang, Y.; Zhang, Y.; Wang, L.; Luo, H. Study on the Treatment of Tuberous Sclerosis with Rapamycin Nanomicelles Based on Abdominal Ultrasound. J. Nanosci. Nanotechnol. 2021, 21, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S. Nanotechnology-Based Targeting of mTOR Signaling in Cancer. Int. J. Nanomed. 2020, 15, 5767–5781. [Google Scholar] [CrossRef]
- Jiang, T.; Du, J.; Raynald; Wang, J.; Li, C. Presurgical Administration of mTOR Inhibitors in Patients with Large Subependymal Giant Cell Astrocytoma Associated with Tuberous Sclerosis Complex. World Neurosurg. 2017, 107, 1053.e1–1053.e6. [Google Scholar] [CrossRef]
- Wagner, A.J.; Ravi, V.; Riedel, R.F.; Ganjoo, K.; Van Tine, B.A.; Chugh, R.; Cranmer, L.; Gordon, E.M.; Hornick, J.L.; Du, H.; et al. Phase II Trial of nab-Sirolimus in Patients with Advanced Malignant Perivascular Epithelioid Cell Tumors (AMPECT): Long-Term Efficacy and Safety Update. J. Clin. Oncol. 2024, 42, 1472–1476. [Google Scholar] [CrossRef]

| Therapeutic Approach for SEGA Tumors | Effect of Therapy | References |
|---|---|---|
| Surgery | After surgical excision, the tumor may grow back. | [32] |
| Radiotherapy | SEGA responds slowly and progressively to fractionated radiotherapy. | [12] |
| Chemotherapy; Everolimus (mTOR kinase inhibitor) | 35% of patients had at least 50% reduction in SEGA volume after 6–9 months of treatment with everolimus. | [54,87,88] |
| Chemotherapy; Sirolimus (mTOR kinase inhibitor) | Reductions in SEGA volume of treatment with sirolimus | [89] |
| Drug/Therapeutic Modality | Experimental/Clinical Study | Major Outcomes | References |
|---|---|---|---|
| Dual PI3K/mTOR inhibitors | clinical studies | May provide survival benefit over standard care for gliomas; to be determined for SEGA | NCT05009992, NCT03970447 [122,123,124] |
| ERK inhibitor | primary human derived SEGA culture | Decreased proliferation in a similar manner to treatment with rapamycin | [125] |
| Gene therapy | SEGA-like cell line | Recombinant lentivirus encoding human TSC1 restored the TSC1 level | [126] |
| Inhibitors of kinase CK2: 4,5,6,7-tetrabromo-1H-benzimidazole (TBI); 2-dimethylamino-4,5,6,7-tetrabromo-1H- benzimidazole (DMAT); 4,5, 6,7-tetrabromo-1H-benzotriazole (TBB) | cell lines established from human SEGA tumor | Reduced SEGA cell growth and viability | [127] |
| Laser-induced interstitial thermotherapy | clinical studies | Tumor shrinkage; less invasive surgical alternative to open resection of SEGAs | [128,129] |
| Metformin | clinical study | The effect of metformin on reducing SEGA volume was observed in patients | [130] |
| MicroRNA-320d mimic | cell culture | Ameliorated MMP/TIMP proteolytic system, dysregulated in SEGA | [131] |
| Radiosurgery | clinical study | Reducing the size of the SEGA tumor | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucko, E.; Sulejczak, D.; Ostrowski, R.P. Subependymal Giant Cell Astrocytoma: The Molecular Landscape and Treatment Advances. Cancers 2024, 16, 3406. https://doi.org/10.3390/cancers16193406
Pucko E, Sulejczak D, Ostrowski RP. Subependymal Giant Cell Astrocytoma: The Molecular Landscape and Treatment Advances. Cancers. 2024; 16(19):3406. https://doi.org/10.3390/cancers16193406
Chicago/Turabian StylePucko, Emanuela, Dorota Sulejczak, and Robert P. Ostrowski. 2024. "Subependymal Giant Cell Astrocytoma: The Molecular Landscape and Treatment Advances" Cancers 16, no. 19: 3406. https://doi.org/10.3390/cancers16193406







