Diffuse-Type Tenosynovial Giant Cell Tumor: What Are the Important Findings on the Initial and Follow-Up MRI?
Abstract
:Simple Summary
Abstract
1. Introduction
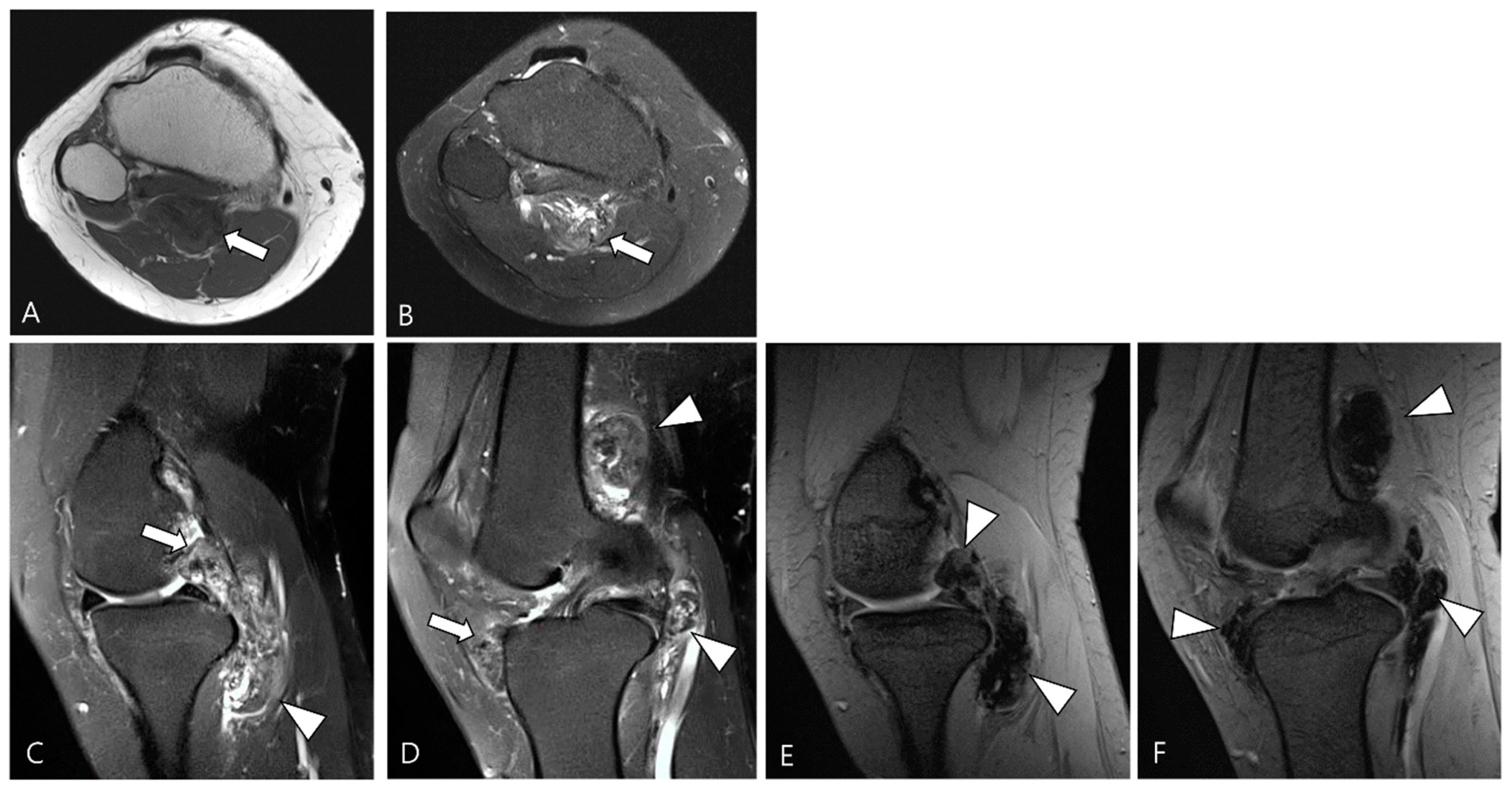
2. MRI Findings for D-TSGCT on Initial MRI
2.1. MRI Protocols for TSGCT
2.2. Signal Intensity (SI) for D-TSGCT
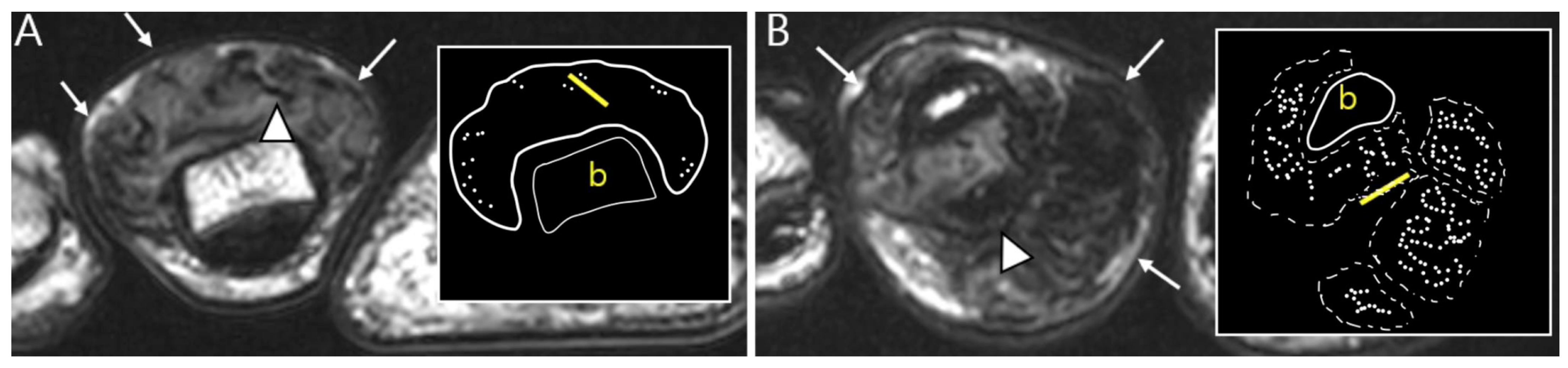
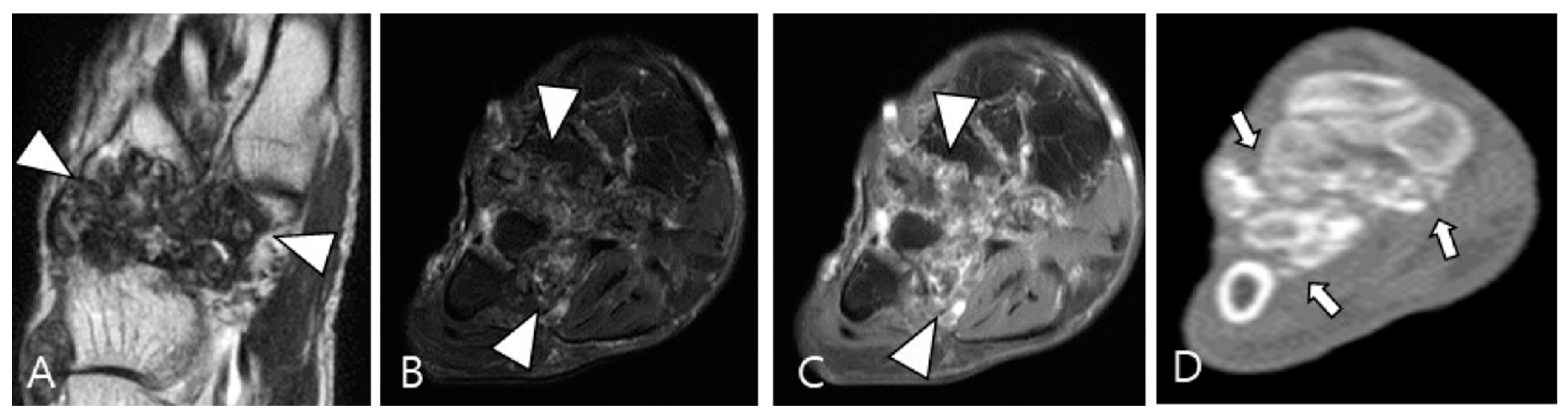
2.3. Morphological Findings for D-TSGCT
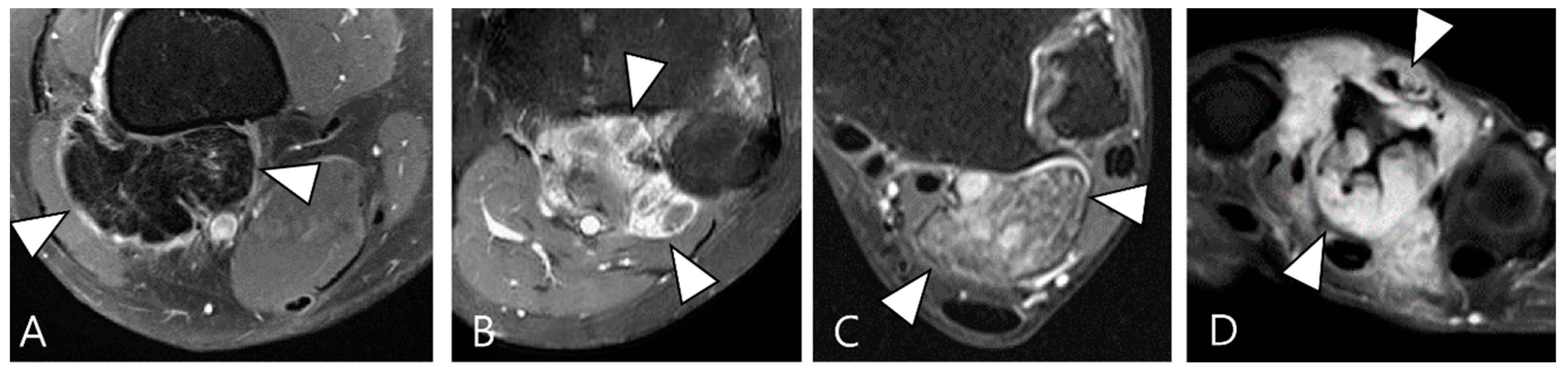
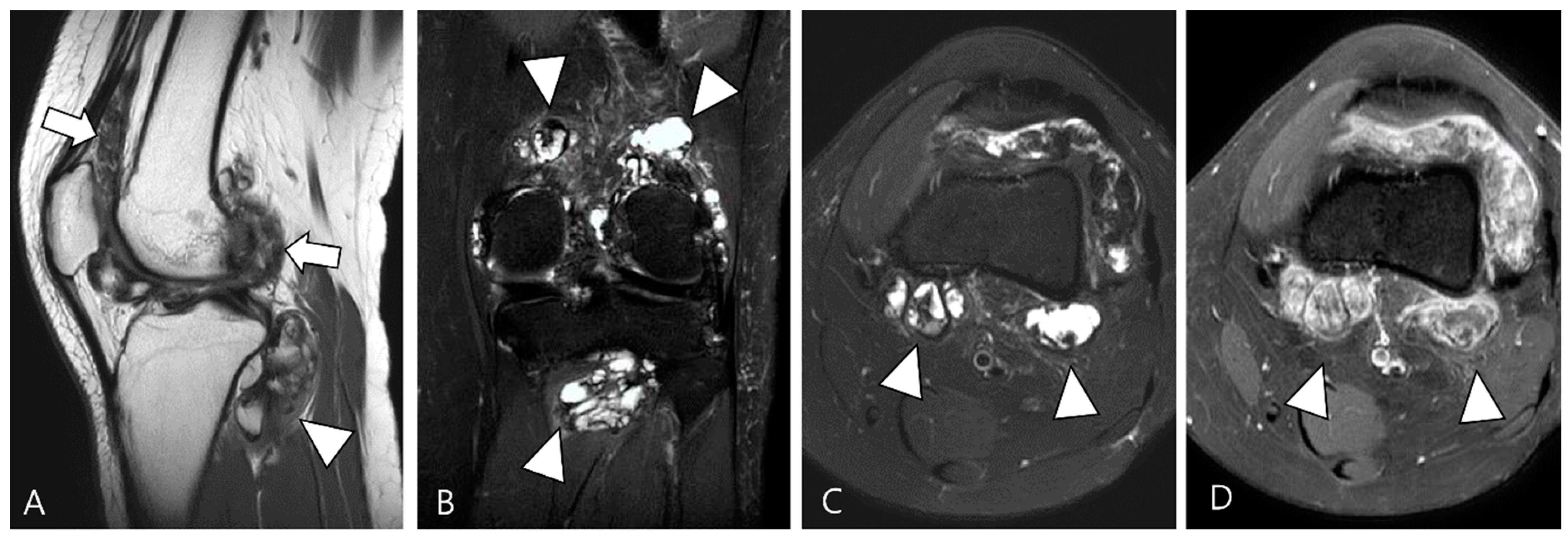
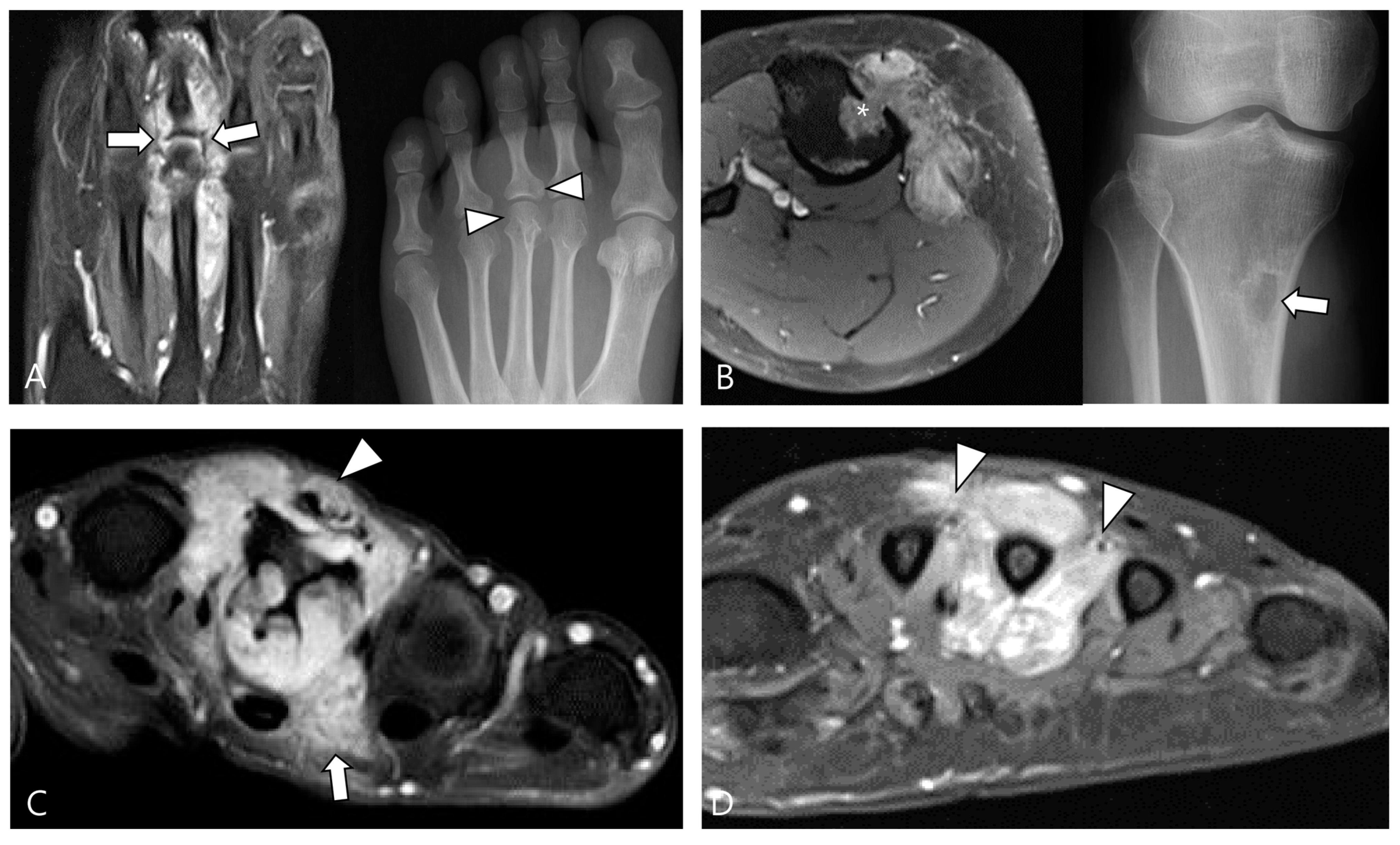
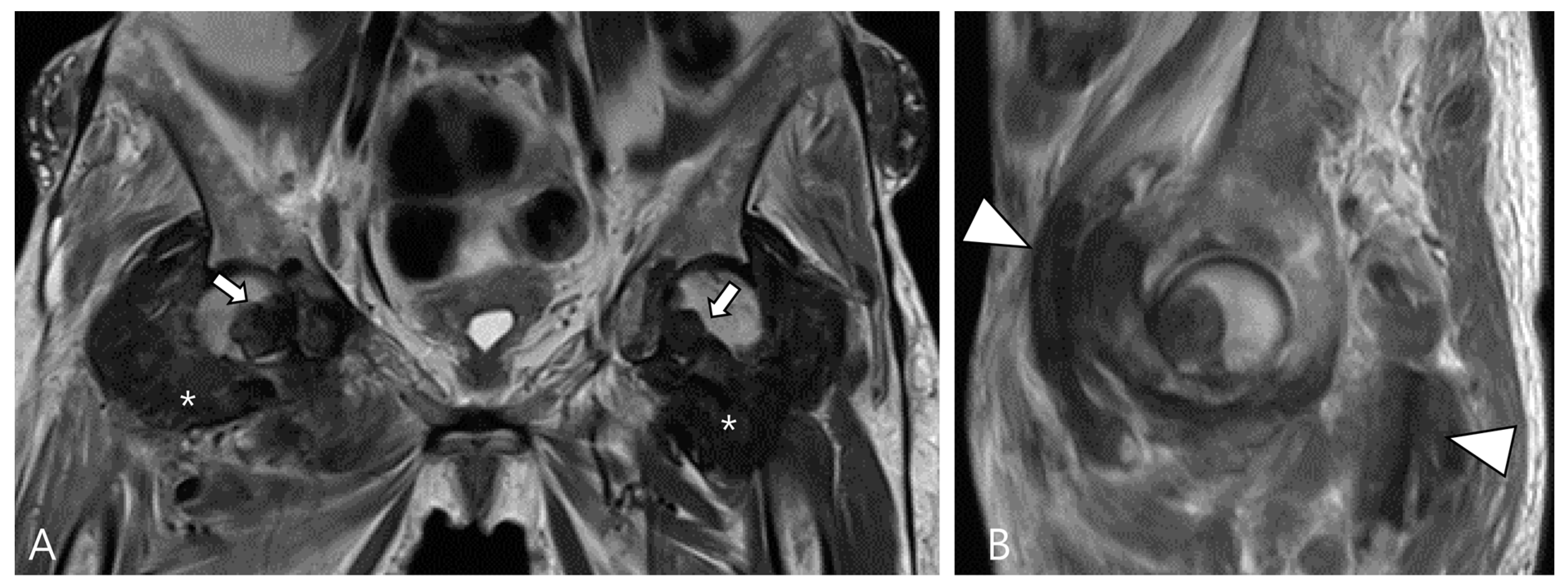
2.4. Relationship to Adjacent Structures of D-TSGCT
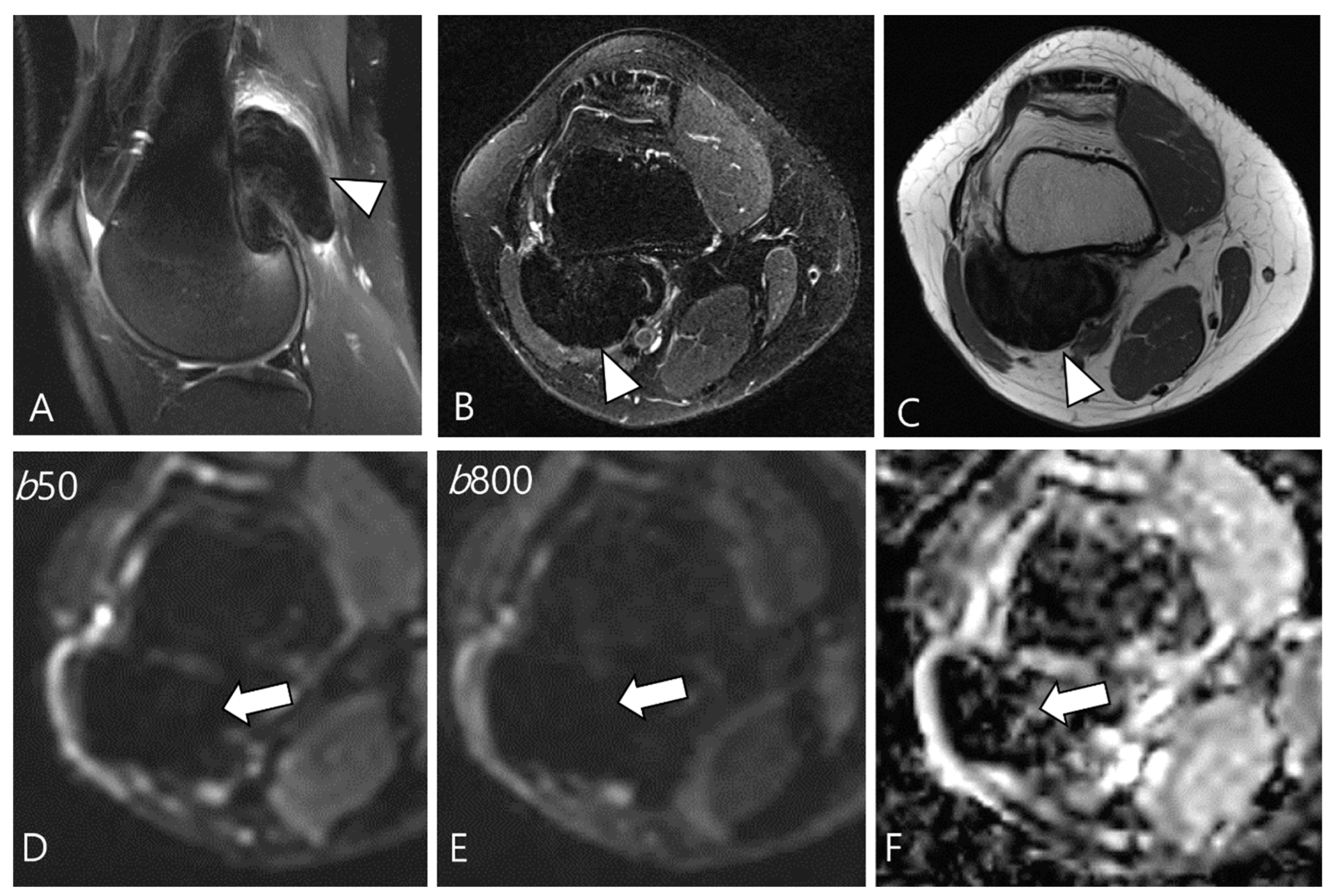
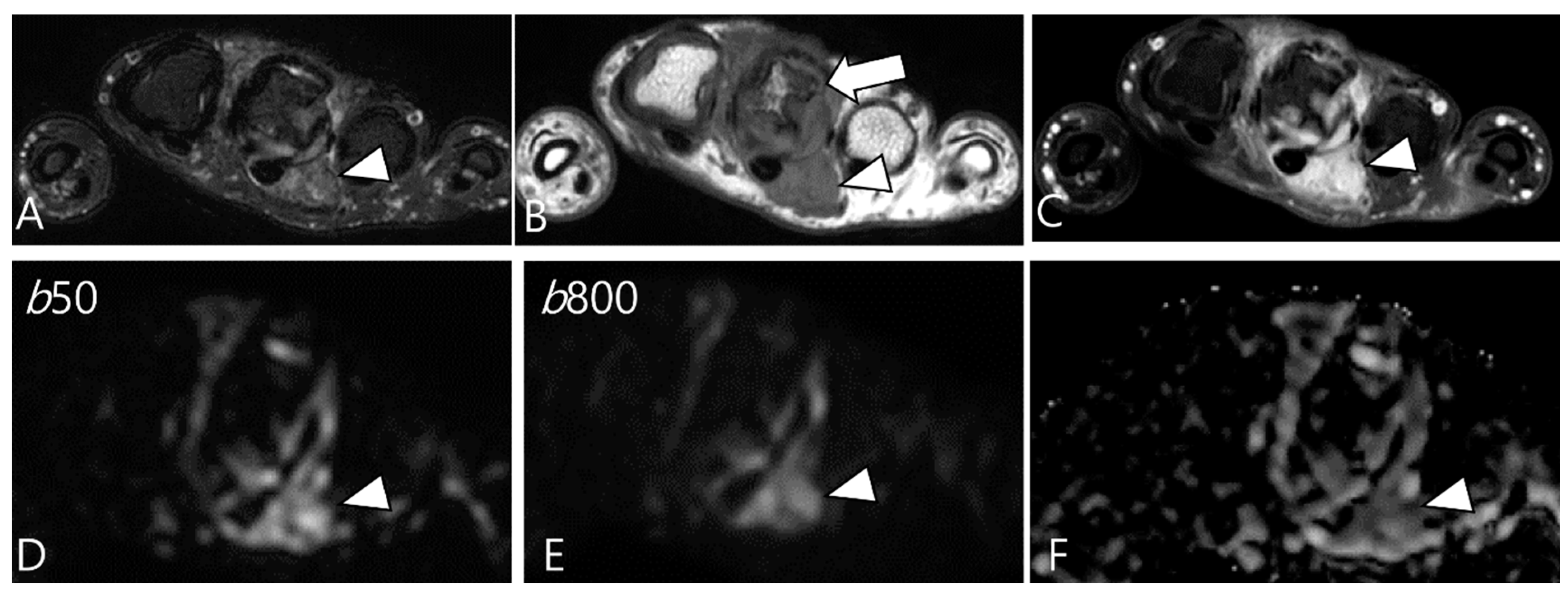
2.5. Advanced MRI Sequences for D-TSGCT
3. Differential Diagnoses for D-TSGCT on Initial MRI
3.1. Differential Diagnoses of Intra-Articular D-TSGCT
3.1.1. Hemosiderotic Synovitis
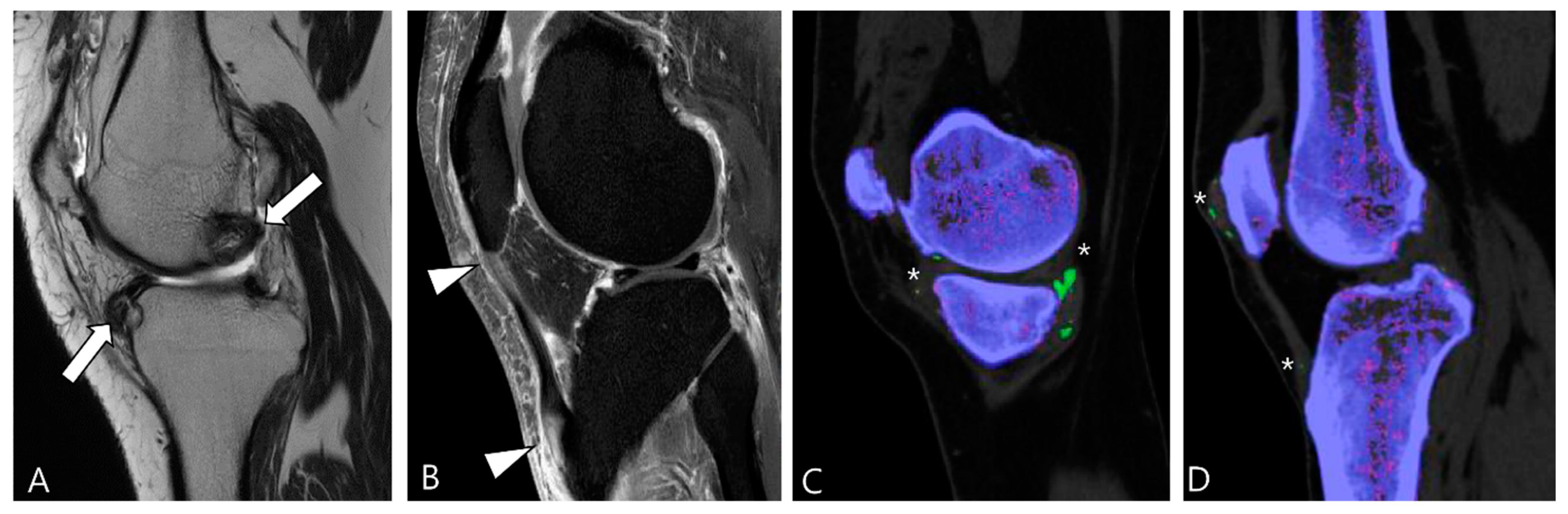
3.1.2. Synovial Chondromatosis
3.1.3. Dialysis-Related Amyloid Arthropathy
3.1.4. Chronic Rheumatoid Arthritis
3.1.5. Tophaceous Gout
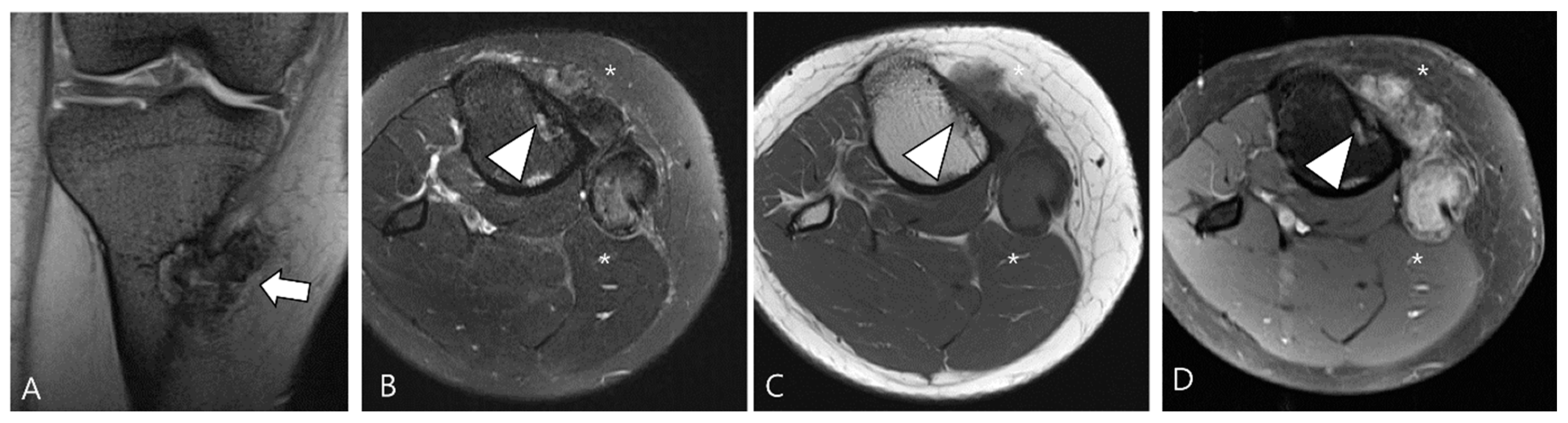
3.2. Differential Diagnoses of Extra-Articular D-TSGCT
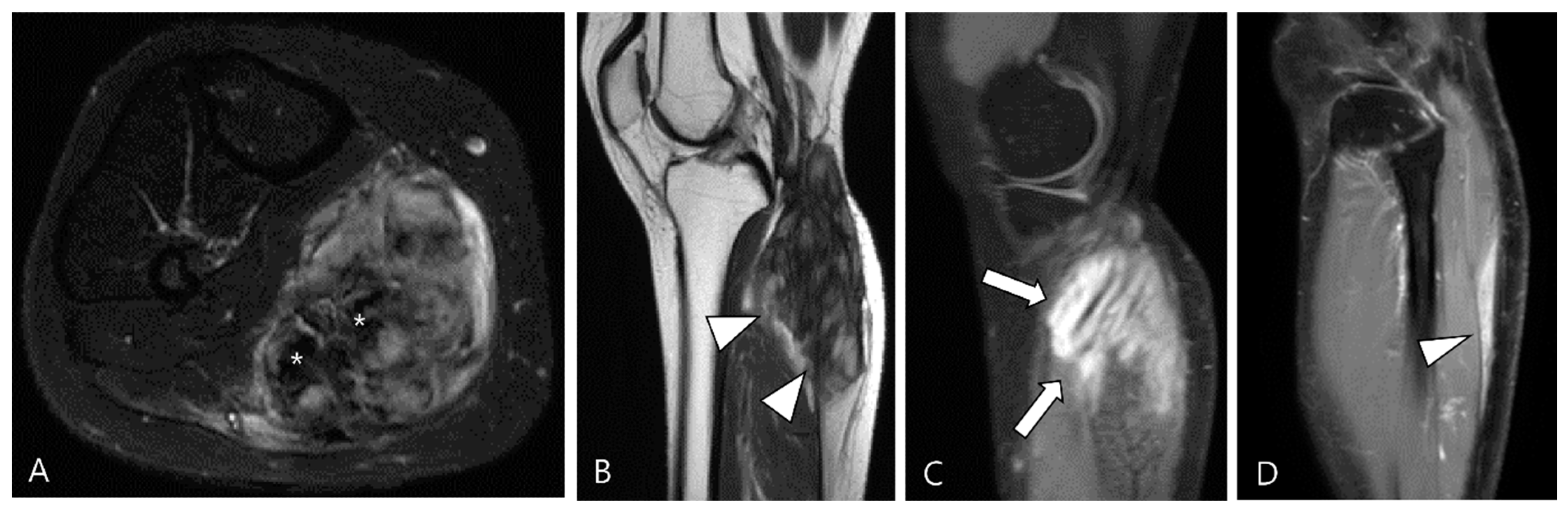
3.2.1. Fibroma of the Tendon Sheath (FTS)
3.2.2. Extra-Abdominal Desmoid-Type Fibromatosis (DF)
3.2.3. Tophaceous Gout
4. MRI Findings for D-TSGCT on Follow-Up MRI
4.1. Treatment Options for D-TSGCT
4.2. Checklists on Follow-Up MRI for D-TSGCT
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Spierenburg, G.; Suevos Ballesteros, C.; Stoel, B.C.; Navas Canete, A.; Gelderblom, H.; van de Sande, M.A.J.; van Langevelde, K. MRI of diffuse-type tenosynovial giant cell tumour in the knee: A guide for diagnosis and treatment response assessment. Insights Imaging 2023, 14, 22. [Google Scholar] [CrossRef]
- Helming, A.; Hansford, B.; Beckett, B. Tenosynovial giant cell tumor-diffuse type, treated with a novel colony-stimulating factor inhibitor, pexidartinib: Initial experience with MRI findings in three patients. Skeletal Radiol. 2022, 51, 1085–1091. [Google Scholar] [CrossRef]
- Murphey, M.D.; Rhee, J.H.; Lewis, R.B.; Fanburg-Smith, J.C.; Flemming, D.J.; Walker, E.A. Pigmented villonodular synovitis: Radiologic-pathologic correlation. Radiographics 2008, 28, 1493–1518. [Google Scholar] [CrossRef] [PubMed]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Sciot, R.; Rosai, J.; Dal Cin, P.; de Wever, I.; Fletcher, C.D.; Mandahl, N.; Mertens, F.; Mitelman, F.; Rydholm, A.; Tallini, G.; et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: A report from the Chromosomes and Morphology (CHAMP) study group. Mod. Pathol. 1999, 12, 576–579. [Google Scholar]
- Mastboom, M.J.L.; Verspoor, F.G.M.; Verschoor, A.J.; Uittenbogaard, D.; Nemeth, B.; Mastboom, W.J.B.; Bovée, J.; Dijkstra, P.D.S.; Schreuder, H.W.B.; Gelderblom, H.; et al. Higher incidence rates than previously known in tenosynovial giant cell tumors. Acta Orthop. 2017, 88, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, V.; Andersen, S.L.; Qazi, I.; Sankar, N.; Pedersen, A.B.; Sikorski, R.; Acquavella, J.F. Tenosynovial Giant Cell Tumor: Incidence, Prevalence, Patient Characteristics, and Recurrence. A Registry-based Cohort Study in Denmark. J. Rheumatol. 2017, 44, 1476–1483. [Google Scholar] [CrossRef]
- Lucas, D.R. Tenosynovial giant cell tumor: Case report and review. Arch. Pathol. Lab. Med. 2012, 136, 901–906. [Google Scholar] [CrossRef]
- Chien, J.C.; Wei, Y.P.; Chen, C.Y.; Hsiang, W.H.; Wang, Y.Y.; Liu, W.S.; Yang, S.W. Long-term functional outcomes of diffuse pigmented villonodular synovitis of knee: The role of adjuvant radiotherapy. Medicine 2021, 100, e23794. [Google Scholar] [CrossRef]
- Somerhausen, N.S.; Fletcher, C.D. Diffuse-type giant cell tumor: Clinicopathologic and immunohistochemical analysis of 50 cases with extraarticular disease. Am. J. Surg. Pathol. 2000, 24, 479–492. [Google Scholar] [CrossRef]
- Darling, J.M.; Goldring, S.R.; Harada, Y.; Handel, M.L.; Glowacki, J.; Gravallese, E.M. Multinucleated cells in pigmented villonodular synovitis and giant cell tumor of tendon sheath express features of osteoclasts. Am. J. Pathol. 1997, 150, 1383–1393. [Google Scholar] [PubMed]
- West, R.B.; Rubin, B.P.; Miller, M.A.; Subramanian, S.; Kaygusuz, G.; Montgomery, K.; Zhu, S.; Marinelli, R.J.; De Luca, A.; Downs-Kelly, E.; et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc. Natl. Acad. Sci. USA 2006, 103, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Spierenburg, G.; van der Heijden, L.; Mastboom, M.J.L.; van Langevelde, K.; van der Wal, R.J.P.; Gelderblom, H.; van de Sande, M.A.J. Surgical management of 144 diffuse-type TGCT patients in a single institution: A 20-year cohort study. J. Surg. Oncol. 2022, 126, 1087–1095. [Google Scholar] [CrossRef]
- Mastboom, M.J.L.; Palmerini, E.; Verspoor, F.G.M.; Rueten-Budde, A.J.; Stacchiotti, S.; Staals, E.L.; Schaap, G.R.; Jutte, P.C.; Aston, W.; Gelderblom, H.; et al. Surgical outcomes of patients with diffuse-type tenosynovial giant-cell tumours: An international, retrospective, cohort study. Lancet Oncol. 2019, 20, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Choong, P.F.; Willén, H.; Nilbert, M.; Mertens, F.; Mandahl, N.; Carlén, B.; Rydholm, A. Pigmented villonodular synovitis. Monoclonality and metastasis—A case for neoplastic origin? Acta Orthop. Scand. 1995, 66, 64–68. [Google Scholar] [CrossRef]
- Folpe, A.L.; Weiss, S.W.; Fletcher, C.D.; Gown, A.M. Tenosynovial giant cell tumors: Evidence for a desmin-positive dendritic cell subpopulation. Mod. Pathol. 1998, 11, 939–944. [Google Scholar] [PubMed]
- Li, C.F.; Wang, J.W.; Huang, W.W.; Hou, C.C.; Chou, S.C.; Eng, H.L.; Lin, C.N.; Yu, S.C.; Huang, H.Y. Malignant diffuse-type tenosynovial giant cell tumors: A series of 7 cases comparing with 24 benign lesions with review of the literature. Am. J. Surg. Pathol. 2008, 32, 587–599. [Google Scholar] [CrossRef]
- Mastboom, M.J.L.; Verspoor, F.G.M.; Hanff, D.F.; Gademan, M.G.J.; Dijkstra, P.D.S.; Schreuder, H.W.B.; Bloem, J.L.; van der Wal, R.J.P.; van de Sande, M.A.J. Severity classification of Tenosynovial Giant Cell Tumours on MR imaging. Surg. Oncol. 2018, 27, 544–550. [Google Scholar] [CrossRef]
- Cho, E.B.; Lee, S.K.; Kim, J.Y.; Kim, Y. Synovial Sarcoma in the Extremity: Diversity of Imaging Features for Diagnosis and Prognosis. Cancers 2023, 15, 4860. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.K.; Kim, J.Y. Prediction of local recurrence in tenosynovial giant cell tumor of the knee: Based on preoperative MRI evaluation into disease subtypes and severity. PLoS ONE 2023, 18, e0287028. [Google Scholar] [CrossRef]
- Crim, J.; Dyroff, S.L.; Stensby, J.D.; Evenski, A.; Layfield, L.J. Limited usefulness of classic MR findings in the diagnosis of tenosynovial giant cell tumor. Skeletal Radiol. 2021, 50, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Guo, G.; You, Y.; Li, Y.; Xuan, Y.; Jin, Z.W.; Yan, G. Magnetic resonance imaging features of fibromas and giant cell tumors of the tendon sheath: Differential diagnosis. Eur. Radiol. 2019, 29, 3441–3449. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ito, H.; Amano, Y.; Sawaizumi, T.; Takeuchi, T. MR imaging for preoperative diagnosis and assessment of local tumor extent on localized giant cell tumor of tendon sheath. Skeletal Radiol. 2003, 32, 633–638. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, A.M.; Hogendoorn, P.C.; Bloem, J.L. Giant cell tumors of the tendon sheath may present radiologically as intrinsic osseous lesions. Eur. Radiol. 2007, 17, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Lee, C.H.; Chan, W.P.; Chen, C.Y.; Yu, J.S.; Resnick, D. Localized nodular synovitis of the knee: MR imaging appearance and clinical correlates in 21 patients. AJR Am. J. Roentgenol. 2003, 181, 539–543. [Google Scholar] [CrossRef]
- Gruber, L.; Loizides, A.; Luger, A.K.; Glodny, B.; Moser, P.; Henninger, B.; Gruber, H. Soft-Tissue Tumor Contrast Enhancement Patterns: Diagnostic Value and Comparison Between Ultrasound and MRI. AJR Am. J. Roentgenol. 2017, 208, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Ashikyan, O.; Chalian, M.; Moore, D.; Xi, Y.; Pezeshk, P.; Chhabra, A. Evaluation of giant cell tumors by diffusion weighted imaging-fractional ADC analysis. Skeletal Radiol. 2019, 48, 1765–1773. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.K.; Kim, J.Y. MRI Prediction Model for Tenosynovial Giant Cell Tumor with Risk of Diffuse-type. Acad. Radiol. 2023, 30, 2616–2624. [Google Scholar] [CrossRef]
- Al-Qattan, M.M. Giant cell tumours of tendon sheath: Classification and recurrence rate. J. Hand Surg. 2001, 26, 72–75. [Google Scholar] [CrossRef]
- Serhal, A.; Samet, J.; Shah, C.; Omar, I.; Youngner, J. MRI evaluation of solid soft tissue masses of the fingers with pathology correlation. Eur. J. Radiol. 2021, 135, 109465. [Google Scholar] [CrossRef]
- Kim, D.E.; Kim, J.M.; Lee, B.S.; Kim, N.K.; Lee, S.H.; Bin, S.I. Distinct extra-articular invasion patterns of diffuse pigmented villonodular synovitis/tenosynovial giant cell tumor in the knee joints. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3508–3514. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kang, Y.-K.; Yoo, C.Y.; Kim, J.Y. Tenosynovial Giant Cell Tumor of the Pes Anserine Bursa with Bone Marrow Extension into the Tibia: A Case Report. J. Korean Soc. Radiol. 2016, 75, 322–326. [Google Scholar] [CrossRef]
- Ushijima, M.; Hashimoto, H.; Tsuneyoshi, M.; Enjoji, M. Giant cell tumor of the tendon sheath (nodular tenosynovitis). A study of 207 cases to compare the large joint group with the common digit group. Cancer 1986, 57, 875–884. [Google Scholar] [CrossRef]
- Zhao, H.; Maheshwari, A.V.; Kumar, D.; Malawer, M.M. Giant cell tumor of the pes anserine bursa (extra-articular pigmented villonodular bursitis): A case report and review of the literature. Case Rep. Med. 2011, 2011, 491470. [Google Scholar] [CrossRef]
- Hepp, P.; Engel, T.; Marquass, B.; Aigner, T.; Josten, C.; Niederhagen, M. Infiltration of the pes anserinus complex by an extraarticular diffuse-type giant cell tumor (D-TGCT). Arch. Orthop. Trauma Surg. 2008, 128, 155–158. [Google Scholar] [CrossRef]
- Jeong, H.S.; Lee, S.K.; Kim, J.Y.; Yoo, C.; Joo, M.W.; Kim, J.H. Tenosynovial giant cell tumors of digits: MRI differentiation between localized types and diffuse types with pathology correlation. Skeletal Radiol. 2023, 52, 593–603. [Google Scholar] [CrossRef]
- Zheng, K.; Yu, X.C.; Hu, Y.C.; Xu, M.; Zhang, J.Y. A New Simple and Practical Clinical Classification for Tenosynovial Giant Cell Tumors of the Knee. Orthop. Surg. 2022, 14, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Jee, W.H.; Jung, C.K.; Chung, Y.G. Multiparametric quantitative analysis of tumor perfusion and diffusion with 3T MRI: Differentiation between benign and malignant soft tissue tumors. Br. J. Radiol. 2020, 93, 20191035. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.K.; Kim, J.Y.; Kim, J.H. Pitfalls of Diffusion-Weighted Imaging: Clinical Utility of T2 Shine-through and T2 Black-out for Musculoskeletal Diseases. Diagnostics 2023, 13, 1647. [Google Scholar] [CrossRef]
- Anazawa, U.; Hanaoka, H.; Shiraishi, T.; Morioka, H.; Morii, T.; Toyama, Y. Similarities between giant cell tumor of bone, giant cell tumor of tendon sheath, and pigmented villonodular synovitis concerning ultrastructural cytochemical features of multinucleated giant cells and mononuclear stromal cells. Ultrastruct. Pathol. 2006, 30, 151–158. [Google Scholar] [CrossRef]
- Ahlawat, S.; Fayad, L.M. Diffusion weighted imaging demystified: The technique and potential clinical applications for soft tissue imaging. Skeletal Radiol. 2018, 47, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, A.; Ashikyan, O.; Slepicka, C.; Dettori, N.; Hwang, H.; Callan, A.; Sharma, R.R.; Xi, Y. Conventional MR and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: Correlation with histologic grading. Eur. Radiol. 2019, 29, 4485–4494. [Google Scholar] [CrossRef]
- Messina, C.; Bignone, R.; Bruno, A.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Coppolino, P.; Robertis, R.; Gentili, F.; et al. Diffusion-Weighted Imaging in Oncology: An Update. Cancers 2020, 12, 1493. [Google Scholar] [CrossRef] [PubMed]
- Hindman, B.W.; Seeger, L.L.; Stanley, P.; Forrester, D.M.; Schwinn, C.P.; Tan, S.Z. Multicentric giant cell tumor: Report of five new cases. Skeletal Radiol. 1994, 23, 187–190. [Google Scholar] [CrossRef]
- Takahara, T.; Kwee, T.C. Low b-value diffusion-weighted imaging: Emerging applications in the body. J. Magn. Reson. Imaging 2012, 35, 1266–1273. [Google Scholar] [CrossRef]
- Iima, M.; Le Bihan, D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology 2016, 278, 13–32. [Google Scholar] [CrossRef]
- Drapé, J.L. Advances in magnetic resonance imaging of musculoskeletal tumours. Orthop. Traumatol. Surg. Res. 2013, 99, S115–S123. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.Y.; Hong, S.J.; Woo, O.H.; Kang, C.H.; Ahn, K.S.; Kim, B.H.; Shim, E. T2 Black Synovitis in Musculoskeletal MRI: Disease Spectrum and Imaging Characteristics of Joint Diseases. Curr. Med. Imaging 2024, 20, e010623217546. [Google Scholar] [CrossRef]
- Narváez, J.A.; Narváez, J.; Ortega, R.; De Lama, E.; Roca, Y.; Vidal, N. Hypointense synovial lesions on T2-weighted images: Differential diagnosis with pathologic correlation. AJR Am. J. Roentgenol. 2003, 181, 761–769. [Google Scholar] [CrossRef]
- Finkelstein, D.; Foremny, G.; Singer, A.; Clifford, P.; Pretell-Mazzini, J.; Kerr, D.A.; Subhawong, T.K. Differential diagnosis of T2 hypointense masses in musculoskeletal MRI. Skeletal Radiol. 2021, 50, 1981–1994. [Google Scholar] [CrossRef]
- Ando, T.; Kato, H.; Kawaguchi, M.; Nagano, A.; Hyodo, F.; Matsuo, M. MR imaging findings for differentiating nonhemophilic hemosiderotic synovitis from diffuse-type tenosynovial giant cell tumor of the knee. Jpn. J. Radiol. 2021, 39, 76–83. [Google Scholar] [CrossRef]
- Maheshwari, A.V.; Muro-Cacho, C.A.; Pitcher, J.D., Jr. Pigmented villonodular bursitis/diffuse giant cell tumor of the pes anserine bursa: A report of two cases and review of literature. Knee 2007, 14, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Murphey, M.D.; Vidal, J.A.; Fanburg-Smith, J.C.; Gajewski, D.A. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics 2007, 27, 1465–1488. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, V.; Cho, G.; Moore, D.; Pezeshk, P.; Coyner, K.; Chhabra, A. T2 black lesions on routine knee MRI: Differential considerations. Eur. Radiol. 2016, 26, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Otake, S.; Tsuruta, Y.; Yamana, D.; Mizutani, H.; Ohba, S. Amyloid arthropathy of the hip joint: MR demonstration of presumed amyloid lesions in 152 patients with long-term hemodialysis. Eur. Radiol. 1998, 8, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, O.; Isaac, A.; Dalili, D.; Noebauer-Huhmann, I.M. T2-weighted Hypointense Tumors and Tumor-like Lesions. Semin. Musculoskelet. Radiol. 2019, 23, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Llauger, J.; Palmer, J.; Rosón, N.; Bagué, S.; Camins, A.; Cremades, R. Nonseptic monoarthritis: Imaging features with clinical and histopathologic correlation. Radiographics 2000, 20, S263–S278. [Google Scholar] [CrossRef]
- Girish, G.; Glazebrook, K.N.; Jacobson, J.A. Advanced imaging in gout. AJR Am. J. Roentgenol. 2013, 201, 515–525. [Google Scholar] [CrossRef]
- Christiansen, S.N.; Müller, F.C.; Østergaard, M.; Slot, O.; Møller, J.M.; Børgesen, H.F.; Gosvig, K.K.; Terslev, L. Dual-energy CT in gout patients: Do all colour-coded lesions actually represent monosodium urate crystals? Arthritis Res. Ther. 2020, 22, 212. [Google Scholar] [CrossRef]
- De Beuckeleer, L.; De Schepper, A.; De Belder, F.; Van Goethem, J.; Marques, M.C.; Broeckx, J.; Verstraete, K.; Vermaut, F. Magnetic resonance imaging of localized giant cell tumour of the tendon sheath (MRI of localized GCTTS). Eur. Radiol. 1997, 7, 198–201. [Google Scholar] [CrossRef]
- Hitora, T.; Yamamoto, T.; Akisue, T.; Marui, T.; Nagira, K.; Ohta, R.; Kurosaka, M. Fibroma of tendon sheath originating from the knee joint capsule. Clin. Imaging 2002, 26, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Staals, E.L.; Maki, R.G.; Pengo, S.; Cioffi, A.; Gambarotti, M.; Picci, P.; Daolio, P.A.; Parafioriti, A.; Morris, C.; et al. Tenosynovial giant cell tumour/pigmented villonodular synovitis: Outcome of 294 patients before the era of kinase inhibitors. Eur. J. Cancer 2015, 51, 210–217. [Google Scholar] [CrossRef]
- Okuda, M.; Yoshida, K.; Kobayashi, S.; Gabata, T. Desmoid-type fibromatosis: Imaging features and course. Skeletal Radiol. 2023, 52, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, D.; Amini, B.; Nikolaidis, P.; Assing, M.; Vikram, R. Current Update on Desmoid Fibromatosis. J. Comput. Assist. Tomogr. 2019, 43, 29–38. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.S.; Yi, E.S.; Wenger, D.E. Best cases from the AFIP: Extraabdominal desmoid-type fibromatosis. Radiographics 2008, 28, 901–906. [Google Scholar] [CrossRef]
- Rosa, F.; Martinetti, C.; Piscopo, F.; Buccicardi, D.; Schettini, D.; Neumaier, C.E.; Gandolfo, N.; Grazioli, L.; Gastaldo, A. Multimodality imaging features of desmoid tumors: A head-to-toe spectrum. Insights Imaging 2020, 11, 103. [Google Scholar] [CrossRef]
- Braschi-Amirfarzan, M.; Keraliya, A.R.; Krajewski, K.M.; Tirumani, S.H.; Shinagare, A.B.; Hornick, J.L.; Baldini, E.H.; George, S.; Ramaiya, N.H.; Jagannathan, J.P. Role of Imaging in Management of Desmoid-type Fibromatosis: A Primer for Radiologists. Radiographics 2016, 36, 767–782. [Google Scholar] [CrossRef]
- Gondim Teixeira, P.A.; Biouichi, H.; Abou Arab, W.; Rios, M.; Sirveaux, F.; Hossu, G.; Blum, A. Evidence-based MR imaging follow-up strategy for desmoid-type fibromatosis. Eur. Radiol. 2020, 30, 895–902. [Google Scholar] [CrossRef]
- Lee, S.K.; Jung, J.Y.; Jee, W.H.; Lee, J.J.; Park, S.H. Combining non-contrast and dual-energy CT improves diagnosis of early gout. Eur. Radiol. 2019, 29, 1267–1275. [Google Scholar] [CrossRef]
- Patel, K.H.; Gikas, P.D.; Pollock, R.C.; Carrington, R.W.; Cannon, S.R.; Skinner, J.A.; Briggs, T.W.; Aston, W.J.S. Pigmented villonodular synovitis of the knee: A retrospective analysis of 214 cases at a UK tertiary referral centre. Knee 2017, 24, 808–815. [Google Scholar] [CrossRef]
- Chin, K.R.; Barr, S.J.; Winalski, C.; Zurakowski, D.; Brick, G.W. Treatment of advanced primary and recurrent diffuse pigmented villonodular synovitis of the knee. J. Bone Jt. Surg. Am. 2002, 84, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Dines, J.S.; DeBerardino, T.M.; Wells, J.L.; Dodson, C.C.; Shindle, M.; DiCarlo, E.F.; Warren, R.F. Long-term follow-up of surgically treated localized pigmented villonodular synovitis of the knee. Arthroscopy 2007, 23, 930–937. [Google Scholar] [CrossRef] [PubMed]
- De Ponti, A.; Sansone, V.; Malcherè, M. Result of arthroscopic treatment of pigmented villonodular synovitis of the knee. Arthroscopy 2003, 19, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Mollon, B.; Lee, A.; Busse, J.W.; Griffin, A.M.; Ferguson, P.C.; Wunder, J.S.; Theodoropoulos, J. The effect of surgical synovectomy and radiotherapy on the rate of recurrence of pigmented villonodular synovitis of the knee: An individual patient meta-analysis. Bone Jt. J. 2015, 97, 550–557. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Cummings, B.; Catton, C.; Bell, R.; Davis, A.; Fornasier, V.; Goldberg, R. Outcome following radiation treatment for high-risk pigmented villonodular synovitis. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 777–786. [Google Scholar] [CrossRef]
- Stamatiou, A.; Nguyen-Ngoc, T.; Wetterwald, L.; Dolcan, A.-M.; Dei Tos, G.; Cherix, S.; Omoumi, P.; Digklia, A. Overview of Pharmacological Therapies for Diffuse Tenosynovial Giant Cell Tumor. Future Pharmacol. 2023, 3, 926–937. [Google Scholar] [CrossRef]
- Brahmi, M.; Vinceneux, A.; Cassier, P.A. Current Systemic Treatment Options for Tenosynovial Giant Cell Tumor/Pigmented Villonodular Synovitis: Targeting the CSF1/CSF1R Axis. Curr. Treat. Options Oncol. 2016, 17, 10. [Google Scholar] [CrossRef]
- Healey, J.H.; Bernthal, N.M.; van de Sande, M. Management of Tenosynovial Giant Cell Tumor: A Neoplastic and Inflammatory Disease. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e20.00028. [Google Scholar] [CrossRef]
- Van De Sande, M.; Tap, W.D.; Gelhorn, H.L.; Ye, X.; Speck, R.M.; Palmerini, E.; Stacchiotti, S.; Desai, J.; Wagner, A.J.; Alcindor, T.; et al. Pexidartinib improves physical functioning and stiffness in patients with tenosynovial giant cell tumor: Results from the ENLIVEN randomized clinical trial. Acta Orthop. 2021, 92, 493–499. [Google Scholar] [CrossRef]
- Machado, V.; San-Julián, M. Risk factors for early osteoarthritis in tenosynovial giant cell tumour. Rev. Esp. Cir. Ortop. Traumatol. 2020, 64, 199–205. [Google Scholar] [CrossRef]
- Lin, F.; Wilson, K.; Kwong, W.J.; Abraham, J.A. Understanding the Effect of Osteoarthritis on Surgical Treatment Patterns, Healthcare Resource Utilization, and Costs Among Patients With Tenosynovial Giant Cell Tumors. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2023, 7, e23.00047. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Jiang, W.; Qiu, C.; Xiao, N.; Liang, J. Image-guided, intensity-modulated radiotherapy for the treatment of diffuse-type tenosynovial giant cell tumor of the knee: Case report and review of the literature. Medicine 2021, 100, e26659. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Dürr, H.R.; Schaefer, I.M.; Woertler, K.; Haas, R.; Trama, A.; Caraceni, A.; Bajpai, J.; Baldi, G.G.; Bernthal, N.; et al. Best clinical management of tenosynovial giant cell tumour (TGCT): A consensus paper from the community of experts. Cancer Treat. Rev. 2023, 112, 102491. [Google Scholar] [CrossRef]
- Robert, M.; Farese, H.; Miossec, P. Update on Tenosynovial Giant Cell Tumor, an Inflammatory Arthritis With Neoplastic Features. Front. Immunol. 2022, 13, 820046. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Cropet, C.; Chevreau, C.; Boyle, R.; Tattersall, M.; Stacchiotti, S.; Italiano, A.; Piperno-Neumann, S.; Le Cesne, A.; Ferraresi, V.; et al. Nilotinib in locally advanced pigmented villonodular synovitis: A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2018, 19, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Tap, W. ENLIVEN study: Pexidartinib for tenosynovial giant cell tumor (TGCT). Future Oncol. 2020, 16, 1875–1878. [Google Scholar] [CrossRef]
- Peterfy, C.; Chen, Y.; Countryman, P.; Chmielowski, B.; Anthony, S.P.; Healey, J.H.; Wainberg, Z.A.; Cohn, A.L.; Shapiro, G.I.; Keedy, V.L.; et al. CSF1 receptor inhibition of tenosynovial giant cell tumor using novel disease-specific MRI measures of tumor burden. Future Oncol. 2022, 18, 1449–1459. [Google Scholar] [CrossRef]





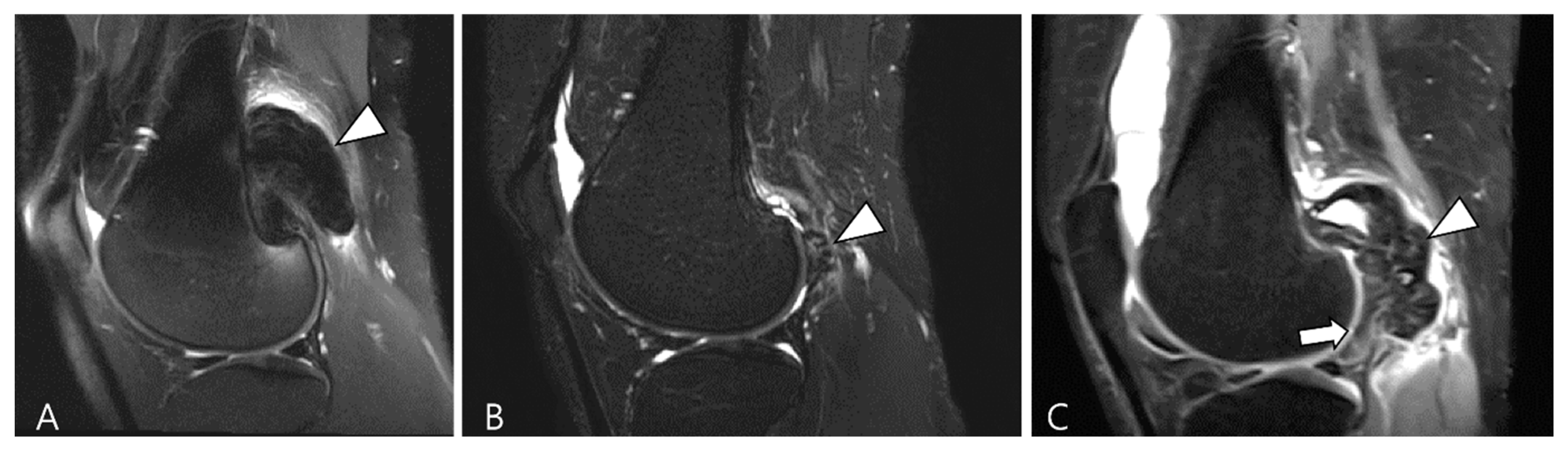
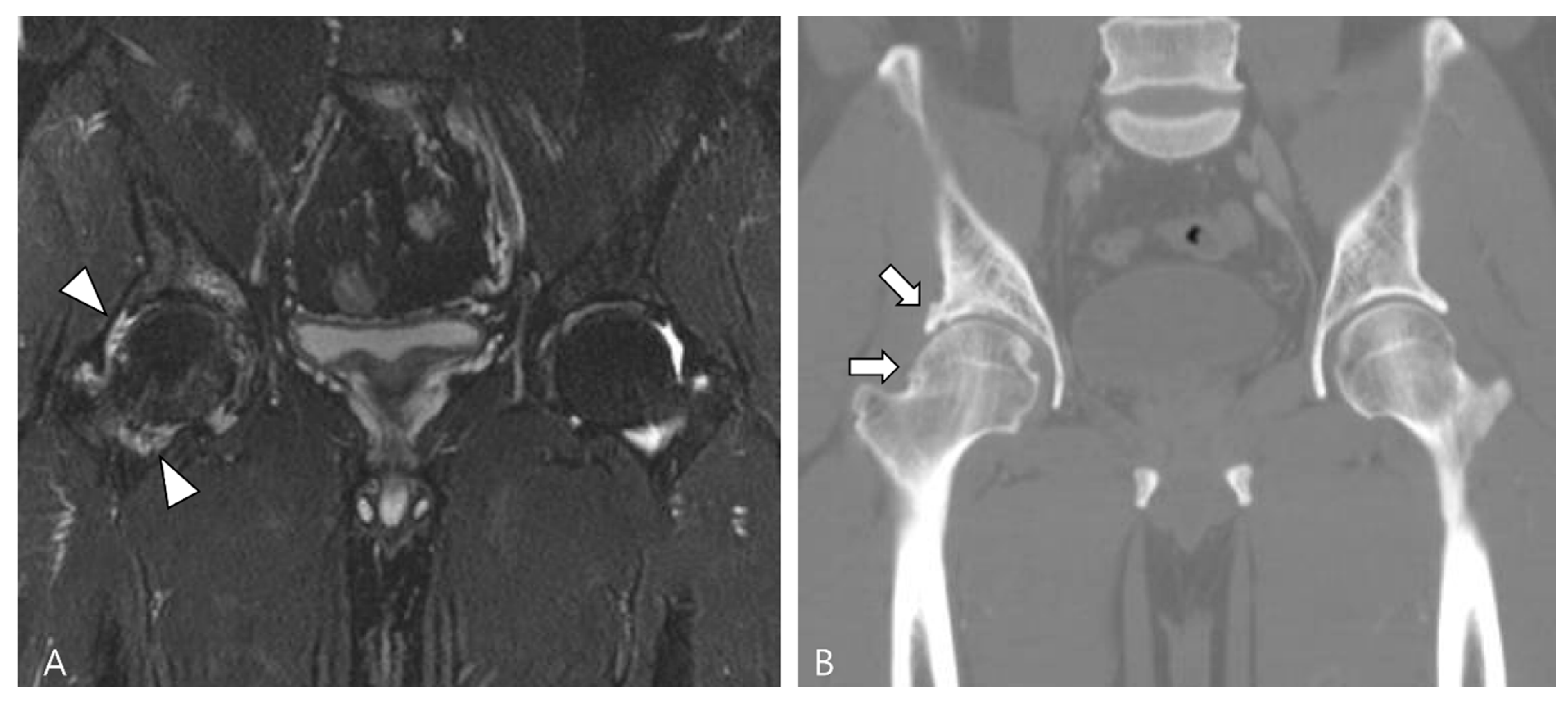
| Localized Type | Diffuse Type |
|---|---|
| A more common form affecting only a portion of the synovium | A less frequent form involving substantial parts of the synovium |
| Predominantly involving the digits and wrists | Primarily affecting large joints (knee, hip, ankle, elbow) |
| Systematically benign | More aggressive and destructive and may exceptionally include a malignant component |
| Localized Type | Diffuse Type | |
|---|---|---|
| Incidence | 45 per million person-years | 5 per million person-years |
| Recurrence rate | <15% | 50% with intra-articular disease 33 to 50% with extra-articular disease |
| MRI Findings | Localized Type TSGCT | Diffuse Type TSGCT |
|---|---|---|
| Signal intensity on T1WI, T2WI and contrast enhancement | Hypo-to-iso SI on T1WI and heterogeneous SI on T2WI with variable degrees of contrast enhancement | |
| GRE images | Presence of blooming artifact due to hemosiderin deposition | |
| Low SI areas (due to bleeding and hemosiderin deposition) | Speckled | Extensive and granular |
| Nodularity | Single | Multinodular |
| Margin | Circumscribed | Infiltrative |
| Peripheral hypointensity (capsule) | Present | Absent |
| Articular or cartilage involvement | Not frequent | Frequent |
| Bone involvement | Uncertain difference between two subtypes | |
| Muscle or tendon involvement | Not frequent | Frequent |
| Neurovascular involvement | Uncertain difference between two subtypes | |
| Location | Disease | Key Component | MR Imaging Features | Clinical Importance |
|---|---|---|---|---|
| Intra-articular | Intra-articular D-TSGCT | Hemosiderin |
|
|
| Hemosiderotic Synovitis | Hemosiderin |
|
| |
| Synovial Chondromatosis | Calcification |
|
| |
| Dialysis-Related Amyloid Arthropathy | Amyloid |
|
| |
| Chronic Rheumatoid Arthritis | Rice bodies |
|
| |
| Tophaceous Gout | Tophi |
|
| |
| Extra-articular | Extra-articular D-TSGCT | Hemosiderin |
|
|
| Fibroma of the Tendon Sheath | Dense collagen |
|
| |
| Extra-abdominal Desmoid-Type Fibromatosis | Fibrous tissue/collagen bands |
|
| |
| Tophaceous Gout | Tophi |
|
|
| Treatment Types | Checklists on Follow-Up MRI |
|---|---|
| Surgical excision | • Local recurrence |
| • Early development of osteoarthritis | |
| Radiotherapy | • Local recurrence |
| • Skin necrosis | |
| • Malignant transformation | |
| CSF1-receptor inhibitors | • Semiquantitative tumor volume change |
| • Decrease in SI along synovium with reduction in capsular distension and joint effusion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.S.; Lee, S.K.; Kim, J.-Y.; Kim, Y. Diffuse-Type Tenosynovial Giant Cell Tumor: What Are the Important Findings on the Initial and Follow-Up MRI? Cancers 2024, 16, 402. https://doi.org/10.3390/cancers16020402
Choi WS, Lee SK, Kim J-Y, Kim Y. Diffuse-Type Tenosynovial Giant Cell Tumor: What Are the Important Findings on the Initial and Follow-Up MRI? Cancers. 2024; 16(2):402. https://doi.org/10.3390/cancers16020402
Chicago/Turabian StyleChoi, Woo Suk, Seul Ki Lee, Jee-Young Kim, and Yuri Kim. 2024. "Diffuse-Type Tenosynovial Giant Cell Tumor: What Are the Important Findings on the Initial and Follow-Up MRI?" Cancers 16, no. 2: 402. https://doi.org/10.3390/cancers16020402
APA StyleChoi, W. S., Lee, S. K., Kim, J. -Y., & Kim, Y. (2024). Diffuse-Type Tenosynovial Giant Cell Tumor: What Are the Important Findings on the Initial and Follow-Up MRI? Cancers, 16(2), 402. https://doi.org/10.3390/cancers16020402






