HER2-Low Expression in Male Breast Cancer: Results from a Multicenter Series in Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
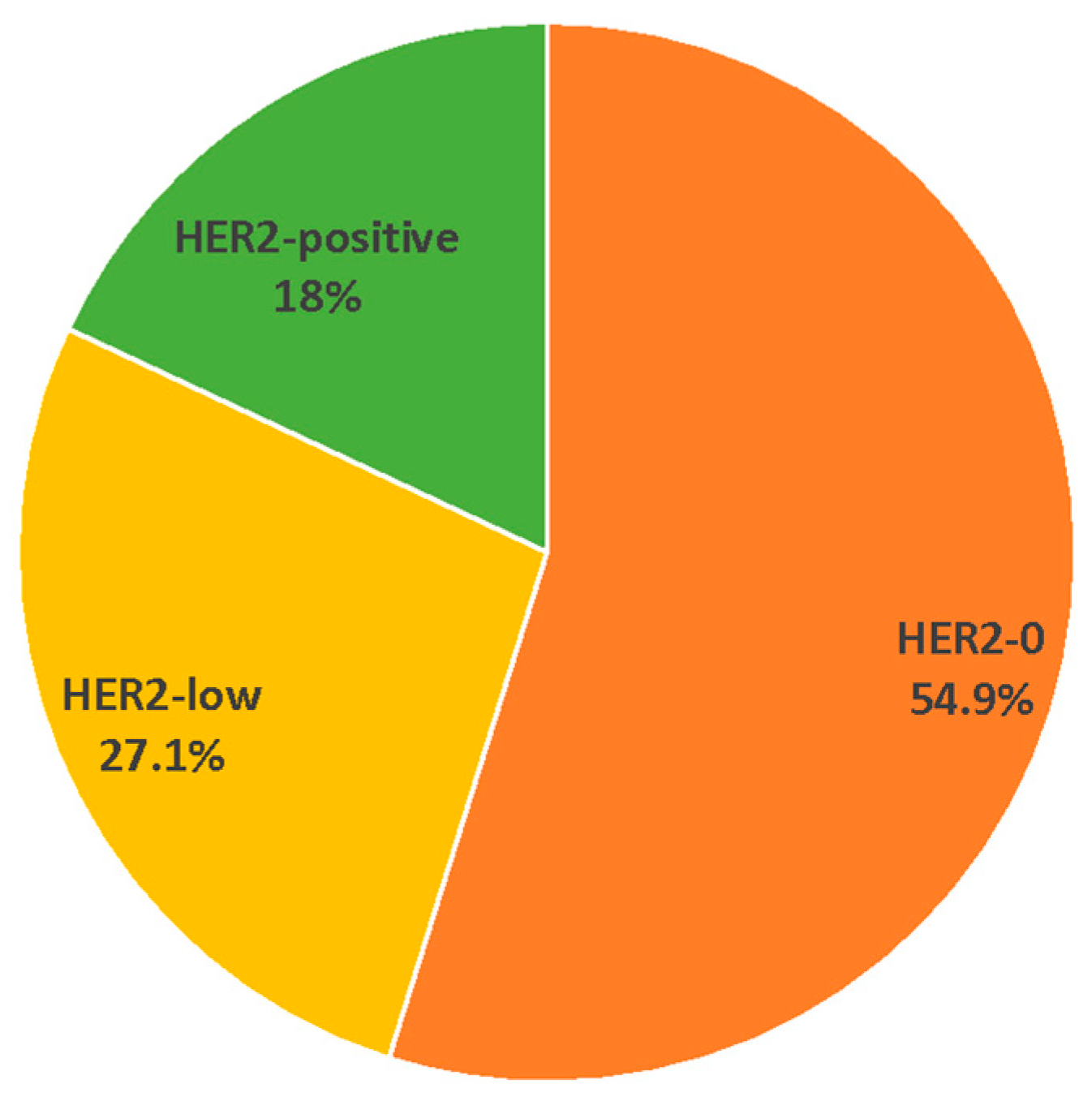
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarantino, P.; Curigliano, G.; Tolaney, S.M. Navigating the HER2-Low Paradigm in Breast Oncology: New Standards, Future Horizons. Cancer Discov. 2022, 12, 2026–2030. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. DESTINY-Breast04 Trial Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, C.; Jacobs, F.; Marchiò, C.; Pitto, F.; Cosso, M.; Spinaci, S.; de Azambuja, E.; Schettini, F.; Agostinetto, E.; Lambertini, M. HER2-Low Breast Cancer: Where Are We? Breast Care 2022, 17, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Park, W.K.; Nam, S.J.; Kim, S.W.; Lee, J.E.; Yu, J.; Ryu, J.M.; Chae, B.J. The Impact of HER2-Low Expression on Oncologic Outcomes in Hormone Receptor-Positive Breast Cancer. Cancers 2023, 15, 5361. [Google Scholar] [CrossRef]
- Won, H.S.; Ahn, J.; Kim, Y.; Kim, J.S.; Song, J.Y.; Kim, H.K.; Lee, J.; Park, H.K.; Kim, Y.S. Clinical significance of HER2-low expression in early breast cancer: A nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. 2022, 24, 22. [Google Scholar] [CrossRef] [PubMed]
- Gampenrieder, S.P.; Rinnerthaler, G.; Tinchon, C.; Petzer, A.; Balic, M.; Heibl, S.; Schmitt, C.; Zabernigg, A.F.; Egle, D.; Sandholzer, M.; et al. Landscape of HER2-low metastatic breast cancer (MBC): Results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021, 23, 112. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, D.S.; Zhao, F.; Chen, N.; Hahn, O.M.; Nanda, R.; Olopade, O.I.; Huo, D.; Howard, F.M. Clinicopathologic Characteristics and Prognosis of ERBB2-Low Breast Cancer Among Patients in the National Cancer Database. JAMA Oncol. 2023, 9, 500–510. [Google Scholar] [CrossRef]
- Ottini, L. Male breast cancer: A rare disease that might uncover underlying pathways of breast cancer. Nat. Rev. Cancer. 2014, 14, 643. [Google Scholar] [CrossRef]
- Giordano, S.H. Breast Cancer in Men. N. Engl. J. Med. 2018, 378, 2311–2320. [Google Scholar] [CrossRef]
- Mangone, L.; Ferrari, F.; Mancuso, P.; Carrozzi, G.; Michiara, M.; Falcini, F.; Piffer, S.; Filiberti, R.A.; Caldarella, A.; Vitale, F.; et al. Epidemiology and biological characteristics of male breast cancer in Italy. Breast Cancer 2020, 27, 724–731. [Google Scholar] [CrossRef]
- Ottini, L.; Capalbo, C.; Rizzolo, P.; Silvestri, V.; Bronte, G.; Rizzo, S.; Russo, A. HER2-positive male breast cancer: An update. Breast Cancer (Dove Med. Press.) 2010, 2, 45–58. [Google Scholar] [CrossRef]
- Kornegoor, R.; Verschuur-Maes, A.H.; Buerger, H.; Hogenes, M.C.; De Bruin, P.C.; Oudejans, J.J.; Van Der Groep, P.; Hinrichs, B.; Van Diest, P.J. Molecular subtyping of male breast cancer by immunohistochemistry. Mod. Pathol. 2012, 25, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.P.; Sundara Rajan, S.; Honarpisheh, H.; Cserni, G.; Dent, J.; Fulford, L.; Jordan, L.B.; Jones, J.L.; Kanthan, R.; Litwiniuk, M.; et al. Characterisation of male breast cancer: A descriptive biomarker study from a large patient series. Sci. Rep. 2017, 7, 45293. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.A.; Slaets, L.; Cardoso, F.; Giordano, S.H.; Tryfonidis, K.; van Diest, P.J.; Dijkstra, N.H.; Schröder, C.P.; van Asperen, C.J.; Linderholm, B.; et al. Pathological characterisation of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Eur. J. Cancer 2017, 82, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Chatterji, S.; Krzoska, E.; Thoroughgood, C.W.; Saganty, J.; Liu, P.; Elsberger, B.; Abu-Eid, R.; Speirs, V. Defining genomic, transcriptomic, proteomic, epigenetic, and phenotypic biomarkers with prognostic capability in male breast cancer: A systematic review. Lancet Oncol. 2023, 24, e74–e85. [Google Scholar] [CrossRef]
- Kornegoor, R.; van Diest, P.J.; Buerger, H.; Korsching, E. Tracing differences between male and female breast cancer: Both diseases own a different biology. Histopathology 2015, 67, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Bucalo, A.; Conti, G.; Valentini, V.; Capalbo, C.; Bruselles, A.; Tartaglia, M.; Bonanni, B.; Calistri, D.; Coppa, A.; Cortesi, L.; et al. Male breast cancer risk associated with pathogenic variants in genes other than BRCA1/2: An Italian case-control study. Eur. J. Cancer 2023, 188, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Coppa, A.; Nicolussi, A.; D’Inzeo, S.; Capalbo, C.; Belardinilli, F.; Colicchia, V.; Petroni, M.; Zani, M.; Ferraro, S.; Rinaldi, C.; et al. Optimizing the identification of risk-relevant mutations by multigene panel testing in selected hereditary breast/ovarian cancer families. Cancer Med. 2018, 7, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Ottini, L.; Silvestri, V.; Rizzolo, P.; Falchetti, M.; Zanna, I.; Saieva, C.; Masala, G.; Bianchi, S.; Manoukian, S.; Barile, M.; et al. Clinical and pathologic characteristics of BRCA-positive and BRCA-negative male breast cancer patients: Results from a collaborative multicenter study in Italy. Breast Cancer Res. Treat. 2012, 134, 411–418. [Google Scholar] [CrossRef]
- Valentini, V.; Silvestri, V.; Bucalo, A.; Conti, G.; Karimi, M.; Di Francesco, L.; Pomati, G.; Mezi, S.; Cerbelli, B.; Pignataro, M.G.; et al. Molecular profiling of male breast cancer by multigene panel testing: Implications for precision oncology. Front. Oncol. 2023, 12, 1092201. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Brcic, I.; Kluba, A.M.; Godschachner, T.M.; Suppan, C.; Regitnig, P.; Dandachi, N.; Lax, S.F.; Balić, M. Tumor Microenvironment in Male Breast Carcinoma with Emphasis on Tumor Infiltrating Lymphocytes and PD-L1 Expression. Int. J. Mol. Sci. 2023, 24, 818. [Google Scholar] [CrossRef]
- Bloom, K.J.; Govil, H.; Gattuso, P.; Reddy, V.; Francescatti, D. Status of HER-2 in male and female breast carcinoma. Am. J. Surg. 2001, 182, 389–392. [Google Scholar] [CrossRef]
- Nilsson, C.; Johansson, I.; Ahlin, C.; Thorstenson, S.; Amini, R.M.; Holmqvist, M.; Bergkvist, L.; Hedenfalk, I.; Fjällskog, M.L. Molecular subtyping of male breast cancer using alternative definitions and its prognostic impact. Acta Oncol. 2013, 52, 102–109. [Google Scholar] [CrossRef]
- Horisawa, N.; Adachi, Y.; Takatsuka, D.; Nozawa, K.; Endo, Y.; Ozaki, Y.; Sugino, K.; Kataoka, A.; Kotani, H.; Yoshimura, A.; et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer 2022, 29, 234–241. [Google Scholar] [CrossRef]
- Li, Y.; Tsang, J.Y.; Tam, F.; Loong, T.; Tse, G.M. Comprehensive characterization of HER2-low breast cancers: Implications in prognosis and treatment. EBioMedicine 2023, 91, 104571. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, J.; Liu, L.; Kong, X.; Lin, D.; Zhou, H.; Gao, J. Clinicopathological characteristics of HER2-low breast cancer: A retrospective study. Sci. Rep. 2023, 13, 12382. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Sun, X.; Xu, Z.; Cai, L.; Liu, C.; Wu, S.; Liu, Y. Evolution and clinical significance of HER2-low status after neoadjuvant therapy for breast cancer. Front. Oncol. 2023, 13, 1086480. [Google Scholar] [CrossRef] [PubMed]
- Polidorio, N.; Montagna, G.; Sevilimedu, V.; Le, T.; Morrow, M. Do HER2-Low Tumors Have a Distinct Clinicopathologic Phenotype? Ann. Surg. Oncol. 2023, 30, 1–13. [Google Scholar] [CrossRef]
- Silvestri, V.; Barrowdale, D.; Mulligan, A.M.; Neuhausen, S.L.; Fox, S.; Karlan, B.Y.; Mitchell, G.; James, P.; Thull, D.L.; Zorn, K.K.; et al. Male breast cancer in BRCA1 and BRCA2 mutation carriers: Pathology data from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Res. 2016, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Nilsson, C.; Berglund, P.; Lauss, M.; Ringnér, M.; Olsson, H.; Luts, L.; Sim, E.; Thorstensson, S.; Fjällskog, M.L.; et al. Gene expression profiling of primary male breast cancers reveals two unique subgroups and identifies N-acetyltransferase-1 (NAT1) as a novel prognostic biomarker. Breast Cancer Res. 2012, 14, R31. [Google Scholar] [CrossRef]
- Zelli, V.; Silvestri, V.; Valentini, V.; Bucalo, A.; Rizzolo, P.; Zanna, I.; Bianchi, S.; Coppa, A.; Giannini, G.; Cortesi, L.; et al. Transcriptome of Male Breast Cancer Matched with Germline Profiling Reveals Novel Molecular Subtypes with Possible Clinical Relevance. Cancers 2021, 13, 4515. [Google Scholar] [CrossRef]
- Li, X.; Lee, J.H.; Gao, Y.; Zhang, J.; Bates, K.M.; Rimm, D.L.; Zhang, H.; Smith, G.H.; Lawson, D.; Meisel, J.; et al. Correlation of HER2 Protein Level With mRNA Level Quantified by RNAscope in Breast Cancer. Mod. Pathol. 2023, 37, 100408. [Google Scholar] [CrossRef] [PubMed]
- Marchiò, C.; Criscitiello, C.; Scatena, C.; Santinelli, A.; Graziano, P.; Malapelle, U.; Cursano, G.; Venetis, K.; Fanelli, G.N.; Pepe, F.; et al. Think “HER2” different: Integrative diagnostic approaches for HER2-low breast cancer. Pathologica 2023, 115, 292–301. [Google Scholar] [CrossRef] [PubMed]
| Characteristics 1 | HER2-0 (N = 73) | HER2-Low (N = 39) | p-Value 2 | |
|---|---|---|---|---|
| Histological type | Ductal | 68 (93.2%) | 36 (92.3%) | 0.87 |
| Other | 5 (6.8%) | 3 (7.7%) | ||
| Stage | I | 15 (26.8%) | 11 (37.9%) | 0.29 |
| ≥II | 41 (73.2%) | 18 (62.1%) | ||
| Nodes | Negative | 33 (45%) | 7 (26%) | 0.01 |
| Positive | 27 (55%) | 20 (74%) | ||
| Grading | 1 | 3 (4.4%) | 3 (8.1%) | 0.44 |
| ≥2 | 65 (95.6%) | 34 (91.9%) | ||
| Ki67 | Low | 24 (34.8%) | 13 (35.1%) | 0.97 |
| High | 45 (65.2%) | 24 (64.9%) | ||
| Germline BRCA1/2 PVs | Negative | 60 (82.2%) | 34 (87.2%) | 0.49 |
| Positive | 13 (17.8%) | 5 (12.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestri, V.; Valentini, V.; Bucalo, A.; Conti, G.; Manzella, L.; Turchetti, D.; Russo, A.; Capalbo, C.; Ottini, L. HER2-Low Expression in Male Breast Cancer: Results from a Multicenter Series in Italy. Cancers 2024, 16, 548. https://doi.org/10.3390/cancers16030548
Silvestri V, Valentini V, Bucalo A, Conti G, Manzella L, Turchetti D, Russo A, Capalbo C, Ottini L. HER2-Low Expression in Male Breast Cancer: Results from a Multicenter Series in Italy. Cancers. 2024; 16(3):548. https://doi.org/10.3390/cancers16030548
Chicago/Turabian StyleSilvestri, Valentina, Virginia Valentini, Agostino Bucalo, Giulia Conti, Livia Manzella, Daniela Turchetti, Antonio Russo, Carlo Capalbo, and Laura Ottini. 2024. "HER2-Low Expression in Male Breast Cancer: Results from a Multicenter Series in Italy" Cancers 16, no. 3: 548. https://doi.org/10.3390/cancers16030548





