The Vascular Endothelial Growth Factor-A121/Vascular Endothelial Growth Factor-A165 Ratio as a Predictor of the Therapeutic Response to Immune Checkpoint Inhibitors in Gastric Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Sample Collection
2.3. Measurement of Target Protein Levels in Serum
2.4. Evaluation of Clinical Indicators and Statistical Analysis
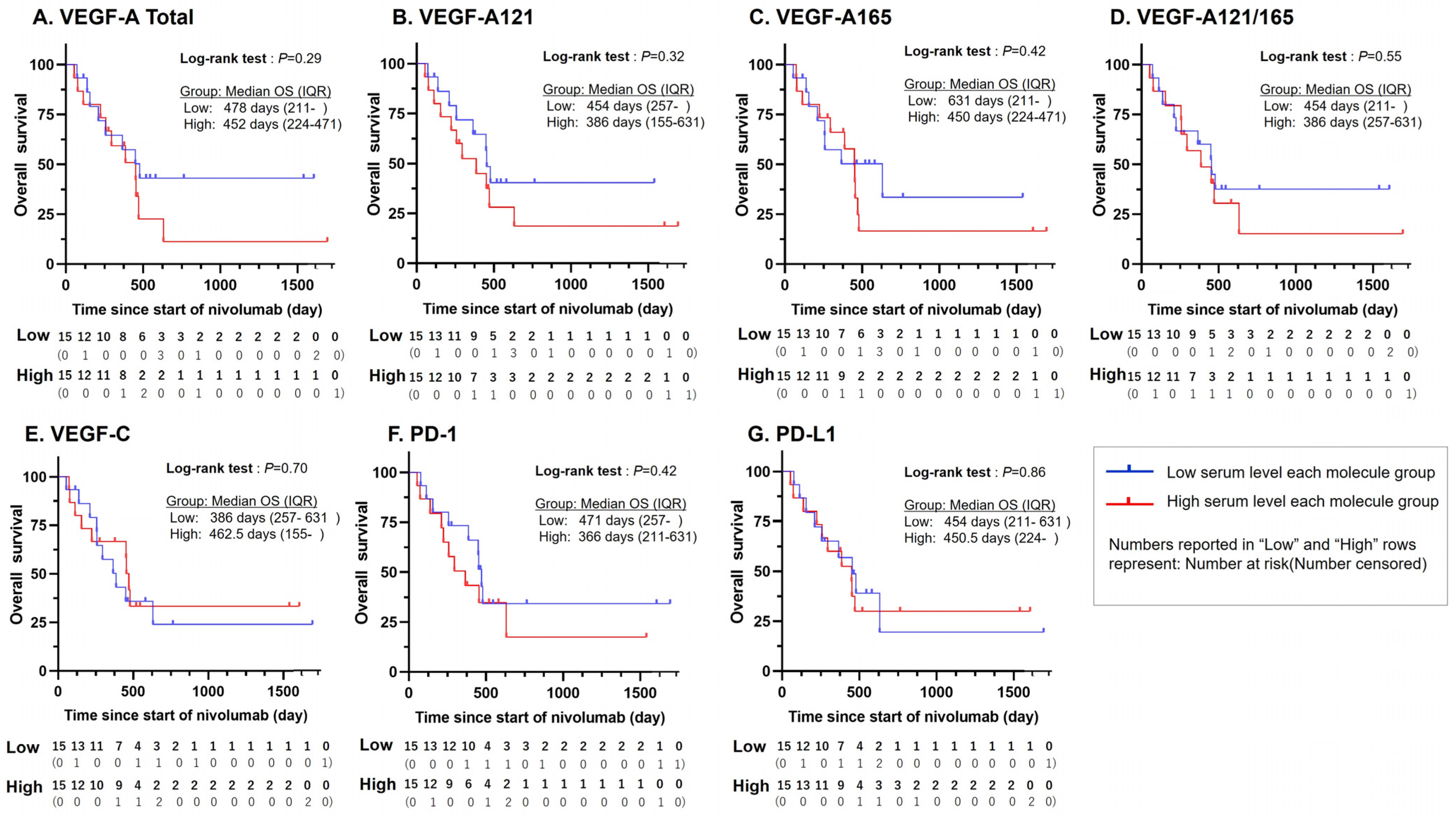
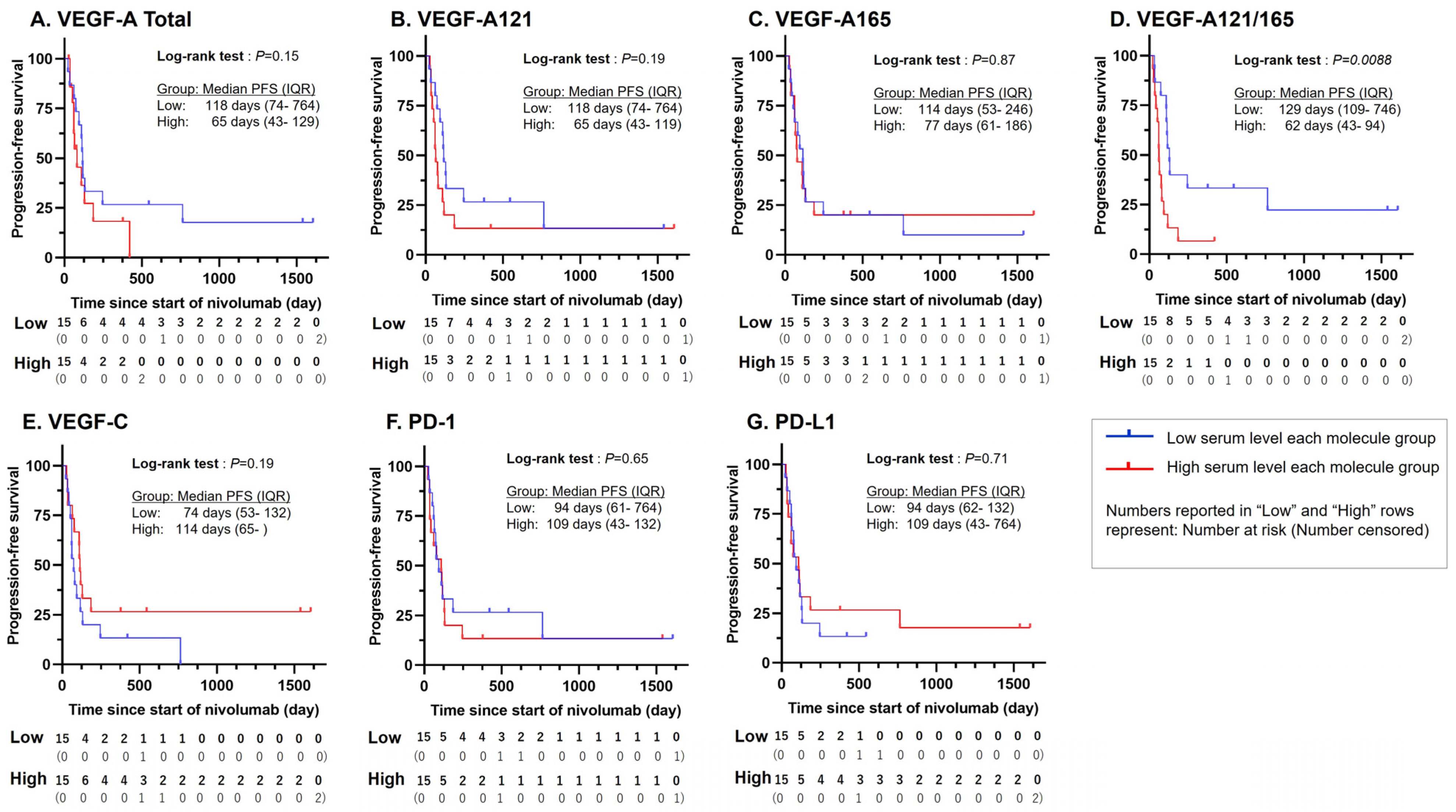
3. Results
3.1. Clinical Characteristics of Subjects
3.2. Statistically Significant PFS Difference for VEGF-A121/165 Ratio
3.3. Statistically Significant Association Between VEGF-A121/165 Ratio and ICI Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Goodman, A.M.; Cohen, P.R.; Jensen, T.J.; Ellison, C.K.; Frampton, G.; Miller, V.; Patel, S.P.; Kurzrock, R. Metastatic basal cell carcinoma with amplification of PD-L1: Exceptional response to anti-PD1 therapy. NPJ Genom. Med. 2016, 1, 16037. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.H.; Fornaro, L.; Olesiński, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomized, open-label, controlled, phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Siefker-Radtke, A.O.; Apolo, A.B.; Bivalacqua, T.J.; Spiess, P.E.; Black, P.C. Immunotherapy with checkpoint blockade in the treatment of urothelial carcinoma. J. Urol. 2018, 199, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Meng, X.; Kong, L.; Yu, J. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: A systematic review. Cancer Lett. 2018, 414, 166–173. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Dudley, J.C.; Lin, M.T.; Le, D.T.; Eshleman, J.R. Microsatellite instability as a biomarker for PD-1 blockade. Clin. Cancer Res. 2016, 22, 813–820. [Google Scholar] [CrossRef]
- Büttner, R.; Longshore, J.W.; López-Ríos, F.; Merkelbach-Bruse, S.; Normanno, N.; Rouleau, E.; Penault-Llorca, F. Implementing TMB measurement in clinical practice: Considerations on assay requirements. ESMO Open 2019, 4, e000442. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Langer, C.J.; Novello, S.; Halmos, B.; Cheng, Y.; Gadgeel, S.M.; Hui, R.; Sugawara, S.; Borghaei, H.; Cristescu, R.; et al. Pembrolizumab (pembro) plus platinum-based chemotherapy (chemo) for metastatic NSCLC: Tissue TMB (tTMB) and outcomes in KEYNOTE-021, 189, and 407. Ann. Oncol. 2019, 30, v917–v918. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, Y.; Zhu, B.; Wang, J.; Zhang, B. Predictive biomarkers of immune checkpoint inhibitors-related toxicities. Front. Immunol. 2020, 11, 2023. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Rasco, D.; Vogelzang, N.J.; Brose, M.S.; Cohn, A.L.; Mier, J.; Di Simone, C.; Hyman, D.M.; Stepan, D.E.; Dutcus, C.E.; et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Arkenau, H.T.; Santana-Davila, R.; Calvo, E.; Paz-Ares, L.; Cassier, P.A.; Bendell, J.; Penel, N.; Krebs, M.G.; Martin-Liberal, J.; et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): A multicohort, non-randomized, open-label, phase 1a/b trial. Lancet Oncol. 2019, 20, 1109–1123. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhou, J.; Dong, Z.; Tandon, S.; Kuk, D.; Panageas, K.S.; Wong, P.; Wu, X.; Naidoo, J.; Page, D.B.; et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab. Cancer Immunol. Res. 2014, 2, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Shibaki, R.; Murakami, S.; Shinno, Y.; Matsumoto, Y.; Yoshida, T.; Goto, Y.; Kanda, S.; Horinouchi, H.; Fujiwara, Y.; Yamamoto, N.; et al. Predictive value of serum VEGF levels for elderly patients or for patients with poor performance status receiving anti-PD-1 antibody therapy for advanced non-small-cell lung cancer. Cancer Immunol. Immunother. 2020, 69, 1229–1236. [Google Scholar] [CrossRef]
- Tozer, G.M.; Akerman, S.; Cross, N.A.; Barber, P.R.; Björndahl, M.A.; Greco, O.; Harris, S.; Hill, S.A.; Honess, D.J.; Ireson, C.R.; et al. Blood vessel maturation and response to vascular-disrupting therapy in single vascular endothelial growth factor-A isoform-producing tumors. Cancer Res. 2008, 68, 2301–2311. [Google Scholar] [CrossRef]
- Yu, J.L.; Rak, J.W.; Klement, G.; Kerbel, R.S. Vascular endothelial growth factor isoform expression as a determinant of blood vessel patterning in human melanoma xenografts. Cancer Res. 2002, 62, 1838–1846. [Google Scholar]
- Yamakuchi, M.; Okawa, M.; Takenouchi, K.; Bibek, A.; Yamada, S.; Inoue, K.; Higurashi, K.; Tabaru, A.; Tanoue, K.; Oyama, Y.; et al. VEGF-A165 is the predominant VEGF-A isoform in platelets, while VEGF-A121 is abundant in serum and plasma from healthy individuals. PLoS ONE 2023, 18, e0284131. [Google Scholar] [CrossRef]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.H.; Chen, K.Y.; Huang, Y.C.; Luo, C.S.; Wu, S.M.; Chen, T.T.; Lee, C.N.; Yeh, C.T.; Chuang, H.C.; Han, C.L.; et al. Bevacizumab reduces S100A9-positive MDSCs linked to intracranial control in patients with EGFR-mutant lung adenocarcinoma. J. Thorac Oncol. 2018, 13, 958–967. [Google Scholar] [CrossRef]
- Ziogas, A.C.; Gavalas, N.G.; Tsiatas, M.; Tsitsilonis, O.; Politi, E.; Terpos, E.; Rodolakis, A.; Vlahos, G.; Thomakos, N.; Haidopoulos, D.; et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int. J. Cancer 2012, 130, 857–864. [Google Scholar] [CrossRef]
- Voron, T.; Marcheteau, E.; Pernot, S.; Colussi, O.; Tartour, E.; Taieb, J.; Terme, M. Control of the immune response by pro-angiogenic factors. Front. Oncol. 2014, 4, 70. [Google Scholar] [CrossRef]
- Oyama, T.; Ran, S.; Ishida, T.; Nadaf, S.; Kerr, L.; Carbone, D.P.; Gabrilovich, D.I. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J. Immunol. 1998, 160, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Chen, H.L.; Girgis, K.R.; Cunningham, H.T.; Meny, G.M.; Nadaf, S.; Kavanaugh, D.; Carbone, D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996, 2, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Nefedova, Y.; Huang, M.; Kusmartsev, S.; Bhattacharya, R.; Cheng, P.; Salup, R.; Jove, R.; Gabrilovich, D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J. Immunol. 2004, 172, 464–474. [Google Scholar] [CrossRef]
- Hamada, Y.; Tanoue, K.; Kita, Y.; Tanabe, K.; Hokonohara, K.; Wada, M.; Hozaka, Y.; Oi, H.; Nakayama, C.; Higashi, M.; et al. Vascular endothelial growth factor inhibitors promote antitumor responses via tumor microenvironment immunosuppression in advanced colorectal cancer. Scand. J. Gastroenterol. 2023, 58, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef]
- Malik, A.K.; Baldwin, M.E.; Peale, F.; Fuh, G.; Liang, W.C.; Lowman, H.; Meng, G.; Ferrara, N.; Gerber, H.P. Redundant roles of VEGF-B and PlGF during selective VEGF-A blockade in mice. Blood 2006, 107, 550–557. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Longatto-Filho, A.; Martins, S.F. Gastric cancer and angiogenesis: Is VEGF a useful biomarker to assess progression and remission? J. Gastric Cancer 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; de Haas, S.; Kang, Y.K.; Ohtsu, A.; Tebbutt, N.C.; Ming Xu, J.; Peng Yong, W.; Langer, B.; Delmar, P.; Scherer, S.J.; et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 2012, 30, 2119–2127. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Muro, K.; Cunningham, D.; Bodoky, G.; Sobrero, A.; Cascinu, S.; Ajani, J.; Oh, S.C.; Al-Batran, S.E.; Wainberg, Z.A.; et al. Biomarker analyses of second-line ramucirumab in patients with advanced gastric cancer from RAINBOW, a global, randomized, double-blind, phase 3 study. Eur. J. Cancer 2020, 127, 150–157. [Google Scholar] [CrossRef]
- Kang, Y.K.; Chen, L.T.; Ryu, M.H.; Oh, D.Y.; Oh, S.C.; Chung, H.C.; Lee, K.W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [CrossRef]
- Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef]
- Kazemi, M.; Carrer, A.; Moimas, S.; Zandonà, L.; Bussani, R.; Casagranda, B.; Palmisano, S.; Prelazzi, P.; Giacca, M.; Zentilin, L.; et al. VEGF121 and VEGF165 differentially promote vessel maturation and tumor growth in mice and humans. Cancer Gene Ther. 2016, 23, 125–132. [Google Scholar] [CrossRef]
- Martini, M.; de Pascalis, I.; D’Alessandris, Q.G.; Fiorentino, V.; Pierconti, F.; Marei, H.E.S.; Ricci-Vitiani, L.; Pallini, R.; Larocca, L.M. VEGF-121 plasma level as biomarker for response to anti-angiogenetic therapy in recurrent glioblastoma. BMC Cancer 2018, 18, 553. [Google Scholar] [CrossRef]
- Mahner, S.; Woelber, L.; Eulenburg, C.; Schwarz, J.; Carney, W.; Jaenicke, F.; Milde-Langosch, K.; Mueller, V. TIMP-1 and VEGF-165 serum concentration during first-line therapy of ovarian cancer patients. BMC Cancer 2010, 10, 139. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef] [PubMed]
- Ramjiawan, R.R.; Griffioen, A.W.; Duda, D.G. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017, 20, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Shirian, J.D.; Shukla, P.; Singh, R.P. Exploring new horizons in neovascular age-related macular degeneration: Novel mechanisms of action and future therapeutic avenues. Eye. 2024; ahead of print. [Google Scholar] [CrossRef]
- Blann, A.D.; Belgore, F.M.; McCollum, C.N.; Silverman, S.; Lip, P.L.; Lip, G.Y. Vascular endothelial growth factor and its receptor, Flt-1, in the plasma of patients with coronary or peripheral atherosclerosis, or Type II diabetes. Clin. Sci. 2002, 102, 187–194. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, K.; Weng, W.; Huang, S.; Zhou, T. Association of vascular endothelial growth factor (VEGF) protein levels and gene polymorphism with the risk of chronic kidney disease. Libyan J. Med. 2023, 18, 2156675. [Google Scholar] [CrossRef]
| Clinical and Pathological Factors | n = 30 (%) |
|---|---|
| Sex | |
| Male | 22 (73.3) |
| Age | |
| Median (Range) | 65 (35–78) |
| PS | |
| 0 | 11 (36.7) |
| 1 | 19 (63.3) |
| Borrmann type | |
| Type 4 | 5 (16.7) |
| Dissemination | |
| Yes | 11 (36.7) |
| Liver metastasis | |
| Yes | 6 (20) |
| Distant lymph node metastasis | |
| Yes | 11 (36.7) |
| Histological diagnosis | |
| Undifferentiated type | 23 (76.7) |
| Number of nivolumab treatment courses | |
| Median (Range) | 5.5 (1–26) |
| Number of chemotherapies pre nivolumab treatment | |
| 2 | 23 (76.7) |
| ≧3 | 7 (23.3) |
| Ramucirumab previous treatment | |
| Yes | 27 (90) |
| Univariable Analysis | |||
|---|---|---|---|
| HR | 95%CI | p-Value | |
| Clinical and pathological factors | |||
| Age | 0.99 | [0.95, 1.03] | 0.65 |
| ECOG PS, 0 | 1.09 | [0.47, 2.44] | 0.84 |
| Histological diagnosis, Undifferentiated type | 1.6 | [0.64, 4.83] | 0.33 |
| Ramucirumab previous treatment, Yes | 1.08 | [0.32, 6.74] | 0.92 |
| Liver metastasis, Yes | 0.93 | [0.30, 2.35] | 0.88 |
| Distant lymph node metastasis, Yes | 1.83 | [0.79, 4.08] | 0.16 |
| Dissemination, Yes | 1.88 | [0.80, 4.38] | 0.14 |
| Serum Biomarker | |||
| VEGF-A total | 1.00 | [1.00, 1.01] | 0.33 |
| VEGF-A 121 type | 1.00 | [1.00, 1.00] | 0.27 |
| VEGF-A 165 type | 1.00 | [0.99, 1.01] | 0.75 |
| VEGF-A 121/165 ratio | 1.08 | [1.00, 1.17] | 0.0454 |
| VEGF-C | 1.00 | [1.00, 1.00] | 0.52 |
| PD-1 | 1.00 | [1.00, 1.00] | 0.76 |
| PD-L1 | 1.00 | [1.00, 1.02] | 0.17 |
| Pre-treatment blood test values | |||
| mGPS, 0 vs. 1–2 | 0.83 | [0.54, 2.68] | 0.65 |
| NLR | 0.96 | [0.04, 5.40] | 0.72 |
| PLR | 1.00 | [0.99, 1.01] | 0.45 |
| LMR | 1.05 | [0.80, 1.36] | 0.71 |
| All Patients | Target Lesion | |||||
|---|---|---|---|---|---|---|
| Low 121/165 Group (n = 15) | High 121/165 Group (n = 15) | p-Value | Low 121/165 Group (n = 8) | High 121/165 Group (n = 11) | p-Value | |
| Best overall response, n (%) | ||||||
| Complete response | 3 (20) | 0 (0) | 2 (25) | 0 (0) | ||
| Partial response | 2 (13) | 0 (0) | 2 (25) | 0 (0) | ||
| Stable disease | 3 (20) | 2 (13) | 3 (38) | 2 (18) | ||
| Progressive disease | 3 (20) | 10 (67) | 1 (12) | 9 (82) | ||
| non-CR/non-PD | 4 (27) | 3 (20) | - | - | ||
| Objective Response Rate (%) | - | - | 50 | 0 | 0.0181 | |
| Disease Control Rate (%) | 80 | 33 | 0.0253 | 88 | 18 | 0.0055 |
| Univariable Analysis | |||
|---|---|---|---|
| RR | 95%CI | p-Value | |
| Clinical and pathological factors | |||
| Age | 1 | [0.93, 1.08] | 0.95 |
| ECOG PS, 0 | 1.15 | [0.25, 5.20] | 0.86 |
| Histological diagnosis, Undifferentiated type | 6.55 | [0.68, 63.33] | 0.06 |
| Ramucirumab previous treatment, Yes | 0.34 | [0.01, 4.02] | 0.39 |
| Liver metastasis, Yes | 1.4 | [0.22, 9.01] | 0.71 |
| Distant lymph node metastasis, Yes | 3.79 | [0.83, 19.81] | 0.09 |
| Dissemination, Yes | 1.15 | [0.25, 5.20] | 0.86 |
| Serum Biomarker | |||
| VEGF-A total | 1.00 | [1.00, 1.01] | 0.0032 |
| VEGF-A 121 type | 1.00 | [1.00, 1.01] | 0.0043 |
| VEGF-A 165 type | 1.00 | [0.98, 1.02] | 0.96 |
| VEGF-A 121/165 ratio | 1.2 | [1.01, 1.38] | 0.0319 |
| VEGF-C | 1.00 | [0.99, 1.00] | 0.63 |
| PD-1 | 1.00 | [0.99, 1.00] | 0.73 |
| PD-L1 | 1.02 | [1.00, 1.06] | 0.06 |
| Pre-treatment blood test values | |||
| mGPS, 0 vs. 1–2 | 0.96 | [0.24, 4.51] | 0.96 |
| NLR | 1.06 | [0.71, 1.61] | 0.77 |
| PLR | 1.00 | [0.004, 3.60] | 0.26 |
| LMR | 0.79 | [0.44, 1.31] | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamada, Y.; Tanoue, K.; Arigami, T.; Yamakuchi, M.; Okawa, M.; Matsushita, D.; Takenouchi, K.; Yamada, S.; Maywar, D.N.; Nakayama, C.; et al. The Vascular Endothelial Growth Factor-A121/Vascular Endothelial Growth Factor-A165 Ratio as a Predictor of the Therapeutic Response to Immune Checkpoint Inhibitors in Gastric Cancer. Cancers 2024, 16, 3958. https://doi.org/10.3390/cancers16233958
Hamada Y, Tanoue K, Arigami T, Yamakuchi M, Okawa M, Matsushita D, Takenouchi K, Yamada S, Maywar DN, Nakayama C, et al. The Vascular Endothelial Growth Factor-A121/Vascular Endothelial Growth Factor-A165 Ratio as a Predictor of the Therapeutic Response to Immune Checkpoint Inhibitors in Gastric Cancer. Cancers. 2024; 16(23):3958. https://doi.org/10.3390/cancers16233958
Chicago/Turabian StyleHamada, Yuki, Kiyonori Tanoue, Takaaki Arigami, Munekazu Yamakuchi, Masashi Okawa, Daisuke Matsushita, Kazunori Takenouchi, Shingo Yamada, Drew N. Maywar, Chieri Nakayama, and et al. 2024. "The Vascular Endothelial Growth Factor-A121/Vascular Endothelial Growth Factor-A165 Ratio as a Predictor of the Therapeutic Response to Immune Checkpoint Inhibitors in Gastric Cancer" Cancers 16, no. 23: 3958. https://doi.org/10.3390/cancers16233958
APA StyleHamada, Y., Tanoue, K., Arigami, T., Yamakuchi, M., Okawa, M., Matsushita, D., Takenouchi, K., Yamada, S., Maywar, D. N., Nakayama, C., Oyama, Y., Higashi, S., Fujisaki, C., Hozaka, Y., Kita, Y., Hashiguchi, T., & Ohtsuka, T. (2024). The Vascular Endothelial Growth Factor-A121/Vascular Endothelial Growth Factor-A165 Ratio as a Predictor of the Therapeutic Response to Immune Checkpoint Inhibitors in Gastric Cancer. Cancers, 16(23), 3958. https://doi.org/10.3390/cancers16233958






