Evaluating CDK4/6 Inhibitor Therapy in Elderly Patients with Metastatic Hormone Receptor-Positive, HER2-Negative Breast Cancer: A Retrospective Real-World Multicenter Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Study Endpoints
2.3. Statistical Analysis
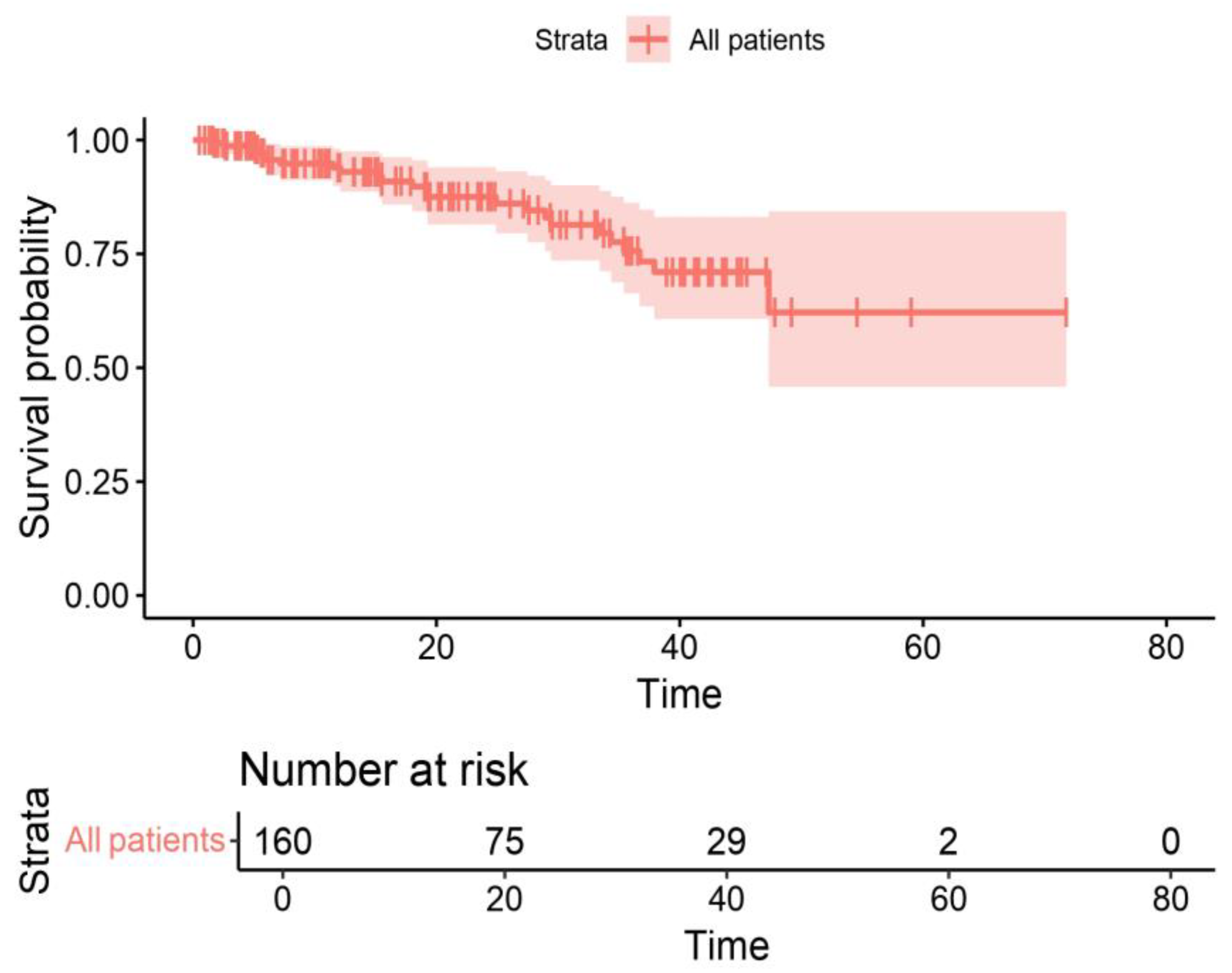
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; André, F.; Barrios, C.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Muñoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor positive, human epidermal growth factor 2-negative, aromatase inhibitor- resistant metastatic breast cancer: A phase III randomised controlled trial-PEARL. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, T.Y.; Kim, G.M.; Kang, S.Y.; Park, I.H.; Kim, J.H.; Lee, K.E.; Ahn, H.K.; Lee, M.H.; Kim, H.J.; et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2- negative metastatic breast cancer (KCSG-BR15-10): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019, 20, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Gelmon, K.; Walshe, J.M.; Mahtani, R.; Joy, A.A.; Karuturi, M.; Neven, P.; Lu, D.R.; Kim, S.; Schnell, P.; Bananis, E.; et al. Efficacy and safety of palbociclib in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with preexisting conditions: A post hoc analysis of PALOMA-2. Breast 2021, 59, 321–326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439, Erratum in Lancet Oncol. 2016, 17, e136; Erratum in Lancet Oncol. 2016, 17, e270. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor- Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Sonke, G.S.; Hart, L.L.; Campone, M.; Erdkamp, F.; Janni, W.; Verma, S.; Villanueva, C.; Jakobsen, E.; Alba, E.; Wist, E.; et al. Ribociclib with letrozole vs letrozole alone in elderly patients with hormone receptor-positive, HER2-negative breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res. Treat. 2018, 167, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; O’shaughnessy, J.; Martin, M.; Huober, J.; Toi, M.; Sohn, J.; André, V.A.M.; Martin, H.R.; Hardebeck, M.C.; Goetz, M.P. Abemaciclib as initial therapy for advanced breast cancer: MONARCH 3 updated results in prognostic subgroups. NPJ Breast Cancer 2021, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Toi, M.; Huober, J.; Sohn, J.; Trédan, O.; Park, I.; Campone, M.; Chen, S.-C.; Manso, L.; Paluch-Shimon, S.; et al. Abemaciclib plus a nonsteroidal aromatase inhibitor as initial therapy for HR+, HER2- advanced breast cancer: Final overall survival results of MONARCH 3. Ann. Oncol. 2024, 35, 718–727. [Google Scholar] [CrossRef] [PubMed]
- De Santis, P.; Sanna, V.; Perrone, M.; Guarini, C.; Santoro, A.N.; Laface, C.; Carrozzo, D.; Oliva, G.R.; Fancellu, A.; Fedele, P. Antibody-Drug Conjugates in HR+ Breast Cancer: Where Are We Now and Where Are We Heading? J. Clin. Med. 2023, 12, 7325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Biganzoli, L.; Battisti, N.M.L.; Wildiers, H.; McCartney, A.; Colloca, G.; Kunkler, I.H.; Cardoso, M.-J.; Cheung, K.-L.; de Glas, N.A.; Trimboli, R.M.; et al. Updated recommendations regarding the management of older patients with breast cancer: A joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021, 22, E327–E340. [Google Scholar] [CrossRef] [PubMed]
- Laface, C.; Giuliani, F.; Melaccio, A.; Pappagallo, M.N.; Santoro, A.N.; Perrone, M.; De Santis, P.; Guarini, C.; Carrozzo, D.; Fedele, P. The Treatment Landscape of Elderly Patients with Hormone Receptor-Positive Her2 Negative Advanced Breast Cancer: Current Perspectives and Future Directions. J. Clin. Med. 2023, 12, 6012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J. Tailoring therapies—Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torregrosa-Maicas, M.D.; del Barco-Berrón, S.; Cotes-Sanchís, A.; Lema-Roso, L.; Servitja-Tormo, S.; Gironés-Sarrió, R. Expert consensus to optimize the treatment of elderly patients with luminal metastatic breast cancer. Clin. Transl. Oncol. 2022, 24, 1033–1046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fountzilas, E.; Koliou, G.-A.; Vozikis, A.; Rapti, V.; Nikolakopoulos, A.; Boutis, A.; Christopoulou, A.; Kontogiorgos, I.; Karageorgopoulou, S.; Lalla, E.; et al. Real-world clinical outcome and toxicity data and economic aspects in patients with advanced breast cancer treated with cyclindependent kinase 4/6 (CDK4/6) inhibitors combined with endocrine therapy: The experience of the Hellenic Cooperative Oncology Group. ESMO Open 2020, 5, e000774. [Google Scholar] [CrossRef] [PubMed]
- Tinterri, C.; Sagona, A.; Barbieri, E.; Grimaldi, S.D.M.; Jacobs, F.; Zambelli, A.; Trimboli, R.M.; Bernardi, D.; Vinci, V.; Gentile, D. Loco-Regional Treatment of the Primary Tumor in De Novo Metastatic Breast Cancer Patients Undergoing Front-Line Chemotherapy. Cancers 2022, 14, 6237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| N° of Patients out of 160 (%) | Characteristics |
|---|---|
| Age at the start of CDK4/6i therapy | |
| 82 (51.26%) | 70–75 |
| 78 (48.74%) | >75 |
| Age at the diagnosis | |
| 89 (55.63%) | ≤75 |
| 71 (44.37%) | >75 |
| Stage at the diagnosis | |
| 51 (31.87%) | I–III |
| 109 (68.13%) | IV |
| N° metastasis sites | |
| 55 (34.37%) | Soft tissue/bone |
| 105 (65.63%) | Visceral |
| BC subtype | |
| 90 (56.25%) | Luminal A |
| 70 (43.75%) | Luminal B |
| Comorbidities | |
| 6 (3.76%) | 0 |
| 123 (76.87%) | 1 |
| 31 (19.37%) | 2 or more |
| ECOG PS | |
| 40 (25.00%) | 0 |
| 81 (50.63%) | 1 |
| 39 (24.37%) | 2 |
| G8 score | |
| 87 (54.37%) | >14 |
| 73 (45.63%) | ≤14 |
| Endocrine therapy | |
| 100 (62.50%) | Letrozole or Anastrozole |
| 60 (37.50%) | Fulvestrant |
| CDK4/6i | |
| 49 (30.62%) | Abemaciclib |
| 58 (36.25%) | Palbociclib |
| 53 (33.13%) | Ribociclib |
| Starting dose | |
| 86 (53.75%) | Standard |
| 74 (46.25%) | Reduced |
| Dose reduction | |
| 61 (38.13%) | Yes |
| 99 (61.87%) | No |
| Toxicities | |
| 35 (21.87%) | G1 |
| 44 (27.50%) | G2 |
| 46 (28.75%) | G3 |
| 75 (46.87%) | Temporary suspensions |
| 28 (17.5%) | 1 |
| 23(14.37%) | 2 |
| 30 (18.75%) | 3 |
| BMI | |
| 60 (37.5%) | BMI < 25 |
| 100 (62.5%) | BMI ≥ 25 |
| 15 | MEDIAN PFS |
| 19 | MEDIAN OS |
| p | 95% CI | HR | Variables | ||
|---|---|---|---|---|---|
| 0.185 | 0.97–1.16 | 1.06 | Age * | ||
| 0.963 | 0.55–1.76 | 0.99 | ≥25 vs. <25 | BMI | |
| <0.001 | 2.11–8.21 | 4.16 | B vs. A | Luminal subtype | |
| 0.874 | 0.61–1.78 | 1.04 | Yes vs. No | Visceral disease | |
| 0.470 | 0.42–1.49 | 0.79 | ≤14 vs. >14 | G8 score | |
| 0.077 | 0.93–3.83 | 1.89 | 2 vs. 0–1 | ECOG PS | |
| 0.267 | 0.32–1.36 | 0.67 | Fulvestrant vs. AI | Type of hormone therapy | |
| 0.255 | 0.74–3.07 | 1.51 | vs. Palbociclib | Ribociclib | Type of CDK4/6inh |
| 0.918 | 0.50–1.85 | 0.97 | Abemaciclib | ||
| AEs | Any Grade No (%) | Grade ≥ 3 No (%) | Total Patients No 160 (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Abemaciclib No 49 | Ribociclib No 53 | Palbociclib No 58 | Abemaciclib No 49 | Ribociclib No 53 | Palbociclib No 58 | Any Grades | Grade ≥ 3 | |
| Anemia | ||||||||
| No | 44 (89.7) | 51 (96.2) | 51 (87.9) | 48 (97.9) | 53 (100) | 54 (93.1) | 146 (91.3) | 155 (96.8) |
| Yes | 5 (10.3) | 2 (3.8) | 7 (12.1) | 1 (2.1) | 0 (0) | 4 (6.9) | 14 (8.7) | 5 (3.2) |
| Neutropenia | ||||||||
| No | 38 (77.5) | 29 (54.7) | 24 (41.3) | 42 (85.7) | 43 (81.1) | 43 (74.1) | 92 (57.5) | 128 (80.0) |
| Yes | 10 (22.5) | 24 (45.3) | 34 (58.7) | 7 (14.3) | 10 (8.9) | 15 (25.9) | 68 (42.5) | 32 (20.0) |
| Thrombocytopenia | ||||||||
| No | 49 (100) | 53 (100) | 55 (94.8) | 49 (100) | 53 (100) | 56 (96) | 157 (98.1) | 158 (98.7) |
| Yes | 0 (0) | 0 (0) | 3 (5.2) | 0 (0) | 0 (0) | 2 (4) | 3 (1.9) | 2 (1.3) |
| Asthenia | ||||||||
| No | 37 (75.5) | 37 (69.8) | 48 (82.7) | 42 (85.7) | 49 (92.4) | 56 (96.5) | 122 (76.2) | 147 (91.9) |
| Yes | 12 (24.5) | 16 (30.2) | 10 (17.3) | 7 (14.3) | 4 (7.6) | 2 (3.5) | 38 (23.8) | 13 (8.1) |
| Diarrhea | ||||||||
| No | 24 (48.9) | 53 (100) | 58 (100) | 42 (85) | 53 (100) | 58 (100) | 135 (84.4) | 153 (95.7) |
| Yes | 25 (51.1) | 0 (0) | 0 (0) | 7 (5) | 0 (0) | 0 (0) | 25 (15.6) | 7 (4.3) |
| ALT/AST increased | ||||||||
| No | 49 (100) | 51 (96.2) | 55 (94.8) | 49 (100) | 52 (98) | 57 (98) | 155 (96.7) | 158 (98.7) |
| Yes | 0 (0) | 2 (3.8) | 3 (5.2) | 0 (0) | 1 (2) | 1 (2) | 5 (3.3) | 2 (1.3) |
| Qtc prolongation | ||||||||
| No | 49 (100) | 51 (96.2) | 58 (100) | 49 (100) | 51 (96.2) | 58 (100) | 158 (98.7) | 158 (98.7) |
| Yes | 0 (0) | 2 (3.8) | 0 (0) | 0 (0) | 2 (3.8) | 0 (0) | 2 (1.3) | 2 (1.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedele, P.; Landriscina, M.; Moraca, L.; Cusmai, A.; Gnoni, A.; Licchetta, A.; Guarini, C.; Lanotte, L.; Pappagallo, M.N.; Melaccio, A.; et al. Evaluating CDK4/6 Inhibitor Therapy in Elderly Patients with Metastatic Hormone Receptor-Positive, HER2-Negative Breast Cancer: A Retrospective Real-World Multicenter Study. Cancers 2024, 16, 3442. https://doi.org/10.3390/cancers16203442
Fedele P, Landriscina M, Moraca L, Cusmai A, Gnoni A, Licchetta A, Guarini C, Lanotte L, Pappagallo MN, Melaccio A, et al. Evaluating CDK4/6 Inhibitor Therapy in Elderly Patients with Metastatic Hormone Receptor-Positive, HER2-Negative Breast Cancer: A Retrospective Real-World Multicenter Study. Cancers. 2024; 16(20):3442. https://doi.org/10.3390/cancers16203442
Chicago/Turabian StyleFedele, Palma, Matteo Landriscina, Lucia Moraca, Antonio Cusmai, Antonio Gnoni, Antonella Licchetta, Chiara Guarini, Laura Lanotte, Maria Nicla Pappagallo, Assunta Melaccio, and et al. 2024. "Evaluating CDK4/6 Inhibitor Therapy in Elderly Patients with Metastatic Hormone Receptor-Positive, HER2-Negative Breast Cancer: A Retrospective Real-World Multicenter Study" Cancers 16, no. 20: 3442. https://doi.org/10.3390/cancers16203442







