Human Cripto-1 and Cripto-3 Protein Expression in Normal and Malignant Settings That Conflicts with Established Conventions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Recombinant Proteins
2.2. Primary/Secondary Antibodies
2.3. Pathological Human Tumor Tissue/Cancer Patient Serum Specimens
2.4. Human Tumor Cell Lines and Immortalized Human Endothelial Cells
2.5. Generating Selective Murine MoAbs Targeting Human CR1 vs. Human CR3
2.6. ELISA Assessment of Antibody Binding Characterization and Serial Titration Studies
2.7. Biacore/Surface Plasmon Resonance
2.8. Immunohistochemical (IHC) Staining of Normal and Malignant Human Tissue
2.9. Protein Extraction and Western Blot Analysis
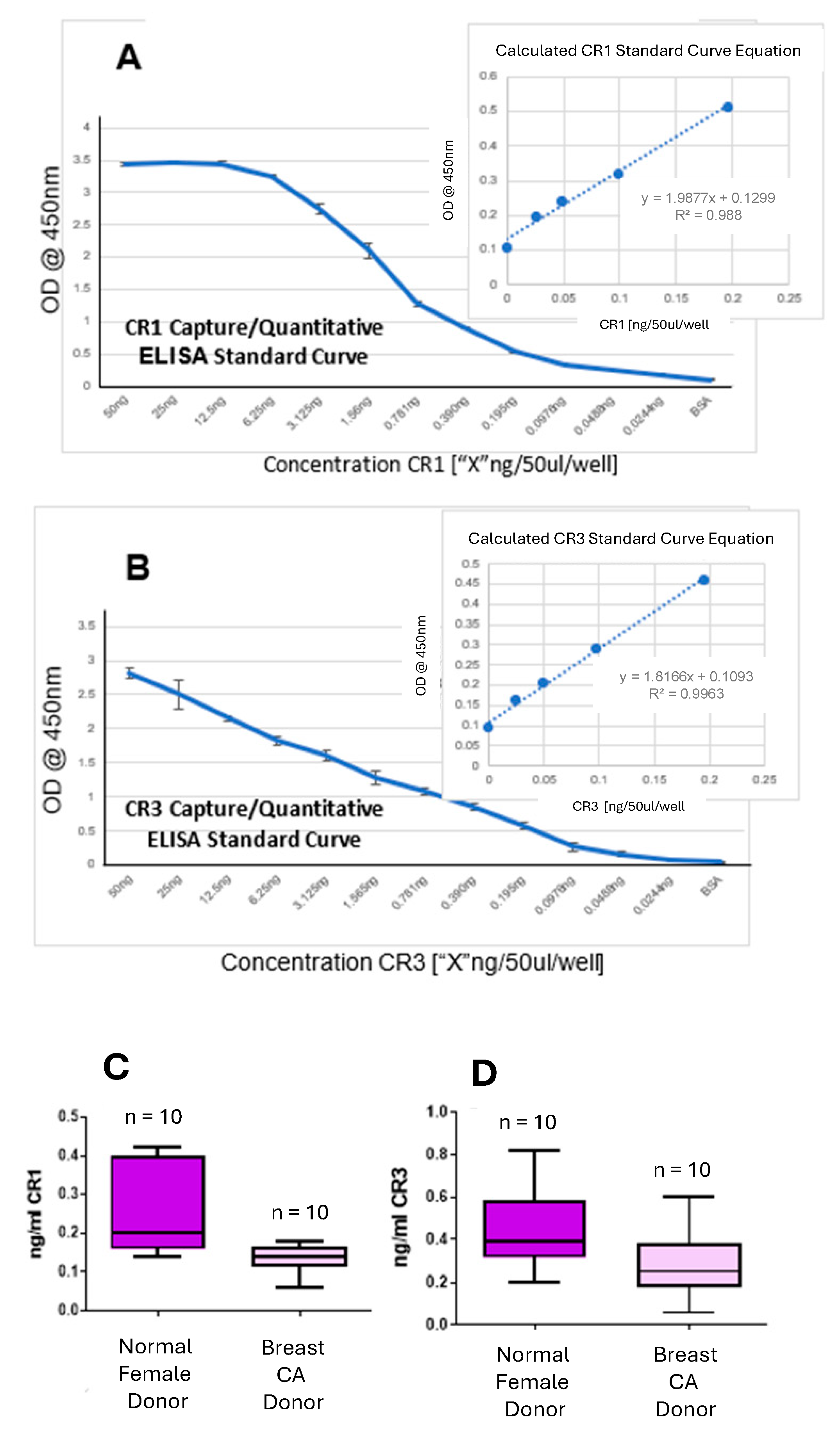
2.10. CR1/CR3 Capture/Quantitative ELISA for Assessing Patient Sera
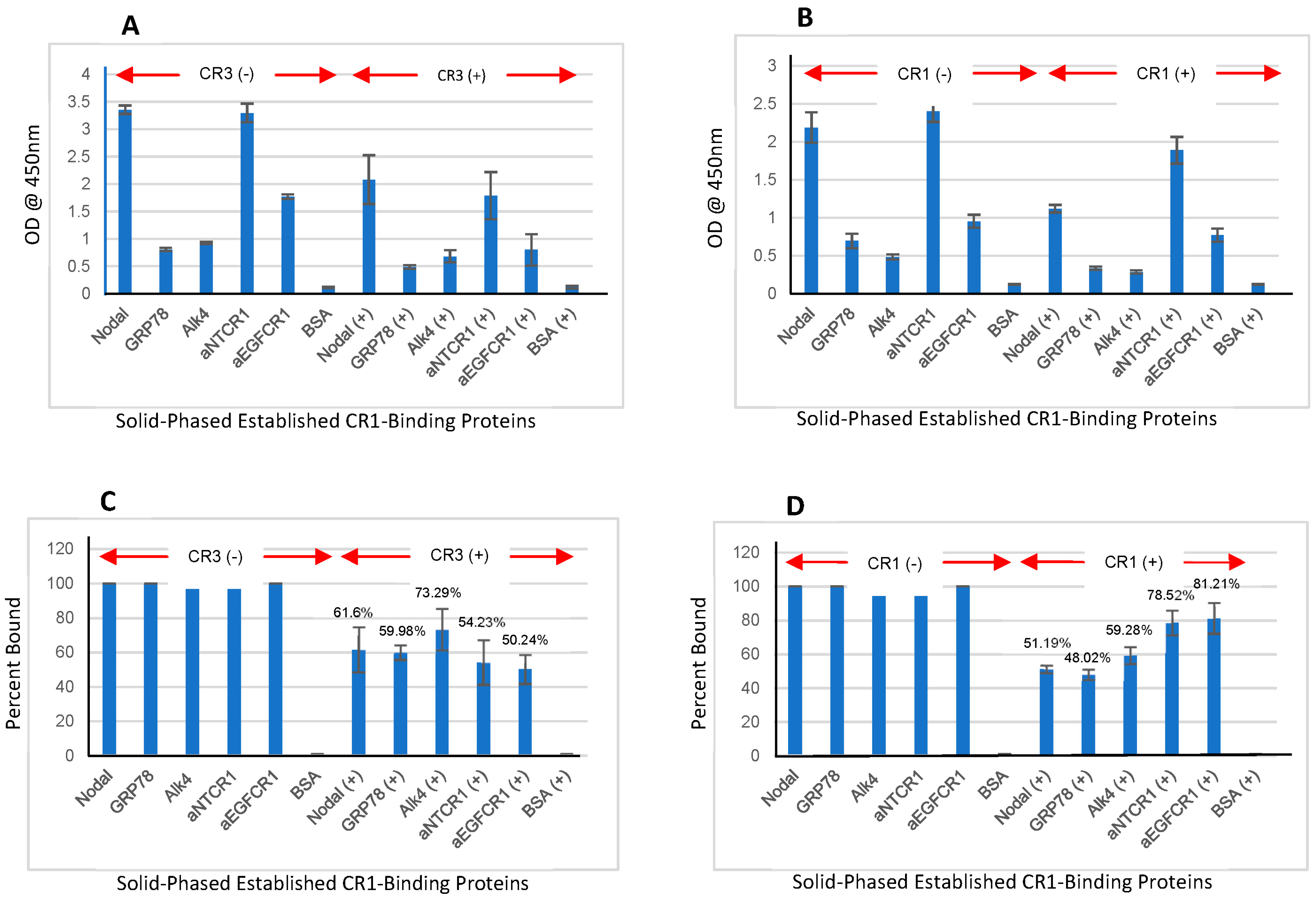
2.11. ELISA Evaluation of CR1/CR3 Interaction to Established Binding Proteins (Nodal, GRP78, and Alk4)
2.12. Statistics
3. Results
3.1. Generation of Selective Mouse Anti-CR MoAbs That Differentiate CR1 and CR3
3.2. Determination of kon/koff/KD Values by Biacore/Surface Plasmon Resonance for NCI Anti-CR1 and NCI Anti-CR3 MoAbs
3.3. Comparison Between Anti-CR1/Anti-CR3 NCI MoAbs and Commercially Available Anti-CR Products
3.4. Immunohistochemistry (IHC) Results
3.5. IHC Detection of CR1/CR3 in Human Tumor Tissue and Ranking of TMN-N/TMN-M/Grade/Stage/Progesterone Receptor Expression Based on Staining Intensity
3.6. Western Blot Analysis of Human Tumor Cell Lines
3.7. CR1/CR3 Capture/Quantitative ELISA Evaluation of Serum from Normal Female Donors Versus Serum from Breast Cancer Patients
3.8. CR1/CR3 Interaction with Solid-Phased Established CR1-Binding Proteins (Nodal, GRP78, and Alk4)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciccodicola, A.; Dono, R.; Obici, S.; Simeone, A.; Zollo, M.; Persico, M.G. Molecular characterization of a gene of the ‘EGF family’ expressed in undifferentiated NTERA2 teratocarcinoma cells. EMBO J. 1989, 8, 1987–1991. [Google Scholar] [CrossRef] [PubMed]
- Saccone, S.; Rapisarda, A.; Motta, S.; Dono, R.; Persico, M.G.; Della Valle, G. Regional localization of the human EGF-Like growth factor CRIPTO gene (TDGF-1) to chromosome 3p21. Hum. Genet. 1995, 95, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Dono, R.; Montuori, N.; Rocchi, M.; De Ponti-Zilli, L.; Ciccodicola, A.; Persico, M.G. Isolation and characterization of the CRIPTO autosomal gene and its X-linked related sequence. Am. J. Hum. Genet. 1991, 49, 555–565. [Google Scholar] [PubMed]
- Scognamiglio, B.; Baldassarre, G.; Cassano, C.; Tucci, M.; Montuori, N.; Dono, R.; Lembo, G.; Barra, A.; Lago, C.T.; Viglietto, G.; et al. Assignment of human teratocarcinoma derived growth factor (TDGF) sequences to chromosomes 2q37, 3q22, 6p25, 19q13.1. Cytogenet. Cell Genet. 1999, 84, 220–224. [Google Scholar] [CrossRef]
- Sun, C.; Orozco, O.; Olson, D.L.; Choi, E.; Garber, E.; Tizard, R.; Szak, S.; Sanicola, M.; Carulli, J.P. CRIPTO3, a presumed pseudogene, is expressed in cancer. Biochem. Biophys. Res. Commun. 2008, 377, 215–220. [Google Scholar] [CrossRef]
- Liguori, G.; Tucci, M.; Montuori, N.; Dono, R.; Lago, C.T.; Pacifico, F.; Armenante, F.; Persico, M.G. Characterization of the mouse Tdgf1 gene and Tdgf pseudogenes. Mamm. Genome 1996, 7, 344–348. [Google Scholar] [CrossRef]
- Ni, Q.; An, M.; Luo, S.; Li, X.; He, G.; Shen, M.; Xu, L.; Huang, J.; Yan, M.; Fan, Y.; et al. Pseudogene TDGF1P3 regulates the proliferation and metastasis of colorectal cancer cells via the miR-338-3p-PKM2 axis. Biochem. Biophys. Res. Commun. 2023, 638, 7–13. [Google Scholar] [CrossRef]
- Gershon, E.; Hadas, R.; Elbaz, M.; Booker, E.; Muchnik, M.; Kleinjan-Elazary, A.; Karasenti, S.; Genin, O.; Cinnamon, Y.; Gray, P.C. Identification of trophectoderm-derived cripto as an essential mediator of embryo implantation. Endocrinology 2018, 159, 1793–1807. [Google Scholar] [CrossRef]
- Shafiei, S.; Farah, O.; Dufort, D. Maternal Cripto is required for proper uterine decidualization and peri-implantation uterine remodeling. Biol. Reprod. 2021, 104, 1045–1057. [Google Scholar] [CrossRef]
- Ding, J.; Yang, L.; Yan, Y.T.; Chen, A.; Desia, N.; Wynshaw-Boris, A.; Shan, M.M. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature 1998, 395, 702–707. [Google Scholar] [CrossRef]
- Jin, J.Z.; Ding, J. Cripto is required for mesoderm and endoderm cell allocation during mouse gastrulation. Dev. Biol. 2013, 381, 170–178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, C.; Liguori, G.; Persico, M.G.; Adamson, E.D. Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development 1999, 126, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Fiorenzano, A.; Pascale, E.; D’Aniello, C.; Acampora, D.; Bassalert, C.; Russo, F.; Andolfi, G.; Biffoni, M.; Francescangeli, F.; Zeyber, A.; et al. Cripto is essential to capture mouse epiblast stem cells and human embryonic stem cell pluripotency. Nat. Commun. 2015, 7, 12589. [Google Scholar] [CrossRef]
- Francescangeli, F.; Contavalli, P.; De Angelis, M.L.; Baiocchi, M.; Gambara, G.; Pagliuca, A.; Fiorenzano, A.; Prezioso, C.; Boe, A.; Todaro, M.; et al. Dynamic regulation of the cancer stem cell compartment by Cripto-1 in colorectal cancer. Cell Death Diff. 2015, 22, 1700–1713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castro, N.P.; Rangel, M.C.; Merchant, A.S.; Mackinnon, G.; Cuttitta, F.; Salomon, D.S.; Kim, Y.S. Sulforaphane suppresses the growth of triple-negative breast cancer stem-like cells in vitro and in vivo. Cancer Prev. Res. 2019, 12, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, O.; Iavarone, F.; Nicoletti, C.; Ventre, M.; Rodriguez, C.; Pisapia, L.; Andolfi, G.; Saccone, V.; Patrìarca, E.J.; Puri, P.L.; et al. CRIPO-based micro-heterogeneity of mouse muscle satellite cells enables adaptive response to regenerative mircoenvironment. Dev. Cell 2023, 58, 2896–2913. [Google Scholar] [CrossRef]
- Kenney, N.J.; Huang, R.P.; Johnson, G.R.; Wu, J.X.; Okamura, D.; Matheny, W.; Kordon, E.; Gullick, W.J.; Plowman, G.; Smith, G.H.; et al. Detection and location of amphiregulin and cripto-1 expression in the developing postnatal mouse mammary gland. Mol. Repro. Dev. 1995, 41, 277–286. [Google Scholar] [CrossRef]
- Niemeyer, C.C.; Persico, M.G.; Adamson, E.D. Cripto: Roles in mammary cell growth, survival, differentiation, and transformation. Cell Death Differ. 1998, 5, 440–449. [Google Scholar] [CrossRef][Green Version]
- Klauzinska, M.; McCurdy, D.; Rangel, M.C.; Vaidyanath, A.; Castro, N.P.; Shen, M.M.; Gonzales, M.; Bertolette, D.; Blanco, C.; Callahan, R.; et al. Cripto-1 ablation disrupts alveolar development in mouse mammary gland through a progesterone receptor-mediated pathway. Am. J. Path. 2015, 185, 2907–2922. [Google Scholar] [CrossRef]
- Bianco, C.; Wechselberger, C.; Ebert, A.; Khan, N.J.; Sun, Y.; Salomon, D.S. Identification of Cripto-1 in human milk. Breast Cancer Res. Treat. 2001, 66, 1–7. [Google Scholar] [CrossRef]
- Klauzinska, M.; Bertolette, D.; Tippireddy, S.; Strizzi, L.; Gray, P.C.; Gonzales, M.; Duroux, M.; Ruvo, M.; Wechselberger, C.; Castro, N.P.; et al. Cripto-1: An extracellular protein—Connecting the sequestered biological dots. Connect. Tissue Res. 2015, 56, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Bao, Y.L.; Yu, C.L.; Wang, Y.M.; Song, Z.B. Cripto-1 modulates macrophage cytokine secretion and phagocytic activity via NF-kB signaling. Immunol. Res. 2016, 64, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Iavarone, F.; Guardiola, O.; Scagliola, A.; Andolfi, G.; Esposito, F.; Perdiguero, E.; Brunelli, S.; Muñoz-Cánoves, P.; Minchiotti, G. Cripto shapes macrophage plasticity and restricts EndMT in injured and diseased skeletal muscle. EMBO Rep. 2020, 21, e49075. [Google Scholar] [CrossRef] [PubMed]
- Marro, J.; Chetwynd, A.J.; Wright, R.D.; Dliso, S.; Oni, L. Urinary protein array analysis to identify key inflammatory markers in children with IgA vasculitis nephritis. Children 2022, 9, 622. [Google Scholar] [CrossRef]
- Guardiola, O.; Lafuste, P.; Brunelli, S.; Iaconis, S.; Tourvier, T.; Mourikis, P.; De Bock, K.; Lonardo, E.; Andolfi, G.; Bouché, A.; et al. Cripto regulates skeletal muscle regeneration and modulates satellite cell determination by antagonizing myostatin. Proc. Natl. Acad. Sci. USA 2012, 109, E3231–E3240. [Google Scholar] [CrossRef]
- Hoover, M.; Runa, F.; Booker, E.; Diedrich, J.K.; Duell, E.; Williams, B.; Areliano-Garcia, C.; Uhlendorf, T.; Kim, S.L.; Fischer, W.; et al. Identification of myosin II as a cripto binding protein and regulator of cripto functions in stem cells and tissue regeneration. Biochem. Biophys. Res. Commun. 2019, 509, 69–75. [Google Scholar] [CrossRef]
- Freeman, D.W.; Sousa, E.R.; Karkampouna, S.; Zoni, E.; Gray, P.C.; Salomon, D.S.; Keurhod-de Julio, M.; Spike, B.T. Whence CRIPTO: The reemergence of an oncofetal factor in ‘Wounds’ that fail to heal. Int. J. Mol. Sci. 2021, 22, 10164. [Google Scholar] [CrossRef]
- Karkampouna, S.; van der Helm, D.; Scarpa, M.; van Hoek, B.; Verspaget, H.W.; Goumans, M.J.; Coenraad, M.J.; Kruithof, B.P.T.; Kruithof-de Julio, M. Oncofetal protein CRIPTO is involved in wound healing and fibrogenesis in the regenerating liver and is associated with the initial stages of cardiac fibrosis. Cells 2021, 10, 3325. [Google Scholar] [CrossRef]
- Bianco, C.; Strizzi, L.; Ebert, A.; Chang, C.; Rehman, A.; Normanno, N.; Guedez, L.; Salloum, R.; Ginsburg, E.; Sun, Y.; et al. Role of human Cripto-1 in tumor angiogenesis. J. Natl. Cancer Inst. 2005, 97, 132–141. [Google Scholar] [CrossRef]
- Bianco, C.; Strizzi, L.; Normanno, N.; Khan, N.; Salomon, D.S. Cripto-1: An oncofetal gene with many faces. Curr. Top. Dev. Biol. 2005, 67, 85–133. [Google Scholar]
- De Luca, A.; Lamura, L.; Strizzi, L.; Roma, C.; D’Antonio, A.; Margaryan, N.; Pirozzi, G.; Hsu, M.-Y.; Botti, G.; Mari, E.; et al. Expression and functional role of CRIPTO-1 in cutaneous melanoma. Br. J. Cancer 2011, 105, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Sandomenico, A.; Ruvo, M. Targeting Nodal and Cripto-1: Perspectives inside dual potential theranostic cancer biomarkers. Curr. Med. Chem. 2019, 26, 1994–2050. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Gao, Y.; Zhou, Y. Understanding the role of Cripto-1 in cancer progression and therapeutic strategies. Clin. Transl. Oncol. 2023, 25, 1135–1144. [Google Scholar] [CrossRef]
- Xu, C.; Zou, J.; Li, L.; Yuan, Q.; Wang, W. Elevated serum Cripto-1 and VEGF levels in patients with non-small cell lung cancer. FASEB Bioadv. 2022, 4, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Bianco, C.; Strizzi, L.; Hamada, S.; Mancino, M.; Bailly, V.; Mo, W.; Wen, D.; Miatkowski, K.; Gonzales, M.; et al. Growth factor induction of cripto-1 shedding by glycosylphosphatidylinositol-phospholipase D and enhancement of endothelial cell migration. J. Biol. Chem. 2007, 282, 31643–31655. [Google Scholar] [CrossRef]
- Lee, G.H.; Fujita, M.; Takaoka, K.; Murakami, Y.; Fujihara, Y.; Kanzawa, N.; Murakami, K.I.; Kajikawa, E.; Takada, Y.; Saito, K.; et al. A GPI processing phospholipase A2, PGAP6, modulates Nodal signaling in embryos by shedding CRIPTO. J. Bio. Chem. 2016, 215, 705–718. [Google Scholar] [CrossRef]
- Gray, P.C.; Vale, W. Cripto/GRP78 modulation of TGF-B pathway in development and oncogenesis. FEBS Lett. 2012, 586, 1836–1845. [Google Scholar] [CrossRef]
- Saeki, T.; Stromberg, K.; Qi, C.F.; Gullick, W.J.; Tahara, E.; Normanno, N.; Ciardiello, F.; Kenney, N.; Johnson, G.R.; Salomon, D.S. Differential immunohistochemical detection of amphiregulin and cripto in human normal colon and colorectal tumors. Cancer Res. 1992, 52, 3467–3473. [Google Scholar]
- Saeki, T.; Salomon, D.; Gullick, W.; Mandai, K.; Yamagami, K.; Moriwaki, S.; Takashima, S.; Nishikawa, Y.; Tahara, E. Expression of cripto-1 in human colorectal adenomas and carcinomas is related to the degree of dysplasia. Int. J. Oncol. 1994, 5, 445–451. [Google Scholar] [CrossRef]
- Normanno, N.; Qi, C.; Gullick, W.; Persico, G.; Yarden, Y.; Wen, D.; Plowman, G.; Kenney, N.; Johnson, G.; Kim, N.; et al. Expression of amphiregulin, cripto-1 and heregulin-alpha in human breast-cancer cells. Int. J. Oncol. 1993, 2, 903–911. [Google Scholar] [CrossRef]
- Qi, C.F.; Liscia, D.S.; Normanno, N.; Merio, G.; Johnson, G.R.; Gullick, W.J.; Ciardiello, F.; Saeki, T.; Brandt, R.; Kim, N. Expression of transforming growth factor a, amphiregulin and cripto-1 in human breast carcinoma. Br. J. Cancer 1994, 69, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Saeki, T.; Salomon, D.; Normanno, N.; Johnson, G.; Gullick, W.; Mandai, K.; Moriwaki, S.; Takashima, S.; Kuniyasu, M.; Tahara, E.; et al. Immunohistochemical detection of cripto-1, amphiregulin and transforming growth factor alpha in human gastric carcinomas and intestinal metaplasias. Int. J. Oncol. 1994, 5, 215–223. [Google Scholar] [CrossRef]
- Baldassarre, G.; Romano, A.; Armenante, F.; Rambaldi, M.; Paoletti, I.; Sandomenico, C.; Pepe, S.; Staibano, S.; Salvatore, G.; De Rosa, G.; et al. Expression of teratocarcinoma-derived growth factor-1 (TDGF-1) in testis germ cell tumors and its effects on growth and differentiation of embryonal carcinoma cell line NTERA2/D1. Oncogene 1997, 15, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, M.; Yasui, W.; Naito, A.; Ohashi, K.; Kobayashi, E.; Noguchi, O.; Horiguchi, K.; Okita, S.; Tsujiuchi, T.; Kitada, H.; et al. Expression of cripto in human pancreatic tumors. Jpn. J. Cancer Res. 1994, 85, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Friess, H.; Yamanaka, Y.; Büchler, M.; Kobrin, M.S.; Tahara, E.; Korc, M. Cripto, a member of the epidermal growth factor family, is over-expressed in human pancreatic cancer and chronic pancreatitis. Int. J. Cancer 1994, 56, 668–674. [Google Scholar] [CrossRef]
- Fujii, K.; Yasui, W.; Kuniyasu, H.; Itoh, M.; Hanada, K.; Kajiyama, G.; Tahara, E. Expression of cripto in human gall bladder lesions. J. Path. 1996, 180, 166–168. [Google Scholar] [CrossRef]
- Okajima, E.; Tsutsumi, M.; Okajima, E.; Konishi, Y. Cripto expression in human urological tumors. Cancer Lett. 1997, 111, 67–70. [Google Scholar] [CrossRef]
- Xing, P.X.; Hu, X.F.; Pietersz, G.A.; Hosick, H.L.; McKenzie, I.F.C. Cripto: A novel target for antibody-based cancer immunotherapy. Cancer Res. 2004, 64, 4018–4023. [Google Scholar] [CrossRef]
- Gong, Y.P.; Yarrow, P.M.; Carmalt, H.L.; Kwun, S.Y.; Kennedy, C.W.; Lin, B.P.C.; Xing, P.X.; Gillet, D.J. Overexpression of cripto and its prognostic significance in breast cancer: A study with long-term survival. Eur. J. Surg. Oncol. 2007, 33, 438–443. [Google Scholar] [CrossRef]
- Brandt, R.; Normanno, N.; Gullick, W.J.; Lin, J.H.; Harkins, R.; Schneider, D.; Jones, B.W.; Ciardiello, F.; Persico, M.G.; Armenante, F.; et al. Identification and biological characterization of an epidermal growth factor-related protein: Cripto-1. J. Biol. Chem. 1994, 269, 17320–17328. [Google Scholar] [CrossRef]
- Schiffer, S.G.; Foley, S.; Kaffashan, A.; Hronowski, X.; Zichittella, A.E.; Yeo, C.Y.; Miatkowski, K.; Adkins, H.B.; Damon, B.; Whitman, M.; et al. Fucosylation of cripto is required for it ability to facilitate nodal signaling. J. Biol. Chem. 2001, 276, 37769–37778. [Google Scholar] [CrossRef] [PubMed]
- Adkins, H.B.; Bianco, C.; Schiffer, S.G.; Rayhorn, P.; Zafari, M.; Cheung, A.E.; Orozco, O.; Olson, D.; De Luca, A.; Chen, L.L.; et al. Antibody blockage of the cripto CFC domain suppresses tumor cell growth in vivo. J. Clin. Investig. 2003, 112, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Salomon, D.S. Targeting the embryonic gene Cripto-1 in cancer and beyond. Expert Opin. Ther. Pat. 2010, 20, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.K.; Olson, D.L.; Sun, Y.; Wen, D.; Wortham, K.A.; Antognetti, G.; Cheung, A.E.; Orozco, O.E.; Yang, L.; Bailly, V.; et al. An antibody-cytotoxic conjugate, BIIB015, is a new targeted therapy for Cripto positive tumours. Eur. J. Cancer 2011, 47, 1736–1746. [Google Scholar] [CrossRef]
- Gudbergsson, J.M.; Duroux, M. An evaluation of different Cripto-1 antibodies and the variable results. J. Cell Biochem. 2020, 121, 545–556. [Google Scholar] [CrossRef]
- Focà, G.; Iaccarion, E.; Focà, A.; Sanguigno, L.; Untiveros, G.; Cuevas-Nunex, M.; Strizzi, L.; Leonardi, A.; Ruvo, M.; Sandomenico, A. Development of conformational antibodies targeting Cripto-1 with neutralizing effects in vitro. Biochimie 2019, 158, 246–256. [Google Scholar] [CrossRef]
- Ishii, H.; Zahra, M.H.; Takayanagi, A.; Seno, M. A novel artificially humanized anti-Cripto-1 antibody suppressing cancer cell growth. Int. J. Mol. Sci. 2021, 22, 1709. [Google Scholar] [CrossRef]
- King-Underwood, L.; Gudnason, V.; Humphries, S.; Seed, M.; Patel, D.; Knight, B.; Soutar, A. Identification of the 664 proline to leucine mutation in the low-density lipoprotein receptor in four unrelated patients with familial hypercholesterolemia in the UK. Clin. Genet. 1991, 40, 17–28. [Google Scholar] [CrossRef]
- Suzuki, K.; Fukabori, Y.; Nakazato, H.; Hasumi, M.; Matsui, H.; Ito, K.; Kurokawa, K.; Yamanaka, H. Novel amino acid substitutional mutation, tyrosine-739-aspartic acid, in the androgen receptor gene in competed androgen insensitivity syndrome. Int. J. Androl. 2001, 24, 183–188. [Google Scholar] [CrossRef]
- Glassy, M. Creating hybridomas by electrofusion. Nature 1988, 333, 579–780. [Google Scholar] [CrossRef]
- van Duijn, G.; Langedijk, J.P.; de Boer, M.; Tager, J.M. High yields of specific hybriomas obtained by electrofusion of murine lymphocytes immunized in vivo or in vitro. Exp. Cell Res. 1989, 183, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Pilgaard, L.; Mortensen, J.H.; Henriksen, M.; Olesen, P.; Sørensen, P.; Laursen, R.; Vyberg, M.; Agger, R.; Zachar, V.; Moos, T.; et al. Cripto-1 expression in glioblastoma multiforme. Brain Pathol. 2014, 24, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Sugo, S.; Minamino, N.; Kangawa, K.; Miyamoto, K.; Kitamura, K.; Sakata, J.; Eto, T.; Matsuo, H. Endothelial cells actively synthesis and secrete adrenomedullin. Biochem. Biophys. Res. Commun. 1994, 201, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Nikitenko, L.L.; Fox, S.B.; Kehoe, S.; Rees, C.P.; Bicknell, R. Adrenomedullin and tumour angiogenesis. Br. J. Cancer 2006, 94, 1–7. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef]
- Knowles, J.; Loizidou, M.; Taylor, I. Endothelin-1 and angiogenesis in cancer. Curr. Vasc. Pharmacol. 2005, 3, 309–314. [Google Scholar] [CrossRef]
- Abcam (Abcam, Inc., Cambridge, UK). Customer Support Service and In-House WB Analysis Demonstrating an ≅50kDa CR1 Band Observed in Human Embryonic Stem Cell Lysates Using Their Rabbit Anti-CR1 Polyclonal Antisera (ab19917). Personal communication, 25 March 2023.
- Bianco, C.; Strizzi, L.; Mancino, M.; Rehman, A.; Hamada, S.; Watanabe, K.; De Luca, A.; Jones, B.; Balogh, G.; Russo, J.; et al. Identification of cripto-1 as a novel serologic marker for breast and colon cancer. Clin. Cancer Res. 2006, 12, 5158–5164. [Google Scholar] [CrossRef]
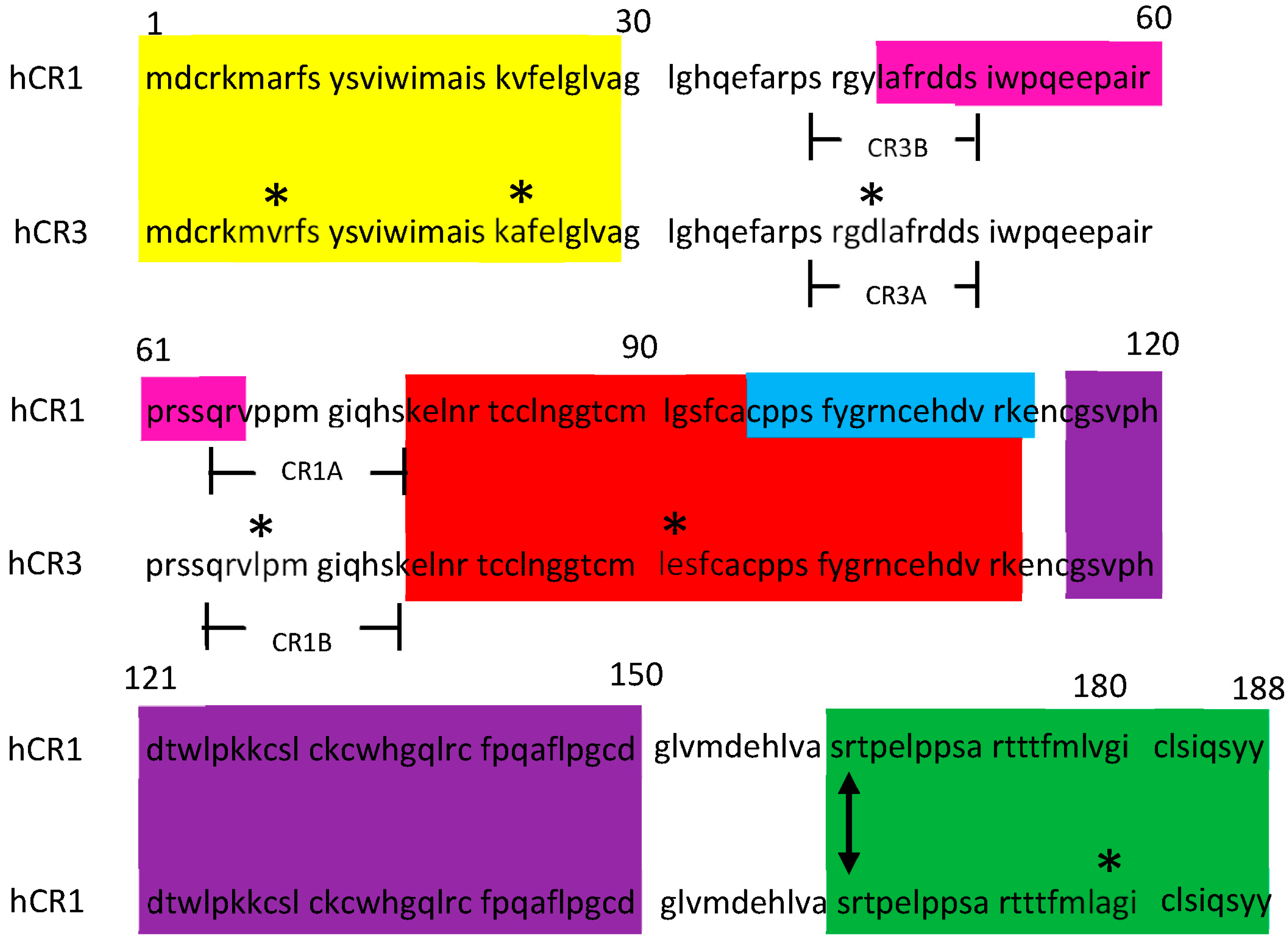

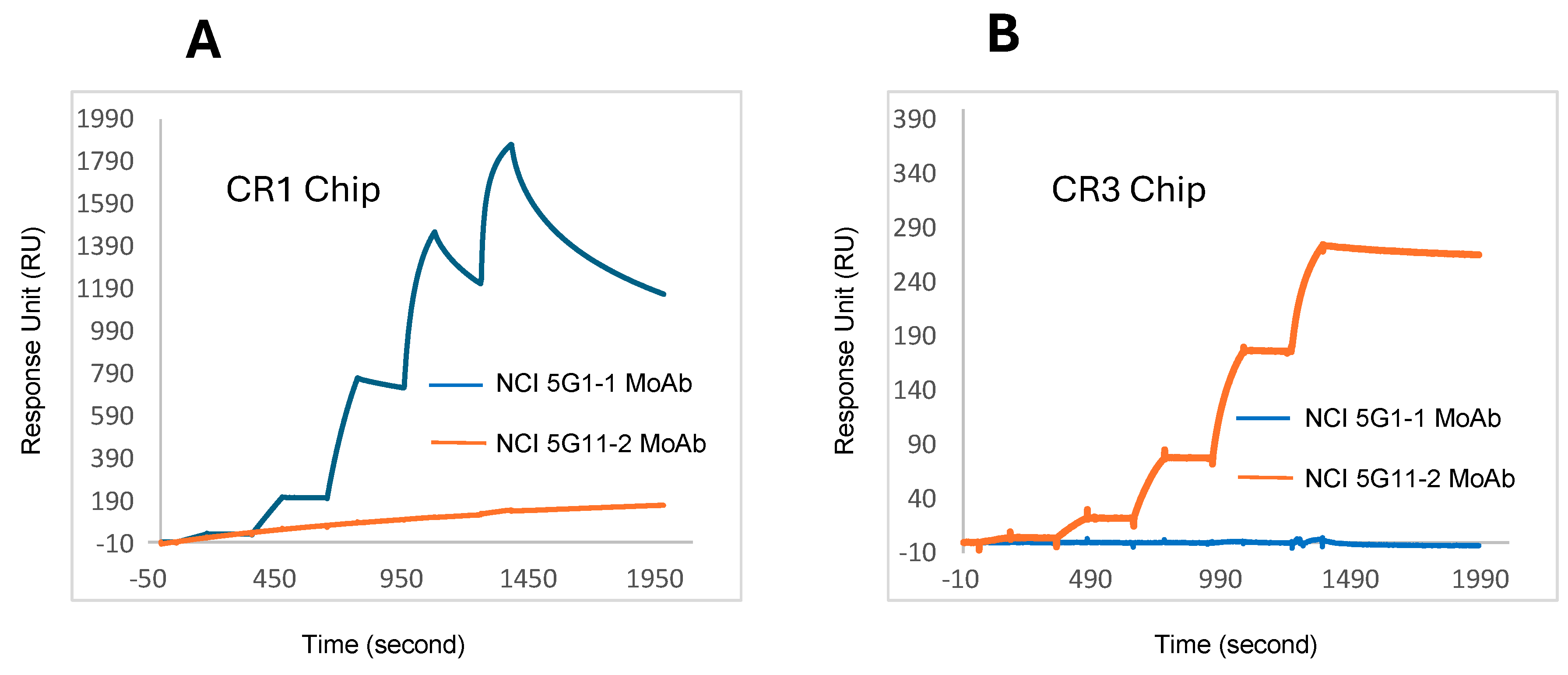

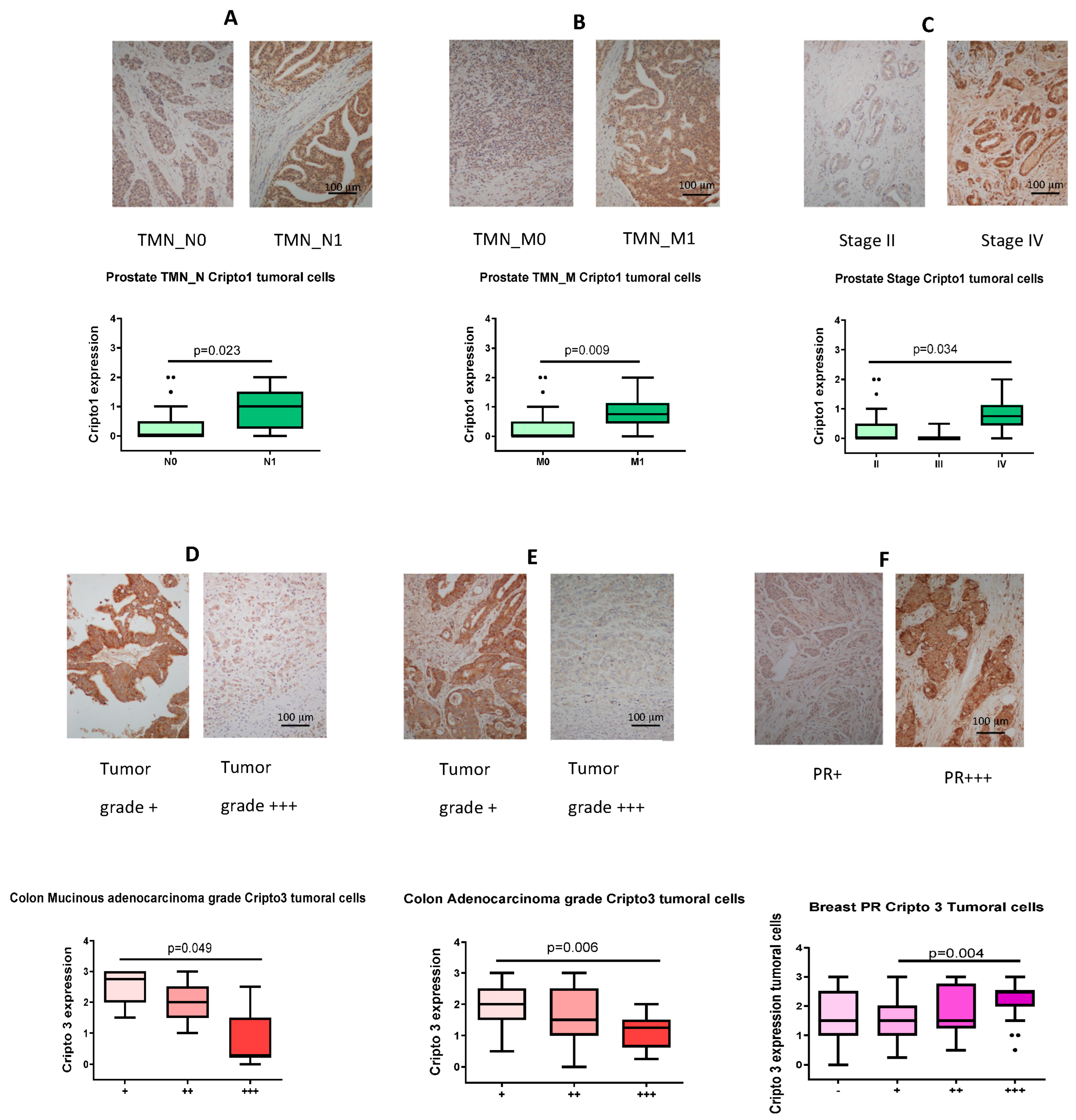
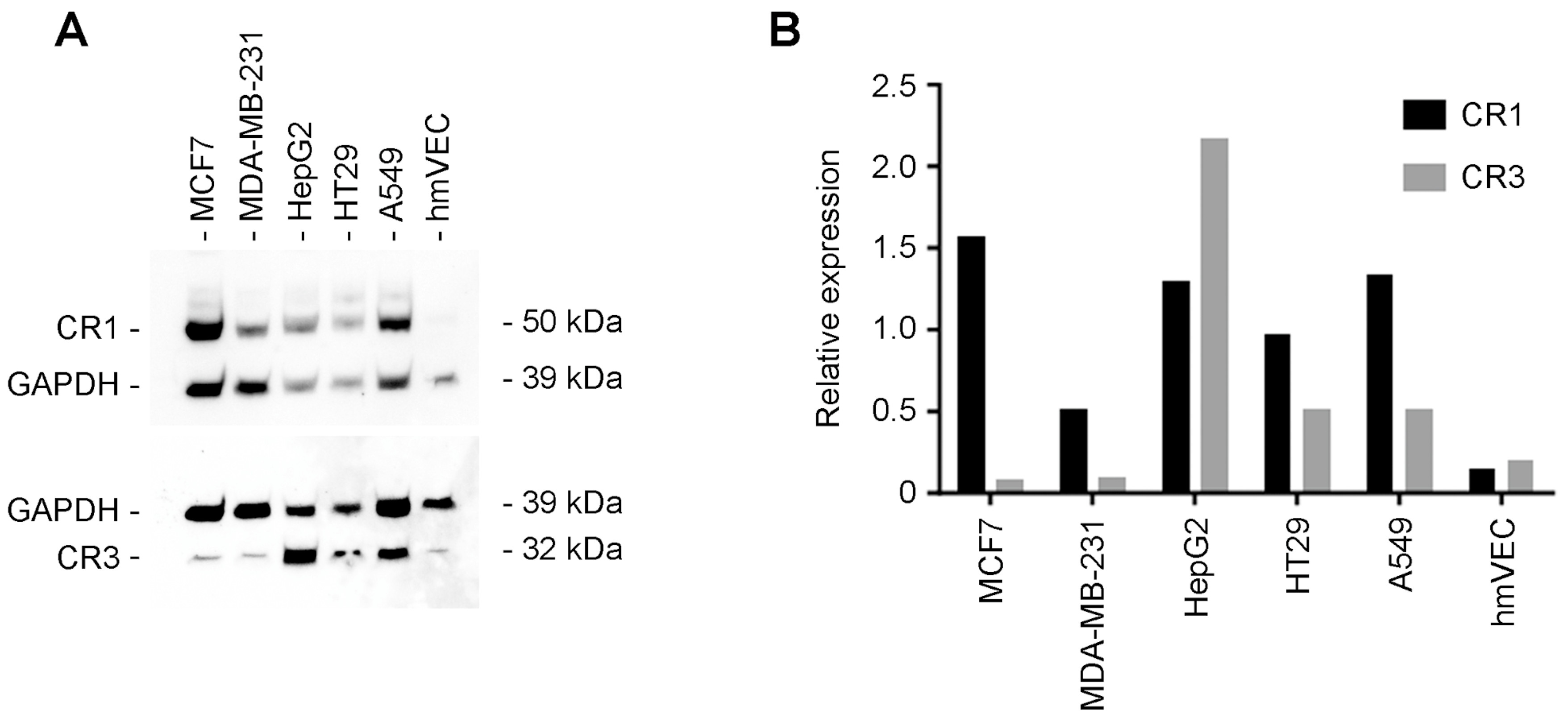
| Screening | Positivity | Positivity in | Positivity | NCI Truncation | ||||
|---|---|---|---|---|---|---|---|---|
| C57BL/6N | Scale 96- | in Primary | Confirmation | 24-Well | Final | Best Hybridomas | ||
| Order No. | Fusion No. | Wells/Plate | Screen | Screen | Plate | Delivery | 5/20 Clones | |
| U7733DJ240 | Best 20 | 5G1-1,10F7-1 | ||||||
| (Anti-CR1) | #B1122 | 15 Plates | 233 Clones | 97 Clones | 28 Clones | Clones | 17G2-1, 18A9-1, | |
| & 18E2-1 | ||||||||
| U6044DJ240 | Best 20 | 1G10-2, 2D9-2 | ||||||
| (Anti-CR3) | #B850 | 15 Plates | 319 Clones | 139 Clones | 66 Clones | Clones | 2G1-2, 3B1-2, | |
| & 5G11-2 | ||||||||
| Monoclonal | Association Rate | Dissociation Rate | Dissociation Constant |
|---|---|---|---|
| Antibody Clone | Kon (1/Ms) | Koff (1/s) | KD (M) |
| NCI 5G1-1 | 7.57 × 104 | 2.45 × 10−3 | 3.23 × 10−8 |
| NCI 5G11-2 | 5.33 × 104 | 3.36 × 10−5 | 6.30 × 10−10 |
| Anti-Cripto | R&D System | GenScript | GenScript | MyBioSource | R&D Systems | R&D Systems | BSA Neg | Binding |
|---|---|---|---|---|---|---|---|---|
| Reagents | Human CR1 | Human CR1 | Human CR3 | Human CR3 | Mouse CR1 | Human Cryptic | Control | Selectivity |
| Baseline | ||||||||
| NCI 5G1-1 | 1:51,200 | 1:51,200 | Binding (BB) | BB | BB | BB | BB | CR1 |
| (1.9 ng/mL) | (1.9 ng/mL) | |||||||
| NCI 511-2 | BB | BB | 1:51,200 | 1:51,200 | BB | BB | BB | CR3 |
| (1.9 ng/mL) | (1.9 ng/mL) | |||||||
| CR1/CR3 | ||||||||
| NCI 17G2-1 | 1:25,000 | 1:12,800 | 1:12,800 | 1:12,800 | BB | BB | BB | (Pan Rx) |
| (3.9 ng/mL) | (7.8 ng/mL) | (7.8 ng/mL) | (7.8 ng/mL) | |||||
| Santa Cruz | ||||||||
| sc376448 | 1:3200 | 1:6400 | 1:6400 | 1:12,800 | BB | BB | BB | Pan Rx |
| (31.3 ng/mL) | (15.6 ng/mL) | (15.6 ng/mL) | (7.8 ng/mL) | |||||
| R&D Systems | ||||||||
| MAB2772 | 1:25,000 | 1:25,000 | 1:25,000 | 1:12,800 | BB | BB | BB | Pan Rx |
| (3.9 ng/mL) | (3.9 ng/mL) | (3.9 ng/mL) | (3.9 ng/mL) | |||||
| Abcam | ||||||||
| ab108391 | 1:102,400 | 1:51,200 | 1:102,400 | 1:204,800 | BB | BB | BB | Pan Rx |
| (0.98 ng/mL) | (1.9 ng/mL) | (0.98 ng/mL) | (0.48 ng/mL) | |||||
| Abcam | ||||||||
| ab133236 | 1:1600 | 1:12,800 | 1:25,000 | 1:51,200 | 1:3200 | BB | BB | Pan Rx |
| (62.5 ng/mL) | (7.8 ng/mL) | (3.9 ng/mL) | (1.95 ng/mL) | (31.2 ng/mL) | ||||
| CUSABIO | ||||||||
| PA302689 | 1:800 | 1:3200 | 1:6400 | 1:12,800 | BB | BB | BB | Pan Rx |
| (125 ng/mL) | (31.2 ng/mL) | (15.6 ng/mL) | (7.8 ng/mL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuttitta, F.; García-Sanmartín, J.; Feng, Y.; Sunday, M.E.; Kim, Y.S.; Martínez, A. Human Cripto-1 and Cripto-3 Protein Expression in Normal and Malignant Settings That Conflicts with Established Conventions. Cancers 2024, 16, 3577. https://doi.org/10.3390/cancers16213577
Cuttitta F, García-Sanmartín J, Feng Y, Sunday ME, Kim YS, Martínez A. Human Cripto-1 and Cripto-3 Protein Expression in Normal and Malignant Settings That Conflicts with Established Conventions. Cancers. 2024; 16(21):3577. https://doi.org/10.3390/cancers16213577
Chicago/Turabian StyleCuttitta, Frank, Josune García-Sanmartín, Yang Feng, Mary Elizabeth Sunday, Young S. Kim, and Alfredo Martínez. 2024. "Human Cripto-1 and Cripto-3 Protein Expression in Normal and Malignant Settings That Conflicts with Established Conventions" Cancers 16, no. 21: 3577. https://doi.org/10.3390/cancers16213577
APA StyleCuttitta, F., García-Sanmartín, J., Feng, Y., Sunday, M. E., Kim, Y. S., & Martínez, A. (2024). Human Cripto-1 and Cripto-3 Protein Expression in Normal and Malignant Settings That Conflicts with Established Conventions. Cancers, 16(21), 3577. https://doi.org/10.3390/cancers16213577







