Common Misconceptions about Diet and Breast Cancer: An Unclear Issue to Dispel
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
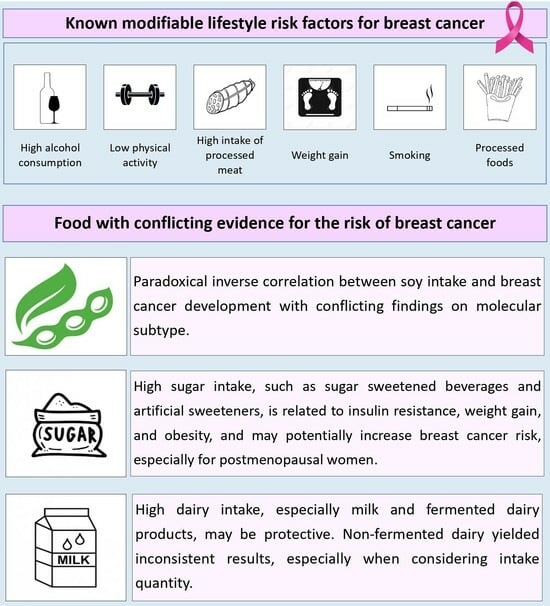
3. Sugar Intake
| Author (Year) [Ref] | Food/Intervention under Investigation | Type of Study | Participants | Assessment of Dietary Intake | Main Results |
|---|---|---|---|---|---|
| Mullie P et al. (2016) [26] | GI and GL | Meta-analysis | 773,971 women | FFQ | Women with a high GI or GL have a 5–6% increased risk of breast cancer |
| Schlesinger S et al. (2017) [27] | Carbohydrate GI, GL diet | Systematic review and dose-response meta-analysis | 892,403 women | FFQ | GL and carbohydrate intake were associated with an increased risk of breast cancer only among hormone receptor–negative tumors, particularly ER-negative. |
| Hodge AM et al. (2018) [28] | Artificially sweetened soft drinks | Prospective cohort study | 35,593 participants | FFQ | The highest risk of breast cancer was associated with 1–6 sweetened soft drinks/week in post-menopausal women. |
| Debras C et al. (2020) [29] | Added sugar intake | Prospective cohort study | 101,279 participants | Repeated 24-h dietary records | Total sugar intake was positively associated with high overall cancer risk, including breast cancer. |
| Li Y et al. (2021) [21] | Sugar-sweetened beverages and fruit juice | Systematic review and dose-response meta-analysis | 8465 cases and 119,153 controls | FFQ | The highest level of sugar-sweetened beverage consumption showed an increased breast cancer risk. |
| Long T et al. (2022) [25] | GI and GL | Meta-analysis | 15,839 cases and 577,538 participants | FFQ | A positive association between breast cancer development and GI was observed only in the post-menopausal setting. |
| Debras C et al. (2022) [30] | Artificial sweeteners | Prospective cohort study | 102,865 adults | Repeated 24-h dietary records | Artificial sweeteners (aspartame and acesulfameK) were associated with increased breast cancer incidence. |
4. Dairy Consumption
| Author (Year) [Ref] | Food/Intervention under Investigation | Type of Study | Participants | Assessment of Dietary Intake | Main Results |
|---|---|---|---|---|---|
| Couto E et al. (2013) [33] | Mediterranean diet | Prospective cohort study | 44,840 women | FFQ | A statistically significant inverse association was reported between dairy consumption and breast cancer risk in all pre-menopausal and post-menopausal women. |
| Genkinger JM et al. (2013) [37] | Dairy, Ca, Vit D, and meat consumption | Prospective cohort study | 52,062 African American women | FFQ | The authors observed no significant association between breast cancer and dairy intake. A negative association was shown between milk consumption and hormone receptor-negative subtypes. |
| Bahadoran Z et al. (2013) [38] | Dairy products | Case-control study | 275 Iranian women | FFQ | An inverse correlation between breast cancer and dairy intake was found, especially for low-fat and fermented dairy products. |
| Li J et al. (2013) [41] | Calcium | Prospective cohort study | 34,028 women | FFQ | A lack of association between calcium intake and breast cancer risk was observed, independently of the source of consumption. |
| Zang J et al. (2015) [32] | Dairy products | Systematic review and meta-analysis | 1,600,312 participants | FFQ, diet questionnaires, and 24-h recall data interview | High and moderate dairy intake reduced breast cancer risk compared to low consumption. |
| Farvid MS et al. (2018) [36] | Dairy products | Observational study | 90,503 pre-menopausal women for early adulthood and 44,264 women for adolescent | FFQ | A positive correlation emerged between dairy intake and hormone receptor-negative breast cancer in contrast to the negative one observed for hormone receptor-positive breast cancer subtypes. |
| Shin WK et al. (2019) [31] | Milk | Prospective cohort study | 78,320 participants | Interviewer-administered semi-quantitive FFQ | In the pre-menopausal setting, a negative association between high daily intake of milk (≥1 serving/day) and breast cancer risk was observed compared to women with low milk consumption (<1 serving/week). |
| Marcondes LH et al. (2019) [35] | Animal food (red meat, poultry, fish, dairy, and egg) | Prospective cohort study | 3209 participants | FFQ and physical examination | No association was observed between breast cancer and dairy consumption in post-menopausal women. |
| Key TJ et al. (2019) [40] | Alcohol, fruit, dietary fiber, meat, fish, milk, cheese, yogurt, eggs, vegetables, dairy protein, fat, carbohydrates, and free sugars | Prospective cohort study | 691,571 post-menopausal UK women without previous cancer history | FFQ | The authors found no association between the consumption of different kinds of dairy products and breast cancer risk. |
| Fraser GE et al. (2020) [34] | Dairy and soy | Prospective cohort study | 52,795 North American women | FFQ and structured 24-h dietary recalls for calibration study subjects | Increased risk of developing breast cancer in the 90th and 10th percentile of consumption of dairy products in both pre- and post-menopausal women. Increased risk of the development of hormone receptor-positive subtypes. |
| He Y et al. (2021) [22] | Dairy products (fermented, non-fermented, low-fat, and high-fat dairy products) | Meta-analysis | 1,019,232 participants | FFQ, diet questionnaires, and home visits or in-depth interviews | The statistically significant protection of fermented dairy products was observed only in post-menopausal women. A statistically significant protective effect of low-fat products was shown solely in pre-menopausal women. |
| Aguilera-Buenosvinos I et al. (2021) [39] | Dairy products | Prospective cohort study | 10,930 women | FFQ | A moderate consumption of dairy products (2–4 servings per day) was associated with decreased breast cancer incidence in the post-menopausal setting. A low intake (1–2 servings per day) of low-fat dairy products consumption reduced breast cancer risk in the pre-menopausal setting. |
5. Soy Intake
| Author (Year) [Ref] | Food/Intervention under Investigation | Type of Study | Participants | Assessment of Dietary Intake | Main Results |
|---|---|---|---|---|---|
| Wada K et al. (2013) [23] | Soy and isoflavones | Prospective cohort study | 15,607 women | FFQ | A negative association between soy and isoflavone intake and breast cancer risk was observed solely in post-menopausal women. |
| Li L et al. (2013) [46] | Isoflavone | Case-control study | 1120 controls | FFQ | A protective effect of dietary isoflavone intake on breast cancer development was reported for both hospital outpatient and population controls. |
| Ko KP et al. (2013) [55] | Soy, vegetables, fruit, meat, and seafood | Case-control study | 2271 women | FFQ | Negative association between soy consumption and breast cancer risk in BRCA carriers |
| Chen M et al. (2014) [51] | Soy and isoflavone | Meta-analysis | 1,391,524 pre-menopausal and 579,33 post-menopausal women | n.d. | An inverse association was found between soy isoflavone intake and breast cancer incidence, independently of menopausal status, solely in Asian women. |
| Woo HD et al. (2014) [52] | Soy products, fruits, and vegetables | Meta-analysis | 8112 participants | n.d. | Different kinds of soy foods were inversely associated with breast cancer risk in both pre-menopausal and post-menopausal women. |
| Wu J et al. (2016) [45] | Meat, soy, milk, yogurt, poultry, fish, eggs, and nuts | Meta-analysis | 452,916 participants | n.d. | Reduced breast cancer risk with high soy consumption. |
| Zhao TT et al. (2017) [48] | Soy and isoflavone | Meta-analysis | 648,913 participants | FFQs, self-administered questionnaires, and mail survey questionnaires | A statistically significant inverse association was shown between high versus low soy consumption and breast cancer risk. |
| Tan MM et al. (2018) [42] | Soy, breastfeeding, and PA | Case-control study | 7663 women | Interviews and FFQs | High soy milk and soy product consumption demonstrated an inverse association with breast cancer incidence. |
| Wei Y et al. (2020) [43] | Soy and isoflavones | Prospective cohort study and meta-analysis | 30,0852 women for the cohort study and 513,313 participants for the meta-analysis | FFQs, physical measurements, resurveys, 24-h dietary recalls | The cohort study revealed no association between moderate or high soy consumption and breast cancer. The meta-analysis showed a 3% reduced risk of breast cancer development with each 10 mg/day increase in isoflavone intake. |
| Wang Q et al. (2020) [49] | Tofu | Meta-analysis | 109,813 participants | n.d. | A protective effect of tofu consumption on breast cancer development was observed independent of menopausal status. |
| Okekunle AP et al. (2020) [53] | Soy and isoflavone | Meta-analysis | 29,810 participants | n.d. | Increased soy consumption reduced breast cancer risk, especially in pre-menopausal women and for ER-negative subtype development. |
| Lu LW et al. (2022) [24] | Isoflavones versus placebo | Clinical trial | 194 pre-menopausal women | N.A. | The authors found a decrease in breast tissue density with higher isoflavone intake, especially in pre-menopausal women. |
| Boutas I et al. (2022) [44] | Soy and isoflavones | Meta-analysis | 485,495 participants | FFQ | High soy consumption reduced the breast cancer risk in pre- and post-menopausal women. |
| Cao S et al. (2022) [54] | Vegetable-fruit-soy dietary pattern | Case-control study | 1753 women | FFQ | Higher soy consumption reduced breast cancer development in post-menopausal women, especially ER- and ER-/PgR-negative subtypes. |
| Shin S et al. (2023) [47] | Fruits, vegetables, meat, soy, green tea, alcohol | Meta-analysis | 216,216 participants | n.d. | A protective effect of soy protein and isoflavone intake on breast cancer incidence was observed, but no correlation was found with soy food consumption. |
| Rajaram N et al. (2023) [50] | Soy isoflavone supplement versus isoflavones from dietary sources | Clinical trial | 90 women | FFQ | Moderate and high intake of soy reduced mammographic density in both pre-menopausal and recently menopausal women. |
6. Discussion
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.V., Jr.; Kreimer, A.R.; Buys, S.S.; Marcus, P.M.; Chang, S.C.; Leitzmann, M.F.; Hoover, R.N.; Prorok, P.C.; Berg, C.D.; Hartge, P.; et al. Breast cancer epidemiology according to recognized breast cancer risk factors in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Cohort. BMC Cancer 2009, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Pal, D.; Sharma, R.; Garg, V.K.; Goel, N.; Koundal, D.; Zaguia, A.; Koundal, S.; Belay, A. Global Increase in Breast Cancer Incidence: Risk Factors and Preventive Measures. Biomed. Res. Int. 2022, 2022, 9605439. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2491–2507. [Google Scholar] [CrossRef]
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer. 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.D.; Agudo, A.; Sánchez, M.J. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef]
- Dandamudi, A.; Tommie, J.; Nommsen-Rivers, L.; Couch, S. Dietary Patterns and Breast Cancer Risk: A Systematic Review. Anticancer Res. 2018, 38, 3209–3222. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- La Vecchia, C.; Altieri, A.; Tavani, A. Vegetables, fruit, antioxidants and cancer: A review of Italian studies. Eur. J. Nutr. 2001, 40, 261–267. [Google Scholar] [CrossRef]
- van Gils, C.H.; Peeters, P.H.; Bueno-de-Mesquita, H.B.; Boshuizen, H.C.; Lahmann, P.H.; Clavel-Chapelon, F.; Thiébaut, A.; Kesse, E.; Sieri, S.; Palli, D.; et al. Consumption of vegetables and fruits and risk of breast cancer. JAMA 2005, 293, 183–193. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, Y.; Chen, Q.; Qiu, W.; Chen, M.; Xue, L.; Lin, M.; Yang, H. The interaction of diet, alcohol, genetic predisposition, and the risk of breast cancer: A cohort study from the UK Biobank. Eur. J. Nutr. 2023. [Google Scholar] [CrossRef]
- Farvid, M.S.; Chen, W.Y.; Rosner, B.A.; Tamimi, R.M.; Willett, W.C.; Eliassen, A.H. Fruit and vegetable consumption and breast cancer incidence: Repeated measures over 30 years of follow-up. Int. J. Cancer 2019, 144, 1496–1510. [Google Scholar] [CrossRef] [PubMed]
- Balaam, S.; Bailey, T.G.; Anderson, D.; Retell, J.; McCarthy, A.L. Alcohol and Breast Cancer: Results from the Women’s Wellness After Cancer Program Randomized Controlled Trial. Cancer Nurs. 2022, 45, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Aragaki, A.K.; Anderson, G.L.; Pan, K.; Neuhouser, M.L.; Manson, J.E.; Thomson, C.A.; Mossavar-Rahmani, Y.; Lane, D.S.; Johnson, K.C.; et al. Dietary Modification and Breast Cancer Mortality: Long-Term Follow-Up of the Women’s Health Initiative Randomized Trial. J. Clin. Oncol. 2020, 38, 1419–1428. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Bonet, C.; Crous-Bou, M.; Katzke, V.; Le Cornet, C.; Jannasch, F.; Schulze, M.B.; Olsen, A.; Tjønneland, A.; Dahm, C.C.; et al. Dietary patterns related to biological mechanisms and survival after breast cancer diagnosis: Results from a cohort study. Br. J. Cancer 2023, 128, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Fortner, R.T.; Katzke, V.; Kühn, T.; Kaaks, R. Obesity and Breast Cancer. Recent Results Cancer Res. 2016, 208, 43–65. [Google Scholar] [CrossRef]
- García-Estévez, L.; Cortés, J.; Pérez, S.; Calvo, I.; Gallegos, I.; Moreno-Bueno, G. Obesity and Breast Cancer: A Paradoxical and Controversial Relationship Influenced by Menopausal Status. Front. Oncol. 2021, 11, 705911. [Google Scholar] [CrossRef]
- Epner, M.; Yang, P.; Wagner, R.W.; Cohen, L. Understanding the Link between Sugar and Cancer: An Examination of the Preclinical and Clinical Evidence. Cancers 2022, 14, 6042. [Google Scholar] [CrossRef]
- Macdonald, I.A. A review of recent evidence relating to sugars, insulin resistance and diabetes. Eur. J. Nutr. 2016, 55, 17–23. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; He, K.; Huang, C.; Tang, S. Consumption of sugar-sweetened beverages and fruit juice and human cancer: A systematic review and dose-response meta-analysis of observational studies. J. Cancer 2021, 12, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tao, Q.; Zhou, F.; Si, Y.; Fu, R.; Xu, B.; Xu, J.; Li, X.; Chen, B. The relationship between dairy products intake and breast cancer incidence: A meta-analysis of observational studies. BMC Cancer 2021, 21, 1109. [Google Scholar] [CrossRef]
- Wada, K.; Nakamura, K.; Tamai, Y.; Tsuji, M.; Kawachi, T.; Hori, A.; Takeyama, N.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; et al. Soy isoflavone intake and breast cancer risk in Japan: From the Takayama study. Int. J. Cancer 2013, 133, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.W.; Chen, N.W.; Brunder, D.G.; Nayeem, F.; Nagamani, M.; Nishino, T.K.; Anderson, K.E.; Khamapirad, T. Soy isoflavones decrease fibroglandular breast tissue measured by magnetic resonance imaging in premenopausal women: A 2-year randomized double-blind placebo controlled clinical trial. Clin. Nutr. ESPEN 2022, 52, 158–168. [Google Scholar] [CrossRef]
- Long, T.; Liu, K.; Long, J.; Li, J.; Cheng, L. Dietary glycemic index, glycemic load and cancer risk: A meta-analysis of prospective cohort studies. Eur. J. Nutr. 2022, 61, 2115–2127. [Google Scholar] [CrossRef]
- Mullie, P.; Koechlin, A.; Boniol, M.; Autier, P.; Boyle, P. Relation between Breast Cancer and High Glycemic Index or Glycemic Load: A Meta-analysis of Prospective Cohort Studies. Crit. Rev. Food Sci. Nutr. 2016, 56, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Chan, D.S.M.; Vingeliene, S.; Vieira, A.R.; Abar, L.; Polemiti, E.; Stevens, C.A.T.; Greenwood, D.C.; Aune, D.; Norat, T. Carbohydrates, glycemic index, glycemic load, and breast cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Nutr. Rev. 2017, 75, 420–441. [Google Scholar] [CrossRef]
- Hodge, A.M.; Bassett, J.K.; Milne, R.L.; English, D.R.; Giles, G.G. Consumption of sugar-sweetened and artificially sweetened soft drinks and risk of obesity-related cancers. Public Health Nutr. 2018, 21, 1618–1626. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Zelek, L.; Agaësse, C.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; et al. Total and added sugar intakes, sugar types, and cancer risk: Results from the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2020, 112, 1267–1279. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo de Edelenyi, F.; Agaësse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med. 2022, 19, e1003950. [Google Scholar] [CrossRef]
- Shin, W.K.; Lee, H.W.; Shin, A.; Lee, J.K.; Kang, D. Milk Consumption Decreases Risk for Breast Cancer in Korean Women under 50 Years of Age: Results from the Health Examinees Study. Nutrients 2019, 12, 32. [Google Scholar] [CrossRef]
- Zang, J.; Shen, M.; Du, S.; Chen, T.; Zou, S. The Association between Dairy Intake and Breast Cancer in Western and Asian Populations: A Systematic Review and Meta-Analysis. J. Breast Cancer 2015, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Couto, E.; Sandin, S.; Löf, M.; Ursin, G.; Adami, H.O.; Weiderpass, E. Mediterranean dietary pattern and risk of breast cancer. PLoS ONE 2013, 8, e55374. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.E.; Jaceldo-Siegl, K.; Orlich, M.; Mashchak, A.; Sirirat, R.; Knutsen, S. Dairy, soy, and risk of breast cancer: Those confounded milks. Int. J. Epidemiol. 2020, 49, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, L.H.; Franco, O.H.; Ruiter, R.; Ikram, M.A.; Mulder, M.; Stricker, B.H.; Kiefte-de Jong, J.C. Animal foods and postmenopausal breast cancer risk: A prospective cohort study. Br. J. Nutr. 2019, 122, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Eliassen, A.H.; Cho, E.; Chen, W.Y.; Willett, W.C. Dairy Consumption in Adolescence and Early Adulthood and Risk of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 575–584. [Google Scholar] [CrossRef]
- Genkinger, J.M.; Makambi, K.H.; Palmer, J.R.; Rosenberg, L.; Adams-Campbell, L.L. Consumption of dairy and meat in relation to breast cancer risk in the Black Women’s Health Study. Cancer Causes Control 2013, 24, 675–684. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Karimi, Z.; Houshiar-rad, A.; Mirzayi, H.R.; Rashidkhani, B. Is dairy intake associated to breast cancer? A case control study of Iranian women. Nutr. Cancer 2013, 65, 1164–1170. [Google Scholar] [CrossRef]
- Aguilera-Buenosvinos, I.; Fernandez-Lazaro, C.I.; Romanos-Nanclares, A.; Gea, A.; Sánchez-Bayona, R.; Martín-Moreno, J.M.; Martínez-González, M.Á.; Toledo, E. Dairy Consumption and Incidence of Breast Cancer in the ‘Seguimiento Universidad de Navarra’ (SUN) Project. Nutrients 2021, 13, 687. [Google Scholar] [CrossRef]
- Key, T.J.; Balkwill, A.; Bradbury, K.E.; Reeves, G.K.; Kuan, A.S.; Simpson, R.F.; Green, J.; Beral, V. Foods, macronutrients and breast cancer risk in postmenopausal women: A large UK cohort. Int. J. Epidemiol. 2019, 48, 489–500. [Google Scholar] [CrossRef]
- Li, J.; Koh, W.P.; Jin, A.Z.; Yuan, J.M.; Yu, M.C.; Butler, L.M. Calcium intake is not related to breast cancer risk among Singapore Chinese women. Int. J. Cancer 2013, 133, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.M.; Ho, W.K.; Yoon, S.Y.; Mariapun, S.; Hasan, S.N.; Lee, D.S.; Hassan, T.; Lee, S.Y.; Phuah, S.Y.; Sivanandan, K.; et al. A case-control study of breast cancer risk factors in 7,663 women in Malaysia. PLoS ONE 2018, 13, e0203469. [Google Scholar] [CrossRef]
- Wei, Y.; Lv, J.; Guo, Y.; Bian, Z.; Gao, M.; Du, H.; Yang, L.; Chen, Y.; Zhang, X.; Wang, T.; et al. Soy intake and breast cancer risk: A prospective study of 300,000 Chinese women and a dose-response meta-analysis. Eur. J. Epidemiol. 2020, 35, 567–578. [Google Scholar] [CrossRef]
- Boutas, I.; Kontogeorgi, A.; Dimitrakakis, C.; Kalantaridou, S.N. Soy Isoflavones and Breast Cancer Risk: A Meta-analysis. Vivo 2022, 36, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zeng, R.; Huang, J.; Li, X.; Zhang, J.; Ho, J.C.; Zheng, Y. Dietary Protein Sources and Incidence of Breast Cancer: A Dose-Response Meta-Analysis of Prospective Studies. Nutrients 2016, 8, 730. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, M.; Holman, C.D. Population versus hospital controls in the assessment of dietary intake of isoflavone for case-control studies on cancers in China. Nutr. Cancer 2013, 65, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Fu, J.; Shin, W.K.; Huang, D.; Min, S.; Kang, D. Association of food groups and dietary pattern with breast cancer risk: A systematic review and meta-analysis. Clin. Nutr. 2023, 42, 282–297. [Google Scholar] [CrossRef]
- Zhao, T.T.; Jin, F.; Li, J.G.; Xu, Y.Y.; Dong, H.T.; Liu, Q.; Xing, P.; Zhu, G.L.; Xu, H.; Miao, Z.F. Dietary isoflavones or isoflavone-rich food intake and breast cancer risk: A meta-analysis of prospective cohort studies. Clin. Nutr. 2019, 38, 136–145. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Ren, S. Tofu intake is inversely associated with risk of breast cancer: A meta-analysis of observational studies. PLoS ONE 2020, 15, e0226745. [Google Scholar] [CrossRef]
- Rajaram, N.; Yap, B.; Eriksson, M.; Mariapun, S.; Tan, L.M.; Sa’at, H.; Ho, E.L.M.; Taib, N.A.M.; Khor, G.L.; Yip, C.H.; et al. Randomized Controlled Trial of Soy Isoflavone Intake on Mammographic Density among Malaysian Women. Nutrients 2023, 15, 299. [Google Scholar] [CrossRef]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.D.; Park, S.; Oh, K.; Kim, H.J.; Shin, H.R.; Moon, H.K.; Kim, J. Diet and cancer risk in the Korean population: A meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 8509–8519. [Google Scholar] [CrossRef]
- Okekunle, A.P.; Gao, J.; Wu, X.; Feng, R.; Sun, C. Higher dietary soy intake appears inversely related to breast cancer risk independent of estrogen receptor breast cancer phenotypes. Heliyon 2020, 6, e04228. [Google Scholar] [CrossRef]
- Cao, S.; Liu, L.; Zhu, Q.; Zhu, Z.; Zhou, J.; Wei, P.; Wu, M. Adherence to the Vegetable-Fruit-Soy Dietary Pattern, a Reference from Mediterranean Diet, Protects Against Postmenopausal Breast Cancer Among Chinese Women. Front. Nutr. 2022, 9, 800996. [Google Scholar] [CrossRef]
- Ko, K.P.; Kim, S.W.; Ma, S.H.; Park, B.; Ahn, Y.; Lee, J.W.; Lee, M.H.; Kang, E.; Kim, L.S.; Jung, Y.; et al. Dietary intake and breast cancer among carriers and noncarriers of BRCA mutations in the Korean Hereditary Breast Cancer Study. Am. J. Clin. Nutr. 2013, 98, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Sanft, T.; Harrigan, M.; McGowan, C.; Cartmel, B.; Zupa, M.; Li, F.Y.; Ferrucci, L.M.; Puklin, L.; Cao, A.; Nguyen, T.H.; et al. Randomized Trial of Exercise and Nutrition on Chemotherapy Completion and Pathologic Complete Response in Women with Breast Cancer: The Lifestyle, Exercise, and Nutrition Early After Diagnosis Study. J. Clin. Oncol. 2023, 41, 5285–5295. [Google Scholar] [CrossRef]
- Tiberio, P.; Antunovic, L.; Gaudio, M.; Viganò, A.; Pastore, M.; Miggiano, C.; Jacobs, F.; Benvenuti, C.; Farina, E.; Chiti, A.; et al. The Relationship among Bowel [18]F-FDG PET Uptake, Pathological Complete Response, and Eating Habits in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Nutrients 2023, 15, 211. [Google Scholar] [CrossRef]
- Castellano, I.; Gallo, F.; Durelli, P.; Monge, T.; Fadda, M.; Metovic, J.; Cassoni, P.; Borella, F.; Raucci, C.; Menischetti, M.; et al. Impact of Caloric Restriction in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: A Prospective Case Control Study. Nutrients 2023, 15, 4677. [Google Scholar] [CrossRef]
- Ligorio, F.; Lobefaro, R.; Fucà, G.; Provenzano, L.; Zanenga, L.; Nasca, V.; Sposetti, C.; Salvadori, G.; Ficchì, A.; Franza, A.; et al. Adding fasting-mimicking diet to first-line carboplatin-based chemotherapy is associated with better overall survival in advanced triple-negative breast cancer patients: A subanalysis of the NCT03340935 trial. Int. J. Cancer 2024, 154, 114–123. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalioti, A.; Verzeletti, L.; Tiberio, P.; Gerosa, R.; Gaudio, M.; Saltalamacchia, G.; Pastore, M.; Zambelli, A.; Santoro, A.; De Sanctis, R. Common Misconceptions about Diet and Breast Cancer: An Unclear Issue to Dispel. Cancers 2024, 16, 306. https://doi.org/10.3390/cancers16020306
Lalioti A, Verzeletti L, Tiberio P, Gerosa R, Gaudio M, Saltalamacchia G, Pastore M, Zambelli A, Santoro A, De Sanctis R. Common Misconceptions about Diet and Breast Cancer: An Unclear Issue to Dispel. Cancers. 2024; 16(2):306. https://doi.org/10.3390/cancers16020306
Chicago/Turabian StyleLalioti, Anastasia, Laura Verzeletti, Paola Tiberio, Riccardo Gerosa, Mariangela Gaudio, Giuseppe Saltalamacchia, Manuela Pastore, Alberto Zambelli, Armando Santoro, and Rita De Sanctis. 2024. "Common Misconceptions about Diet and Breast Cancer: An Unclear Issue to Dispel" Cancers 16, no. 2: 306. https://doi.org/10.3390/cancers16020306
APA StyleLalioti, A., Verzeletti, L., Tiberio, P., Gerosa, R., Gaudio, M., Saltalamacchia, G., Pastore, M., Zambelli, A., Santoro, A., & De Sanctis, R. (2024). Common Misconceptions about Diet and Breast Cancer: An Unclear Issue to Dispel. Cancers, 16(2), 306. https://doi.org/10.3390/cancers16020306