Precision Medicine for Metastatic Colorectal Cancer: Where Do We Stand?
Simple Summary
Abstract
1. Introduction
2. Methods
3. Genomic and Molecular Profiling in Colorectal Cancer
4. Epidermal Growth Factor Pathway Signaling Inhibitors
4.1. EGFR Targeted Therapy
4.2. RAS
4.3. BRAF/MEK
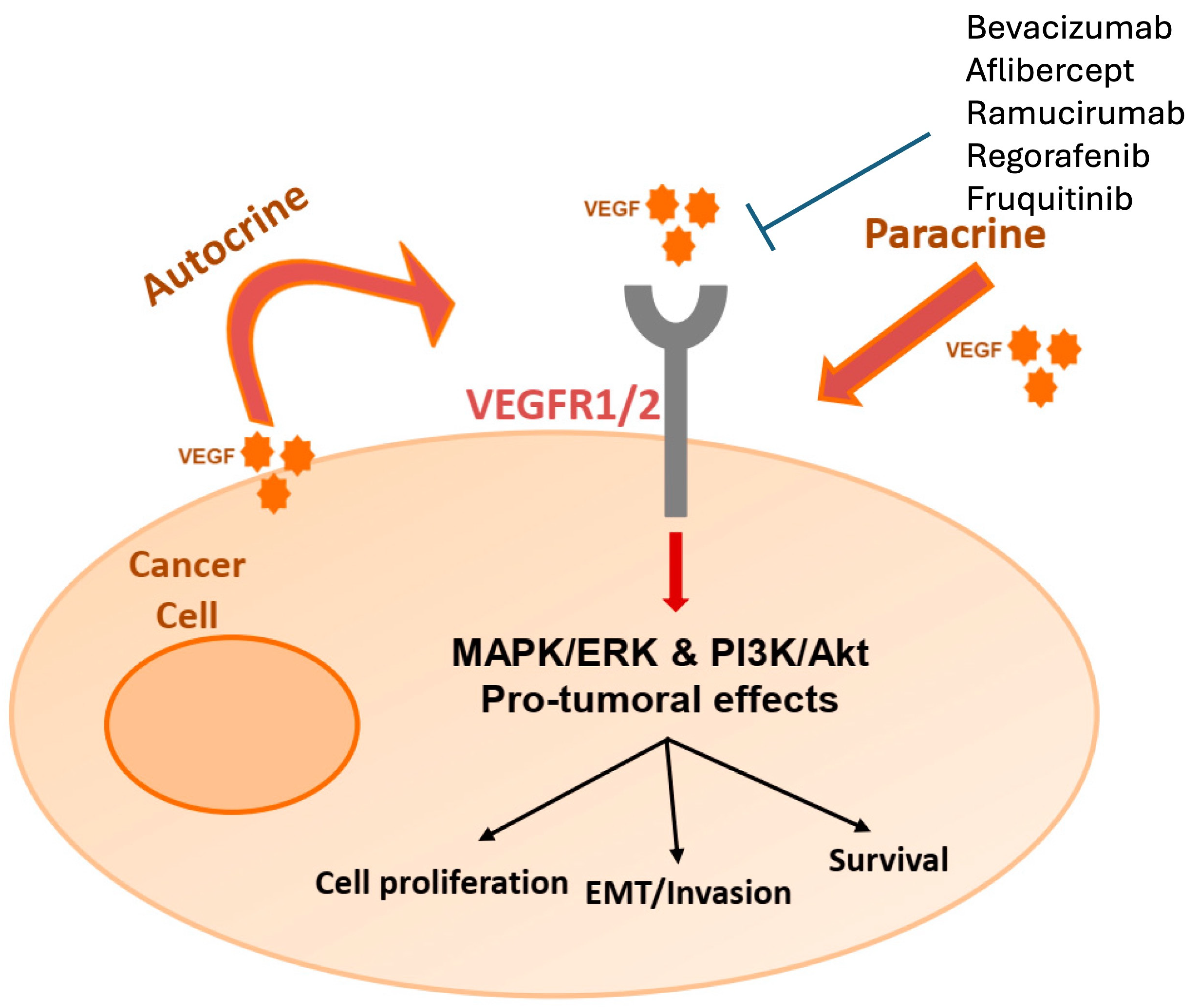
5. Vascular Endothelial Growth Factor Inhibitors
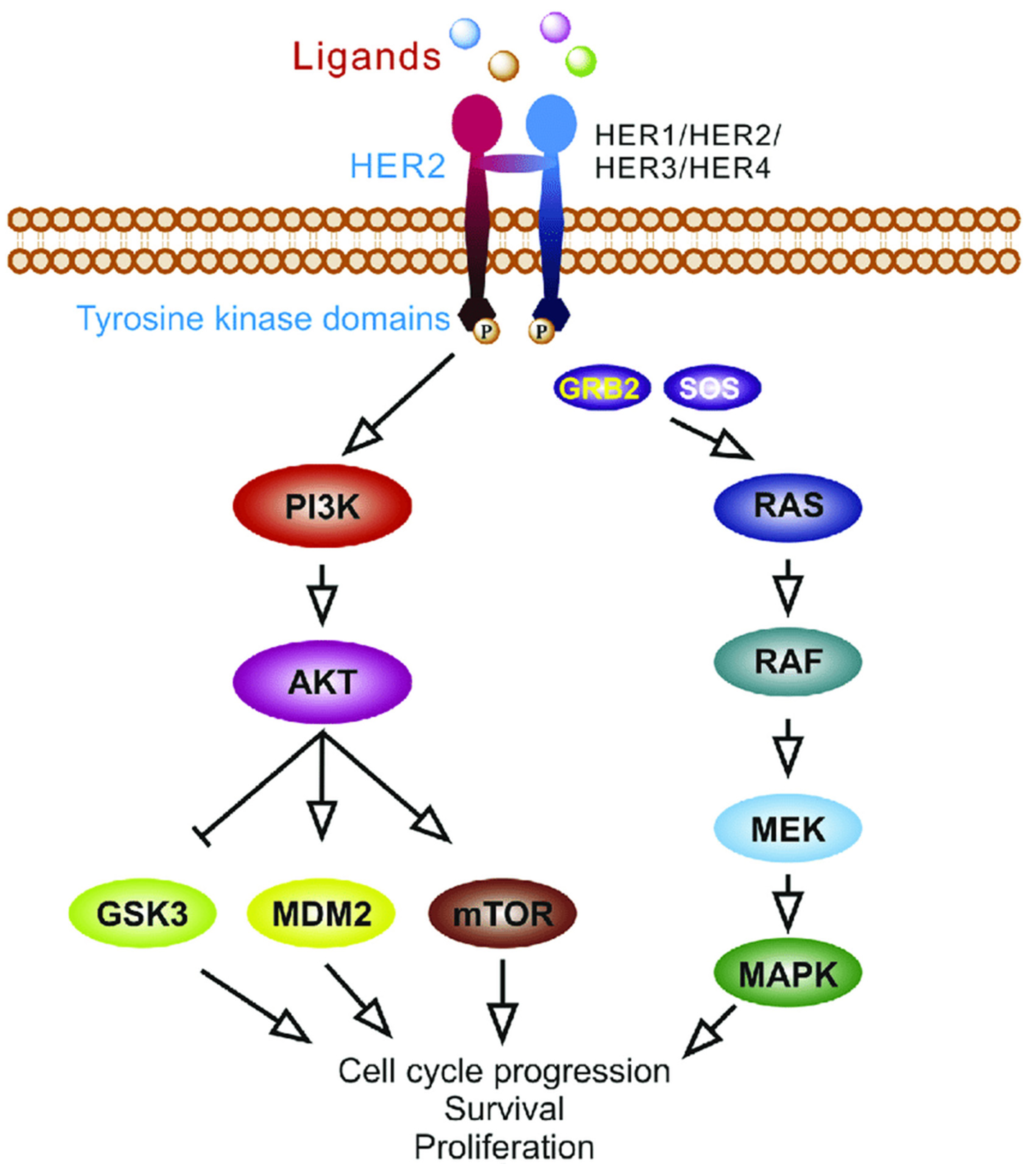
6. HER2 Inhibitors
7. Neurotrophic Receptor Tyrosine Kinase Fusion
8. RET Fusion
9. Deficient Mismatch Repair and Immunotherapy
10. Ongoing Challenges and Future Directions
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Schrijvers, J.J.A.; Greuter, M.J.W.; Kats-Ugurlu, G.; Lu, W.; de Bock, G.H. Effectiveness of Colorectal Cancer (CRC) Screening on All-Cause and CRC-Specific Mortality Reduction: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1948. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.; Saraste, D.; Törnberg, S.; Jonsson, H. Routine Fecal Occult Blood Screening and Colorectal Cancer Mortality in Sweden. JAMA Netw. Open 2024, 7, e240516. [Google Scholar] [CrossRef]
- Machairas, N.; Di Martino, M.; Primavesi, F.; Underwood, P.; de Santibanes, M.; Ntanasis-Stathopoulos, I.; Urban, I.; Tsilimigras, D.I.; Siriwardena, A.K.; Frampton, A.E.; et al. Simultaneous Resection for Colorectal Cancer with Synchronous Liver Metastases: Current State-of-the-Art. J. Gastrointest. Surg. 2024, 28, 577–586. [Google Scholar] [CrossRef]
- Zeineddine, F.A.; Zeineddine, M.A.; Yousef, A.; Gu, Y.; Chowdhury, S.; Dasari, A.; Huey, R.W.; Johnson, B.; Kee, B.; Lee, M.S.; et al. Survival Improvement for Patients with Metastatic Colorectal Cancer over Twenty Years. NPJ Precis. Oncol. 2023, 7, 16. [Google Scholar] [CrossRef]
- Shen, C.; Tannenbaum, D.; Horn, R.; Rogers, J.; Eng, C.; Zhou, S.; Johnson, B.; Kopetz, S.; Morris, V.; Overman, M.; et al. Overall Survival in Phase 3 Clinical Trials and the Surveillance, Epidemiology, and End Results Database in Patients With Metastatic Colorectal Cancer, 1986-2016: A Systematic Review. JAMA Netw. Open 2022, 5, e2213588. [Google Scholar] [CrossRef]
- Sargent, D. Improved Outcomes in Metastatic Colon Cancer: Giving Credit Where Credit Is Due. JAMA Oncol. 2015, 1, 795–796. [Google Scholar] [CrossRef]
- Eng, C.; Yoshino, T.; Ruíz-García, E.; Mostafa, N.; Cann, C.G.; O’Brian, B.; Benny, A.; Perez, R.O.; Cremolini, C. Colorectal Cancer. Lancet 2024, 404, 294–310. [Google Scholar] [CrossRef]
- Chow, F.C.-L.; Chok, K.S.-H. Colorectal Liver Metastases: An Update on Multidisciplinary Approach. World J. Hepatol. 2019, 11, 150–172. [Google Scholar] [CrossRef]
- Peng, D.; Cheng, Y.-X.; Cheng, Y. Improved Overall Survival of Colorectal Cancer under Multidisciplinary Team: A Meta-Analysis. Biomed. Res. Int. 2021, 2021, 5541613. [Google Scholar] [CrossRef] [PubMed]
- Almlöv, K.; Arbman, G.; Björnsson, B.; Elander, N.O.; Hager, J.; Hamid, S.; Landerholm, K.; Loftås, P.; Sandström, P. Assessment by a Multidisciplinary Team Conference Affects Treatment Strategy and Overall Survival in Patients with Synchronous Colorectal Liver Metastases. HPB 2024, 26, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Underwood, P.W.; Ruff, S.M.; Pawlik, T.M. Update on Targeted Therapy and Immunotherapy for Metastatic Colorectal Cancer. Cells 2024, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive Review of Targeted Therapy for Colorectal Cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Dienstmann, R.; Lonardi, S. Is Upfront Full Molecular Profiling Needed in All Patients with Colorectal Cancer in Daily Practice? Lancet Oncol. 2022, 23, 1129–1131. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, H.Y.; Hwang, D.-Y.; Han, H.S. High Concordance of Genomic Profiles between Primary and Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 5561. [Google Scholar] [CrossRef]
- Vignot, S.; Lefebvre, C.; Frampton, G.M.; Meurice, G.; Yelensky, R.; Palmer, G.; Capron, F.; Lazar, V.; Hannoun, L.; Miller, V.A.; et al. Comparative Analysis of Primary Tumour and Matched Metastases in Colorectal Cancer Patients: Evaluation of Concordance between Genomic and Transcriptional Profiles. Eur. J. Cancer 2015, 51, 791–799. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Liao, H.; Cai, S.; Bai, Y.; Zhang, B.; Sheng, Y.; Tong, S.; Cai, J.; Zhao, F.; Zhao, X.; Chen, S.; et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Variants and Somatic Second Hits in Colorectal Cancer. Am. J. Cancer Res. 2021, 11, 5571–5580. [Google Scholar]
- Yurgelun, M.B.; Kulke, M.H.; Fuchs, C.S.; Allen, B.A.; Uno, H.; Hornick, J.L.; Ukaegbu, C.I.; Brais, L.K.; McNamara, P.G.; Mayer, R.J.; et al. Cancer Susceptibility Gene Mutations in Individuals With Colorectal Cancer. J. Clin. Oncol. 2017, 35, 1086–1095. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. EGFR Antagonists in Cancer Treatment. N. Engl. J. Med. 2008, 358, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Soreide, K. EGFR and Downstream Genetic Alterations in KRAS/BRAF and PI3K/AKT Pathways in Colorectal Cancer: Implications for Targeted Therapy. Discov. Med. 2012, 14, 207–214. [Google Scholar] [PubMed]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab Monotherapy and Cetuximab plus Irinotecan in Irinotecan-Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Sobrero, A.F.; Maurel, J.; Fehrenbacher, L.; Scheithauer, W.; Abubakr, Y.A.; Lutz, M.P.; Vega-Villegas, M.E.; Eng, C.; Steinhauer, E.U.; Prausova, J.; et al. EPIC: Phase III Trial of Cetuximab plus Irinotecan after Fluoropyrimidine and Oxaliplatin Failure in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2008, 26, 2311–2319. [Google Scholar] [CrossRef]
- De Roock, W.; Piessevaux, H.; De Schutter, J.; Janssens, M.; De Hertogh, G.; Personeni, N.; Biesmans, B.; Van Laethem, J.-L.; Peeters, M.; Humblet, Y.; et al. KRAS Wild-Type State Predicts Survival and Is Associated to Early Radiological Response in Metastatic Colorectal Cancer Treated with Cetuximab. Ann. Oncol. 2008, 19, 508–515. [Google Scholar] [CrossRef]
- Di Fiore, F.; Blanchard, F.; Charbonnier, F.; Le Pessot, F.; Lamy, A.; Galais, M.P.; Bastit, L.; Killian, A.; Sesboüé, R.; Tuech, J.J.; et al. Clinical Relevance of KRAS Mutation Detection in Metastatic Colorectal Cancer Treated by Cetuximab plus Chemotherapy. Br. J. Cancer 2007, 96, 1166–1169. [Google Scholar] [CrossRef]
- Lièvre, A.; Bachet, J.-B.; Le Corre, D.; Boige, V.; Landi, B.; Emile, J.-F.; Côté, J.-F.; Tomasic, G.; Penna, C.; Ducreux, M.; et al. KRAS Mutation Status Is Predictive of Response to Cetuximab Therapy in Colorectal Cancer. Cancer Res. 2006, 66, 3992–3995. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus Irinotecan, Fluorouracil, and Leucovorin as First-Line Treatment for Metastatic Colorectal Cancer: Updated Analysis of Overall Survival According to Tumor KRAS and BRAF Mutation Status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Wang, L.; Xu, J.; Cheng, Y.; Bai, Y.; Li, W.; Xu, N.; Lin, L.-Z.; Wu, Q.; et al. Efficacy and Tolerability of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) Versus FOLFOX-4 in Patients With RAS Wild-Type Metastatic Colorectal Cancer: The Open-Label, Randomized, Phase III TAILOR Trial. J. Clin. Oncol. 2018, 36, 3031–3039. [Google Scholar] [CrossRef]
- Bridgewater, J.A.; Pugh, S.A.; Maishman, T.; Eminton, Z.; Mellor, J.; Whitehead, A.; Stanton, L.; Radford, M.; Corkhill, A.; Griffiths, G.O.; et al. Systemic Chemotherapy with or without Cetuximab in Patients with Resectable Colorectal Liver Metastasis (New EPOC): Long-Term Results of a Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2020, 21, 398–411. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Peeters, M.; Siena, S.; Humblet, Y.; Hendlisz, A.; Neyns, B.; Canon, J.-L.; Van Laethem, J.-L.; Maurel, J.; Richardson, G.; et al. Open-Label Phase III Trial of Panitumumab plus Best Supportive Care Compared with Best Supportive Care Alone in Patients with Chemotherapy-Refractory Metastatic Colorectal Cancer. J. Clin. Oncol. 2007, 25, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; André, T.; Chan, E.; Lordick, F.; Punt, C.J.A.; et al. Final Results from a Randomized Phase 3 Study of FOLFIRI {+/-} Panitumumab for Second-Line Treatment of Metastatic Colorectal Cancer. Ann. Oncol. 2014, 25, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Final Results from PRIME: Randomized Phase III Study of Panitumumab with FOLFOX4 for First-Line Treatment of Metastatic Colorectal Cancer. Ann. Oncol. 2014, 25, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Price, T.J.; Peeters, M.; Kim, T.W.; Li, J.; Cascinu, S.; Ruff, P.; Suresh, A.S.; Thomas, A.; Tjulandin, S.; Zhang, K.; et al. Panitumumab versus Cetuximab in Patients with Chemotherapy-Refractory Wild-Type KRAS Exon 2 Metastatic Colorectal Cancer (ASPECCT): A Randomised, Multicentre, Open-Label, Non-Inferiority Phase 3 Study. Lancet Oncol. 2014, 15, 569–579. [Google Scholar] [CrossRef]
- Sakai, D.; Taniguchi, H.; Sugimoto, N.; Tamura, T.; Nishina, T.; Hara, H.; Esaki, T.; Denda, T.; Sakamoto, T.; Okuda, H.; et al. Randomised Phase II Study of Panitumumab plus Irinotecan versus Cetuximab plus Irinotecan in Patients with KRAS Wild-Type Metastatic Colorectal Cancer Refractory to Fluoropyrimidine, Irinotecan and Oxaliplatin (WJOG 6510G). Eur. J. Cancer 2020, 135, 11–21. [Google Scholar] [CrossRef]
- Brulé, S.Y.; Jonker, D.J.; Karapetis, C.S.; O’Callaghan, C.J.; Moore, M.J.; Wong, R.; Tebbutt, N.C.; Underhill, C.; Yip, D.; Zalcberg, J.R.; et al. Location of Colon Cancer (Right-Sided versus Left-Sided) as a Prognostic Factor and a Predictor of Benefit from Cetuximab in NCIC CO.17. Eur. J. Cancer 2015, 51, 1405–1414. [Google Scholar] [CrossRef]
- Boeckx, N.; Koukakis, R.; Op de Beeck, K.; Rolfo, C.; Van Camp, G.; Siena, S.; Tabernero, J.; Douillard, J.-Y.; André, T.; Peeters, M. Primary Tumor Sidedness Has an Impact on Prognosis and Treatment Outcome in Metastatic Colorectal Cancer: Results from Two Randomized First-Line Panitumumab Studies. Ann. Oncol. 2017, 28, 1862–1868. [Google Scholar] [CrossRef]
- Punekar, S.R.; Velcheti, V.; Neel, B.G.; Wong, K.-K. The Current State of the Art and Future Trends in RAS-Targeted Cancer Therapies. Nat. Rev. Clin. Oncol. 2022, 19, 637–655. [Google Scholar] [CrossRef]
- Ros, J.; Vaghi, C.; Baraibar, I.; Saoudi González, N.; Rodríguez-Castells, M.; García, A.; Alcaraz, A.; Salva, F.; Tabernero, J.; Elez, E. Targeting KRAS G12C Mutation in Colorectal Cancer, A Review: New Arrows in the Quiver. Int. J. Mol. Sci. 2024, 25, 3304. [Google Scholar] [CrossRef]
- Bteich, F.; Mohammadi, M.; Li, T.; Bhat, M.A.; Sofianidi, A.; Wei, N.; Kuang, C. Targeting KRAS in Colorectal Cancer: A Bench to Bedside Review. Int. J. Mol. Sci. 2023, 24, 12030. [Google Scholar] [CrossRef]
- Fakih, M.G.; Kopetz, S.; Kuboki, Y.; Kim, T.W.; Munster, P.N.; Krauss, J.C.; Falchook, G.S.; Han, S.-W.; Heinemann, V.; Muro, K.; et al. Sotorasib for Previously Treated Colorectal Cancers with KRASG12C Mutation (CodeBreaK100): A Prespecified Analysis of a Single-Arm, Phase 2 Trial. Lancet Oncol. 2022, 23, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Salvatore, L.; Esaki, T.; Modest, D.P.; Lopez-Bravo, D.P.; Taieb, J.; Karamouzis, M.V.; Ruiz-Garcia, E.; Kim, T.-W.; Kuboki, Y.; et al. Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 389, 2125–2139. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.S.; Chan, G.; Deming, D.A.; Chee, C.E. State-of-the-Art Management of Colorectal Cancer: Treatment Advances and Innovation. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e438466. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Weiss, J.; Pelster, M.S.; Spira, A.I.; Barve, M.; Ou, S.-H.I.; Leal, T.A.; Bekaii-Saab, T.S.; Paweletz, C.P.; Heavey, G.A.; et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 388, 44–54. [Google Scholar] [CrossRef]
- Tabernero, J.; Ros, J.; Élez, E. The Evolving Treatment Landscape in BRAF-V600E-Mutated Metastatic Colorectal Cancer. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 349561. [Google Scholar] [CrossRef]
- Huang, T.; Karsy, M.; Zhuge, J.; Zhong, M.; Liu, D. B-Raf and the Inhibitors: From Bench to Bedside. J. Hematol. Oncol. 2013, 6, 30. [Google Scholar] [CrossRef]
- Tosi, D.; Pérez-Gracia, E.; Atis, S.; Vié, N.; Combès, E.; Gabanou, M.; Larbouret, C.; Jarlier, M.; Mollevi, C.; Torro, A.; et al. Rational Development of Synergistic Combinations of Chemotherapy and Molecular Targeted Agents for Colorectal Cancer Treatment. BMC Cancer 2018, 18, 812. [Google Scholar] [CrossRef]
- Akinleye, A.; Furqan, M.; Mukhi, N.; Ravella, P.; Liu, D. MEK and the Inhibitors: From Bench to Bedside. J. Hematol. Oncol. 2013, 6, 27. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef]
- Costigan, D.C.; Dong, F. The Extended Spectrum of RAS-MAPK Pathway Mutations in Colorectal Cancer. Genes. Chromosomes Cancer 2020, 59, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The Biology of VEGF and Its Receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Donnini, S.; Filippelli, A.; Ciccone, V.; Spini, A.; Ristori, E.; Ziche, M.; Morbidelli, L. Antiangiogenic Drugs: Chemosensitizers for Combination Cancer Therapy. In Antiangiogenic Drugs as Chemosensitizers in Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–66. ISBN 978-0-323-90190-1. [Google Scholar]
- Ntellas, P.; Mavroeidis, L.; Gkoura, S.; Gazouli, I.; Amylidi, A.-L.; Papadaki, A.; Zarkavelis, G.; Mauri, D.; Karpathiou, G.; Kolettas, E.; et al. Old Player-New Tricks: Non Angiogenic Effects of the VEGF/VEGFR Pathway in Cancer. Cancers 2020, 12, 3145. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.-S.; Rivera, F.; et al. Bevacizumab in Combination with Oxaliplatin-Based Chemotherapy as First-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O’Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B. Eastern Cooperative Oncology Group Study E3200 Bevacizumab in Combination with Oxaliplatin, Fluorouracil, and Leucovorin (FOLFOX4) for Previously Treated Metastatic Colorectal Cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007, 25, 1539–1544. [Google Scholar] [CrossRef]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; et al. Continuation of Bevacizumab after First Progression in Metastatic Colorectal Cancer (ML18147): A Randomised Phase 3 Trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef]
- Simkens, L.H.J.; van Tinteren, H.; May, A.; ten Tije, A.J.; Creemers, G.-J.M.; Loosveld, O.J.L.; de Jongh, F.E.; Erdkamp, F.L.G.; Erjavec, Z.; van der Torren, A.M.E.; et al. Maintenance Treatment with Capecitabine and Bevacizumab in Metastatic Colorectal Cancer (CAIRO3): A Phase 3 Randomised Controlled Trial of the Dutch Colorectal Cancer Group. Lancet 2015, 385, 1843–1852. [Google Scholar] [CrossRef]
- André, T.; Vernerey, D.; Im, S.A.; Bodoky, G.; Buzzoni, R.; Reingold, S.; Rivera, F.; McKendrick, J.; Scheithauer, W.; Ravit, G.; et al. Bevacizumab as Adjuvant Treatment of Colon Cancer: Updated Results from the S-AVANT Phase III Study by the GERCOR Group. Ann. Oncol. 2020, 31, 246–256. [Google Scholar] [CrossRef]
- Folprecht, G.; Pericay, C.; Saunders, M.P.; Thomas, A.; Lopez Lopez, R.; Roh, J.K.; Chistyakov, V.; Höhler, T.; Kim, J.-S.; Hofheinz, R.-D.; et al. Oxaliplatin and 5-FU/Folinic Acid (Modified FOLFOX6) with or without Aflibercept in First-Line Treatment of Patients with Metastatic Colorectal Cancer: The AFFIRM Study. Ann. Oncol. 2016, 27, 1273–1279. [Google Scholar] [CrossRef]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.-E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus Placebo in Combination with Second-Line FOLFIRI in Patients with Metastatic Colorectal Carcinoma That Progressed during or after First-Line Therapy with Bevacizumab, Oxaliplatin, and a Fluoropyrimidine (RAISE): A Randomised, Double-Blind, Multicentre, Phase 3 Study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, H.; Kitadai, Y.; Shinagawa, K.; Yuge, R.; Higashi, Y.; Tanaka, S.; Yasui, W.; Chayama, K. Multikinase Inhibitor Regorafenib Inhibits the Growth and Metastasis of Colon Cancer with Abundant Stroma. Cancer Sci. 2016, 107, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, S.; Xu, R.; Yau, T.C.C.; Ma, B.; Pan, H.; Xu, J.; Bai, Y.; Chi, Y.; Wang, L.; et al. Regorafenib plus Best Supportive Care versus Placebo plus Best Supportive Care in Asian Patients with Previously Treated Metastatic Colorectal Cancer (CONCUR): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2015, 16, 619–629. [Google Scholar] [CrossRef]
- Argilés, G.; Saunders, M.P.; Rivera, F.; Sobrero, A.; Benson, A.; Guillén Ponce, C.; Cascinu, S.; Van Cutsem, E.; Macpherson, I.R.; Strumberg, D.; et al. Regorafenib plus Modified FOLFOX6 as First-Line Treatment of Metastatic Colorectal Cancer: A Phase II Trial. Eur. J. Cancer 2015, 51, 942–949. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.-H.; Shen, L.; Xu, J.; Bai, Y.; Yang, L.; Deng, Y.; Chen, Z.-D.; Zhong, H.; et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018, 319, 2486–2496. [Google Scholar] [CrossRef]
- Dasari, A.; Lonardi, S.; Garcia-Carbonero, R.; Elez, E.; Yoshino, T.; Sobrero, A.; Yao, J.; García-Alfonso, P.; Kocsis, J.; Cubillo Gracian, A.; et al. Fruquintinib versus Placebo in Patients with Refractory Metastatic Colorectal Cancer (FRESCO-2): An International, Multicentre, Randomised, Double-Blind, Phase 3 Study. Lancet 2023, 402, 41–53. [Google Scholar] [CrossRef]
- Martínez-Sáez, O.; Prat, A. Current and Future Management of HER2-Positive Metastatic Breast Cancer. JCO Oncol. Pract. 2021, 17, 594–604. [Google Scholar] [CrossRef]
- Zheng-Lin, B.; Bekaii-Saab, T.S. Treatment Options for HER2-Expressing Colorectal Cancer: Updates and Recent Approvals. Ther. Adv. Med. Oncol. 2024, 16, 17588359231225037. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes. Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus Trastuzumab for HER2-Amplified Metastatic Colorectal Cancer (MyPathway): An Updated Report from a Multicentre, Open-Label, Phase 2a, Multiple Basket Study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Meric-Bernstam, F.; Rothe, M.; Garrett-Mayer, E.; Mangat, P.K.; D’Andre, S.; Ahn, E.R.; O’Lone, R.; Halabi, S.; Grantham, G.N.; et al. Pertuzumab Plus Trastuzumab in Patients With Colorectal Cancer With ERBB2 Amplification or ERBB2/3 Mutations: Results From the TAPUR Study. JCO Precis. Oncol. 2022, 6, e2200306. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-Targeted Therapy with Trastuzumab and Lapatinib in Treatment-Refractory, KRAS Codon 12/13 Wild-Type, HER2-Positive Metastatic Colorectal Cancer (HERACLES): A Proof-of-Concept, Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Cercek, A.; Siena, S.; André, T.; Ng, K.; Van Cutsem, E.; Wu, C.; Paulson, A.S.; Hubbard, J.M.; Coveler, A.L.; et al. Tucatinib plus Trastuzumab for Chemotherapy-Refractory, HER2-Positive, RAS Wild-Type Unresectable or Metastatic Colorectal Cancer (MOUNTAINEER): A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2023, 24, 496–508. [Google Scholar] [CrossRef]
- Yoshino, T.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Wainberg, Z.; Elez, E.; et al. Final Results of DESTINY-CRC01 Investigating Trastuzumab Deruxtecan in Patients with HER2-Expressing Metastatic Colorectal Cancer. Nat. Commun. 2023, 14, 3332. [Google Scholar] [CrossRef]
- Raghav, K.; Siena, S.; Takashima, A.; Kato, T.; Van den Eynde, M.; Pietrantonio, F.; Komatsu, Y.; Kawakami, H.; Peeters, M.; Andre, T.; et al. Trastuzumab Deruxtecan in Patients with HER2-Positive Advanced Colorectal Cancer (DESTINY-CRC02): Primary Results from a Multicentre, Randomised, Phase 2 Trial. Lancet Oncol. 2024, 25, 1147–1162. [Google Scholar] [CrossRef]
- Theik, N.W.Y.; Muminovic, M.; Alvarez-Pinzon, A.M.; Shoreibah, A.; Hussein, A.M.; Raez, L.E. NTRK Therapy among Different Types of Cancers, Review and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 2366. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.-W.; Ou, Q.; Wu, X.; Nagasaka, M.; Shao, Y.; Ou, S.-H.I.; Yang, Y. NTRK Fusion Positive Colorectal Cancer Is a Unique Subset of CRC with High TMB and Microsatellite Instability. Cancer Med. 2022, 11, 2541–2549. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Drilon, A.; Camidge, D.R.; Lin, J.J.; Kim, S.-W.; Solomon, B.J.; Dziadziuszko, R.; Besse, B.; Goto, K.; de Langen, A.J.; Wolf, J.; et al. Repotrectinib in ROS1 Fusion-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 390, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Brazel, D.; Baca, Y.; Xiu, J.; Al-Hallak, M.N.; Kim, C.; Nieva, J.; Swensen, J.J.; Spetzler, D.; Korn, W.M.; et al. Pan-Tumor Survey of RET Fusions as Detected by next-Generation RNA Sequencing Identified RET Fusion Positive Colorectal Carcinoma as a Unique Molecular Subset. Transl. Oncol. 2023, 36, 101744. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-Agnostic Efficacy and Safety of Selpercatinib in Patients with RET Fusion-Positive Solid Tumours Other than Lung or Thyroid Tumours (LIBRETTO-001): A Phase 1/2, Open-Label, Basket Trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Karapetyan, L.; Kirkwood, J.M. Immunotherapy in Melanoma: Recent Advances and Future Directions. Cancers 2023, 15, 1106. [Google Scholar] [CrossRef]
- Stein, A.; Moehler, M.; Trojan, J.; Goekkurt, E.; Vogel, A. Immuno-Oncology in GI Tumours: Clinical Evidence and Emerging Trials of PD-1/PD-L1 Antagonists. Crit. Rev. Oncol. Hematol. 2018, 130, 13–26. [Google Scholar] [CrossRef]
- Gutierrez, C.; Ogino, S.; Meyerhardt, J.A.; Iorgulescu, J.B. The Prevalence and Prognosis of Microsatellite Instability-High/Mismatch Repair-Deficient Colorectal Adenocarcinomas in the United States. JCO Precis. Oncol. 2023, 7, e2200179. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Diaz, L.A.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus Chemotherapy for Microsatellite Instability-High or Mismatch Repair-Deficient Metastatic Colorectal Cancer (KEYNOTE-177): Final Analysis of a Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in Patients with Metastatic DNA Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer (CheckMate 142): An Open-Label, Multicentre, Phase 2 Study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.-J.; Van Cutsem, E.; Luisa Limon, M.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of Anti-Angiogenic Therapy and Immune Checkpoint Blockade Normalizes Vascular-Immune Crosstalk to Potentiate Cancer Immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Catteau, A.; Salvatore, L.; Lonardi, S.; Boquet, I.; Tamberi, S.; Marmorino, F.; Moretto, R.; et al. Upfront FOLFOXIRI plus Bevacizumab with or without Atezolizumab in the Treatment of Patients with Metastatic Colorectal Cancer (AtezoTRIBE): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2022, 23, 876–887. [Google Scholar] [CrossRef]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Salvatore, L.; Lonardi, S.; Tamberi, S.; Marmorino, F.; Moretto, R.; Prisciandaro, M.; Tamburini, E.; et al. Upfront Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan Plus Bevacizumab With or Without Atezolizumab for Patients With Metastatic Colorectal Cancer: Updated and Overall Survival Results of the ATEZOTRIBE Study. J. Clin. Oncol. 2024, 42, 2637–2644. [Google Scholar] [CrossRef]
- Pan, Q.-Z.; Zhao, J.-J.; Liu, L.; Zhang, D.-S.; Wang, L.-P.; Hu, W.-W.; Weng, D.-S.; Xu, X.; Li, Y.-Z.; Tang, Y.; et al. XELOX (Capecitabine plus Oxaliplatin) plus Bevacizumab (Anti-VEGF-A Antibody) with or without Adoptive Cell Immunotherapy in the Treatment of Patients with Previously Untreated Metastatic Colorectal Cancer: A Multicenter, Open-Label, Randomized, Controlled, Phase 3 Trial. Signal Transduct. Target. Ther. 2024, 9, 79. [Google Scholar] [CrossRef]
- Xue, C.; Zhu, D.; Wang, X.; Jiao, L.; Lu, Y.; Zhang, S.; Lv, J.; Cui, L.; Ruan, M.; Xu, D.; et al. Durable Response to Pembrolizumab in Hepatic Metastasis from Colonic Carcinoma with Lynch Syndrome: A Case Report. Front. Immunol. 2024, 15, 1455907. [Google Scholar] [CrossRef]
- Wei, L.; Lin, Z.; Xie, S.; Ruan, D.; Jiang, W.; Cui, Y.; Liu, S.; Wang, T.; Chen, Z.; Lin, Q. Complete Response With Cetuximab-Based Treatment of Metastatic Colorectal Cancers: Two Case Reports and Literature Review. Front. Oncol. 2022, 12, 798515. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Liu, Y.; Zhang, C.; Li, H.; Lai, B. Metastatic Patterns and Survival Outcomes in Patients with Stage IV Colon Cancer: A Population-Based Analysis. Cancer Med. 2020, 9, 361–373. [Google Scholar] [CrossRef]
- De Roock, W.; Claes, B.; Bernasconi, D.; De Schutter, J.; Biesmans, B.; Fountzilas, G.; Kalogeras, K.T.; Kotoula, V.; Papamichael, D.; Laurent-Puig, P.; et al. Effects of KRAS, BRAF, NRAS, and PIK3CA Mutations on the Efficacy of Cetuximab plus Chemotherapy in Chemotherapy-Refractory Metastatic Colorectal Cancer: A Retrospective Consortium Analysis. Lancet Oncol. 2010, 11, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Karapetis, C.S.; Khambata-Ford, S.; Jonker, D.J.; O’Callaghan, C.J.; Tu, D.; Tebbutt, N.C.; Simes, R.J.; Chalchal, H.; Shapiro, J.D.; Robitaille, S.; et al. K-Ras Mutations and Benefit from Cetuximab in Advanced Colorectal Cancer. N. Engl. J. Med. 2008, 359, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Milano, M.; Brambilla, M.; Mennitto, A.; Maggi, C.; Cona, M.S.; Prisciandaro, M.; Fabbroni, C.; Celio, L.; Mariani, G.; et al. Resistance Mechanisms to Anti-HER2 Therapies in HER2-Positive Breast Cancer: Current Knowledge, New Research Directions and Therapeutic Perspectives. Crit. Rev. Oncol. Hematol. 2019, 139, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Alese, O.B.; Wu, C.; Chapin, W.J.; Ulanja, M.B.; Zheng-Lin, B.; Amankwah, M.; Eads, J. Update on Emerging Therapies for Advanced Colorectal Cancer. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389574. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Samuels, Y.; Li, Q.; Krokowski, D.; Guan, B.-J.; Wang, C.; Jin, Z.; Dong, B.; Cao, B.; Feng, X.; et al. Oncogenic PIK3CA Mutations Reprogram Glutamine Metabolism in Colorectal Cancer. Nat. Commun. 2016, 7, 11971. [Google Scholar] [CrossRef] [PubMed]
- Manic, G.; Signore, M.; Sistigu, A.; Russo, G.; Corradi, F.; Siteni, S.; Musella, M.; Vitale, S.; De Angelis, M.L.; Pallocca, M.; et al. CHK1-Targeted Therapy to Deplete DNA Replication-Stressed, P53-Deficient, Hyperdiploid Colorectal Cancer Stem Cells. Gut 2018, 67, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Hong, D.S.; Patel, M.R.; Pant, S.; Ulahannan, S.V.; Jones, S.; Meric-Bernstam, F.; Wang, J.S.; Aljumaily, R.; Hamilton, E.P.; et al. A Phase 1b Trial of Prexasertib in Combination with Standard-of-Care Agents in Advanced or Metastatic Cancer. Target. Oncol. 2021, 16, 569–589. [Google Scholar] [CrossRef]
- Jahangiri, A.; De Lay, M.; Miller, L.M.; Carbonell, W.S.; Hu, Y.-L.; Lu, K.; Tom, M.W.; Paquette, J.; Tokuyasu, T.A.; Tsao, S.; et al. Gene Expression Profile Identifies Tyrosine Kinase C-Met as a Targetable Mediator of Antiangiogenic Therapy Resistance. Clin. Cancer Res. 2013, 19, 1773–1783. [Google Scholar] [CrossRef]
- Goto, H.; Nishioka, Y. Fibrocytes: A Novel Stromal Cells to Regulate Resistance to Anti-Angiogenic Therapy and Cancer Progression. Int. J. Mol. Sci. 2017, 19, 98. [Google Scholar] [CrossRef]
- Sahin, I.H.; Ciombor, K.K.; Diaz, L.A.; Yu, J.; Kim, R. Immunotherapy for Microsatellite Stable Colorectal Cancers: Challenges and Novel Therapeutic Avenues. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 349811. [Google Scholar] [CrossRef]
- Dienstmann, R.; Vermeulen, L.; Guinney, J.; Kopetz, S.; Tejpar, S.; Tabernero, J. Consensus Molecular Subtypes and the Evolution of Precision Medicine in Colorectal Cancer. Nat. Rev. Cancer 2017, 17, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, B.; El Ghanmi, A.; Kandoussi, S.; Ghouzlani, A.; Badou, A. CAR T-Cells for Colorectal Cancer Immunotherapy: Ready to Go? Front. Immunol. 2022, 13, 978195. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhang, T.; Huang, H.; Feng, H.; Wang, S.; Guo, Z.; Luo, Z.; Ji, X.; Cheng, X.; Zhao, R. Colorectal Cancer Vaccines: The Current Scenario and Future Prospects. Front. Immunol. 2022, 13, 942235. [Google Scholar] [CrossRef] [PubMed]
- Leighl, N.B.; Nirmalakumar, S.; Ezeife, D.A.; Gyawali, B. An Arm and a Leg: The Rising Cost of Cancer Drugs and Impact on Access. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 100028. [Google Scholar] [CrossRef]

| Target | Mutation Prevalence | Therapy |
|---|---|---|
| EGFR | N/A | Cetuximab Panitumumab |
| BRAF V600E | 8–12% | Encorafenib (with binimetinib) |
| RAS | 50% | Sotorasib Adagrasib |
| VEGF | N/A | Bevacizumab Aflibercept Ramucirumab Regorafenib Fruquitinib |
| HER2 | 3–5% | Trastuzumab Pertuzumab Lapatinib Tucatinib Trastuzumab deruxtecan |
| NTRK | 0.7% | Entrectinib Larotrectinib Repotrectinib |
| RET | 0.2% | Selpercatinib |
| MSI-H/dMMR | 15% | Pembrolizumab Nivolumab Ipilimumab |
| Subtype | Prevalence | Features |
|---|---|---|
| CMS1 (Microsatellite instability immune) | 14% | Hypermutated Microsatellite unstable Strong immune activation |
| CMS2 (Canonical) | 37% | Epithelial WNT and MYC signaling activation |
| CMS3 (Metabolic) | 13% | Epithelial Metabolic dysregulation |
| CMS4 (Mesenchymal) | 23% | TGF-β activation Stromal invasion Angiogenesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Underwood, P.W.; Pawlik, T.M. Precision Medicine for Metastatic Colorectal Cancer: Where Do We Stand? Cancers 2024, 16, 3870. https://doi.org/10.3390/cancers16223870
Underwood PW, Pawlik TM. Precision Medicine for Metastatic Colorectal Cancer: Where Do We Stand? Cancers. 2024; 16(22):3870. https://doi.org/10.3390/cancers16223870
Chicago/Turabian StyleUnderwood, Patrick W., and Timothy M. Pawlik. 2024. "Precision Medicine for Metastatic Colorectal Cancer: Where Do We Stand?" Cancers 16, no. 22: 3870. https://doi.org/10.3390/cancers16223870
APA StyleUnderwood, P. W., & Pawlik, T. M. (2024). Precision Medicine for Metastatic Colorectal Cancer: Where Do We Stand? Cancers, 16(22), 3870. https://doi.org/10.3390/cancers16223870








