Cohort Profile: VZNKUL–NMIBC Quality Indicators Program: A Flemish Prospective Cohort to Evaluate the Quality Indicators in the Treatment of Non-Muscle-Invasive Bladder Cancer
Simple Summary
Abstract
1. Introduction
2. Cohort Description
2.1. Demographic and Medical History Information
2.2. Surgical and Adjuvant Treatment Information
2.3. Follow-Up and Survival Information
2.4. Statistical Analysis
3. Findings to Date
3.1. Patient Characteristics
3.2. Surgical Characteristics
3.3. Tumor Characteristics
3.4. Adjuvant Surgical and Intravesical Treatment Characteristics
3.5. MIBC Patient Characteristics
3.6. Follow-Up and Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| ASA | American Society of Anesthesiologists |
| BC | Bladder cancer |
| BCG | Bacillus Calmette–Guérin |
| CCI | Charlson comorbidity index |
| CFS | Cystectomy-free survival |
| CIS | Carcinoma in situ |
| COBLAnCE | A Cohort to Study Bladder Cancer |
| CR | Complete resection |
| CSS | Cancer-specific survival |
| DM | Detrusor muscle |
| EAU | European Association of Urology |
| eCRF | Electronic case report form |
| FDA | Food and Drug Administration |
| HG | High grade |
| IQR | Interquartile range |
| KWS | Klinisch Werkstation |
| LG | Low grade |
| LVI | Lymphovascular invasion |
| MDT | Multidisciplinary team |
| MIBC | Muscle-invasive bladder cancer |
| NAC | Neoadjuvant chemotherapy |
| NMIBC | Non-muscle-invasive bladder cancer |
| OS | Overall survival |
| PFS | Progression-free survival |
| QCI | Quality control indicator |
| RC | Radical cystectomy |
| RESECT | Transurethral REsection and Single-instillation intra-vesical chemotherapy Evaluation in bladder Cancer Treatment |
| reTURBT | Repeat TURBT |
| RFS | Recurrence-free survival |
| SIVIC | Single intravesical instillation of chemotherapy |
| TNM | Tumor-node-metastasis |
| TURBT | Transurethral resection of the bladder tumor |
| UC | Urothelial carcinoma |
| UTUC | Upper tract urothelial carcinoma |
| VZNKUL | Vlaams Ziekenhuisnetwerk–KU Leuven |
| WHO | World Health Organization |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates and incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Belgian Cancer Registry. Cancer Burden in Belgium 2004–2013; Belgian Cancer Registry: Brussels, Belgium, 2015; pp. 191–199. [Google Scholar]
- World Health Organization. World Health Statistics 2016: Monitoring Health for the SDGs, Sustainable Development Goals. Available online: http://www.who.int/publications/i/item/9789241565264 (accessed on 10 June 2024).
- Donsky, H.; Coyle, S.; Scosyrev, E.; Messing, E.M. Sex differences in incidence and mortality of bladder and kidney cancers: National estimates from 49 countries. Urol. Oncol. 2014, 32, 40.e23–40.e31. [Google Scholar] [CrossRef]
- Yee, D.S.; Ishill, N.M.; Lowrance, W.T.; Herr, H.W.; Elkin, E.B. Ethnic differences in bladder cancer survival. Urology 2011, 78, 544–549. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Dominguez Escrig, J.L.D.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Jubber, I.; Ong, S.; Bukavina, L.; Black, P.C.; Comperat, E.; Kamat, A.M.; Kiemeney, L.; Lawrentschuk, N.; Lerner, S.P.; Meeks, J.J.; et al. Epidemiology of bladder cancer in 2023: A systematic review of risk factors. Eur. Urol. 2023, 84, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta, T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–475. [Google Scholar] [CrossRef]
- Fernandez-Gomez, J.; Madero, R.; Solsona, E.; Unda, M.; Martinez-Pineiro, L.; Gonzalez, M.; Portillo, J.; Ojea, A.; Pertusa, C.; Rodriguez-Molina, J.; et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with Bacillus Calmette-Guerin: The CUETO scoring model. J. Urol. 2009, 182, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Lammers, R.J.; Hendriks, J.C.; Rodriguez Faba, O.R.; Witjes, W.P.; Palou, J.; Witjes, J.A. Prediction model for recurrence probabilities after intravesical chemotherapy in patients with intermediate-risk non-muscle-invasive bladder cancer, including external validation. World J. Urol. 2016, 34, 173–180. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerova, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European Association of Urology (EAU) prognostic factor risk groups for non-muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: An update from the EAU NMIBC Guidelines Panel. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef]
- Hendricksen, K.; Aziz, A.; Bes, P.; Chun, F.K.H.; Dobruch, J.; Kluth, L.A.; Gontero, P.; Necchi, A.; Noon, A.P.; van Rhijn, B.W.G.; et al. Discrepancy between European Association of Urology guidelines and daily practice in the management of non-muscle-invasive bladder cancer: Results of a European survey. Eur. Urol. Focus 2019, 5, 681–688. [Google Scholar] [CrossRef]
- Gontero, P.; Oderda, M.; Altieri, V.; Bartoletti, R.; Cai, T.; Colombo, R.; Curotto, A.; Di Stasi, S.; Maffezzini, M.; Tamagno, S.; et al. Are referral centers for non-muscle-invasive bladder cancer compliant to EAU guidelines? A report from the vesical antiblastic therapy Italian study. Urol. Int. 2011, 86, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sano, T.; Kawamura, S.; Ikeda, Y.; Orikasa, K.; Tanaka, T.; Kyan, A.; Ota, S.; Tokuyama, S.; Saito, H.; et al. Improving compliance with guidelines may lead to favorable clinical outcomes for patients with non-muscle-invasive bladder cancer: A retrospective multicenter study. Int. J. Urol. 2023, 30, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.O.; Moro, J.C.; Ribeiro, L.F.B.; Voris, B.R.I.; Sadi, M.V. Are we following the guidelines on non-muscle-invasive bladder cancer? Int. Braz. J. Urol. 2016, 42, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Matulay, J.T.; Tabayoyong, W.; Duplisea, J.J.; Chang, C.; Daneshmand, S.; Gore, J.L.; Holzbeierlein, J.M.; Karsh, L.I.; Kim, S.P.; Konety, B.R.; et al. Variability in adherence to guidelines based management of nonmuscle invasive bladder cancer among Society of Urologic Oncology (SUO) members. Urol. Oncol. 2020, 38, 796.e1–796.e6. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Miura, N.; Babjuk, M.; Karakiewicz, P.I.; Mostafaei, H.; Laukhtina, E.; Quhal, F.; Motlagh, R.S.; Pradere, B.; Kimura, S.; et al. Low compliance to guidelines in nonmuscle-invasive bladder carcinoma: A systematic review. Urol. Oncol. 2020, 38, 774–782. [Google Scholar] [CrossRef]
- Hensley, P.J.; Bree, K.K.; Brooks, N.; Matulay, J.; Li, R.; Nogueras-Gonzalez, G.M.; Nagaraju, S.; Navai, N.; Grossman, H.B.; Dinney, C.P.; et al. Implications of guideline-based, risk-stratified restaging transurethral resection of high-grade Ta urothelial carcinoma on bacillus Calmette-Guérin therapy outcomes. Eur. Urol. Oncol. 2022, 5, 347–356. [Google Scholar] [CrossRef]
- Abushamma, F.; Khayyat, Z.; Soroghle, A.; Zyoud, S.H.; Jaradat, A.; Akkawi, M.; Aburass, H.; Qaddumi, I.K.K.; Odeh, R.; Salameh, H.; et al. The impact of non-compliance to a standardized risk-adjusted protocol on recurrence, progression, and mortality in non-muscle-invasive bladder cancer. Cancer Manag. Res. 2021, 13, 2937–2945. [Google Scholar] [CrossRef]
- Mariappan, P.; Johnston, A.; Trail, A.; Hamid, S.; Hollins, G.; Dreyer, B.A.; Ramsey, S.; Padovani, L.; Garau, R.; Enriquez, J.G.; et al. Achieving benchmarks for national quality indicators reduces recurrence and progression in non-muscle-invasive bladder cancer. Eur. Urol. Oncol. 2024. [Google Scholar] [CrossRef]
- Akand, M.; Muilwijk, T.; Cornelissen, J.; Van Bruwaene, S.; Vander Eeckt, K.; Baekelandt, F.; Mattelaer, P.; Van Reusel, R.; Van Cleynenbreugel, B.; Joniau, S.; et al. Development of a prospective data registry system for non-muscle-invasive bladder cancer patients incorporated in the electronic patient file system. Front Oncol. 2019, 9, 1402. [Google Scholar] [CrossRef]
- Akand, M.; Muilwijk, T.; Cornelissen, J.; Van Bruwaene, S.; Vander Eeckt, K.; Baekelandt, F.; Mattelaer, P.; Van Reusel, R.; Van Cleynenbreugel, B.; Joniau, S.; et al. Quality control indicators for transurethral resection of bladder tumor: Results from an embedded Belgian multicenter prospective registry. Eur. Urol. 2023, 6, 422–430. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (Ta, T1 and carcinoma in situ)–2019 update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Food and Drug Administration Guidance. BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment (Guidance for Industry). 2018. Available online: www.regulations.gov/document/FDA-2018-D-0342-0002 (accessed on 10 June 2024).
- Lin, D.Y.; Wei, L.J. The robust inference for the Cox proportional hazards model. J. Am. Stat. Assoc. 1989, 84, 1074–1078. [Google Scholar] [CrossRef]
- Lebret, T.; Bonastre, J.; Fraslin, A.; Neuzillet, Y.; Droupy, S.; Rebillard, X.; Vordos, D.; Guy, L.; Villers, A.; Schneider, M.; et al. Cohort profile: COBLAnCE: A French prospective cohort to study prognostic and predictive factors in bladder cancer and to generate real-world data on treatment patterns, resource use and quality of life. BMJ Open 2023, 13, e075942. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Steinberg, G.; Witjes, J.A.; Li, R.; Shariat, S.F.; Roupret, M.; Babjuk, M.; Bivalacqua, T.J.; Psutka, S.P.; Williams, S.B.; et al. Intermediate-risk non-muscle-invasive bladder cancer: Updated consensus definition and management recommendations from the International Bladder Cancer Group. Eur. Urol. Oncol. 2022, 5, 505–516. [Google Scholar] [CrossRef]
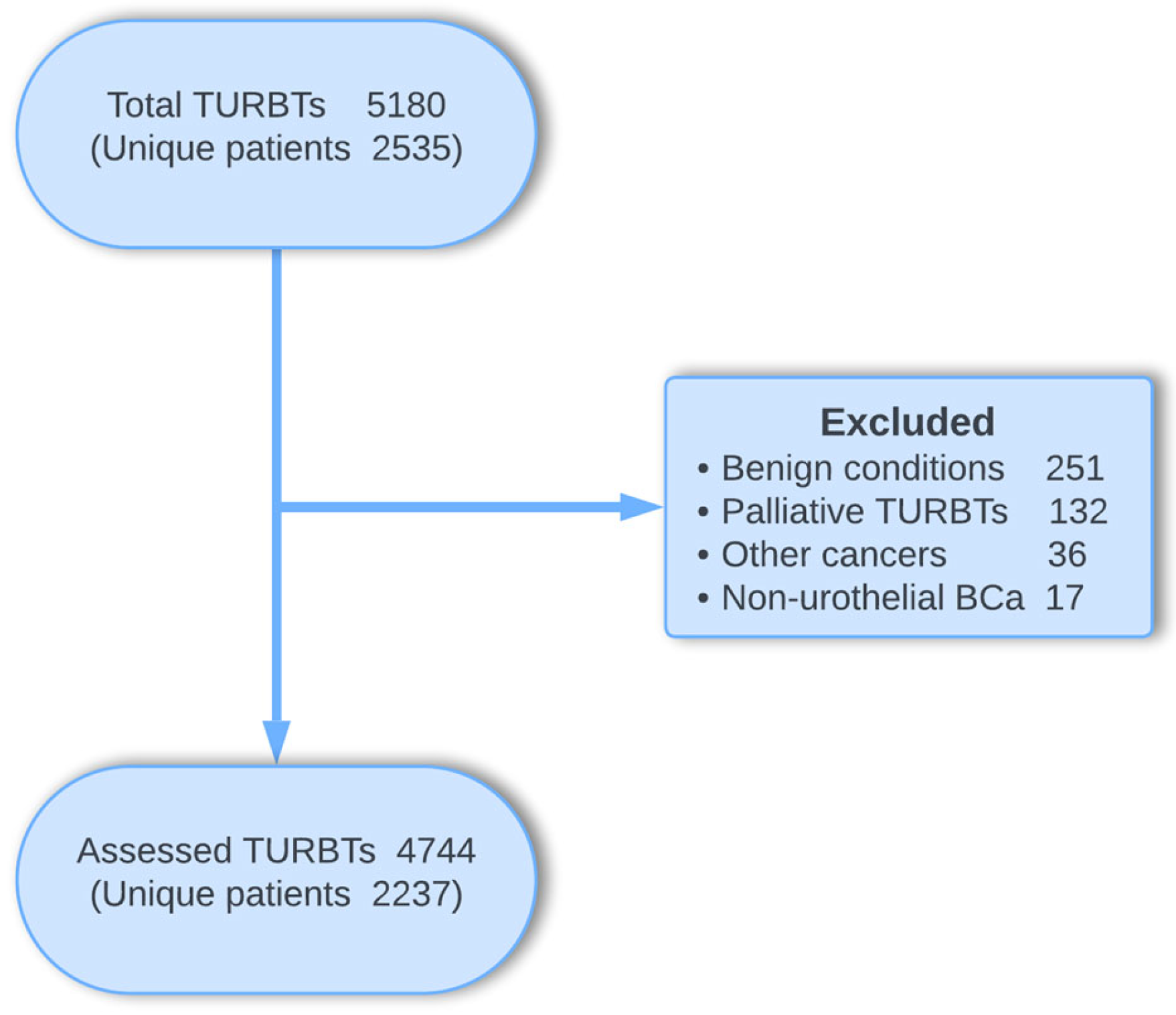
| Total n = 2237 | |||
|---|---|---|---|
| Gender | Male | 1792 | 80.1% |
| Female | 445 | 19.9% | |
| Age | Median (IQR) | 73 | 73–74 |
| >70 years | 1375 | 61.5% | |
| ≤70 years | 862 | 38.5% | |
| ASA score | 1 | 138 | 6.2% |
| 2 | 971 | 43.4% | |
| 3 | 592 | 26.5% | |
| 4 | 27 | 1.2% | |
| Not known | 509 | 22.7% | |
| CCI | 0 | 640 | 33% |
| 1 | 507 | 22.7% | |
| 2 | 416 | 18.6% | |
| 3 | 257 | 11.5% | |
| 4 | 122 | 5.5% | |
| 5 | 49 | 2.2% | |
| ≥6 | 67 | 3% | |
| Not known | 79 | 3.5% | |
| Smoking status | Never smoked | 471 | 21% |
| Former smoker | 1066 | 47.7% | |
| Active smoker | 474 | 21.2% | |
| Not known | 226 | 10.1% | |
| History of pelvic radiotherapy | No | 2106 | 94.2% |
| Yes | 128 | 5.7% | |
| Not known | 3 | 0.1% | |
| History of UTUC | 125 | 5.6% | |
| History of prior BCG | 155 | 6.9% | |
| History of prior chemotherapy | 158 | 7.1% | |
| Total n = 4744 | |||
|---|---|---|---|
| Time from Dx to TURBT (days) median (IQR) (min–max) | 19 (10–37) (0–375) | ||
| Operation duration (minutes) median (IQR) (min–max) | 20 (15–30) (1–301) | ||
| Use of visual enhancement | No | 4231 | 89.2% |
| Yes, Hexvix | 469 | 9.9% | |
| Yes, NBI | 14 | 0.3% | |
| Unknown | 30 | 0.6% | |
| Macroscopical appearance of tumor | Superficial | 3976 | 83.8% |
| Invasive | 619 | 13% | |
| NK/NR | 149 | 3.2% | |
| Macroscopically complete resection a | No | 218 | 7.4% |
| Yes | 2674 | 91% | |
| NK/NR/NA | 47 | 1.6% | |
| reTURBT planned b | No | 619 | 54% |
| Yes | 527 | 46% | |
| reTURBT performed c | No | 46 | 8.7% |
| Yes | 481 | 91.2% | |
| Bladder perforation | 358 | 7.5% | |
| Bleeding | 113 | 2.4% | |
| Postoperative SIVIC_requested d | No | 499 | 32.6% |
| Yes | 956 | 62.4% | |
| NK/NR | 78 | 5% | |
| Postoperative SIVIC_ordered d | No | 39 | 2.5% |
| Yes | 917 | 59.8% | |
| Postoperative SIVIC_received d | No | 45 | 2.9% |
| Yes | 872 | 56.9% | |
| Time to SIVIC e (minutes) median (IQR) (min–max) | 282 (194–467.5) (20–1768) | ||
| Time from TURBT to PR f (days) median (IQR) (min–max) | 5 (3–7) (1–64) | ||
| Cases discussed in MDT g | 2875 | 86.9% | |
| Time from TURBT to MDT h (days) median (IQR) (min–max) | 12 (7–19) (1–139) | ||
| Time from PR to MDT i (days) median (IQR) (min–max) | 7 (3–12) (0–134) | ||
| Follow-up time (months) median (IQR) (min–max) | 57 (35–83) (0–136) | ||
| Reasons | n = 499 | |
|---|---|---|
| Surgeon’s choice | 153 | 30.7% |
| Bladder perforation (overt/suspicious) | 95 | 19% |
| Deep/extensive resection | 65 | 13% |
| Need for continuous irrigation | 45 | 9% |
| Patient comorbidity | 18 | 3.6% |
| Deep resection + continuous irrigation (+/− perforation) | 17 | 3.4% |
| Concomitant TURP | 7 | 1.4% |
| Already received/receiving MMC at the moment | 7 | 1.4% |
| Patient age | 6 | 1.2% |
| Other | 12 | 2.4% |
| Not known | 74 | 14.8% |
| Total n = 4744 | |||
|---|---|---|---|
| Tumor size | ≤1 cm | 1265 | 26.6% |
| 1–3 cm | 1280 | 27% | |
| ≥3 cm | 985 | 20.8% | |
| NA/NK | 1214 | 25.6% | |
| Tumor number median (IQR) (min–max) | 2 (1–3) (1–50) | ||
| Tumor multiplicity | Single | 1760 | 37.1% |
| 2–7 | 2076 | 43.8% | |
| ≥8 | 266 | 5.6% | |
| NA/NK | 642 | 13.5% | |
| Tumor localization a | Base b | 976 | 13.4% |
| Posterior wall | 1201 | 16.5% | |
| Dome | 899 | 12.3% | |
| Anterior wall | 331 | 4.5% | |
| Left wall | 1447 | 19.9% | |
| Right wall | 1448 | 19.9% | |
| Bladder neck | 749 | 10.3% | |
| Prostatic loge | 234 | 3.2% | |
| Total tumor number per localization a | Base b | 1514 | 13% |
| Posterior wall | 2030 | 17.4% | |
| Dome | 1696 | 14.5% | |
| Anterior wall | 595 | 5.1% | |
| Left wall | 2112 | 18.1% | |
| Right wall | 2283 | 19.5% | |
| Bladder neck | 1107 | 9.5% | |
| Prostatic loge | 342 | 2.9% | |
| Tumor shape | NK/NR/NA | 887 | 18.7% |
| Pws | 128 | 2.7% | |
| Pbb/N/S | 3283 | 69.2% | |
| Flat | 446 | 9.4% | |
| Tumor in diverticulum | No | 4035 | 85.1% |
| Yes | 78 | 1.6% | |
| NA | 631 | 13.3% | |
| Tumor stage | T0 | 976 | 20.6% |
| Tis | 293 | 6.2% | |
| Ta | 1891 | 39.8% | |
| T1 | 682 | 14.4% | |
| ≥T2 | 439 | 9.3% | |
| Tx | 6 | 0.1% | |
| Inconclusive c | 30 | 0.6% | |
| No PR | 427 | 9% | |
| Tumor grade (WHO1973) | Grade 1 | 334 | 7% |
| Grade 2 | 445 | 9.4% | |
| Grade 3 | 588 | 12.4% | |
| No tumor | 977 | 20.6% | |
| Not reported | 1973 | 41.6% | |
| No PR | 427 | 9% | |
| Tumor grade (WHO2004/2016) | PUNLMP | 232 | 4.9% |
| LG | 1042 | 21.9% | |
| HG | 1289 | 27.2% | |
| No tumor | 977 | 20.6% | |
| Not reported | 777 | 16.4% | |
| No PR | 427 | 9% | |
| PRs with both grading systems d | 828 | 25% | |
| Tumor-grade reporting e | TaGx | 11 | 0.6% |
| TaPUNLMP | 231 | 12.2% | |
| TaLG | 1010 | 53.4% | |
| TaHG | 506 | 26.8% | |
| TaG1 f | 47 | 2.5% | |
| TaG2 f | 70 | 3.7% | |
| TaG3 f | 16 | 0.8% | |
| T1Gx | 17 | 2.5% | |
| T1PUNLMP | 1 | 0.2% | |
| T1LG | 22 | 3.2% | |
| T1HG | 494 | 72.4% | |
| T1G1 f | 1 | 0.2% | |
| T1G2 f | 35 | 5.1% | |
| T1G3 f | 112 | 16.4% | |
| Risk stratification (at TURBT level) g | Low-risk | 259 | 7.9% |
| Intermediate-risk | 1451 | 44% | |
| High-risk | 1030 | 31.3% | |
| Highest-risk | 554 | 16.8% | |
| Risk stratification (at patient level) h | Low-risk | 259 | 14.3% |
| Intermediate-risk | 670 | 36.9% | |
| High-risk | 520 | 28.6% | |
| Highest-risk | 368 | 20.3% | |
| Concomitant CIS present i | 309 | 10.3% | |
| Detrusor muscle in specimen j | Not present | 338 | 21.1% |
| Present | 1069 | 66.9% | |
| NR | 192 | 12% | |
| LVI k | Not present | 512 | 15.5% |
| Present | 93 | 2.8% | |
| NR | 2700 | 81.7% | |
| Variant histology present l | 160 | 9.7% | |
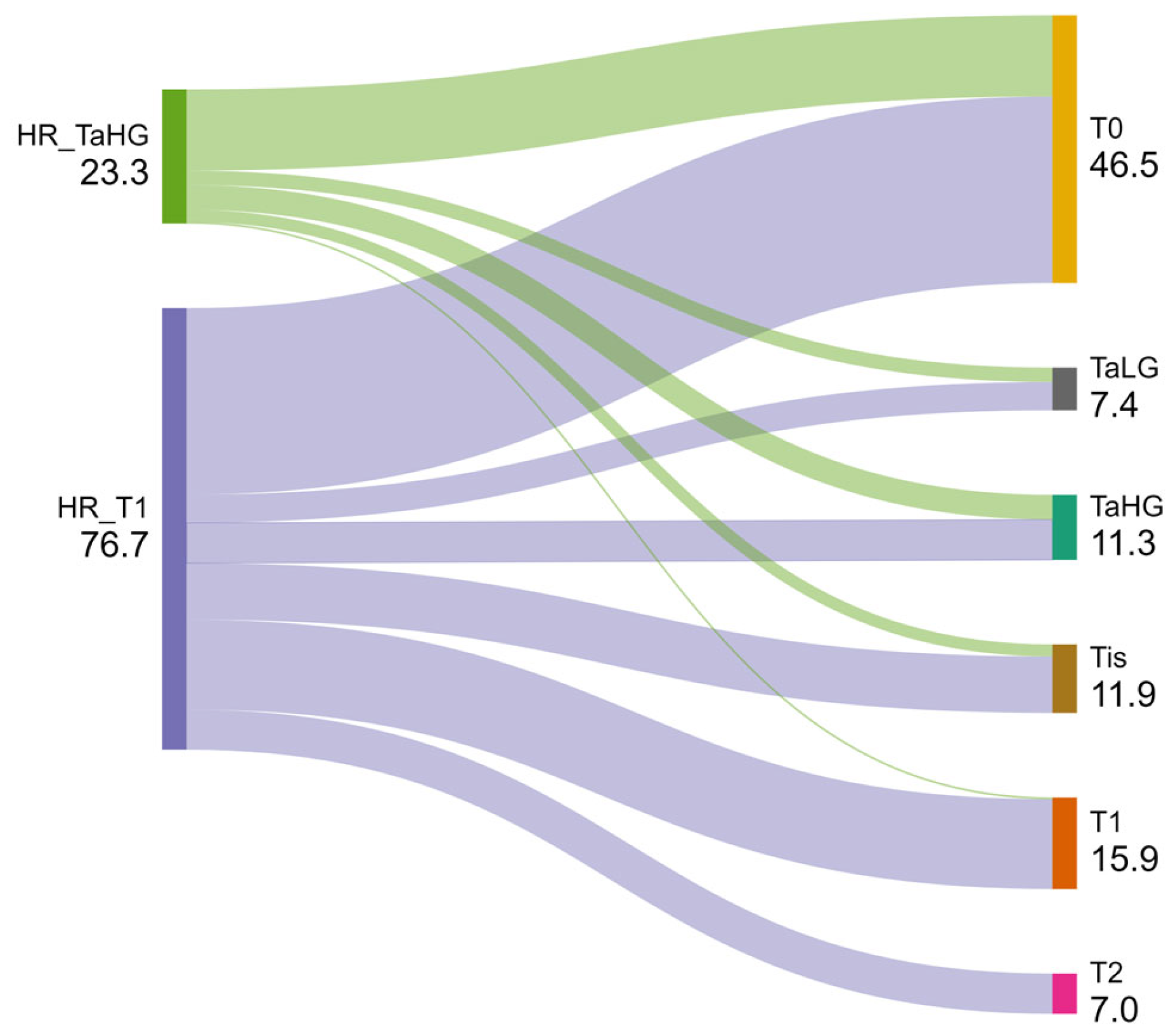
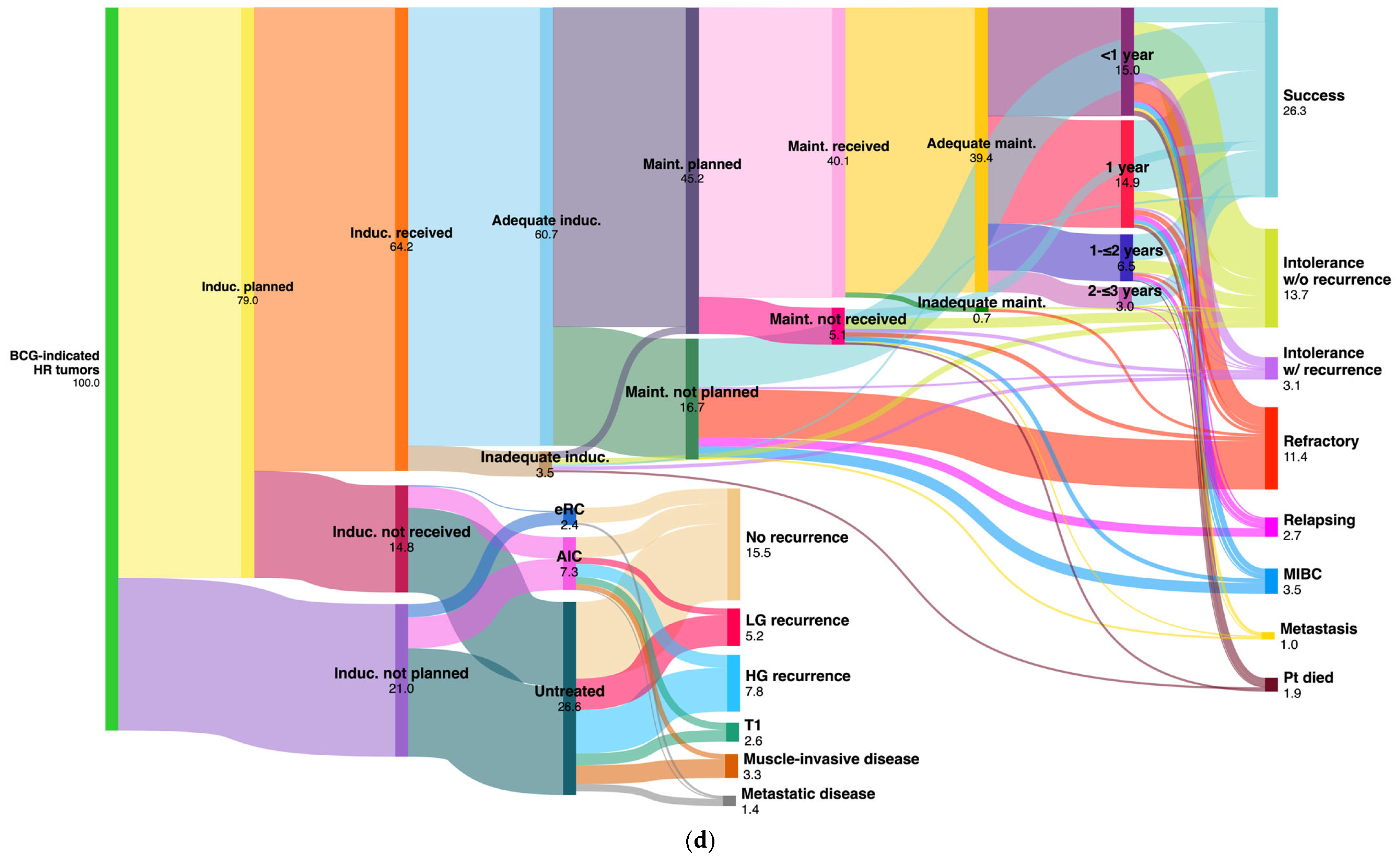
| Treatment | T1 Tumors n = 478 | TaHG Tumors n = 376 | Tis Tumors n = 118 | All HR Tumors n = 972 | All IR Tumors n = 1063 | |
| BCG induction planned | 388 (81.2%) | 276 (73.4%) | 104 (88.2%) | 768 (79%) | 41 (3.9%) | |
| BCG induction received | 327 (68.4%) | 201 (53.5%) | 96 (81.4%) | 624 (64.2%) | 35 (3.3%) | |
| Adequate BCG induction | 311 (65.1%) | 189 (50.3%) | 90 (76.3%) | 590 (60.7%) | 33 (3.1%) | |
| BCG maintenance planned | 246 (51.5%) | 131 (34.8%) | 62 (52.5%) | 439 (45.2%) | 22 (2.1%) | |
| BCG maintenance received | 217 (45.4%) | 116 (30.9%) | 57 (48.3%) | 390 (40.1%) | 22 (2.1%) | |
| Adequate BCG maintenance | 214 (44.8%) | 114 (30.3%) | 55 (46.6%) | 383 (39.4%) | 22 (2.1%) | |
| BCG maintenance <1-year | 83 (17.4%) | 40 (10.6%) | 22 (18.6%) | 146 (15%) | 17 (1.6%) | |
| BCG maintenance 1-year | 75 (15.7%) | 46 (12.2%) | 24 (20.3%) | 145 (14.9%) | 3 (0.3%) | |
| BCG maintenance 2-year a | 38 (8%) | 20 (5.3%) | 5 (4.2%) | 63 (6.5%) | 1 (0.1%) | |
| BCG maintenance 3-year b | 17 (3.6%) | 8 (2.1%) | 4 (3.4%) | 29 (3%) | 1 (0.1%) | |
| Chemotherapy received/1-year c | 23 (4.8%) | 44 (11.7%) | 5 (4.2%) | 72 (7.4%) | 245 (23%) | 14 (1.3%) |
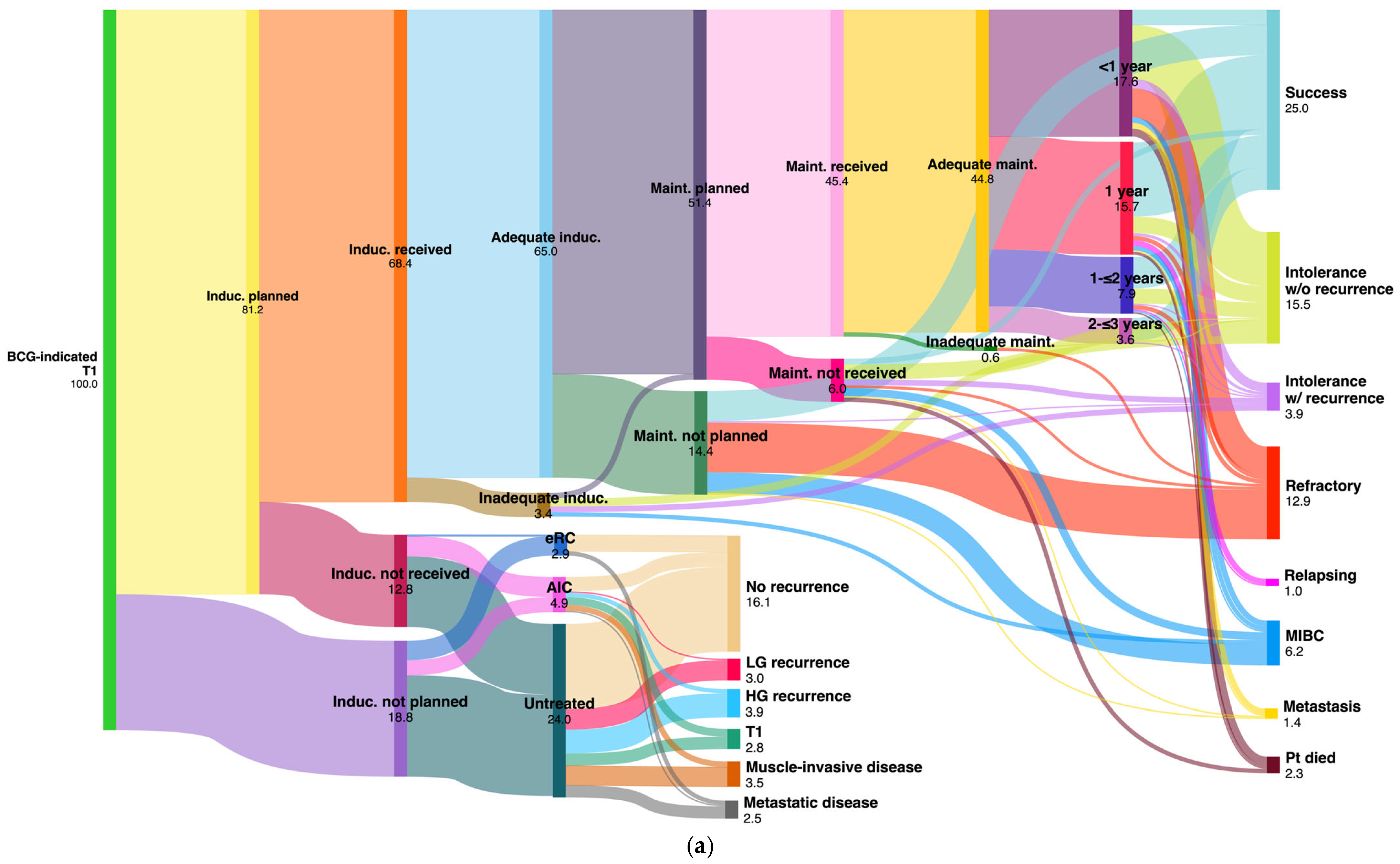
| T1 (n = 478) | BCG Result | Adequate BCG 214 (44.8%) | No maint. 113 (23.6%) | BCG induction not planned/not received 151 (31.6%) | |||||||||
| Success | 95 | (19.8%) | 24 | (5%) | AIC (23; 4.9%) | NT (114; 23.8%) | eRC (14; 2.9%) | ||||||
| Intolerance w/o recurrence | 58 | (12.1%) | 16 | (3.4%) | No recurrence | 9 | (2%) | 56 | (11.8%) | 11 | (2.3%) | ||
| Intolerance w/recurrence | 10 | (2.1%) | 9 | (1.9%) | Recurrence | TaLG | 1 | (0.2%) | 13 | (2.8%) | - | - | |
| Refractory | 25 | (5.2%) | 37 | (7.7%) | TaHG | 1 | (0.2%) | 8 | (1.6%) | - | - | ||
| Relapsing | 5 | (1.1%) | - | - | Tis | 2 | (0.4%) | 8 | (1.6%) | - | - | ||
| MIBC | 8 | (1.7%) | 22 | (4.6%) | T1 | 5 | (1.1%) | 8 | (1.6%) | - | - | ||
| Metastasis | 5 | (1.1%) | 2 | (0.4%) | MIBC | 4 | (0.8%) | 13 | (2.8%) | - | - | ||
| Patient died a | 8 | (1.7%) | 3 | (0.6%) | Metastasis | 1 | (0.2%) | 8 | (1.6%) | 3 | (0.6%) | ||
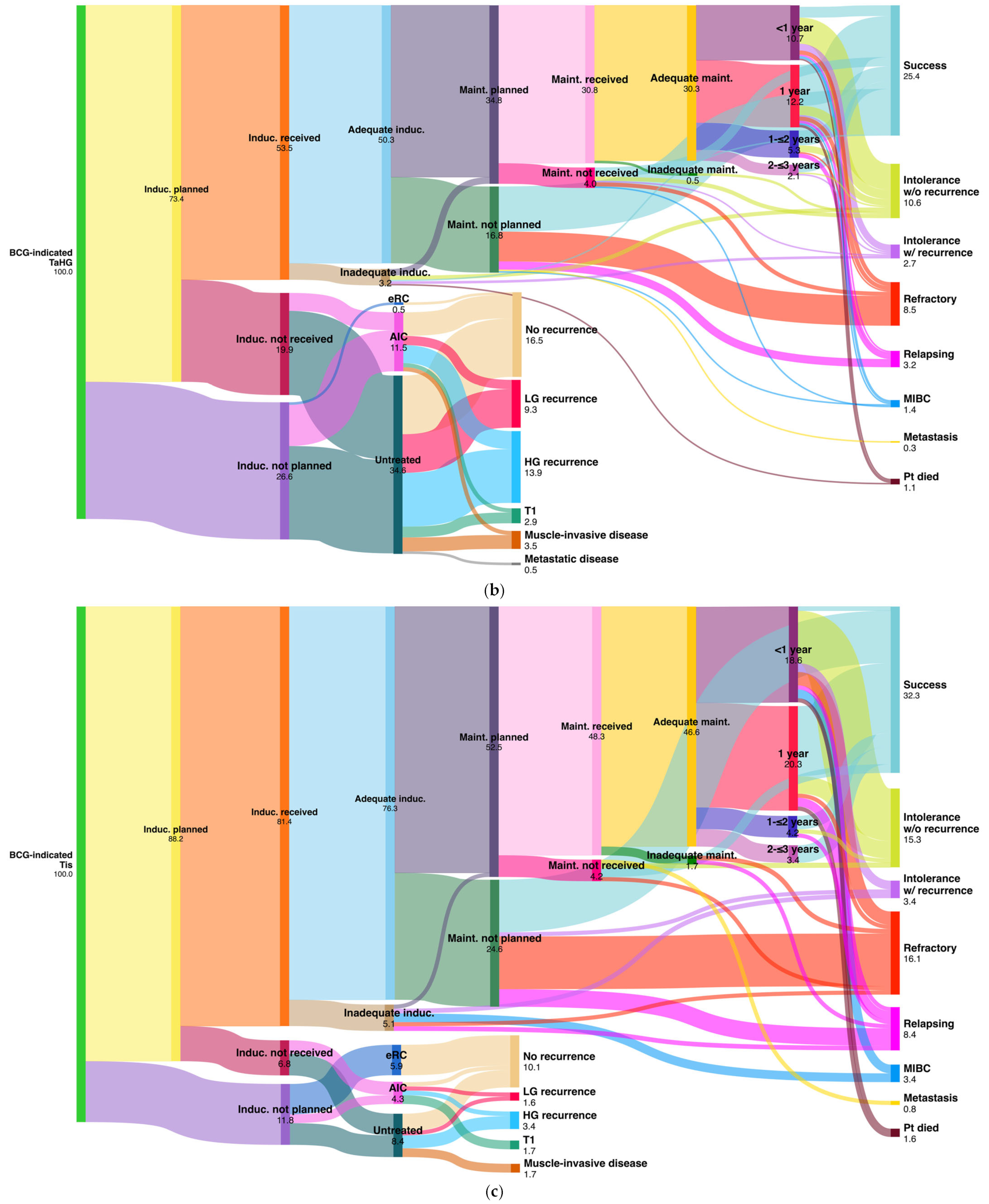
| TaHG (n = 376) | BCG result | Adequate BCG 114 (30.3%) | No maint. 87 (23.1%) | BCG induction not planned/not received 175 (46.5%) | |||||||||
| Success | 55 | (14.6%) | 41 | (10.9%) | AIC (43; 11.5%) | NT (130; 34.6%) | eRC (2; 0.5%) | ||||||
| Intolerance w/o recurrence | 33 | (8.8%) | 8 | (2.1%) | No recurrence | 17 | (4.5%) | 43 | (11.4%) | 2 | (0.5%) | ||
| Intolerance w/recurrence | 6 | (1.6%) | 3 | (0.8%) | Recurrence | TaLG | 7 | (1.9%) | 28 | (7.5%) | - | - | |
| Refractory | 7 | (1.9%) | 25 | (6.7%) | TaHG | 3 | (0.8%) | 33 | (8.8%) | - | - | ||
| Relapsing | 7 | (1.9%) | 6 | (1.6%) | Tis | 10 | (2.7%) | 6 | (1.6%) | - | - | ||
| MIBC | 3 | (0.8%) | 2 | (0.5%) | T1 | 3 | (0.8%) | 8 | (2.1%) | - | - | ||
| Metastasis | - | - | 1 | (0.3%) | MIBC | 3 | (0.8%) | 10 | (2.7%) | - | - | ||
| Patient died a | 3 | (0.8%) | 1 | (0.3%) | Metastasis | - | - | 2 | (0.5%) | - | - | ||
| Tis (n = 118) | BCG result | Adequate BCG 55 (46.6%) | No maint. 40 (33.9%) | BCG induction not planned/not received 22 (18.6%) | |||||||||
| Success | 24 | (20.3%) | 14 | (11.9%) | AIC (5; 4.2%) | NT (10; 8.4%) | eRC (7; 5.9%) | ||||||
| Intolerance w/o recurrence | 17 | (14.4%) | 1 | (0.8%) | No recurrence | 1 | (0.8%) | 4 | (3.4%) | 7 | (5.9%) | ||
| Intolerance w/recurrence | 2 | (1.7%) | 2 | (1.7%) | Recurrence | TaLG | 1 | (0.8%) | 1 | (0.8%) | - | - | |
| Refractory | 4 | (3.4%) | 14 | (11.9%) | TaHG | - | - | - | - | - | - | ||
| Relapsing | 4 | (3.4%) | 6 | (5.1%) | Tis | 1 | (0.9%) | 3 | (2.5%) | - | - | ||
| MIBC | 2 | (1.7%) | 2 | (1.7%) | T1 | 2 | (1.7%) | - | - | - | - | ||
| Metastasis | - | - | 1 | (0.8%) | MIBC | - | - | 2 | (1.7%) | - | - | ||
| Patient died a | 2 | (1.7%) | - | - | Metastasis | - | - | - | - | - | - | ||
| BCG result | Adequate BCG 383 (39.4%) | No maint. 240 (24.7%) | BCG induction not planned/not received 348 (35.8%) | ||||||||||
| All HR tumors (n = 972) | Success | 174 | (17.9%) | 79 | (8.1%) | AIC (71; 7.3%) | NT (254; 26.1%) | eRC (23; 2.4%) | |||||
| Intolerance w/o recurrence | 108 | (11.1%) | 25 | (2.6%) | No recurrence | 27 | (2.8%) | 103 | (10.6%) | 20 | (2.1%) | ||
| Intolerance w/recurrence | 18 | (1.9%) | 14 | (1.4%) | Recurrence | TaLG | 9 | (0.9%) | 42 | (4.3%) | - | - | |
| Refractory | 36 | (3.7%) | 76 | (7.8%) | TaHG | 4 | (0.4%) | 41 | (4.2%) | - | - | ||
| Relapsing | 16 | (1.7%) | 12 | (1.2%) | Tis | 13 | (1.4%) | 17 | (1.8%) | - | - | ||
| MIBC | 13 | (1.3%) | 26 | (2.7%) | T1 | 10 | (1%) | 16 | (1.6%) | - | - | ||
| Metastasis | 5 | (0.5%) | 4 | (0.4%) | MIBC | 7 | (0.7%) | 25 | (2.6%) | - | - | ||
| Patient died a | 13 | (1.3%) | 4 | (0.4%) | Metastasis | 1 | (0.1%) | 10 | (1%) | 3 | (0.3%) | ||
| Disease Group | BCG Failure Type | n | No Active Treatment | BCG RechalLenge | BCG Continuation | Intravesical Chemo | (Early) Cx Proposed |
|---|---|---|---|---|---|---|---|
| T1 n=116 | Intolerance w/rec. | 19 (15.3%) | 3 (15.8%) | 4 (21.1%) | 1 (5.2%) | 3 (15.8%) | 8 (42.1%) |
| Refractory | 62 (53.4%) | 12 (19.4%) | 18 (29%) | 3 (4.8%) | - | 29 (46.8%) | |
| Relapsing | 5 (4.2%) | 1 (20%) | 2 (40%) | - | - | 2 (40%) | |
| MIBC | 30 (27.1%) | 5 (16.7%) | - | - | - | 25 (83.3%) | |
| TaHG n=59 | Intolerance w/rec. | 9 (14.3%) | 5 (55.6%) | 1 (11.1%) | - | - | 3 (33.3%) |
| Refractory | 32 (57.1%) | 7 (21.8%) | 12 (37.5%) | 2 (6.3%) | - | 11 (34.4%) | |
| Relapsing | 13 (20.7%) | 9 (69.2%) | 1 (7.7%) | - | - | 3 (23.1%) | |
| MIBC | 5 (7.9%) | 2 (40%) | - | - | - | 3 (60%) | |
| Tis n=36 | Intolerance w/rec. | 4 (10.3%) | 1 (25%) | 3 (75%) | - | - | - |
| Refractory | 18 (43.6%) | 1 (5.9%) | 10 (58.9%) | - | 1 (5.9%) | 6 (29.4%) | |
| Relapsing | 10 (35.9%) | 3 (30%) | 5 (50%) | - | - | 2 (20%) | |
| MIBC | 4 (10.3%) | 1 (25%) | - | - | - | 3 (75%) | |
| All tumors n=211 | Intolerance w/rec. | 32 (14.1%) | 9 (28.1%) | 8 (25%) | 1 (3.1%) | 3 (9.4%) | 11 (34.4%) |
| Refractory | 112 (52.7%) | 20 (17.8%) | 40 (35.7%) | 5 (4.5%) | 1 (0.9%) | 46 (41.1%) | |
| Relapsing | 28 (14.6%) | 13 (46.3%) | 8 (28.6%) | - | - | 7 (25%) | |
| MIBC | 39 (18.6%) | 8 (20.5%) | - | - | - | 31 (79.5%) | |
| Total | 211 (100%) | 50 (23.7%) | 56 (26.5%) | 6 (2.9%) | 4 (1.9%) | 99 (45%) |
| n (%) | |||
|---|---|---|---|
| Non-metastatic primary MIBC a | 283 | 12.7% | |
| Non-metastatic secondary MIBC a | 117 | 5.2% | |
| de novo metastatic UC a | 39 | 1.7% | |
| Upstaging from T1 disease to MIBC b | 21 | 8.4% | |
| Undergoing RC c | 217 | 54.3% | |
| Receiving NAC d | 100 | 46% | |
| Tumor stage | Tx | 4 | 1.8% |
| T0 | 46 | 21.2% | |
| Tis | 12 | 5.5% | |
| Ta | 2 | 0.9% | |
| T1 | 8 | 3.7% | |
| T2a | 18 | 8.3% | |
| T2b | 22 | 10.1% | |
| T3a | 39 | 18% | |
| T3b | 40 | 18.4% | |
| T4a | 22 | 10.1% | |
| T4b | 4 | 1.8% | |
| Node stage | Nx | 9 | 4.1% |
| N0 | 157 | 72.4% | |
| N1 | 19 | 8.8% | |
| N2 | 27 | 12.4% | |
| N3 | 5 | 2.3% | |
| Variant histology | 75 | 17% | |
| Bi-/trimodality treatment c | 30 | 7.5% | |
| Palliative RT c | 21 | 5.3% | |
| Palliative treatment/BSC/FU c | 123 | 30.8% | |
| Unknown c | 9 | 2.3% | |
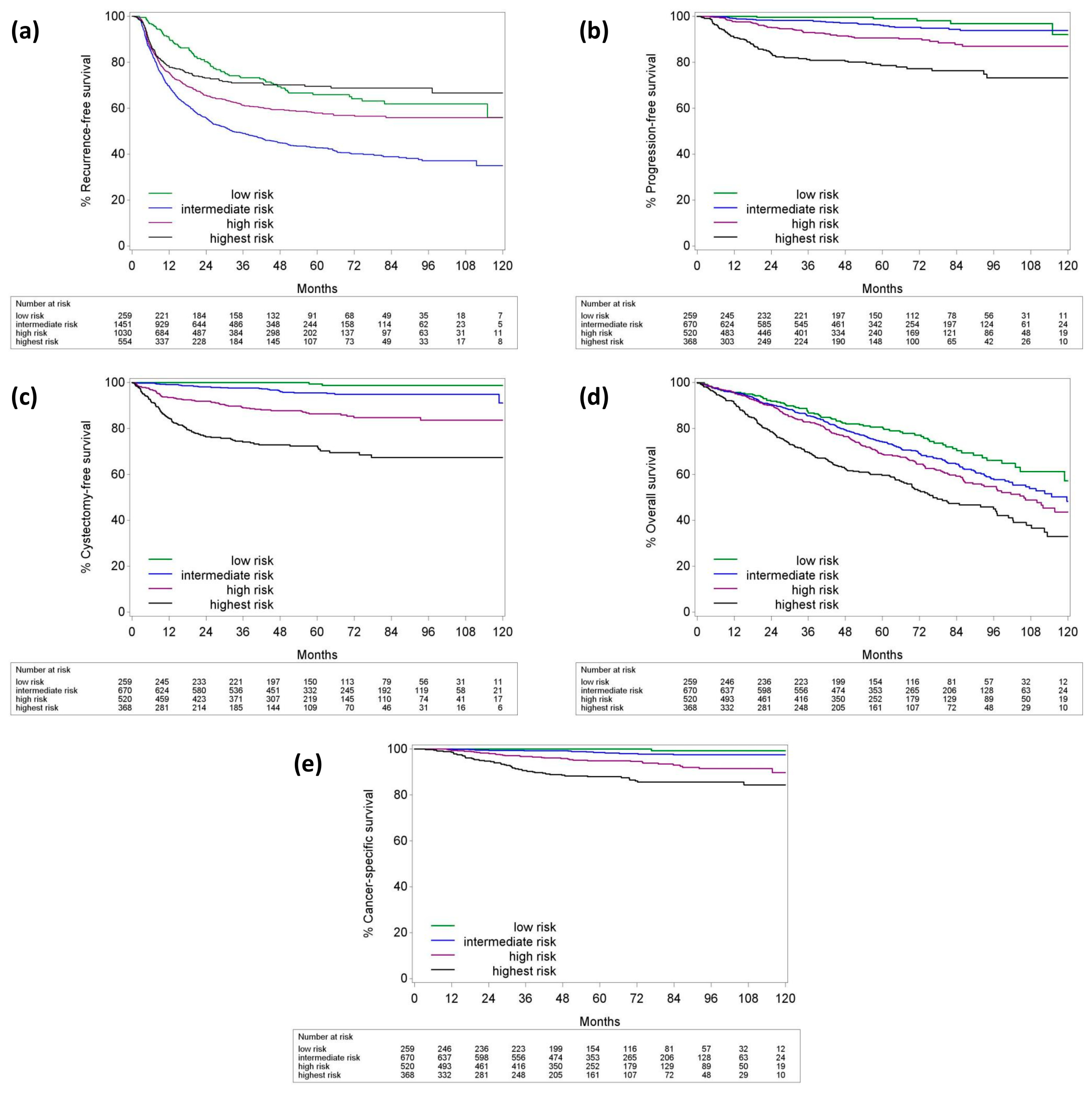
| 2-Year | RFS | PFS | CFS | OS | CSS |
| All tumors | 63.4 (61–65.9) | 94.9 (93.7–95.8) | 92.3 (91–93.5) | 88.2 (86.6–89.6) | 98.3 (97.6–98.8) |
| Low-risk | 80.3 (75.5–85.5) | 99.6 (97.1–99.9) | 100 | 91.8 (87.8–94.6) | 100 |
| Intermediate-risk | 68.6 (65–72.3) | 98.4 (97.1–99.2) | 99.2 (98.1–99.7) | 90.5 (88–92.5) | 99.6 (98.8–99.9) |
| High-risk | 74.3 (70.5–78.4) | 95.3 (93–96.8) | 91.8 (89–93.9) | 90.1 (87.2–92.4) | 98.3 (96.8–99.1) |
| Highest-risk | 80.8 (76.5–85.3) | 84 (79.6–87.6) | 76.4 (71.4–80.6) | 78.6 (74–82.4) | 94.8 (92.1–96.7) |
| 5-year | RFS | PFS | CFS | OS | CSS |
| All tumors | 53 (50.6–55.5) | 91.6 (90–92.9) | 89 (87.3–90.4) | 70.6 (68.4–72.7) | 95.6 (94.5–96.5) |
| Low-risk | 66 (60–72.5) | 99 (95.9–99.8) | 99.4 (95.7–99.9) | 80.6 (75.2–85) | 100 |
| Int-risk | 51.9 (47.9–56.3) | 96.1 (94–97.5) | 97.3 (95.4–98.4) | 74.1 (70.5–77.4) | 98.5 (97.3–99.3) |
| High-risk | 63.8 (59.3–68.7) | 90.7 (87.6–93) | 86.4 (82.7–89.3) | 68.7 (64.3–72.7) | 94.9 (92.7–96.6) |
| Highest-risk | 80.1 (75.7–84.8) | 78.6 (73.5–82.8) | 72.3 (67–77) | 59.6 (54.2–64.5) | 87.9 (84.3 – 91.1) |
| 10-year | RFS | PFS | CFS | OS | CSS |
| All tumors | 47.3 (44–50.8) | 87.6 (84.7–89.9) | 85.8 (82.2–88.7) | 45.1 (41–49.2) | 92.9 (91–94.5) |
| Low-risk | 56 (45–69.6) | 92.1 (74.3–97.7) | 98.7 (94.9–99.7) | 57.2 (45.2–67.4) | 99.3 (96.4–99.9) |
| Int-risk | 42.6 (36–50.4) | 93.9 (91–95.9) | 84.9 (72.3–92.1) | 48.2 (40.1–55.3) | 97.5 (95.8–98.6) |
| High-risk | 62 (57.2–67.2) | 87 (82.5–90.4) | 83.6 (78.9–87.4) | 43.6 (36.1–50.9) | 89.8 (84.8–93.7) |
| Highest-risk | 76 (69–83.8) | 73.2 (65.9–79.1) | 67.3 (60.8–73) | 32.9 (25–41.1) | 84.4 (79.7–88.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akand, M.; Veys, R.; Ost, D.; Vander Eeckt, K.; Baekelandt, F.; Van Reusel, R.; Mattelaer, P.; Baekelandt, L.; Van Cleynenbreugel, B.; Joniau, S.; et al. Cohort Profile: VZNKUL–NMIBC Quality Indicators Program: A Flemish Prospective Cohort to Evaluate the Quality Indicators in the Treatment of Non-Muscle-Invasive Bladder Cancer. Cancers 2024, 16, 3653. https://doi.org/10.3390/cancers16213653
Akand M, Veys R, Ost D, Vander Eeckt K, Baekelandt F, Van Reusel R, Mattelaer P, Baekelandt L, Van Cleynenbreugel B, Joniau S, et al. Cohort Profile: VZNKUL–NMIBC Quality Indicators Program: A Flemish Prospective Cohort to Evaluate the Quality Indicators in the Treatment of Non-Muscle-Invasive Bladder Cancer. Cancers. 2024; 16(21):3653. https://doi.org/10.3390/cancers16213653
Chicago/Turabian StyleAkand, Murat, Ralf Veys, Dieter Ost, Kathy Vander Eeckt, Frederic Baekelandt, Raf Van Reusel, Pieter Mattelaer, Loic Baekelandt, Ben Van Cleynenbreugel, Steven Joniau, and et al. 2024. "Cohort Profile: VZNKUL–NMIBC Quality Indicators Program: A Flemish Prospective Cohort to Evaluate the Quality Indicators in the Treatment of Non-Muscle-Invasive Bladder Cancer" Cancers 16, no. 21: 3653. https://doi.org/10.3390/cancers16213653
APA StyleAkand, M., Veys, R., Ost, D., Vander Eeckt, K., Baekelandt, F., Van Reusel, R., Mattelaer, P., Baekelandt, L., Van Cleynenbreugel, B., Joniau, S., & Van der Aa, F. (2024). Cohort Profile: VZNKUL–NMIBC Quality Indicators Program: A Flemish Prospective Cohort to Evaluate the Quality Indicators in the Treatment of Non-Muscle-Invasive Bladder Cancer. Cancers, 16(21), 3653. https://doi.org/10.3390/cancers16213653







