Strategies to Target Chemoradiotherapy Resistance in Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Small Cell Lung Cancer
1.1. Biology
1.1.1. Recurrent Alterations
1.1.2. Subtypes
1.2. Treatment Options
2. Mechanisms of Resistance to DNA-Damaging Therapy in SCLC
2.1. Neuroendocrine-High to Neuroendocrine-Low Transformation
2.2. Cancer Stem Cells
2.3. Growth Factor Signaling
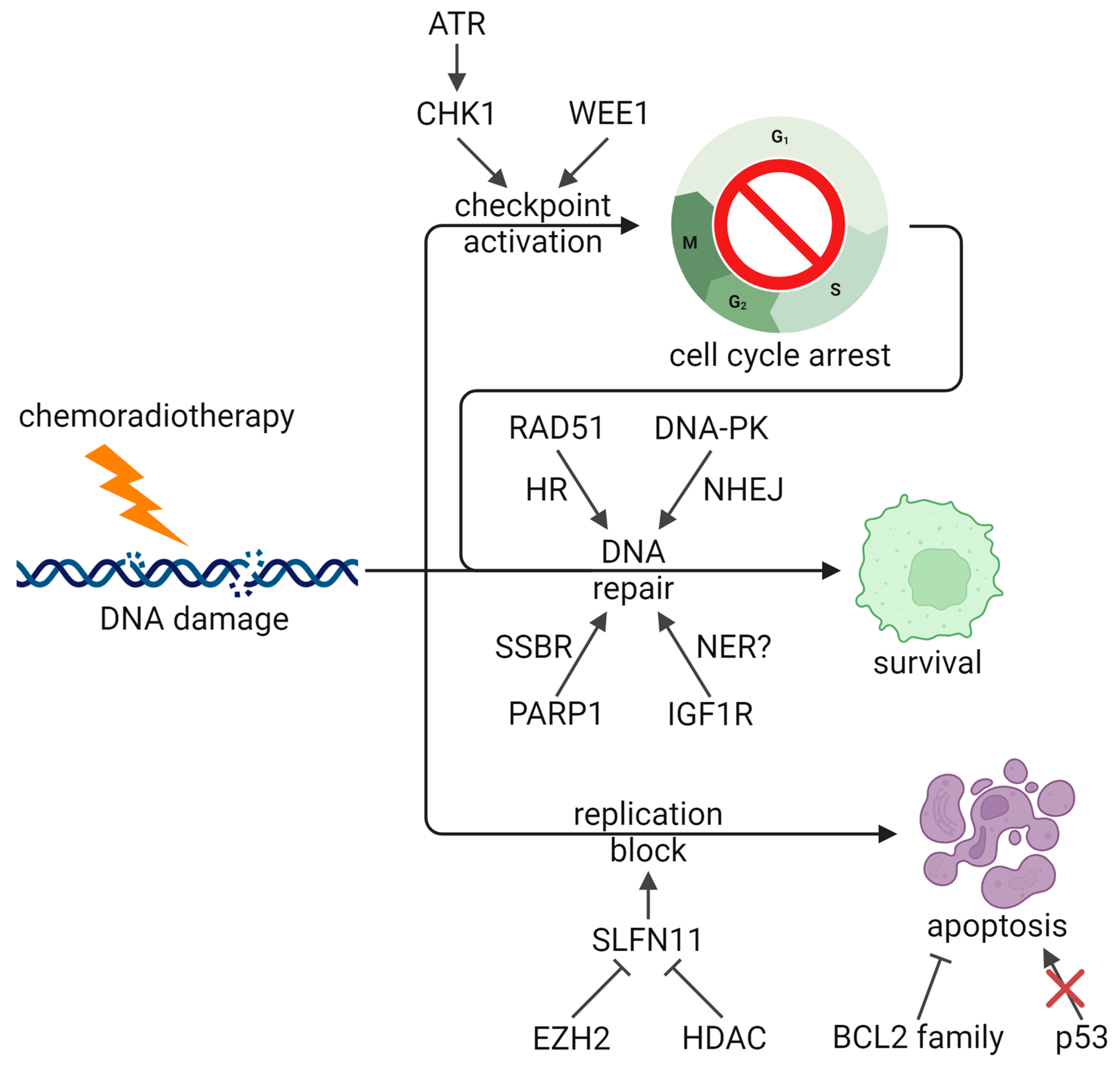
2.4. DNA Damage Response
2.5. Apoptosis Regulation
2.6. Metabolism
2.7. Drug Efflux Pumps
2.8. Epigenetics
2.9. Other Resistance Mechanisms and Therapeutic Opportunities
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-Cell Lung Cancer. Nat. Rev. Dis. Prim. 2021, 7, 3. [Google Scholar] [CrossRef]
- Pesch, B.; Kendzia, B.; Gustavsson, P.; Jöckel, K.-H.; Johnen, G.; Pohlabeln, H.; Olsson, A.; Ahrens, W.; Gross, I.M.; Brüske, I.; et al. Cigarette Smoking and Lung Cancer—Relative Risk Estimates for the Major Histological Types from a Pooled Analysis of Case–Control Studies. Int. J. Cancer 2012, 131, 1210–1219. [Google Scholar] [CrossRef]
- The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Stahel, R.A.; Ginsberg, R.; Havemann, K.; Hirsch, F.R.; Ihde, D.C.; Jassem, J.; Karrer, K.; Herbert Maurer, L.; Osterlind, K.; Van Houtte, P. Staging and Prognostic Factors in Small Cell Lung Cancer: A Consensus Report. Lung Cancer 1989, 5, 119–126. [Google Scholar] [CrossRef]
- Peifer, M.; Fernández-Cuesta, L.; Sos, M.L.; George, J.; Seidel, D.; Kasper, L.H.; Plenker, D.; Leenders, F.; Sun, R.; Zander, T.; et al. Integrative Genome Analyses Identify Key Somatic Driver Mutations of Small-Cell Lung Cancer. Nat. Genet. 2012, 44, 1104–1110. [Google Scholar] [CrossRef]
- Rudin, C.M.; Durinck, S.; Stawiski, E.W.; Poirier, J.T.; Modrusan, Z.; Shames, D.S.; Bergbower, E.A.; Guan, Y.; Shin, J.; Guillory, J.; et al. Comprehensive Genomic Analysis Identifies SOX2 as a Frequently Amplified Gene in Small-Cell Lung Cancer. Nat. Genet. 2012, 44, 1111–1116. [Google Scholar] [CrossRef]
- Umemura, S.; Mimaki, S.; Makinoshima, H.; Tada, S.; Ishii, G.; Ohmatsu, H.; Niho, S.; Yoh, K.; Matsumoto, S.; Takahashi, A.; et al. Therapeutic Priority of the PI3K/AKT/MTOR Pathway in Small Cell Lung Cancers as Revealed by a Comprehensive Genomic Analysis. J. Thorac. Oncol. 2014, 9, 1324–1331. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Meuwissen, R.; Linn, S.C.; Linnoila, R.I.; Zevenhoven, J.; Mooi, W.J.; Berns, A. Induction of Small Cell Lung Cancer by Somatic Inactivation of Both Trp53 and Rb1 in a Conditional Mouse Model. Cancer Cell 2003, 4, 181–189. [Google Scholar] [CrossRef]
- Udagawa, H.; Umemura, S.; Murakami, I.; Mimaki, S.; Makinoshima, H.; Ishii, G.; Miyoshi, T.; Kirita, K.; Matsumoto, S.; Yoh, K.; et al. Genetic Profiling-Based Prognostic Prediction of Patients with Advanced Small-Cell Lung Cancer in Large Scale Analysis. Lung Cancer 2018, 126, 182–188. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Bonneville, R.; Paruchuri, A.; Reeser, J.W.; Wing, M.R.; Samorodnitsky, E.; Krook, M.A.; Smith, A.M.; Dao, T.; Miya, J.; et al. Genomic and Transcriptomic Characterization of Relapsed SCLC Through Rapid Research Autopsy. JTO Clin. Res. Rep. 2021, 2, 100164. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Gay, C.M.; Xi, Y.; Sivajothi, S.; Sivakamasundari, V.; Fujimoto, J.; Bolisetty, M.; Hartsfield, P.M.; Balasubramaniyan, V.; Chalishazar, M.D.; et al. Single-Cell Analyses Reveal Increased Intratumoral Heterogeneity after the Onset of Therapy Resistance in Small-Cell Lung Cancer. Nat. Cancer 2020, 1, 423–436. [Google Scholar] [CrossRef]
- George, J.; Maas, L.; Abedpour, N.; Cartolano, M.; Kaiser, L.; Fischer, R.N.; Scheel, A.H.; Weber, J.-P.; Hellmich, M.; Bosco, G.; et al. Evolutionary Trajectories of Small Cell Lung Cancer under Therapy. Nature 2024, 627, 880–889. [Google Scholar] [CrossRef]
- Baine, M.K.; Hsieh, M.-S.; Lai, W.V.; Egger, J.V.; Jungbluth, A.A.; Daneshbod, Y.; Beras, A.; Spencer, R.; Lopardo, J.; Bodd, F.; et al. SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A Comprehensive Immunohistochemical and Histopathologic Characterization. J. Thorac. Oncol. 2020, 15, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular Subtypes of Small Cell Lung Cancer: A Synthesis of Human and Mouse Model Data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef]
- Heeke, S.; Gay, C.M.; Estecio, M.R.; Tran, H.; Morris, B.B.; Zhang, B.; Tang, X.; Raso, M.G.; Rocha, P.; Lai, S.; et al. Tumor- and Circulating-Free DNA Methylation Identifies Clinically Relevant Small Cell Lung Cancer Subtypes. Cancer Cell 2024, 42, 225–237. [Google Scholar] [CrossRef]
- Mollaoglu, G.; Guthrie, M.R.; Böhm, S.; Brägelmann, J.; Can, I.; Ballieu, P.M.; Marx, A.; George, J.; Heinen, C.; Chalishazar, M.D.; et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition. Cancer Cell 2017, 31, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Ireland, A.S.; Micinski, A.M.; Kastner, D.W.; Guo, B.; Wait, S.J.; Spainhower, K.B.; Conley, C.C.; Chen, O.S.; Guthrie, M.R.; Soltero, D.; et al. MYC Drives Temporal Evolution of Small Cell Lung Cancer Subtypes by Reprogramming Neuroendocrine Fate. Cancer Cell 2020, 38, 60–78. [Google Scholar] [CrossRef]
- Borromeo, M.D.; Savage, T.K.; Kollipara, R.K.; He, M.; Augustyn, A.; Osborne, J.K.; Girard, L.; Minna, J.D.; Gazdar, A.F.; Cobb, M.H.; et al. ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs. Cell Rep. 2016, 16, 1259–1272. [Google Scholar] [CrossRef]
- Lim, J.S.; Ibaseta, A.; Fischer, M.M.; Cancilla, B.; O’Young, G.; Cristea, S.; Luca, V.C.; Yang, D.; Jahchan, N.S.; Hamard, C.; et al. Intratumoural Heterogeneity Generated by Notch Signalling Promotes Small-Cell Lung Cancer. Nature 2017, 545, 360–364. [Google Scholar] [CrossRef]
- Shue, Y.T.; Drainas, A.P.; Li, N.Y.; Pearsall, S.M.; Morgan, D.; Sinnott-Armstrong, N.; Hipkins, S.Q.; Coles, G.L.; Lim, J.S.; Oro, A.E.; et al. A Conserved YAP/Notch/REST Network Controls the Neuroendocrine Cell Fate in the Lungs. Nat. Commun. 2022, 13, 2690. [Google Scholar] [CrossRef]
- Olsen, R.R.; Ireland, A.S.; Kastner, D.W.; Groves, S.M.; Spainhower, K.B.; Pozo, K.; Kelenis, D.P.; Whitney, C.P.; Guthrie, M.R.; Wait, S.J.; et al. ASCL1 Represses a SOX9(+) Neural Crest Stem-like State in Small Cell Lung Cancer. Genes. Dev. 2021, 35, 847–869. [Google Scholar] [CrossRef]
- Wu, Q.; Guo, J.; Liu, Y.; Zheng, Q.; Li, X.; Wu, C.; Fang, D.; Chen, X.; Ma, L.; Xu, P.; et al. YAP Drives Fate Conversion and Chemoresistance of Small Cell Lung Cancer. Sci. Adv. 2021, 7, eabg1850. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Klingbeil, O.; He, X.-Y.; Wu, X.S.; Arun, G.; Lu, B.; Somerville, T.D.D.; Milazzo, J.P.; Wilkinson, J.E.; Demerdash, O.E.; et al. POU2F3 Is a Master Regulator of a Tuft Cell-like Variant of Small Cell Lung Cancer. Genes. Dev. 2018, 32, 915–928. [Google Scholar] [CrossRef]
- Pearsall, S.M.; Humphrey, S.; Revill, M.; Morgan, D.; Frese, K.K.; Galvin, M.; Kerr, A.; Carter, M.; Priest, L.; Blackhall, F.; et al. The Rare YAP1 Subtype of SCLC Revisited in a Biobank of 39 Circulating Tumor Cell Patient Derived Explant Models: A Brief Report. J. Thorac. Oncol. 2020, 15, 1836–1843. [Google Scholar] [CrossRef]
- Sonkin, D.; Thomas, A.; Teicher, B.A. Are Neuroendocrine Negative Small Cell Lung Cancer and Large Cell Neuroendocrine Carcinoma with WT RB1 Two Faces of the Same Entity? Lung Cancer Manag. 2019, 8, LMT13. [Google Scholar] [CrossRef]
- Ng, J.; Cai, L.; Girard, L.; Prall, O.W.J.; Rajan, N.; Khoo, C.; Batrouney, A.; Byrne, D.J.; Boyd, D.K.; Kersbergen, A.J.; et al. Molecular and Pathologic Characterization of YAP1-Expressing Small Cell Lung Cancer Cell Lines Leads to Reclassification as SMARCA4-Deficient Malignancies. Clin. Cancer Res. 2024, OF1–OF13. [Google Scholar] [CrossRef]
- Gay, C.M.; Stewart, C.A.; Park, E.M.; Diao, L.; Groves, S.M.; Heeke, S.; Nabet, B.Y.; Fujimoto, J.; Solis, L.M.; Lu, W.; et al. Patterns of Transcription Factor Programs and Immune Pathway Activation Define Four Major Subtypes of SCLC with Distinct Therapeutic Vulnerabilities. Cancer Cell 2021, 39, 346–360.e7. [Google Scholar] [CrossRef]
- Nabet, B.Y.; Hamidi, H.; Lee, M.C.; Banchereau, R.; Morris, S.; Adler, L.; Gayevskiy, V.; Elhossiny, A.M.; Srivastava, M.K.; Patil, N.S.; et al. Immune Heterogeneity in Small-Cell Lung Cancer and Vulnerability to Immune Checkpoint Blockade. Cancer Cell 2024, 42, 429–443.e4. [Google Scholar] [CrossRef]
- Johnson, B.E.; Grayson, J.; Makuch, R.W.; Linnoila, R.I.; Anderson, M.J.; Cohen, M.H.; Glatstein, E.; Minna, J.D.; Ihde, D.C. Ten-Year Survival of Patients with Small-Cell Lung Cancer Treated with Combination Chemotherapy with or without Irradiation. J. Clin. Oncol. 1990, 8, 396–401. [Google Scholar] [CrossRef]
- Warde, P.; Payne, D. Does Thoracic Irradiation Improve Survival and Local Control in Limited-Stage Small-Cell Carcinoma of the Lung? A Meta-Analysis. J. Clin. Oncol. 1992, 10, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.-P.; Arriagada, R.; Ihde, D.C.; Johnson, D.H.; Perry, M.C.; Souhami, R.L.; Brodin, O.; Joss, R.A.; Kies, M.S.; Lebeau, B.; et al. A Meta-Analysis of Thoracic Radiotherapy for Small-Cell Lung Cancer. N. Engl. J. Med. 1992, 327, 1618–1624. [Google Scholar] [CrossRef]
- Martucci, N.; Morabito, A.; La Rocca, A.; De Luca, G.; De Cecio, R.; Botti, G.; Totaro, G.; Muto, P.; Picone, C.; Esposito, G.; et al. Surgery in Small-Cell Lung Cancer. Cancers 2021, 13, 390. [Google Scholar] [CrossRef] [PubMed]
- Nugent, J.L.; Bunn, P.A., Jr.; Matthews, M.J.; Ihde, D.C.; Cohen, M.H.; Gazdar, A.; Minna, J.D. CNS Metastases in Small Cell Bronchogenic Carcinoma. Increasing Frequency and Changing Pattern with Lengthening Survival. Cancer 1979, 44, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A.; Arriagada, R.; Pignon, J.-P.; Le Péchoux, C.; Gregor, A.; Stephens, R.J.; Kristjansen, P.E.G.; Johnson, B.E.; Ueoka, H.; Wagner, H.; et al. Prophylactic Cranial Irradiation for Patients with Small-Cell Lung Cancer in Complete Remission. N. Engl. J. Med. 1999, 341, 476–484. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus Platinum–Etoposide versus Platinum–Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Higgins, K.A.; Curran, W.J.; Liu, S.V.; Yu, W.; Brockman, M.; Johnson, A.; Bara, I.; Bradley, J.D. Patterns of Disease Progression after Carboplatin/Etoposide + Atezolizumab in Extensive-Stage Small-Cell Lung Cancer (ES-SCLC). Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1398. [Google Scholar] [CrossRef]
- Chen, Y.; Paz-Ares, L.; Reinmuth, N.; Garassino, M.C.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Verderame, F.; Havel, L.; Losonczy, G.; et al. Impact of Brain Metastases on Treatment Patterns and Outcomes with First-Line Durvalumab Plus Platinum-Etoposide in Extensive-Stage SCLC (CASPIAN): A Brief Report. JTO Clin. Res. Rep. 2022, 3, 100330. [Google Scholar] [CrossRef]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csőszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.-H.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Park, K.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodríguez-Cid, J.; Schenker, M.; et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J. Clin. Oncol. 2021, 39, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Han, L.; Wu, L.; Chen, J.; Sun, H.; Wen, G.; Ji, Y.; Dvorkin, M.; Shi, J.; Pan, Z.; et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients with Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022, 328, 1223–1232. [Google Scholar] [CrossRef]
- Spigel, D.R.; Cheng, Y.; Cho, B.C.; Laktionov, K.K.; Fang, J.; Chen, Y.; Zenke, Y.; Lee, K.H.; Wang, Q.; Navarro, A.; et al. ADRIATIC: Durvalumab (D) as Consolidation Treatment (Tx) for Patients (Pts) with Limited-Stage Small-Cell Lung Cancer (LS-SCLC). J. Clin. Oncol. 2024, 42 (Suppl. S17), LBA5. [Google Scholar] [CrossRef]
- Noda, K.; Nishiwaki, Y.; Kawahara, M.; Negoro, S.; Sugiura, T.; Yokoyama, A.; Fukuoka, M.; Mori, K.; Watanabe, K.; Tamura, T.; et al. Irinotecan plus Cisplatin Compared with Etoposide plus Cisplatin for Extensive Small-Cell Lung Cancer. N. Engl. J. Med. 2002, 346, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Bunn, P.A.; Langer, C.; Einhorn, L.; Guthrie, T.; Beck, T.; Ansari, R.; Ellis, P.; Byrne, M.; Morrison, M.; et al. Randomized Phase III Trial Comparing Irinotecan/Cisplatin with Etoposide/Cisplatin in Patients with Previously Untreated Extensive-Stage Disease Small-Cell Lung Cancer. J. Clin. Oncol. 2006, 24, 2038–2043. [Google Scholar] [CrossRef]
- Lara, P.N.; Natale, R.; Crowley, J.; Lenz, H.J.; Redman, M.W.; Carleton, J.E.; Jett, J.; Langer, C.J.; Kuebler, J.P.; Dakhil, S.R.; et al. Phase III Trial of Irinotecan/Cisplatin Compared with Etoposide/Cisplatin in Extensive-Stage Small-Cell Lung Cancer: Clinical and Pharmacogenomic Results from SWOG S0124. J. Clin. Oncol. 2009, 27, 2530–2535. [Google Scholar] [CrossRef]
- Slotman, B.J.; van Tinteren, H.; Praag, J.O.; Knegjens, J.L.; El Sharouni, S.Y.; Hatton, M.; Keijser, A.; Faivre-Finn, C.; Senan, S. Use of Thoracic Radiotherapy for Extensive Stage Small-Cell Lung Cancer: A Phase 3 Randomised Controlled Trial. Lancet 2015, 385, 36–42. [Google Scholar] [CrossRef]
- Slotman, B.; Faivre-Finn, C.; Kramer, G.; Rankin, E.; Snee, M.; Hatton, M.; Postmus, P.; Collette, L.; Musat, E.; Senan, S. Prophylactic Cranial Irradiation in Extensive Small-Cell Lung Cancer. N. Engl. J. Med. 2007, 357, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamanaka, T.; Seto, T.; Harada, H.; Nokihara, H.; Saka, H.; Nishio, M.; Kaneda, H.; Takayama, K.; Ishimoto, O.; et al. Prophylactic Cranial Irradiation versus Observation in Patients with Extensive-Disease Small-Cell Lung Cancer: A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 663–671. [Google Scholar] [CrossRef]
- Lattuca-Truc, M.; Timsit, J.-F.; Levra, M.G.; Ruckly, S.; Villa, J.; Dumas, I.; Pinsolle, J.; Ferrer, L.; Guillem, P.; Moro-Sibilot, D.; et al. Trends in Response Rate and Survival in Small-Cell Lung Cancer Patients between 1997 and 2017. Lung Cancer 2019, 131, 122–127. [Google Scholar] [CrossRef]
- Karacz, C.M.; Yan, J.; Zhu, H.; Gerber, D.E. Timing, Sites, and Correlates of Lung Cancer Recurrence. Clin. Lung Cancer 2020, 21, 127–135.e3. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.R.; Ciuleanu, T.-E.; Tsekov, H.; Shparyk, Y.; Čučeviá, B.; Juhasz, G.; Thatcher, N.; Ross, G.A.; Dane, G.C.; Crofts, T. Phase III Trial Comparing Supportive Care Alone with Supportive Care With Oral Topotecan in Patients With Relapsed Small-Cell Lung Cancer. J. Clin. Oncol. 2006, 24, 5441–5447. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.J.; Johnson, D.H.; Einhorn, L.H.; Schacter, L.P.; Cherng, N.C.; Cohen, H.J.; Crawford, J.; Randolph, J.A.; Goodlow, J.L.; Broun, G.O. Randomized Study of Cyclophosphamide, Doxorubicin, and Vincristine versus Etoposide and Cisplatin versus Alternation of These Two Regimens in Extensive Small-Cell Lung Cancer: A Phase III Trial of the Southeastern Cancer Study Group. J. Clin. Oncol. 1992, 10, 282–291. [Google Scholar] [CrossRef] [PubMed]
- von Pawel, J.; Schiller, J.H.; Shepherd, F.A.; Fields, S.Z.; Kleisbauer, J.P.; Chrysson, N.G.; Stewart, D.J.; Clark, P.I.; Palmer, M.C.; Depierre, A.; et al. Topotecan Versus Cyclophosphamide, Doxorubicin, and Vincristine for the Treatment of Recurrent Small-Cell Lung Cancer. J. Clin. Oncol. 1999, 17, 658. [Google Scholar] [CrossRef]
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; López, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernández, C.; Alfaro, V.; et al. Lurbinectedin as Second-Line Treatment for Patients with Small-Cell Lung Cancer: A Single-Arm, Open-Label, Phase 2 Basket Trial. Lancet Oncol. 2020, 21, 645–654. [Google Scholar] [CrossRef]
- Myung-Ju, A.; Chul, C.B.; Enriqueta, F.; Ippokratis, K.; Kadoaki, O.; Margarita, M.; Oscar, J.-V.; Sabin, H.; Hiroki, I.; Jong-Seok, L.; et al. Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 2063–2075. [Google Scholar] [CrossRef]
- Besse, B.; Paz-Ares, L.G.; Peters, S.; Cappuzzo, F.; Reck, M.; Calles, A.; Califano, R.; Lopez-Vilariño, J.A.; Veramendi, S.; Kahatt, C.M.; et al. A Phase III Study of Lurbinectedin Alone or in Combination with Irinotecan vs Investigator’s Choice (Topotecan or Irinotecan) in Patients with Relapsed Small Cell Lung Cancer (SCLC; LAGOON Trial). J. Clin. Oncol. 2023, 41 (Suppl. S16), TPS8613. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Reck, M.; Peters, S.; Borghaei, H.; Herbst, R.; Siddiqui, M.; Cuchelkar, V.; Bhatt, K.; Chakrabarti, D.; Wang, L.; et al. EP14.01-015 IMforte: A Phase III Study of Lurbinectedin and Atezolizumab Versus Atezolizumab as Maintenance Therapy in ES-SCLC. J. Thorac. Oncol. 2022, 17, S532–S533. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Felip, E.; Ahn, M.-J.; Blackhall, F.H.; Borghaei, H.; Cho, B.C.; Johnson, M.L.; Ramalingam, S.S.; Reck, M.; Zhang, A.; et al. Randomized Phase 3 Study of Tarlatamab, a DLL3-Targeting Bispecific T-Cell Engager (BiTE), Compared to Standard of Care in Patients with Relapsed Small Cell Lung Cancer (DeLLphi-304). J. Clin. Oncol. 2023, 41 (Suppl. S16), TPS8611. [Google Scholar] [CrossRef]
- Baize, N.; Monnet, I.; Greillier, L.; Geier, M.; Lena, H.; Janicot, H.; Vergnenegre, A.; Crequit, J.; Lamy, R.; Auliac, J.-B.; et al. Carboplatin plus Etoposide versus Topotecan as Second-Line Treatment for Patients with Sensitive Relapsed Small-Cell Lung Cancer: An Open-Label, Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2020, 21, 1224–1233. [Google Scholar] [CrossRef]
- Goto, K.; Ohe, Y.; Shibata, T.; Seto, T.; Takahashi, T.; Nakagawa, K.; Tanaka, H.; Takeda, K.; Nishio, M.; Mori, K.; et al. Combined Chemotherapy with Cisplatin, Etoposide, and Irinotecan versus Topotecan Alone as Second-Line Treatment for Patients with Sensitive Relapsed Small-Cell Lung Cancer (JCOG0605): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2016, 17, 1147–1157. [Google Scholar] [CrossRef]
- Masuda, N.; Fukuoka, M.; Kusunoki, Y.; Matsui, K.; Takifuji, N.; Kudoh, S.; Negoro, S.; Nishioka, M.; Nakagawa, K.; Takada, M. CPT-11: A New Derivative of Camptothecin for the Treatment of Refractory or Relapsed Small-Cell Lung Cancer. J. Clin. Oncol. 1992, 10, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.F.; Fokkema, E.; Biesma, B.; Groen, H.J.; Snoek, W.; Postmus, P.E. A Phase II Study of Paclitaxel in Heavily Pretreated Patients with Small-Cell Lung Cancer. Br. J. Cancer 1998, 77, 347–351. [Google Scholar] [CrossRef]
- Yamamoto, N.; Tsurutani, J.; Yoshimura, N.; Asai, G.Y.O.; Moriyama, A.; Nakagawa, K.; Kudoh, S.; Takada, M.; Minato, Y.; Fukuoka, M. Phase II Study of Weekly Paclitaxel for Relapsed and Refractory Small Cell Lung Cancer. Anticancer Res. 2006, 26, 777–781. [Google Scholar]
- Pietanza, M.C.; Kadota, K.; Huberman, K.; Sima, C.S.; Fiore, J.J.; Sumner, D.K.; Travis, W.D.; Heguy, A.; Ginsberg, M.S.; Holodny, A.I.; et al. Phase II Trial of Temozolomide in Patients with Relapsed Sensitive or Refractory Small Cell Lung Cancer, with Assessment of Methylguanine-DNA Methyltransferase as a Potential Biomarker. Clin. Cancer Res. 2012, 18, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- von Pawel, J.; Jotte, R.; Spigel, D.R.; O’Brien, M.E.R.; Socinski, M.A.; Mezger, J.; Steins, M.; Bosquée, L.; Bubis, J.; Nackaerts, K.; et al. Randomized Phase III Trial of Amrubicin Versus Topotecan as Second-Line Treatment for Patients with Small-Cell Lung Cancer. J. Clin. Oncol. 2014, 32, 4012–4019. [Google Scholar] [CrossRef]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab Alone and Nivolumab plus Ipilimumab in Recurrent Small-Cell Lung Cancer (CheckMate 032): A Multicentre, Open-Label, Phase 1/2 Trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Piha-Paul, S.A.; Lopez-Martin, J.; Schellens, J.H.M.; Kao, S.; Miller, W.H.; Delord, J.-P.; Gao, B.; Planchard, D.; Gottfried, M.; et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients with Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies. J. Thorac. Oncol. 2020, 15, 618–627. [Google Scholar] [CrossRef]
- Spigel, D.R.; Vicente, D.; Ciuleanu, T.E.; Gettinger, S.; Peters, S.; Horn, L.; Audigier-Valette, C.; Pardo Aranda, N.; Juan-Vidal, O.; Cheng, Y.; et al. Second-Line Nivolumab in Relapsed Small-Cell Lung Cancer: CheckMate 331. Ann. Oncol. 2021, 32, 631–641. [Google Scholar] [CrossRef]
- Daniel, D.B.; Rudin, C.M.; Hart, L.; Spigel, D.R.; Edelman, M.J.; Goldschmidt, J.; Bordoni, R.; Glisson, B.; Burns, T.F.; Dowlati, A.; et al. Results of a Randomized, Placebo-Controlled, Phase 2 Study of Tarextumab (TRXT, Anti-Notch2/3) in Combination with Etoposide and Platinum (EP) in Patients (Pts) with Untreated Extensive-Stage Small-Cell Lung Cancer (ED-SCLC). Ann. Oncol. 2017, 28 (Suppl. S5), v540. [Google Scholar] [CrossRef]
- Melichar, B.; Adenis, A.; Lockhart, A.C.; Bennouna, J.; Dees, E.C.; Kayaleh, O.; Obermannova, R.; DeMichele, A.; Zatloukal, P.; Zhang, B.; et al. Safety and Activity of Alisertib, an Investigational Aurora Kinase A Inhibitor, in Patients with Breast Cancer, Small-Cell Lung Cancer, Non-Small-Cell Lung Cancer, Head and Neck Squamous-Cell Carcinoma, and Gastro-Oesophageal Adenocarcinoma: A Five-Arm Ph. Lancet Oncol. 2015, 16, 395–405. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Niu, H.; Nackaerts, K.; Csoszi, T.; Ostoros, G.; Mark, Z.; Baik, C.; Joy, A.A.; Chouaid, C.; Jaime, J.C.; et al. Randomized Phase II Study of Paclitaxel plus Alisertib versus Paclitaxel plus Placebo as Second-Line Therapy for SCLC: Primary and Correlative Biomarker Analyses. J. Thorac. Oncol. 2020, 15, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shim, J.; Mortimer, P.G.S.; Smith, S.A.; Godin, R.E.; Hollingsworth, S.J.; Kim, H.-J.; Jung, H.A.; Sun, J.-M.; Park, W.-Y.; et al. Biomarker-Driven Phase 2 Umbrella Trial Study for Patients with Recurrent Small Cell Lung Cancer Failing Platinum-Based Chemotherapy. Cancer 2020, 126, 4002–4012. [Google Scholar] [CrossRef] [PubMed]
- Pietanza, M.C.; Litvak, A.M.; Varghese, A.M.; Krug, L.M.; Fleisher, M.; Teitcher, J.B.; Holodny, A.I.; Sima, C.S.; Woo, K.M.; Ng, K.K.; et al. A Phase I Trial of the Hedgehog Inhibitor, Sonidegib (LDE225), in Combination with Etoposide and Cisplatin for the Initial Treatment of Extensive Stage Small Cell Lung Cancer. Lung Cancer 2016, 99, 23–30. [Google Scholar] [CrossRef]
- Belani, C.P.; Dahlberg, S.E.; Rudin, C.M.; Fleisher, M.; Chen, H.X.; Takebe, N.; Ramalingam, S.S.; Schiller, J.H. Three-Arm Randomized Phase II Study of Cisplatin and Etoposide (CE) versus CE with Either Vismodegib (V) or Cixutumumab (Cx) for Patients with Extensive Stage-Small Cell Lung Cancer (ES-SCLC) (ECOG 1508). J. Clin. Oncol. 2013, 31 (Suppl. S15), 7508. [Google Scholar] [CrossRef]
- Pandya, K.J.; Dahlberg, S.; Hidalgo, M.; Cohen, R.B.; Lee, M.W.; Schiller, J.H.; Johnson, D.H. A Randomized, Phase II Trial of Two Dose Levels of Temsirolimus (CCI-779) in Patients with Extensive-Stage Small-Cell Lung Cancer Who Have Responding or Stable Disease after Induction Chemotherapy: A Trial of the Eastern Cooperative Oncology Group (E1500). J. Thorac. Oncol. 2007, 2, 1036–1041. [Google Scholar] [CrossRef]
- Tarhini, A.; Kotsakis, A.; Gooding, W.; Shuai, Y.; Petro, D.; Friedland, D.; Belani, C.P.; Dacic, S.; Argiris, A. Phase II Study of Everolimus (RAD001) in Previously Treated Small Cell Lung Cancer. Clin. Cancer Res. 2010, 16, 5900–5907. [Google Scholar] [CrossRef]
- Sun, J.M.; Kim, J.R.; Do, I.G.; Lee, S.Y.; Lee, J.; Choi, Y.L.; Ahn, J.S.; Ahn, M.J.; Park, K. A Phase-1b Study of Everolimus plus Paclitaxel in Patients with Small-Cell Lung Cancer. Br. J. Cancer 2013, 109, 1482–1487. [Google Scholar] [CrossRef][Green Version]
- Thomas, A.; Takahashi, N.; Rajapakse, V.N.; Zhang, X.; Sun, Y.; Ceribelli, M.; Wilson, K.M.; Zhang, Y.; Beck, E.; Sciuto, L.; et al. Therapeutic Targeting of ATR Yields Durable Regressions in Small Cell Lung Cancers with High Replication Stress. Cancer Cell 2021, 39, 566–579.e7. [Google Scholar] [CrossRef]
- Byers, L.A.; Navarro, A.; Schaefer, E.; Johnson, M.; Özgüroğlu, M.; Han, J.-Y.; Bondarenko, I.; Cicin, I.; Dragnev, K.H.; Abel, A.; et al. A Phase II Trial of Prexasertib (LY2606368) in Patients with Extensive-Stage Small-Cell Lung Cancer. Clin. Lung Cancer 2021, 22, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Moore, K.N.; Rader, J.S.; Simpkins, F.; Mita, A.C.; Beck, J.T.; Hart, L.; Chu, Q.; Oza, A.; Tinker, A.V.; et al. A Phase Ib Study Assessing the Safety, Tolerability, and Efficacy of the First-in-Class Wee1 Inhibitor Adavosertib (AZD1775) as Monotherapy in Patients with Advanced Solid Tumors. Target. Oncol. 2023, 18, 517–530. [Google Scholar] [CrossRef]
- von Pawel, J.; Vynnychenko, I.; Jiang, H.; Huang, Y.; Dennis, P.A. A Phase II, Open-Label, Multi-Arm Study of Novel Combinations of Immunotherapies or DDR Inhibitors in Platinum-Refractory, Extensive Disease Small-Cell Lung Cancer (ED-SCLC): BALTIC. J. Clin. Oncol. 2017, 35 (Suppl. S15), TPS8585. [Google Scholar] [CrossRef]
- Mita, M.; Gordon, M.; Rosen, L.; Kapoor, N.; Choy, G.; Redkar, S.; Taverna, P.; Oganesian, A.; Sahai, A.; Azab, M.; et al. Phase 1B Study of Amuvatinib in Combination with Five Standard Cancer Therapies in Adults with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2014, 74, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Byers, L.A.; Horn, L.; Ghandi, J.; Kloecker, G.; Owonikoko, T.; Naheed Waqar, S.; Krzakowski, M.; Cardnell, R.J.; Fujimoto, J.; Taverna, P.; et al. A Phase 2, Open-Label, Multi-Center Study of Amuvatinib in Combination with Platinum Etoposide Chemotherapy in Platinum-Refractory Small Cell Lung Cancer Patients. Oncotarget 2017, 8, 81441–81454. [Google Scholar] [CrossRef]
- de Bono, J.; Ramanathan, R.K.; Mina, L.; Chugh, R.; Glaspy, J.; Rafii, S.; Kaye, S.; Sachdev, J.; Heymach, J.; Smith, D.C.; et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov. 2017, 7, 620–629. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.J.; Min, Y.J.; Mortimer, P.G.S.; Kim, H.-J.; Smith, S.A.; Dean, E.; Jung, H.A.; Sun, J.-M.; Park, W.-Y.; et al. Biomarker-Driven Phase 2 Umbrella Trial: Clinical Efficacy of Olaparib Monotherapy and Combination with Ceralasertib (AZD6738) in Small Cell Lung Cancer. Cancer 2024, 130, 541–552. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Dahlberg, S.E.; Sica, G.L.; Wagner, L.I.; Wade, J.L.; Srkalovic, G.; Lash, B.W.; Leach, J.W.; Leal, T.B.; Aggarwal, C.; et al. Randomized Phase II Trial of Cisplatin and Etoposide in Combination with Veliparib or Placebo for Extensive-Stage Small-Cell Lung Cancer: ECOG-ACRIN 2511 Study. J. Clin. Oncol. 2018, 37, 222–229. [Google Scholar] [CrossRef]
- Byers, L.A.; Bentsion, D.; Gans, S.; Penkov, K.; Son, C.; Sibille, A.; Owonikoko, T.K.; Groen, H.J.M.; Gay, C.M.; Fujimoto, J.; et al. Veliparib in Combination with Carboplatin and Etoposide in Patients with Treatment-Naïve Extensive-Stage Small Cell Lung Cancer: A Phase 2 Randomized Study. Clin. Cancer Res. 2021, 27, 3884–3895. [Google Scholar] [CrossRef]
- Chiappori, A.A.; Otterson, G.A.; Dowlati, A.; Traynor, A.M.; Horn, L.; Owonikoko, T.K.; Ross, H.J.; Hann, C.L.; Abu Hejleh, T.; Nieva, J.; et al. A Randomized Phase II Study of Linsitinib (OSI-906) Versus Topotecan in Patients with Relapsed Small-Cell Lung Cancer. Oncologist 2016, 21, 1163–1164. [Google Scholar] [CrossRef]
- Lara, P.N.; Chansky, K.; Davies, A.M.; Franklin, W.A.; Gumerlock, P.H.; Guaglianone, P.P.; Atkins, J.N.; Farneth, N.; Mack, P.C.; Crowley, J.J.; et al. Bortezomib (PS-341) in Relapsed or Refractory Extensive Stage Small Cell Lung Cancer: A Southwest Oncology Group Phase II Trial (S0327). J. Thorac. Oncol. 2006, 1, 996–1001. [Google Scholar] [CrossRef]
- Baggstrom, M.Q.; Qi, Y.; Koczywas, M.; Argiris, A.; Johnson, E.A.; Millward, M.J.; Murphy, S.C.; Erlichman, C.; Rudin, C.M.; Govindan, R. A Phase II Study of AT-101 (Gossypol) in Chemotherapy-Sensitive Recurrent Extensive-Stage Small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Hann, C.L.; Garon, E.B.; Ribeiro de Oliveira, M.; Bonomi, P.D.; Camidge, D.R.; Chu, Q.; Giaccone, G.; Khaira, D.; Ramalingam, S.S.; et al. Phase II Study of Single-Agent Navitoclax (ABT-263) and Biomarker Correlates in Patients with Relapsed Small Cell Lung Cancer. Clin. Cancer Res. 2012, 18, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Suk Heist, R.; Fain, J.; Chinnasami, B.; Khan, W.; Molina, J.R.; Sequist, L.V.; Temel, J.S.; Fidias, P.; Brainerd, V.; Leopold, L.; et al. Phase I/II Study of AT-101 with Topotecan in Relapsed and Refractory Small Cell Lung Cancer. J. Thorac. Oncol. 2010, 5, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Rudin, C.M.; Pietanza, M.C.; Brown, A.; Rizvi, N.A.; Takebe, N.; Travis, W.; James, L.; Ginsberg, M.S.; Juergens, R.; et al. A Phase II Study of Obatoclax Mesylate, a Bcl-2 Antagonist, plus Topotecan in Relapsed Small Cell Lung Cancer. Lung Cancer 2011, 74, 481–485. [Google Scholar] [CrossRef]
- Rudin, C.M.; Salgia, R.; Wang, X.; Hodgson, L.D.; Masters, G.A.; Green, M.; Vokes, E.E. Randomized Phase II Study of Carboplatin and Etoposide with or Without the Bcl-2 Antisense Oligonucleotide Oblimersen for Extensive-Stage Small-Cell Lung Cancer: CALGB 30103. J. Clin. Oncol. 2008, 26, 870–876. [Google Scholar] [CrossRef]
- Langer, C.J.; Albert, I.; Ross, H.J.; Kovacs, P.; Blakely, L.J.; Pajkos, G.; Somfay, A.; Zatloukal, P.; Kazarnowicz, A.; Moezi, M.M.; et al. Randomized Phase II Study of Carboplatin and Etoposide with or without Obatoclax Mesylate in Extensive-Stage Small Cell Lung Cancer. Lung Cancer 2014, 85, 420–428. [Google Scholar] [CrossRef]
- Schelman, W.R.; Mohammed, T.A.; Traynor, A.M.; Kolesar, J.M.; Marnocha, R.M.; Eickhoff, J.; Keppen, M.; Alberti, D.B.; Wilding, G.; Takebe, N.; et al. A Phase I Study of AT-101 with Cisplatin and Etoposide in Patients with Advanced Solid Tumors with an Expanded Cohort in Extensive-Stage Small Cell Lung Cancer. Investig. New Drugs 2014, 32, 295–302. [Google Scholar] [CrossRef]
- Qin, A.; Kalemkerian, G.P.; Mohindra, N.A.; Patel, J.D.; Karapetis, C.S.; Carlisle, J.W.; Sands, J.; Spira, A.I.; Gao, B.; Amin, H.; et al. First-in-Human Study of Pelcitoclax (APG-1252) in Combination with Paclitaxel in Patients (Pts) with Relapsed/Refractory Small-Cell Lung Cancer (R/R SCLC). J. Clin. Oncol. 2022, 40 (Suppl. S16), e20612. [Google Scholar] [CrossRef]
- Lai, C.-H.; Park, K.-S.; Lee, D.-H.; Alberobello, A.T.; Raffeld, M.; Pierobon, M.; Pin, E.; Petricoin III, E.F.; Wang, Y.; Giaccone, G. HSP-90 Inhibitor Ganetespib Is Synergistic with Doxorubicin in Small Cell Lung Cancer. Oncogene 2014, 33, 4867–4876. [Google Scholar] [CrossRef]
- Gutierrez, M.; Guo, R.; Giaccone, G.; Liu, S.V.; Hao, Z.; Hilton, C.; Hinson, J.M.; Kris, M.G.; Orlemans, E.O.; Drilon, A. Phase 1 Multicenter Study of the HSP90 Inhibitor SNX-5422 plus Carboplatin and Paclitaxel in Patients with Lung Cancers. Lung Cancer 2021, 162, 23–28. [Google Scholar] [CrossRef]
- Hall, P.E.; Ready, N.; Johnston, A.; Bomalaski, J.S.; Venhaus, R.R.; Sheaff, M.; Krug, L.; Szlosarek, P.W. Phase II Study of Arginine Deprivation Therapy with Pegargiminase in Patients with Relapsed Sensitive or Refractory Small-Cell Lung Cancer. Clin. Lung Cancer 2020, 21, 527–533. [Google Scholar] [CrossRef]
- Han, J.-Y.; Lim, K.Y.; Yu, S.Y.; Yun, T.; Kim, H.T.; Lee, J.S. A Phase 2 Study of Irinotecan, Cisplatin, and Simvastatin for Untreated Extensive-Disease Small Cell Lung Cancer. Cancer 2011, 117, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Seckl, M.J.; Ottensmeier, C.H.; Cullen, M.; Schmid, P.; Ngai, Y.; Muthukumar, D.; Thompson, J.; Harden, S.; Middleton, G.; Fife, K.M.; et al. Multicenter, Phase III, Randomized, Double-Blind, Placebo-Controlled Trial of Pravastatin Added to First-Line Standard Chemotherapy in Small-Cell Lung Cancer (LUNGSTAR). J. Clin. Oncol. 2017, 35, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.-H.; Lee, G.K.; Lim, E.J.; Han, J.-Y. A Randomized Phase II Study of Irinotecan Plus Cisplatin with or without Simvastatin in Ever-Smokers with Extended Disease Small Cell Lung Cancer. Cancer Res. Treat. 2023, 55, 885–893. [Google Scholar] [CrossRef]
- Gandhi, L.; Harding, M.W.; Neubauer, M.; Langer, C.J.; Moore, M.; Ross, H.J.; Johnson, B.E.; Lynch, T.J. A Phase II Study of the Safety and Efficacy of the Multidrug Resistance Inhibitor VX-710 Combined with Doxorubicin and Vincristine in Patients with Recurrent Small Cell Lung Cancer. Cancer 2007, 109, 924–932. [Google Scholar] [CrossRef]
- Schrump, D.S.; Fischette, M.R.; Nguyen, D.M.; Zhao, M.; Li, X.; Kunst, T.F.; Hancox, A.; Hong, J.A.; Chen, G.A.; Kruchin, E.; et al. Clinical and Molecular Responses in Lung Cancer Patients Receiving Romidepsin. Clin. Cancer Res. 2008, 14, 188–198. [Google Scholar] [CrossRef]
- Otterson, G.A.; Hodgson, L.; Pang, H.; Vokes, E.E. Phase II Study of the Histone Deacetylase Inhibitor Romidepsin in Relapsed Small Cell Lung Cancer (Cancer and Leukemia Group B 30304). J. Thorac. Oncol. 2010, 5, 1644–1648. [Google Scholar] [CrossRef]
- de Marinis, F.; Atmaca, A.; Tiseo, M.; Giuffreda, L.; Rossi, A.; Gebbia, V.; Antonio, C.D.; Zotto, L.D.; Al-Batran, S.-E.; Marsoni, S.; et al. A Phase II Study of the Histone Deacetylase Inhibitor Panobinostat (LBH589) in Pretreated Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 1091–1094. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Zahoor, H.; McLaughlin, B.; Gooding, W.E.; Schmitz, J.C.; Siegfried, J.M.; Socinski, M.A.; Argiris, A. Phase I Trial of Carboplatin and Etoposide in Combination with Panobinostat in Patients with Lung Cancer. Anticancer Res. 2013, 33, 4475–4481. [Google Scholar]
- Gentzler, R.D.; Villaruz, L.C.; Rhee, J.C.; Horton, B.; Mock, J.; Hanley, M.; Kim, K.; Rudek, M.A.; Phelps, M.A.; Carducci, M.A.; et al. Phase I Study of Entinostat, Atezolizumab, Carboplatin, and Etoposide in Previously Untreated Extensive-Stage Small Cell Lung Cancer, ETCTN 10399. Oncologist 2023, 28, 1007–e1107. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Redon, C.E.; Peer, C.J.; Bryla, C.; Lee, M.-J.; Trepel, J.B.; Tomita, Y.; Rajan, A.; Giaccone, G.; Bonner, W.M.; et al. Phase I Trial of Belinostat with Cisplatin and Etoposide in Advanced Solid Tumors, with a Focus on Neuroendocrine and Small Cell Cancers of the Lung. Anticancer Drugs 2018, 29, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Besse, B.; Martinez-Marti, A.; Trigo, J.M.; Moreno, V.; Garrido, P.; Ferron-Brady, G.; Wu, Y.; Park, J.; Collingwood, T.; et al. Phase I, Open-Label, Dose-Escalation Study of the Safety, Pharmacokinetics, Pharmacodynamics, and Efficacy of GSK2879552 in Relapsed/Refractory SCLC. J. Thorac. Oncol. 2019, 14, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Niell, H.B.; Herndon, J.E.; Miller, A.A.; Watson, D.M.; Sandler, A.B.; Kelly, K.; Marks, R.S.; Perry, M.C.; Ansari, R.H.; Otterson, G.; et al. Randomized Phase III Intergroup Trial of Etoposide and Cisplatin with or Without Paclitaxel and Granulocyte Colony-Stimulating Factor in Patients With Extensive-Stage Small-Cell Lung Cancer: Cancer and Leukemia Group B Trial 9732. J. Clin. Oncol. 2005, 23, 3752–3759. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sun, Y.; Lei, Y.; Yang, K.; Tang, R. YAP1 Promotes Multidrug Resistance of Small Cell Lung Cancer by CD74-Related Signaling Pathways. Cancer Med. 2020, 9, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Ouyang, S.; Chen, R.; Huang, J.; Guo, L. MYCN-Mediated Regulation of the HES1 Promoter Enhances the Chemoresistance of Small-Cell Lung Cancer by Modulating Apoptosis. Am. J. Cancer Res. 2019, 9, 1938–1956. [Google Scholar]
- Kumari, A.; Gesumaria, L.; Liu, Y.-J.; Hughitt, V.K.; Zhang, X.; Ceribelli, M.; Wilson, K.M.; Klumpp-Thomas, C.; Chen, L.; McKnight, C.; et al. MTOR Inhibition Overcomes RSK3-Mediated Resistance to BET Inhibitors in Small Cell Lung Cancer. JCI Insight 2023, 8, e156657. [Google Scholar] [CrossRef]
- Li, J.-M.; Hsu, P.-C.; Kuan, F.-C.; Shi, C.-S.; Yang, C.-T. The Cancer Stemness Inhibitor Napabucasin Suppresses Small Cell Lung Cancer Growth through SOX2 Expression. Am. J. Cancer Res. 2022, 12, 4637–4651. [Google Scholar]
- Deng, H.; Chen, Y.; Wang, L.; Zhang, Y.; Hang, Q.; Li, P.; Zhang, P.; Ji, J.; Song, H.; Chen, M.; et al. PI3K/MTOR Inhibitors Promote G6PD Autophagic Degradation and Exacerbate Oxidative Stress Damage to Radiosensitize Small Cell Lung Cancer. Cell Death Dis. 2023, 14, 652. [Google Scholar] [CrossRef]
- Quintanal-Villalonga, A.; Taniguchi, H.; Hao, Y.; Chow, A.; Zhan, Y.A.; Chavan, S.S.; Uddin, F.; Allaj, V.; Manoj, P.; Shah, N.S.; et al. Inhibition of XPO1 Sensitizes Small Cell Lung Cancer to First- and Second-Line Chemotherapy. Cancer Res. 2022, 82, 472–483. [Google Scholar] [CrossRef]
- Sen, T.; Tong, P.; Stewart, C.A.; Cristea, S.; Valliani, A.; Shames, D.S.; Redwood, A.B.; Fan, Y.H.; Li, L.; Glisson, B.S.; et al. CHK1 Inhibition in Small-Cell Lung Cancer Produces Single-Agent Activity in Biomarker-Defined Disease Subsets and Combination Activity with Cisplatin or Olaparib. Cancer Res. 2017, 77, 3870–3884. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Zhao, X.; Zhu, J.; Kim, I.-K.; Rao, G.; McCutcheon, J.; Hsu, S.-T.; Teicher, B.; Kallakury, B.; Dowlati, A.; et al. Checkpoint Kinase 1 Inhibition Enhances Cisplatin Cytotoxicity and Overcomes Cisplatin Resistance in SCLC by Promoting Mitotic Cell Death. J. Thorac. Oncol. 2019, 14, 1032–1045. [Google Scholar] [CrossRef]
- Ran, X.; Wu, B.X.; Shi, M.; Song, L.; Nixon, K.; Philip, V.; He, H.H.; Tsao, M.-S.; Lok, B.H. CRISPR Screen of Druggable Targets in Small Cell Lung Cancer Identified ATM Inhibitor (AZD1390) as a Radiosensitizer. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 1308–1314. [Google Scholar] [CrossRef]
- Kodama, M.; Toyokawa, G.; Sugahara, O.; Sugiyama, S.; Haratake, N.; Yamada, Y.; Wada, R.; Takamori, S.; Shimokawa, M.; Takenaka, T.; et al. Modulation of Host Glutamine Anabolism Enhances the Sensitivity of Small Cell Lung Cancer to Chemotherapy. Cell Rep. 2023, 42, 112899. [Google Scholar] [CrossRef]
- Bola, B.M.; Chadwick, A.L.; Michopoulos, F.; Blount, K.G.; Telfer, B.A.; Williams, K.J.; Smith, P.D.; Critchlow, S.E.; Stratford, I.J. Inhibition of Monocarboxylate Transporter-1 (MCT1) by AZD3965 Enhances Radiosensitivity by Reducing Lactate Transport. Mol. Cancer Ther. 2014, 13, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Thirusangu, P.; Ray, U.; Sarkar Bhattacharya, S.; Oien, D.B.; Jin, L.; Staub, J.; Kannan, N.; Molina, J.R.; Shridhar, V. PFKFB3 Regulates Cancer Stemness through the Hippo Pathway in Small Cell Lung Carcinoma. Oncogene 2022, 41, 4003–4017. [Google Scholar] [CrossRef]
- Wang, J.; Lin, W.; Li, R.; Cheng, H.; Sun, S.; Shao, F.; Yang, Y.; Zhang, L.; Feng, X.; Gao, S.; et al. The Deubiquitinase USP13 Maintains Cancer Cell Stemness by Promoting FASN Stability in Small Cell Lung Cancer. Front. Oncol. 2022, 12, 899987. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Bian, X.; Lv, Y.; Lin, W. FK228 Sensitizes Radioresistant Small Cell Lung Cancer Cells to Radiation. Clin. Epigenetics 2021, 13, 41. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, W.; Hu, S.; Lyu, Q.; Wang, Q.; Wei, T.; Zhu, W.; Zhang, J. METTL3 Promotes Chemoresistance in Small Cell Lung Cancer by Inducing Mitophagy. J. Exp. Clin. Cancer Res. 2023, 42, 65. [Google Scholar] [CrossRef]
- Fukuoka, K.; Arioka, H.; Iwamoto, Y.; Fukumoto, H.; Kurokawa, H.; Ishida, T.; Tomonari, A.; Suzuki, T.; Usuda, J.; Kanzawa, F.; et al. Mechanism of Vinorelbine-Induced Radiosensitization of Human Small Cell Lung Cancer Cells. Cancer Chemother. Pharmacol. 2002, 49, 385–390. [Google Scholar] [CrossRef]
- Locke, V.L.; Davey, R.A.; Davey, M.W. Modulation of Drug and Radiation Resistance in Small Cell Lung Cancer Cells by Paclitaxel. Anticancer. Drugs 2003, 14, 523–531. [Google Scholar] [CrossRef]
- Patel, K.; Doudican, N.A.; Schiff, P.B.; Orlow, S.J. Albendazole Sensitizes Cancer Cells to Ionizing Radiation. Radiat. Oncol. 2011, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Yang, L.; Zhu, S.; Li, M.; Wang, Y.; Cao, X.; Wang, Q.; Guo, L. Identifying CDC7 as a Synergistic Target of Chemotherapy in Resistant Small-Cell Lung Cancer via CRISPR/Cas9 Screening. Cell Death Discov. 2023, 9, 40. [Google Scholar] [CrossRef]
- Majeed, S.; Aparnathi, M.K.; Nixon, K.C.J.; Venkatasubramanian, V.; Rahman, F.; Song, L.; Weiss, J.; Barayan, R.; Sugumar, V.; Barghout, S.H.; et al. Targeting the Ubiquitin–Proteasome System Using the UBA1 Inhibitor TAK-243 Is a Potential Therapeutic Strategy for Small-Cell Lung Cancer. Clin. Cancer Res. 2022, 28, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhao, Y.; Du, Y.; Tang, R. Evaluation of Hippo Pathway and CD133 in Radiation Resistance in Small-Cell Lung Cancer. J. Oncol. 2021, 2021, 8842554. [Google Scholar] [CrossRef]
- Pearsall, S.M.; Williamson, S.C.; Humphrey, S.; Hughes, E.; Morgan, D.; García Marqués, F.J.; Awanis, G.; Carroll, R.; Burks, L.; Shue, Y.T.; et al. Lineage Plasticity in SCLC Generates Non-Neuroendocrine Cells Primed for Vasculogenic Mimicry. J. Thorac. Oncol. 2023, 18, 1362–1385. [Google Scholar] [CrossRef]
- Jimenez, L.; Stolzenbach, V.; Ozawa, P.M.M.; Ramirez-Solano, M.; Liu, Q.; Sage, J.; Weaver, A.M. Extracellular Vesicles from Non-Neuroendocrine SCLC Cells Promote Adhesion and Survival of Neuroendocrine SCLC Cells. Proteomics 2023, 2300030. [Google Scholar] [CrossRef]
- Williamson, S.C.; Metcalf, R.L.; Trapani, F.; Mohan, S.; Antonello, J.; Abbott, B.; Leong, H.S.; Chester, C.P.E.; Simms, N.; Polanski, R.; et al. Vasculogenic Mimicry in Small Cell Lung Cancer. Nat. Commun. 2016, 7, 13322. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Park, S.-Y.; Lee, G.K.; Lim, K.Y.; Kim, J.Y.; Hwang, J.-A.; Yu, N.; Kang, E.H.; Hwang, M.; et al. Molecular Subtypes and Tumor Microenvironment Characteristics of Small-Cell Lung Cancer Associated with Platinum-Resistance. Cancers 2023, 15, 3568. [Google Scholar] [CrossRef]
- Schenk, M.W.; Humphrey, S.; Hossain, A.S.M.M.; Revill, M.; Pearsall, S.; Lallo, A.; Brown, S.; Bratt, S.; Galvin, M.; Descamps, T.; et al. Soluble Guanylate Cyclase Signalling Mediates Etoposide Resistance in Progressing Small Cell Lung Cancer. Nat. Commun. 2021, 12, 6652. [Google Scholar] [CrossRef]
- Wagner, A.H.; Devarakonda, S.; Skidmore, Z.L.; Krysiak, K.; Ramu, A.; Trani, L.; Kunisaki, J.; Masood, A.; Waqar, S.N.; Spies, N.C.; et al. Recurrent WNT Pathway Alterations Are Frequent in Relapsed Small Cell Lung Cancer. Nat. Commun. 2018, 9, 3787. [Google Scholar] [CrossRef]
- Reinhold, M.I.; Kapadia, R.M.; Liao, Z.; Naski, M.C. The Wnt-Inducible Transcription Factor Twist1 Inhibits Chondrogenesis. J. Biol. Chem. 2006, 281, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Gardner, E.E.; Lok, B.H.; Schneeberger, V.E.; Desmeules, P.; Miles, L.A.; Arnold, P.K.; Ni, A.; Khodos, I.; de Stanchina, E.; Nguyen, T.; et al. Chemosensitive Relapse in Small Cell Lung Cancer Proceeds through an EZH2-SLFN11 Axis. Cancer Cell 2017, 31, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.C.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-Mesenchymal Transition Is Not Required for Lung Metastasis but Contributes to Chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.-C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-Mesenchymal Transition Is Dispensable for Metastasis but Induces Chemoresistance in Pancreatic Cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Bahar, E.; Kim, J.-Y.; Kim, H.-S.; Yoon, H. Establishment of Acquired Cisplatin Resistance in Ovarian Cancer Cell Lines Characterized by Enriched Metastatic Properties with Increased Twist Expression. Int. J. Mol. Sci. 2020, 21, 7613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qi, J.; Wei, H.; Lei, Y.; Yu, H.; Liu, N.; Zhao, L.; Wang, P. TGFβ1 in Cancer-Associated Fibroblasts Is Associated with Progression and Radiosensitivity in Small-Cell Lung Cancer. Front. Cell Dev. Biol. 2021, 9, 667645. [Google Scholar] [CrossRef]
- Grunblatt, E.; Wu, N.; Zhang, H.; Liu, X.; Norton, J.P.; Ohol, Y.; Leger, P.; Hiatt, J.B.; Eastwood, E.C.; Thomas, R.; et al. MYCN Drives Chemoresistance in Small Cell Lung Cancer While USP7 Inhibition Can Restore Chemosensitivity. Genes Dev. 2020, 34, 1210–1226. [Google Scholar] [CrossRef]
- Chen, J.; Guanizo, A.C.; Jakasekara, W.S.N.; Inampudi, C.; Luong, Q.; Garama, D.J.; Alamgeer, M.; Thakur, N.; DeVeer, M.; Ganju, V.; et al. MYC Drives Platinum Resistant SCLC That Is Overcome by the Dual PI3K-HDAC Inhibitor Fimepinostat. J. Exp. Clin. Cancer Res. 2023, 42, 100. [Google Scholar] [CrossRef]
- Drapkin, B.J.; George, J.; Christensen, C.L.; Mino-Kenudson, M.; Dries, R.; Sundaresan, T.; Phat, S.; Myers, D.T.; Zhong, J.; Igo, P.; et al. Genomic and Functional Fidelity of Small Cell Lung Cancer Patient-Derived Xenografts. Cancer Discov. 2018, 8, 600–615. [Google Scholar] [CrossRef]
- Rygaard, K.; Slebos, R.J.C.; Spang-Thomsen, M. Radiosensitivity of Small-Cell Lung Cancer Xenografts Compared with Activity of c-Myc, N-Myc, L-Myc, c-Raf-1 and K-Ras Proto-Oncogenes. Int. J. Cancer 1991, 49, 279–284. [Google Scholar] [CrossRef]
- Chalishazar, M.D.; Wait, S.J.; Huang, F.; Ireland, A.S.; Mukhopadhyay, A.; Lee, Y.; Schuman, S.S.; Guthrie, M.R.; Berrett, K.C.; Vahrenkamp, J.M.; et al. MYC-Driven Small-Cell Lung Cancer Is Metabolically Distinct and Vulnerable to Arginine Depletion. Clin. Cancer Res. 2019, 25, 5107–5121. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, S.; Angelucci, S.; Montemurro, L.; Bartolucci, D.; Raieli, S.; Lampis, S.; Amadesi, C.; Scardovi, A.; Nieddu, G.; Cerisoli, L.; et al. Antigene MYCN Silencing by BGA002 Inhibits SCLC Progression Blocking MTOR Pathway and Overcomes Multidrug Resistance. Cancers 2023, 15, 990. [Google Scholar] [CrossRef] [PubMed]
- Nassar, D.; Blanpain, C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Sarvi, S.; Mackinnon, A.C.; Avlonitis, N.; Bradley, M.; Rintoul, R.C.; Rassl, D.M.; Wang, W.; Forbes, S.J.; Gregory, C.D.; Sethi, T. CD133+ Cancer Stem-like Cells in Small Cell Lung Cancer Are Highly Tumorigenic and Chemoresistant but Sensitive to a Novel Neuropeptide Antagonist. Cancer Res. 2014, 74, 1554–1565. [Google Scholar] [CrossRef]
- Kubo, T.; Takigawa, N.; Osawa, M.; Harada, D.; Ninomiya, T.; Ochi, N.; Ichihara, E.; Yamane, H.; Tanimoto, M.; Kiura, K. Subpopulation of Small-Cell Lung Cancer Cells Expressing CD133 and CD87 Show Resistance to Chemotherapy. Cancer Sci. 2013, 104, 78–84. [Google Scholar] [CrossRef]
- Gutova, M.; Najbauer, J.; Gevorgyan, A.; Metz, M.Z.; Weng, Y.; Shih, C.-C.; Aboody, K.S. Identification of UPAR-Positive Chemoresistant Cells in Small Cell Lung Cancer. PLoS ONE 2007, 2, e243. [Google Scholar] [CrossRef]
- Voigt, E.; Wallenburg, M.; Wollenzien, H.; Thompson, E.; Kumar, K.; Feiner, J.; McNally, M.; Friesen, H.; Mukherjee, M.; Afeworki, Y.; et al. Sox2 Is an Oncogenic Driver of Small-Cell Lung Cancer and Promotes the Classic Neuroendocrine Subtype. Mol. Cancer Res. 2021, 19, 2015–2025. [Google Scholar] [CrossRef]
- Tripathi, S.C.; Fahrmann, J.F.; Celiktas, M.; Aguilar, M.; Marini, K.D.; Jolly, M.K.; Katayama, H.; Wang, H.; Murage, E.N.; Dennison, J.B.; et al. MCAM Mediates Chemoresistance in Small-Cell Lung Cancer via the PI3K/AKT/SOX2 Signaling Pathway. Cancer Res. 2017, 77, 4414–4425. [Google Scholar] [CrossRef]
- Liang, S.; Wang, Q.; Wen, Y.; Wang, Y.; Li, M.; Wang, Q.; Peng, J.; Guo, L. Ligand-Independent EphA2 Contributes to Chemoresistance in Small-Cell Lung Cancer by Enhancing PRMT1-Mediated SOX2 Methylation. Cancer Sci. 2023, 114, 921–936. [Google Scholar] [CrossRef]
- Ferté, C.; Loriot, Y.; Clémenson, C.; Commo, F.; Gombos, A.; Bibault, J.-E.; Fumagalli, I.; Hamama, S.; Auger, N.; Lahon, B.; et al. IGF-1R Targeting Increases the Antitumor Effects of DNA-Damaging Agents in SCLC Model: An Opportunity to Increase the Efficacy of Standard Therapy. Mol. Cancer Ther. 2013, 12, 1213–1222. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Y.; Tang, H.; Hu, X.; Hubert, S.M.; Li, Q.; Su, D.; Xu, H.; Fan, Y.; Yu, X.; et al. Activation of PI3K/AKT Pathway Is a Potential Mechanism of Treatment Resistance in Small Cell Lung Cancer. Clin. Cancer Res. 2022, 28, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Tsurutani, J.; West, K.A.; Sayyah, J.; Gills, J.J.; Dennis, P.A. Inhibition of the Phosphatidylinositol 3-Kinase/Akt/Mammalian Target of Rapamycin Pathway but Not the MEK/ERK Pathway Attenuates Laminin-Mediated Small Cell Lung Cancer Cellular Survival and Resistance to Imatinib Mesylate or Chemotherapy. Cancer Res. 2005, 65, 8423–8432. [Google Scholar] [CrossRef] [PubMed]
- Fridman, R.; Giaccone, G.; Kanemoto, T.; Martin, G.R.; Gazdar, A.F.; Mulshine, J.L. Reconstituted Basement Membrane (Matrigel) and Laminin Can Enhance the Tumorigenicity and the Drug Resistance of Small Cell Lung Cancer Cell Lines. Proc. Natl. Acad. Sci. USA 1990, 87, 6698–6702. [Google Scholar] [CrossRef]
- Sethi, T.; Rintoul, R.C.; Moore, S.M.; MacKinnon, A.C.; Salter, D.; Choo, C.; Chilvers, E.R.; Dransfield, I.; Donnelly, S.C.; Strieter, R.; et al. Extracellular Matrix Proteins Protect Small Cell Lung Cancer Cells against Apoptosis: A Mechanism for Small Cell Lung Cancer Growth and Drug Resistance in Vivo. Nat. Med. 1999, 5, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, P.S.; Elliott, T.; Wong, W.S.; Rintoul, R.C.; Mackinnon, A.C.; Haslett, C.; Sethi, T. ECM Overrides DNA Damage-Induced Cell Cycle Arrest and Apoptosis in Small-Cell Lung Cancer Cells through Β1 Integrin-Dependent Activation of PI3-Kinase. Cell Death Differ. 2006, 13, 1776–1788. [Google Scholar] [CrossRef]
- Güçlü, E.; Eroğlu Güneş, C.; Kurar, E.; Vural, H. Knockdown of LncRNA HIF1A-AS2 Increases Drug Sensitivity of SCLC Cells in Association with Autophagy. Med. Oncol. 2021, 38, 113. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shan, W.; Hua, Y.; Chao, F.; Cui, Y.; Lv, L.; Dou, X.; Bian, X.; Zou, J.; Li, H.; et al. Exosomal MiR-92b-3p Promotes Chemoresistance of Small Cell Lung Cancer Through the PTEN/AKT Pathway. Front. Cell Dev. Biol. 2021, 9, 661602. [Google Scholar] [CrossRef]
- Wang, C.; Yang, D.; Zhang, X.; Zhang, X.; Yang, L.; Wang, P.; Zhou, W.; Li, H.; Li, Y.; Nie, H.; et al. Association of PTEN Gene SNPs Rs2299939 with PFS in Patients with Small Cell Lung Cancer Treated with Early Radiotherapy. Front. Genet. 2020, 11, 298. [Google Scholar] [CrossRef]
- Doerr, F.; George, J.; Schmitt, A.; Beleggia, F.; Rehkämper, T.; Hermann, S.; Walter, V.; Weber, J.-P.; Thomas, R.K.; Wittersheim, M.; et al. Targeting a Non-Oncogene Addiction to the ATR/CHK1 Axis for the Treatment of Small Cell Lung Cancer. Sci. Rep. 2017, 7, 15511. [Google Scholar] [CrossRef]
- Byers, L.A.; Wang, J.; Nilsson, M.B.; Fujimoto, J.; Saintigny, P.; Yordy, J.; Giri, U.; Peyton, M.; Fan, Y.H.; Diao, L.; et al. Proteomic Profiling Identifies Dysregulated Pathways in Small Cell Lung Cancer and Novel Therapeutic Targets Including PARP1. Cancer Discov. 2012, 2, 798–811. [Google Scholar] [CrossRef]
- Kundu, K.; Cardnell, R.J.; Zhang, B.; Shen, L.; Stewart, C.A.; Ramkumar, K.; Cargill, K.R.; Wang, J.; Gay, C.M.; Byers, L.A. SLFN11 Biomarker Status Predicts Response to Lurbinectedin as a Single Agent and in Combination with ATR Inhibition in Small Cell Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 4095–4105. [Google Scholar] [CrossRef]
- Schultz, C.W.; Zhang, Y.; Elmeskini, R.; Zimmermann, A.; Fu, H.; Murai, Y.; Wangsa, D.; Kumar, S.; Takahashi, N.; Atkinson, D.; et al. ATR Inhibition Augments the Efficacy of Lurbinectedin in Small-cell Lung Cancer. EMBO Mol. Med. 2023, 15, e17313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kim, I.-K.; Kallakury, B.; Chahine, J.J.; Iwama, E.; Pierobon, M.; Petricoin, E.; McCutcheon, J.N.; Zhang, Y.-W.; Umemura, S.; et al. Acquired Small Cell Lung Cancer Resistance to Chk1 Inhibitors Involves Wee1 Up-Regulation. Mol. Oncol. 2021, 15, 1130–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wen, X.; Liu, B. Wee1 Epigenetically Modulates H2B Mono-Ubiquitination at K120 Lysine and DNA Double-Strand Break Repair through Phosphorylation of H2BY37-Dependent Manner in Small-Cell Lung Cancer. Thorac. Cancer 2023, 14, 1420–1429. [Google Scholar] [CrossRef]
- Sen, T.; Tong, P.; Diao, L.; Li, L.; Fan, Y.; Hoff, J.; Heymach, J.V.; Wang, J.; Byers, L.A. Targeting AXL and MTOR Pathway Overcomes Primary and Acquired Resistance to WEE1 Inhibition in Small-Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 6239–6253. [Google Scholar] [CrossRef]
- Tang, S.-W.; Thomas, A.; Murai, J.; Trepel, J.B.; Bates, S.E.; Rajapakse, V.N.; Pommier, Y. Overcoming Resistance to DNA-Targeted Agents by Epigenetic Activation of Schlafen 11 (SLFN11) Expression with Class I Histone Deacetylase Inhibitors. Clin. Cancer Res. 2018, 24, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ma, L.; Cao, G.; Hua, J.; Lv, X.; Lin, W. FK228 Potentiates Topotecan Activity against Small Cell Lung Cancer Cells via Induction of SLFN11. Acta Pharmacol. Sin. 2022, 43, 2119–2127. [Google Scholar] [CrossRef]
- Poirier, J.T.; Gardner, E.E.; Connis, N.; Moreira, A.L.; de Stanchina, E.; Hann, C.L.; Rudin, C.M. DNA Methylation in Small Cell Lung Cancer Defines Distinct Disease Subtypes and Correlates with High Expression of EZH2. Oncogene 2015, 34, 5869–5878. [Google Scholar] [CrossRef]
- Hansen, L.T.; Lundin, C.; Helleday, T.; Poulsen, H.S.; Sørensen, C.S.; Petersen, L.N.; Spang-Thomsen, M. DNA Repair Rate and Etoposide (VP16) Resistance of Tumor Cell Subpopulations Derived from a Single Human Small Cell Lung Cancer. Lung Cancer 2003, 40, 157–164. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Zhang, G.; Deng, X.; Rossi, M.R.; Switchenko, J.M.; Doho, G.H.; Chen, Z.; Kim, S.; Strychor, S.; Christner, S.M.; et al. Poly (ADP) Ribose Polymerase Enzyme Inhibitor, Veliparib, Potentiates Chemotherapy and Radiation in Vitro and in Vivo in Small Cell Lung Cancer. Cancer Med. 2014, 3, 1579–1594. [Google Scholar] [CrossRef]
- Lok, B.H.; Gardner, E.E.; Schneeberger, V.E.; Ni, A.; Desmeules, P.; Rekhtman, N.; de Stanchina, E.; Teicher, B.A.; Riaz, N.; Powell, S.N.; et al. PARP Inhibitor Activity Correlates with SLFN11 Expression and Demonstrates Synergy with Temozolomide in Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.H.; Lok, B.H.; Ma, J.; Bell, A.; de Stanchina, E.; Poirier, J.T.; Rudin, C.M. Talazoparib Is a Potent Radiosensitizer in Small Cell Lung Cancer Cell Lines and Xenografts. Clin. Cancer Res. 2018, 24, 5143–5152. [Google Scholar] [CrossRef]
- Noyes, C.; Kitajima, S.; Li, F.; Suita, Y.; Miriyala, S.; Isaac, S.; Ahsan, N.; Knelson, E.; Vajdi, A.; Tani, T.; et al. The Germline Factor DDX4 Contributes to the Chemoresistance of Small Cell Lung Cancer Cells. Commun. Biol. 2023, 6, 65. [Google Scholar] [CrossRef]
- Hansen, L.T.; Thykjaer, T.; Ørntoft, T.F.; Rasmussen, L.J.; Keller, P.; Spang-Thomsen, M.; Bocker Edmonston, T.; Schmutte, C.; Fishel, R.; Nørgård Petersen, L. The Role of Mismatch Repair in Small-Cell Lung Cancer Cells. Eur. J. Cancer 2003, 39, 1456–1467. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Jiang, H.; Kang, Z.; Guan, M. Biomarkers for Chemotherapy and Drug Resistance in the Mismatch Repair Pathway. Clin. Chim. Acta 2023, 544, 117338. [Google Scholar] [CrossRef]
- Dingemans, A.M.; Witlox, M.A.; Stallaert, R.A.; van der Valk, P.; Postmus, P.E.; Giaccone, G. Expression of DNA Topoisomerase IIα and Topoisomerase IIβ Genes Predicts Survival and Response to Chemotherapy in Patients with Small Cell Lung Cancer. Clin. Cancer Res. 1999, 5, 2048–2058. [Google Scholar]
- Li, H.; Wang, H.; Deng, K.; Han, W.; Hong, B.; Lin, W. The Ratio of Bcl-2/Bim as a Predictor of Cisplatin Response Provides a Rational Combination of ABT-263 with Cisplatin or Radiation in Small Cell Lung Cancer. Cancer Biomark. 2019, 24, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Valko, Z.; Megyesfalvi, Z.; Schwendenwein, A.; Lang, C.; Paku, S.; Barany, N.; Ferencz, B.; Horvath-Rozsas, A.; Kovacs, I.; Schlegl, E.; et al. Dual Targeting of BCL-2 and MCL-1 in the Presence of BAX Breaks Venetoclax Resistance in Human Small Cell Lung Cancer. Br. J. Cancer 2023, 128, 1850–1861. [Google Scholar] [CrossRef]
- Loriot, Y.; Mordant, P.; Dugue, D.; Geneste, O.; Gombos, A.; Opolon, P.; Guegan, J.; Perfettini, J.-L.; Pierre, A.; Berthier, L.K.; et al. Radiosensitization by a Novel Bcl-2 and Bcl-XL Inhibitor S44563 in Small-Cell Lung Cancer. Cell Death Dis. 2014, 5, e1423. [Google Scholar] [CrossRef]
- Ramkumar, K.; Tanimoto, A.; Della Corte, C.M.; Stewart, C.A.; Wang, Q.; Shen, L.; Cardnell, R.J.; Wang, J.; Polanska, U.M.; Andersen, C.; et al. Targeting BCL2 Overcomes Resistance and Augments Response to Aurora Kinase B Inhibition by AZD2811 in Small Cell Lung Cancer. Clin. Cancer Res. 2023, 29, 3237–3249. [Google Scholar] [CrossRef]
- Kaiser, U.; Schilli, M.; Haag, U.; Neumann, K.; Kreipe, H.; Kogan, E.; Havemann, K. Expression of Bcl-2—Protein in Small Cell Lung Cancer. Lung Cancer 1996, 15, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Henness, S.; Davey, M.W.; Harvie, R.M.; Davey, R.A. Fractionated Irradiation of H69 Small-Cell Lung Cancer Cells Causes Stable Radiation and Drug Resistance with Increased MRP1, MRP2, and Topoisomerase IIα Expression. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 895–902. [Google Scholar] [CrossRef]
- Zandi, R.; Selivanova, G.; Christensen, C.L.; Gerds, T.A.; Willumsen, B.M.; Poulsen, H.S. PRIMA-1Met/APR-246 Induces Apoptosis and Tumor Growth Delay in Small Cell Lung Cancer Expressing Mutant P53. Clin. Cancer Res. 2011, 17, 2830–2841. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kang, K.; Ng, K.P.; Radivoyevitch, T.; Schalper, K.; Zhang, H.; Lindner, D.J.; Thomas, A.; MacPherson, D.; Gastman, B.; et al. Neuroendocrine Lineage Commitment of Small Cell Lung Cancers Can Be Leveraged into P53-Independent Non-Cytotoxic Therapy. Cell Rep. 2023, 42, 113016. [Google Scholar] [CrossRef]
- Neely, V.; Manchikalapudi, A.; Nguyen, K.; Dalton, K.; Hu, B.; Koblinski, J.E.; Faber, A.C.; Deb, S.; Harada, H. Targeting Oncogenic Mutant P53 and BCL-2 for Small Cell Lung Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 13082. [Google Scholar] [CrossRef]
- Rodríguez-Salas, N.; Palacios, J.; Moreno, G.; de Castro, J.; González-Barón, M.; Gamallo, C. Correlation of P53 Oncoprotein Expression with Chemotherapy Response in Small Cell Lung Carcinomas. Lung Cancer 2001, 34, 67–74. [Google Scholar] [CrossRef]
- Akeno, N.; Reece, A.L.; Callahan, M.; Miller, A.L.; Kim, R.G.; He, D.; Lane, A.; Moulton, J.S.; Wikenheiser-Brokamp, K.A. TRP53 Mutants Drive Neuroendocrine Lung Cancer Through Loss-of-Function Mechanisms with Gain-of-Function Effects on Chemotherapy Response. Mol. Cancer Ther. 2017, 16, 2913–2926. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, M.-H.; Park, I.; Jeon, H.; Choi, J.; Seo, S.; Kim, S.-W.; Koh, G.Y.; Park, K.-S.; Lee, D.H. HSP90 Inhibitor (NVP-AUY922) Enhances the Anti-Cancer Effect of BCL-2 Inhibitor (ABT-737) in Small Cell Lung Cancer Expressing BCL-2. Cancer Lett. 2017, 411, 19–26. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, W.; Fu, B.; Shi, L.; Wang, X.; Kuca, K. JNK Signaling in Cancer Cell Survival. Med. Res. Rev. 2019, 39, 2082–2104. [Google Scholar] [CrossRef]
- Butterfield, L.; Storey, B.; Maas, L.; Heasley, L.E. C-Jun NH2-Terminal Kinase Regulation of the Apoptotic Response of Small Cell Lung Cancer Cells to Ultraviolet Radiation. J. Biol. Chem. 1997, 272, 10110–10116. [Google Scholar] [CrossRef]
- Levresse, V.; Marek, L.; Blumberg, D.; Heasley, L.E. Regulation of Platinum-Compound Cytotoxicity by the c-Jun N-Terminal Kinase and c-Jun Signaling Pathway in Small-Cell Lung Cancer Cells. Mol. Pharmacol. 2002, 62, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.P.; Jungbluth, A.A.; Wu, B.-W.; Bomalaski, J.; Old, L.J.; Ritter, G. Arginine Deiminase PEG20 Inhibits Growth of Small Cell Lung Cancers Lacking Expression of Argininosuccinate Synthetase. Br. J. Cancer 2012, 106, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Agnello, G.; Alters, S.E.; Rowlinson, S.W. Preclinical Safety and Antitumor Activity of the Arginine-Degrading Therapeutic Enzyme Pegzilarginase, a PEGylated, Cobalt-Substituted Recombinant Human Arginase 1. Transl. Res. 2020, 217, 11–22. [Google Scholar] [CrossRef]
- Xu, S.; Lam, S.-K.; Cheng, P.N.-M.; Ho, J.C.-M. Recombinant Human Arginase Induces Apoptosis through Oxidative Stress and Cell Cycle Arrest in Small Cell Lung Cancer. Cancer Sci. 2018, 109, 3471–3482. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Oshikawa, K.; Shimizu, H.; Yoshioka, S.; Takahashi, M.; Izumi, Y.; Bamba, T.; Tateishi, C.; Tomonaga, T.; Matsumoto, M.; et al. A Shift in Glutamine Nitrogen Metabolism Contributes to the Malignant Progression of Cancer. Nat. Commun. 2020, 11, 1320. [Google Scholar] [CrossRef]
- Obrist, F.; Michels, J.; Durand, S.; Chery, A.; Pol, J.; Levesque, S.; Joseph, A.; Astesana, V.; Pietrocola, F.; Wu, G.S.; et al. Metabolic Vulnerability of Cisplatin-Resistant Cancers. EMBO J. 2018, 37, e98597. [Google Scholar] [CrossRef]
- Brodin, O.; Arnberg, H.; Bergh, J.; Nilsson, S. Increased Radioresistance of an in Vitro Transformed Human Small Cell Lung Cancer Cell Line. Lung Cancer 1995, 12, 183–198. [Google Scholar] [CrossRef]
- Pedersen, M.W.B.; Holm, S.; Lund, E.L.; Hojgaard, L.; Kristjansen, P.E.G. Coregulation of Glucose Uptake and Vascular Endothelial Growth Factor (VEGF) in Two Small-Cell Lung Cancer (SCLC) Sublines In Vivo and In Vitro. Neoplasia 2001, 3, 80–87. [Google Scholar] [CrossRef]
- Polański, R.; Hodgkinson, C.L.; Fusi, A.; Nonaka, D.; Priest, L.; Kelly, P.; Trapani, F.; Bishop, P.W.; White, A.; Critchlow, S.E.; et al. Activity of the Monocarboxylate Transporter 1 Inhibitor AZD3965 in Small Cell Lung Cancer. Clin. Cancer Res. 2014, 20, 926–937. [Google Scholar] [CrossRef]
- Cargill, K.R.; Stewart, C.A.; Park, E.M.; Ramkumar, K.; Gay, C.M.; Cardnell, R.J.; Wang, Q.; Diao, L.; Shen, L.; Fan, Y.-H.; et al. Targeting MYC-Enhanced Glycolysis for the Treatment of Small Cell Lung Cancer. Cancer Metab. 2021, 9, 33. [Google Scholar] [CrossRef]
- Kang, H.; Kim, B.; Park, J.; Youn, H.; Youn, B. The Warburg Effect on Radioresistance: Survival beyond Growth. Biochim. Biophys. Acta - Rev. Cancer 2023, 1878, 188988. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wan, R.; He, Y.; Lin, S.-H.; Cao, J.; Qiu, Y.; Zhang, T.; Zhao, Q.; Niu, Y.; Jin, Y.; et al. Therapeutic Targeting of the Mevalonate–Geranylgeranyl Diphosphate Pathway with Statins Overcomes Chemotherapy Resistance in Small Cell Lung Cancer. Nat. Cancer 2022, 3, 614–628. [Google Scholar] [CrossRef]
- Cole, S.P.C.; Bhardwaj, G.; Gerlach, J.H.; Mackie, J.E.; Grant, C.E.; Almquist, K.C.; Stewart, A.J.; Kurz, E.U.; Duncan, A.M.V.; Deeley, R.G. Overexpression of a Transporter Gene in a Multidrug-Resistant Human Lung Cancer Cell Line. Science 1992, 258, 1650–1654. [Google Scholar] [CrossRef] [PubMed]
- Holzmayer, T.A.; Hilsenbeck, S.; Von Hoff, D.D.; Roninson, I.B. Clinical Correlates of MDR1 (P-Glycoprotein) Gene Expression in Ovarian and Small-Cell Lung Carcinomas. JNCI J. Natl. Cancer Inst. 1992, 84, 1486–1491. [Google Scholar] [CrossRef]
- Poupon, M.F.; Arvelo, F.; Goguel, A.F.; Bourgeois, Y.; Jacrot, M.; Hanania, N.; Arriagada, R.; Chevalier, T.L. Response of Small-Cell Lung Cancer Xenografts to Chemotherapy: Multidrug Resistance and Direct Clinical Correlates. JNCI J. Natl. Cancer Inst. 1993, 85, 2023–2029. [Google Scholar] [CrossRef][Green Version]
- Campling, B.G.; Young, L.C.; Baer, K.A.; Lam, Y.M.; Deeley, R.G.; Cole, S.P.; Gerlach, J.H. Expression of the MRP and MDR1 Multidrug Resistance Genes in Small Cell Lung Cancer. Clin. Cancer Res. 1997, 3, 115–122. [Google Scholar]
- Savaraj, N.; Wu, C.J.; Xu, R.; Lampidis, T.; Lai, S.; Donnelly, E.; Solomon, J.; Feun, L.G. Multidrug-Resistant Gene Expression in Small-Cell Lung Cancer. Am. J. Clin. Oncol. 1997, 20, 398–403. [Google Scholar] [CrossRef]
- Yeh, J.-J.; Hsu, N.-Y.; Hsu, W.-H.; Tsai, C.-H.; Lin, C.-C.; Liang, J.-A. Comparison of Chemotherapy Response with P-Glycoprotein, Multidrug Resistance-Related Protein-1, and Lung Resistance-Related Protein Expression in Untreated Small Cell Lung Cancer. Lung 2005, 183, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Omori, M.; Noro, R.; Seike, M.; Matsuda, K.; Hirao, M.; Fukuizumi, A.; Takano, N.; Miyanaga, A.; Gemma, A. Inhibitors of ABCB1 and ABCG2 Overcame Resistance to Topoisomerase Inhibitors in Small Cell Lung Cancer. Thorac. Cancer 2022, 13, 2142–2151. [Google Scholar] [CrossRef]
- Solta, A.; Boettiger, K.; Kovács, I.; Lang, C.; Megyesfalvi, Z.; Ferk, F.; Mišík, M.; Hoetzenecker, K.; Aigner, C.; Kowol, C.R.; et al. Entinostat Enhances the Efficacy of Chemotherapy in Small Cell Lung Cancer Through S-Phase Arrest and Decreased Base Excision Repair. Clin. Cancer Res. 2023, 29, 4644–4659. [Google Scholar] [CrossRef]
- Mohammad, H.P.; Smitheman, K.N.; Kamat, C.D.; Soong, D.; Federowicz, K.E.; Van Aller, G.S.; Schneck, J.L.; Carson, J.D.; Liu, Y.; Butticello, M.; et al. A DNA Hypomethylation Signature Predicts Antitumor Activity of LSD1 Inhibitors in SCLC. Cancer Cell 2015, 28, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Ishikawa, Y.; Mizutani, A.; Iwasaki, S.; Matsumoto, S.; Kamada, Y.; Nomura, T.; Nakamura, K. LSD1 Inhibitor T-3775440 Inhibits SCLC Cell Proliferation by Disrupting LSD1 Interactions with SNAG Domain Proteins INSM1 and GFI1B. Cancer Res. 2017, 77, 4652–4662. [Google Scholar] [CrossRef] [PubMed]
- Augert, A.; Eastwood, E.; Ibrahim, A.H.; Wu, N.; Grunblatt, E.; Basom, R.; Liggitt, D.; Eaton, K.D.; Martins, R.; Poirier, J.T.; et al. Targeting NOTCH Activation in Small Cell Lung Cancer through LSD1 Inhibition. Sci. Signal. 2019, 12, eaau2922. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Chung, C.-Y.; Xie, T.; Ozeck, M.; Nichols, T.C.; Frey, J.; Udyavar, A.R.; Sharma, S.; Paul, T.A. Intrinsic and Acquired Drug Resistance to LSD1 Inhibitors in Small Cell Lung Cancer Occurs through a TEAD4-Driven Transcriptional State. Mol. Oncol. 2022, 16, 1309–1328. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Yang, Z.; Zhang, G.; Wu, P.; Luo, Y.; Zeng, Q.; Wang, L.; Xue, Q.; Zhang, Y.; et al. M6A Regulators as Predictive Biomarkers for Chemotherapy Benefit and Potential Therapeutic Targets for Overcoming Chemotherapy Resistance in Small-Cell Lung Cancer. J. Hematol. Oncol. 2021, 14, 190. [Google Scholar] [CrossRef]
- Jia, D.; Augert, A.; Kim, D.-W.; Eastwood, E.; Wu, N.; Ibrahim, A.H.; Kim, K.-B.; Dunn, C.T.; Pillai, S.P.S.; Gazdar, A.F.; et al. Crebbp Loss Drives Small Cell Lung Cancer and Increases Sensitivity to HDAC Inhibition. Cancer Discov. 2018, 8, 1422–1437. [Google Scholar] [CrossRef]
- Krushkal, J.; Silvers, T.; Reinhold, W.C.; Sonkin, D.; Vural, S.; Connelly, J.; Varma, S.; Meltzer, P.S.; Kunkel, M.; Rapisarda, A.; et al. Epigenome-Wide DNA Methylation Analysis of Small Cell Lung Cancer Cell Lines Suggests Potential Chemotherapy Targets. Clin. Epigenetics 2020, 12, 93. [Google Scholar] [CrossRef]
- Wollenzien, H.; Afeworki Tecleab, Y.; Szczepaniak-Sloane, R.; Restaino, A.; Kareta, M.S. Single-Cell Evolutionary Analysis Reveals Drivers of Plasticity and Mediators of Chemoresistance in Small Cell Lung Cancer. Mol. Cancer Res. 2023, 21, 892–907. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, F.; Sun, Y.; Qiu, Q.; Zhang, J.; Huang, W.; Huang, J.; Huang, X.; Guo, L. Etk Interaction with PFKFB4 Modulates Chemoresistance of Small-Cell Lung Cancer by Regulating Autophagy. Clin. Cancer Res. 2018, 24, 950–962. [Google Scholar] [CrossRef]
- Shen, W.; Luo, P.; Sun, Y.; Zhang, W.; Zhou, N.; Zhan, H.; Zhang, Q.; Shen, J.; Lin, A.; Cheng, Q.; et al. NRBF2 Regulates the Chemoresistance of Small Cell Lung Cancer by Interacting with the P62 Protein in the Autophagy Process. iScience 2022, 25, 104471. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Wang, C.-J.; Xiao, D.-S.; He, B.-M.; Li, M.; Yi, X.-P.; Zhang, W.; Yin, J.-Y.; Liu, Z.-Q. EIF3a R803K Mutation Mediates Chemotherapy Resistance by Inducing Cellular Senescence in Small Cell Lung Cancer. Pharmacol. Res. 2021, 174, 105934. [Google Scholar] [CrossRef] [PubMed]
- Hyer, M.L.; Milhollen, M.A.; Ciavarri, J.; Fleming, P.; Traore, T.; Sappal, D.; Huck, J.; Shi, J.; Gavin, J.; Brownell, J.; et al. A Small-Molecule Inhibitor of the Ubiquitin Activating Enzyme for Cancer Treatment. Nat. Med. 2018, 24, 186–193. [Google Scholar] [CrossRef] [PubMed]

| Mechanism | Therapeutic Strategy | Treatment Regimen | Trial Phase | Participants | Outcome | NCT Number |
|---|---|---|---|---|---|---|
| Neuroendocrine-High to Neuroendocrine-Low Transformation | NOTCH pathway inhibition | Platinum and etoposide plus placebo vs. tarextumab | Ib/II | Untreated extensive-stage SCLC | PFS: 5.5 vs. 5.5 months OS: 10.3 vs. 9.3 months ORR: 70.8% vs. 68.6% | NCT01859741 [70] |
| AURKA inhibition | Alisertib | I/II | Relapsed/refractory SCLC and other advanced solid tumors | PFS: 2.1 months ORR: 21% | NCT01045421 [71] | |
| II | Relapsed/refractory extensive-stage SCLC | Recruiting | NCT06095505 | |||
| Paclitaxel plus placebo vs. alisertib | II | Relapsed/refractory SCLC | PFS: 2.17 vs. 3.32 months OS: 5.42 vs. 6.11 months ORR: 18% vs. 22% | NCT02038647 [72] | ||
| AURKB inhibition | Barasertib-HQPA | II | Relapsed/refractory SCLC | PFS: 1.6 months OS: 5.3 months ORR: 0% | NCT03366675 [73] | |
| Cancer Stem Cells | Hedgehog pathway inhibition | Cisplatin and etoposide plus sonidegib | I | Untreated extensive-stage SCLC | PFS: 5.5 months OS: 19.7 months ORR: 79% | NCT01579929 [74] |
| Cisplatin and etoposide vs. cisplatin and etoposide plus vismodegib vs. other treatments | II | Untreated extensive-stage SCLC | PFS: 4.7 vs. 4.4 months OS: 9.1 vs. 9.8 months ORR: 43% vs. 52% | NCT00887159 [75] | ||
| Growth Factor Signaling | PI3K inhibition | Cisplatin and etoposide plus buparlisib | I | Extensive-stage SCLC and other advanced solid tumors | Completed, awaiting results | NCT02194049 |
| mTOR inhibition | Temsirolimus | II | Extensive-stage SCLC, responding or stable disease after chemotherapy | PFS: 2.2 months OS: 8 months ORR: 1.2% | NCT00028028 [76] | |
| Everolimus | II | SCLC progressing after chemotherapy | PFS: 1.3 months OS: 6.7 months ORR: 3% | NCT00374140 [77] | ||
| Paclitaxel plus everolimus | Ib | Relapsed/refractory SCLC | ORR: 28% | NCT01079481 [78] | ||
| Vistusertib | II | Relapsed/refractory SCLC with RICTOR amplification | PFS: 1.2 months OS: 11.0 months ORR: 0% | NCT03106155 [73] | ||
| XPO1 inhibition | Selinexor | II | Relapsed SCLC | Terminated—slow patient accrual | NCT02351505 | |
| DNA Damage Response | ATR inhibition | Topotecan plus berzosertib | I/II | Relapsed SCLC and other small cell cancers | PFS: 4.8 months OS: 8.5 months ORR: 36.0% Expanded trial in progress | NCT02487095 [79] |
| II | Relapsed platinum-resistant SCLC | Completed, awaiting results | NCT04768296 | |||
| Topotecan vs. topotecan plus berzosertib | II | SCLC and other small cell cancers | In progress | NCT03896503 | ||
| Lurbinectedin plus berzosertib | I/II | SCLC and other small cell and high-grade neuroendocrine cancers | Recruiting | NCT04802174 | ||
| CHK1 inhibition | Prexasertib | II | Extensive-stage SCLC | Platinum-sensitive: PFS: 1.41 months OS: 5.42 months ORR 5.2% Platinum-refractory: PFS: 1.36 months OS: 3.15 months ORR: 0% | NCT02735980 [80] | |
| WEE1 inhibition | Adavosertib | Ib | SCLC and other advanced solid tumors | CCNE1 and/or MYC family amplifications: PFS: 1.2 months ORR: 0% Others: PFS: 1.3 months ORR: 8.3% | NCT02482311 [81] | |
| II | Relapsed/refractory SCLC | Unselected: PFS: 1.3 months OS: 7.8 months ORR: 0% MYC family amplification or CDKN2A and TP53 mutations: PFS: 1.2 months OS: 7.7 months ORR: 0% | NCT02593019 [73] | |||
| Adavosertib plus carboplatin or other therapies | II | Refractory extensive-stage SCLC | Completed, awaiting results | NCT02937818 [82] | ||
| EZH2 inhibition | Topotecan and pembrolizumab plus tazemetostat | I | Relapsed SCLC | Recruiting | NCT05353439 | |
| PF-06821497 or standard of care plus PF-06821497 | I | Relapsed/refractory SCLC and other cancers | Recruiting | NCT03460977 | ||
| DNA-PK inhibition | Cisplatin and etoposide plus M3814 | Ib/II | Untreated extensive-stage SCLC | Terminated—slow patient accrual | NCT03116971 | |
| RAD51 inhibition | Standard of care plus amuvatinib | Ib | SCLC and other solid tumors | ORR: 33% | NCT00881166 [83] | |
| Platinum and etoposide plus amuvatinib | II | Refractory SCLC | PFS: 68 days OS: 119 days ORR: 17.4% | NCT01357395 [84] | ||
| PARP inhibition | Talazoparib | I | Advanced/relapsed SCLC and other solid tumors | PFS: 11.1 weeks ORR: 8.7% | NCT01286987 [85] | |
| Olaparib | II | Relapsed SCLC with HR deficiency | PFS: 1.4 months OS: 8.6 months ORR: 6.7% | NCT03009682 [86] | ||
| Cisplatin and etoposide plus placebo vs. veliparib | I/II | Extensive-stage SCLC | PFS: 5.5 vs. 6.1 months OS: 8.9 vs. 10.3 months ORR: 65.6% vs. 71.9% | NCT01642251 [87] | ||
| Carboplatin and etoposide plus placebo and placebo maintenance vs. veliparib and placebo maintenance vs. veliparib and veliparib maintenance | I/II | Untreated extensive-stage SCLC | PFS: 5.6 vs. 5.7 vs. 5.8 months OS: 12.4 vs. 10.0 vs. 10.1 months ORR: 64% vs. 59% vs. 77% | NCT02289690 [88] | ||
| Radiotherapy plus olaparib | I | SCLC | In progress | NCT03532880 | ||
| Thoracic radiotherapy plus talazoparib | I | Extensive-stage SCLC | Recruiting | NCT04170946 | ||
| Chemoradiotherapy and pembrolizumab followed by pembrolizumab plus placebo vs. olaparib | III | Untreated limited stage SCLC | Recruiting | NCT04624204 | ||
| Chemoradiotherapy plus durvalumab and olaparib | I/II | Extensive-stage SCLC | Recruiting | NCT04728230 | ||
| IGF1R inhibition | Cisplatin and etoposide vs. cisplatin and etoposide plus cixutumumab vs. other treatments | II | Untreated extensive-stage SCLC | PFS: 4.7 vs. 4.6 months OS: 9.1 vs. 10.1 months ORR: 43% vs. 49% | NCT00887159 [75] | |
| Topotecan vs. linsitinib | II | Relapsed SCLC | PFS: 3.0 vs. 1.2 months OS: 5.3 vs. 3.4 months ORR: 13% vs. 0% | NCT01533181 [89] | ||
| Apoptosis Regulation | BCL2 inhibition | Bortezomib | II | Relapsed/refractory extensive-stage SCLC | PFS: 1 month OS: 3 months ORR: 4% | NCT00068289 [90] |
| Gossypol | II | Sensitive relapsed extensive-stage SCLC | TTP: 1.7 months OS: 8.5 months ORR: 0% | NCT00773955 [91] | ||
| Navitoclax | I/IIa | SCLC and other solid tumors | PFS: 1.5 months OS: 3.2 months ORR: 2.6% | NCT00445198 [92] | ||
| Topotecan plus gossypol | I/II | Relapsed/refractory SCLC | Platinum-sensitive: TTP: 17.4 weeks ORR 17% Platinum-refractory: TTP: 11.7 weeks ORR: 0% | NCT00397293 [93] | ||
| Topotecan plus obatoclax | I/II | Relapsed SCLC | PFS: 2 months ORR: 0% | NCT00521144 [94] | ||
| Carboplatin and etoposide vs. carboplatin and etoposide plus oblimersen | II | Untreated extensive-stage SCLC | FFS: 7.6 vs. 6.0 months OS: 10.6 vs. 8.6 months ORR: 60% vs. 61% | NCT00042978 [95] | ||
| Carboplatin and etoposide vs. carboplatin and etoposide plus obatoclax | I/II | Untreated extensive-stage SCLC | PFS: 5.2 vs. 5.8 months OS: 9.8 vs. 10.5 months ORR: 53% vs. 62% | NCT00682981 [96] | ||
| Cisplatin and etoposide plus gossypol | I | Untreated extensive-stage SCLC and other solid tumors | ORR: 85.7% | NCT00544596 [97] | ||
| Paclitaxel plus pelcitoclax | Ib/II | Relapsed/refractory SCLC | ORR: 25% | NCT04210037 [98] | ||
| HSP90 inhibition | Doxorubicin plus ganetespib | I/II | Refractory SCLC and other solid tumors | Terminated—no significant activity | NCT02261805 [99] | |
| Carboplatin and paclitaxel plus SNX-5422 | I | SCLC and other lung cancers | ORR: 0% | NCT01892046 [100] | ||
| Metabolism | Arginine depletion | Pegargiminase | II | Relapsed SCLC with low ASS1 | Terminated—slow patient accrual and no significant activity | NCT01266018 [101] |
| Pembrolizumab plus pegzilarginase | I/II | Relapsed/refractory extensive-stage SCLC | Completed, awaiting results | NCT03371979 | ||
| Statin treatment | Cisplatin and irinotecan plus simvastatin | II | Untreated extensive-stage SCLC | PFS: 6.1 months OS: 11 months ORR: 75% | NCT00452634 [102] | |
| Chemoradiotherapy plus placebo vs. pravastatin | III | Untreated SCLC | PFS: 7.3 vs. 7.7 months OS: 10.6 vs. 10.7 months ORR: 69.1% vs. 69.0% | NCT00433498 [103] | ||
| Cisplatin and irinotecan vs. cisplatin and irinotecan plus simvastatin | II | Untreated extensive-stage SCLC | PFS: 6.4 vs. 6.3 months OS: 15.2 vs. 14.4 months ORR: 84.7% vs. 88.5% | NCT01441349 [104] | ||
| Drug Efflux Pumps | Drug efflux pump inhibition | Doxorubicin and vincristine plus biricodar | II | Relapsed SCLC | ORR: 19% | NCT00003847 [105] |
| Epigenetics | HDAC inhibition | Romidepsin | II | Refractory SCLC and other lung cancers | ORR: 0% | NCT00020202 [106] |
| II | Sensitive relapsed SCLC | PFS: 1.8 months OS: 6 months ORR: 0% | NCT00086827 [107] | |||
| Panobinostat | II | Relapsed SCLC | TTP: 1.41 months ORR: 0% | NCT01222936 [108] | ||
| Carboplatin and etoposide plus panobinostat | I/II | SCLC and other lung cancers | Terminated—treatment toxicity | NCT00958022 [109] | ||
| Carboplatin, etoposide, and atezolizumab plus entinostat | I | Untreated extensive-stage SCLC | Terminated—treatment toxicity | NCT04631029 [110] | ||
| Cisplatin and etoposide plus belinostat | I | SCLC and other advanced cancers | ORR: 57% | NCT00926640 [111] | ||
| DNMT and HDAC inhibition | Decitabine and romidepsin vs. decitabine and romidepsin plus celecoxib | I | SCLC and other pulmonary and pleural malignancies | Completed, not reported | NCT00037817 | |
| LSD1 inhibition | GSK2879552 | I | Relapsed/refractory SCLC | Terminated—treatment toxicity and no significant activity | NCT02034123 [112] | |
| Platinum, etoposide, and nivolumab plus pulrodemstat | Ib | Untreated extensive-stage SCLC | In progress | NCT03850067 | ||
| Atezolizumab maintenance plus bomedemstat | I/II | Extensive-stage SCLC | Recruiting | NCT05191797 | ||
| LSD1 and microtubule inhibition | Paclitaxel plus iadademstat | II | Relapsed/refractory SCLC and extrapulmonary high-grade neuroendocrine carcinoma | Recruiting | NCT05420636 | |
| Autophagy | Autophagy inhibition | Chloroquine | I | Stage 4 SCLC | Terminated—slow patient accrual | NCT00969306 |
| Radiotherapy plus chloroquine | I | Stage 1-3 SCLC | Terminated—slow patient accrual | NCT01575782 | ||
| Carboplatin and etoposide vs. carboplatin, gemcitabine, and hydroxychloroquine | II | Untreated stage 4 SCLC | Terminated—slow patient accrual, treatment toxicity, and no significant activity | NCT02722369 | ||
| Cell Cycle | Microtubule inhibition | Cisplatin and etoposide vs. cisplatin and etoposide plus paclitaxel | III | Untreated extensive-stage SCLC | FFS: 5.9 vs. 6.4 months OS: 9.9 vs. 10.6 months ORR: 68% vs. 75% | NCT00003299 [113] |
| Gemcitabine plus paclitaxel | II | Relapsed SCLC | In progress | NCT02769832 | ||
| Carboplatin, paclitaxel, and durvalumab | II | Untreated extensive-stage SCLC | Recruiting | NCT05856695 | ||
| Nelmastobart plus paclitaxel | Ib/II | Relapsed/refractory extensive-stage SCLC | Recruiting | NCT06016270 |
| Mechanism | Target | Therapy | Stage of Development |
|---|---|---|---|
| Neuroendocrine-High to Neuroendocrine-Low Transformation | YAP1 | YAP1 Inhibitor + Chemotherapy | Preclinical [114] |
| NOTCH | NOTCH Inhibitor + Chemotherapy | Clinical [70] | |
| HES1 Inhibition + Chemotherapy | Preclinical [115] | ||
| WNT, PI3K | BET Inhibitor + mTOR Inhibitor | Preclinical [116] | |
| MYC | AURKA Inhibitor + Paclitaxel | Clinical [72] | |
| Cancer Stem Cells | MYC, SOX2 | STAT3 Inhibitor + Chemotherapy | Preclinical [117] |
| SOX2 | Hedgehog Inhibitor + Chemotherapy | Clinical [74,75] | |
| Growth Factor Signaling | PI3K | PI3K Inhibitor + Chemotherapy | Clinical (NCT02194049) |
| mTOR Inhibitor + Paclitaxel | Clinical [78] | ||
| PI3K or mTOR Inhibitor + Radiotherapy | Preclinical [118] | ||
| XPO1 Inhibitor + Chemotherapy | Preclinical [119] | ||
| DNA Damage Response | ATR | ATR Inhibitor + Topotecan | Clinical (NCT04768296, NCT03896503) [79] |
| ATR Inhibitor + Lurbinectedin | Clinical (NCT04802174) | ||
| CHK1 Inhibitor + Chemotherapy | Preclinical [120,121] | ||
| WEE1 | WEE1 Inhibitor + Chemotherapy | Clinical [82] | |
| ATM | ATM Inhibitor + Radiotherapy | Preclinical [122] | |
| SLFN11 | EZH2 Inhibitor + Topotecan + Immunotherapy | Clinical (NCT05353439) | |
| EZH2 Inhibitor + Chemotherapy | Clinical (NCT03460977) | ||
| NHEJ | DNA-PK Inhibitor + Chemotherapy | Clinical (NCT03116971) | |
| HR | RAD51 Inhibitor + Chemotherapy | Clinical [83,84] | |
| PARP1 | PARP Inhibitor + Chemotherapy | Clinical [87,88] | |
| PARP Inhibitor + Radiotherapy | Clinical (NCT03532880, NCT04170946) | ||
| PARP Inhibitor + Chemotherapy + Radiotherapy + Immunotherapy | Clinical (NCT04624204, NCT04728230) | ||
| NER | IGF1R Inhibitor + Chemotherapy | Clinical [75] | |
| Apoptosis Regulation | BCL2 | BCL2 Inhibitor + Chemotherapy | Clinical [93,94,95,96,97,98] |
| p53 | HSP90 Inhibitor + Chemotherapy | Clinical [99,100] | |
| Metabolism | Arginine | Arginase + Immunotherapy | Clinical (NCT03371979) |
| Glutamine | Glutamine Synthesis Inhibitor + Antimetabolite | Preclinical [123] | |
| Glycolysis | MCT1 Inhibitor + Radiotherapy | Preclinical [124] | |
| PFKFB3 Inhibitor + Chemotherapy | Preclinical [125] | ||
| MVA-GGPS | Statin + Chemotherapy | Clinical [102,104] | |
| Statin + Chemotherapy + Radiotherapy | Clinical [103] | ||
| Lipogenesis | FASN Inhibition + Chemotherapy | Preclinical [126] | |
| Drug Efflux Pumps | Drug Efflux Pumps | Drug Efflux Pump Inhibitor + Chemotherapy | Clinical [105] |
| Epigenetics | MYC, SLFN11 | HDAC Inhibitor + Chemotherapy | Clinical [109,111] |
| HDAC Inhibitor + Chemotherapy + Immunotherapy | Clinical [110] | ||
| Dual PI3K/HDAC Inhibitor + Radiotherapy | Preclinical [127] | ||
| p53, MYC, SLFN11 | DNMT Inhibitor + HDAC Inhibitor | Clinical (NCT00037817) | |
| MYCN, ASCL1 | LSD1 Inhibition + Chemotherapy + Immunotherapy | Clinical (NCT03850067) | |
| LSD1 Inhibition + Immunotherapy | Clinical (NCT05191797) | ||
| MYCN, ASCL1, Cell Cycle Arrest | LSD1 Inhibition + Paclitaxel | Clinical (NCT05420636) | |
| Mitophagy | METTL3 Inhibition + Chemotherapy | Preclinical [128] | |
| Autophagy | Autophagy | Autophagy Inhibitor + Radiotherapy | Clinical (NCT01575782) |
| Autophagy Inhibitor + Chemotherapy | Clinical (NCT02722369) | ||
| Cell Cycle | Cell Cycle Arrest | Microtubule Inhibitor + Radiotherapy | Preclinical [129,130,131] |
| CDC7 Inhibitor + Chemotherapy | Preclinical [132] | ||
| Paclitaxel + Chemotherapy | Clinical (NCT02769832) [113] | ||
| Paclitaxel + Chemotherapy + Immunotherapy | Clinical (NCT05856695) | ||
| Paclitaxel + Immunotherapy | Clinical (NCT06016270) | ||
| Protein Degradation | Protein Degradation | UBA1 Inhibitor + Chemotherapy or Radiotherapy or PARP Inhibitor | Preclinical [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Lok, B.H. Strategies to Target Chemoradiotherapy Resistance in Small Cell Lung Cancer. Cancers 2024, 16, 3438. https://doi.org/10.3390/cancers16203438
Yu T, Lok BH. Strategies to Target Chemoradiotherapy Resistance in Small Cell Lung Cancer. Cancers. 2024; 16(20):3438. https://doi.org/10.3390/cancers16203438
Chicago/Turabian StyleYu, Tony, and Benjamin H. Lok. 2024. "Strategies to Target Chemoradiotherapy Resistance in Small Cell Lung Cancer" Cancers 16, no. 20: 3438. https://doi.org/10.3390/cancers16203438
APA StyleYu, T., & Lok, B. H. (2024). Strategies to Target Chemoradiotherapy Resistance in Small Cell Lung Cancer. Cancers, 16(20), 3438. https://doi.org/10.3390/cancers16203438






