Triptolide Reduces Neoplastic Progression in Hepatocellular Carcinoma by Downregulating the Lipid Lipase Signaling Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human HCC Samples and Cell Lines
2.2. Natural Small Molecule
2.3. Animals
2.4. Cell Proliferation and Colony Formation Assay
2.5. Transwell Migration Assay
2.6. Cell Transfection
2.7. Quantitative Real-Time PCR (q-RT-PCR)
2.8. Western Blot Assay (WB)
2.9. Immunohistochemistry (IHC)
2.10. In Vivo Tumor Growth Assay
2.11. Statistical Analysis
3. Results
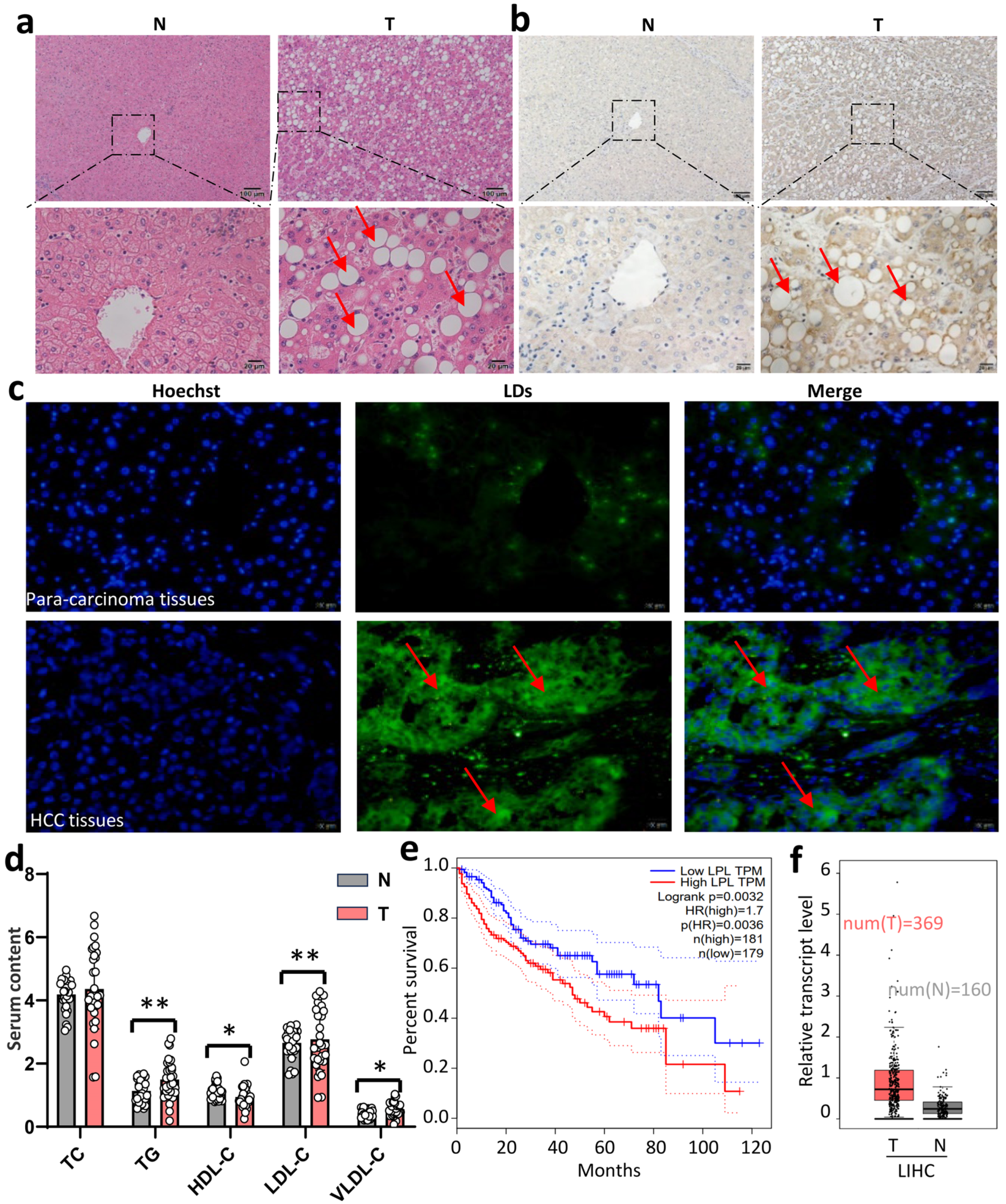
3.1. High LPL Expression Predicted an Unfavorable Prognosis in HCC Patients
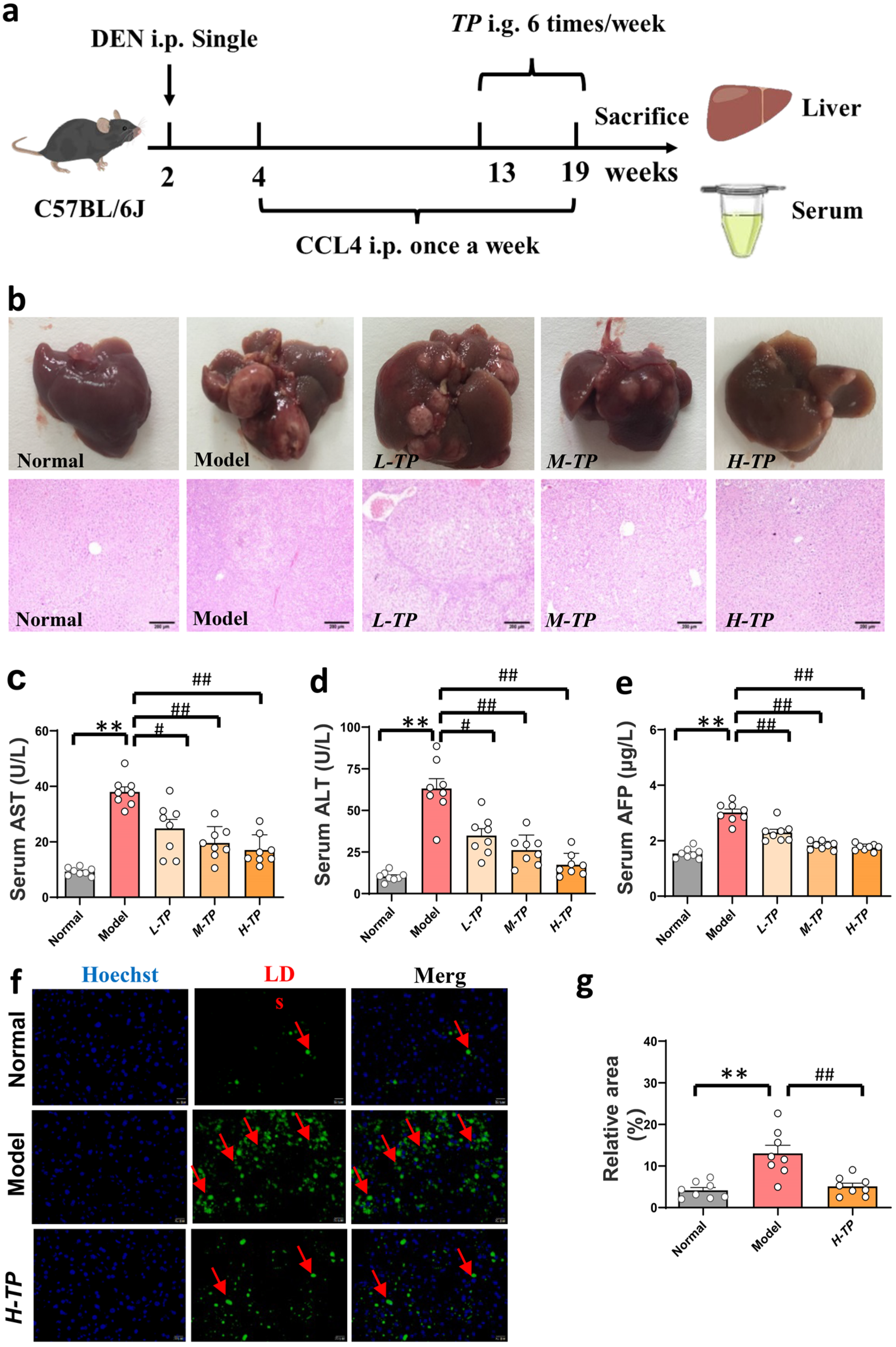
3.2. Triptolide Ameliorates DEN- and CCl4-Induced HCC in Mice
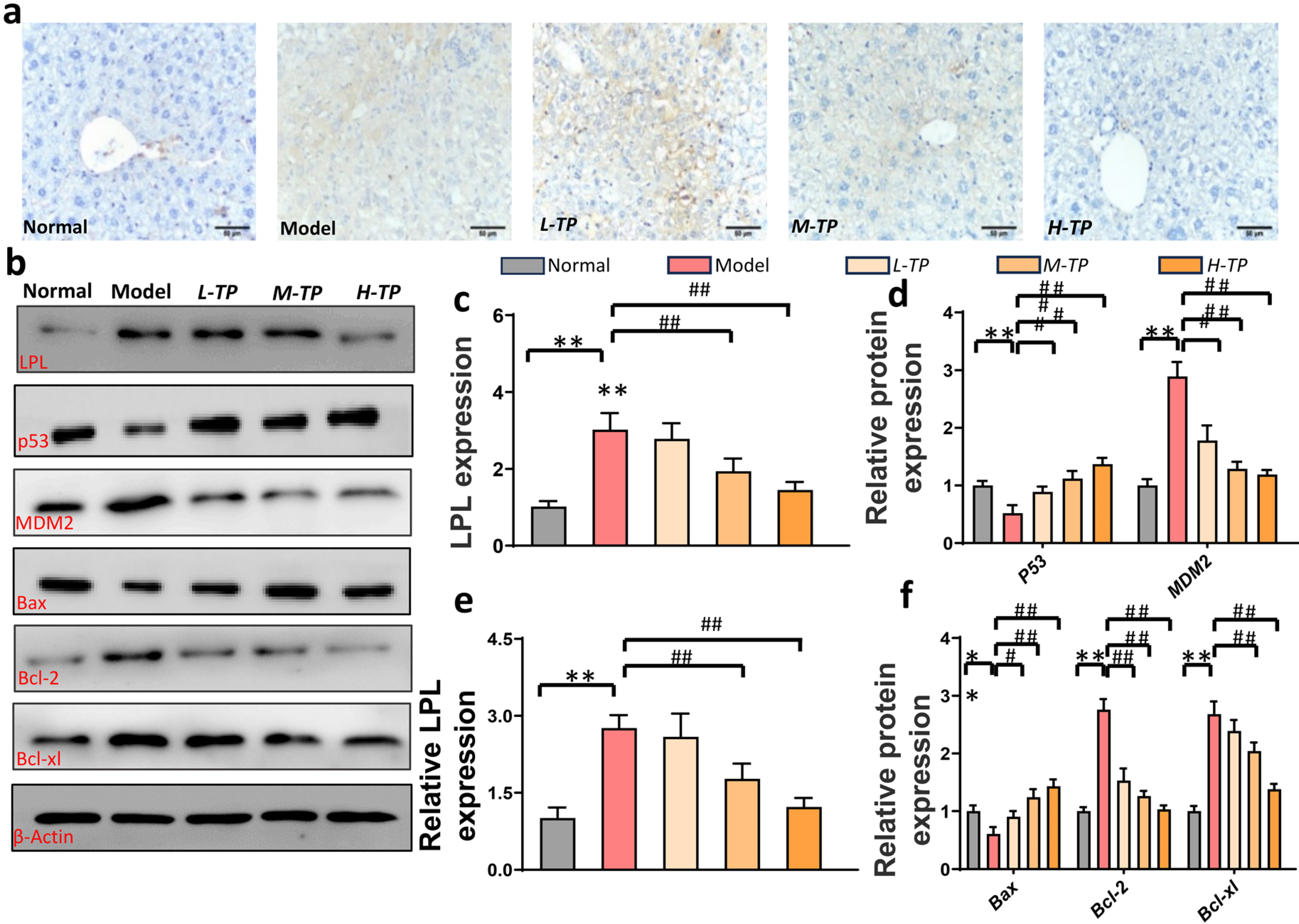
3.3. Triptolide Triggers HCC Cells Apoptosis by LPL and p53-Bax Pathways in Mice
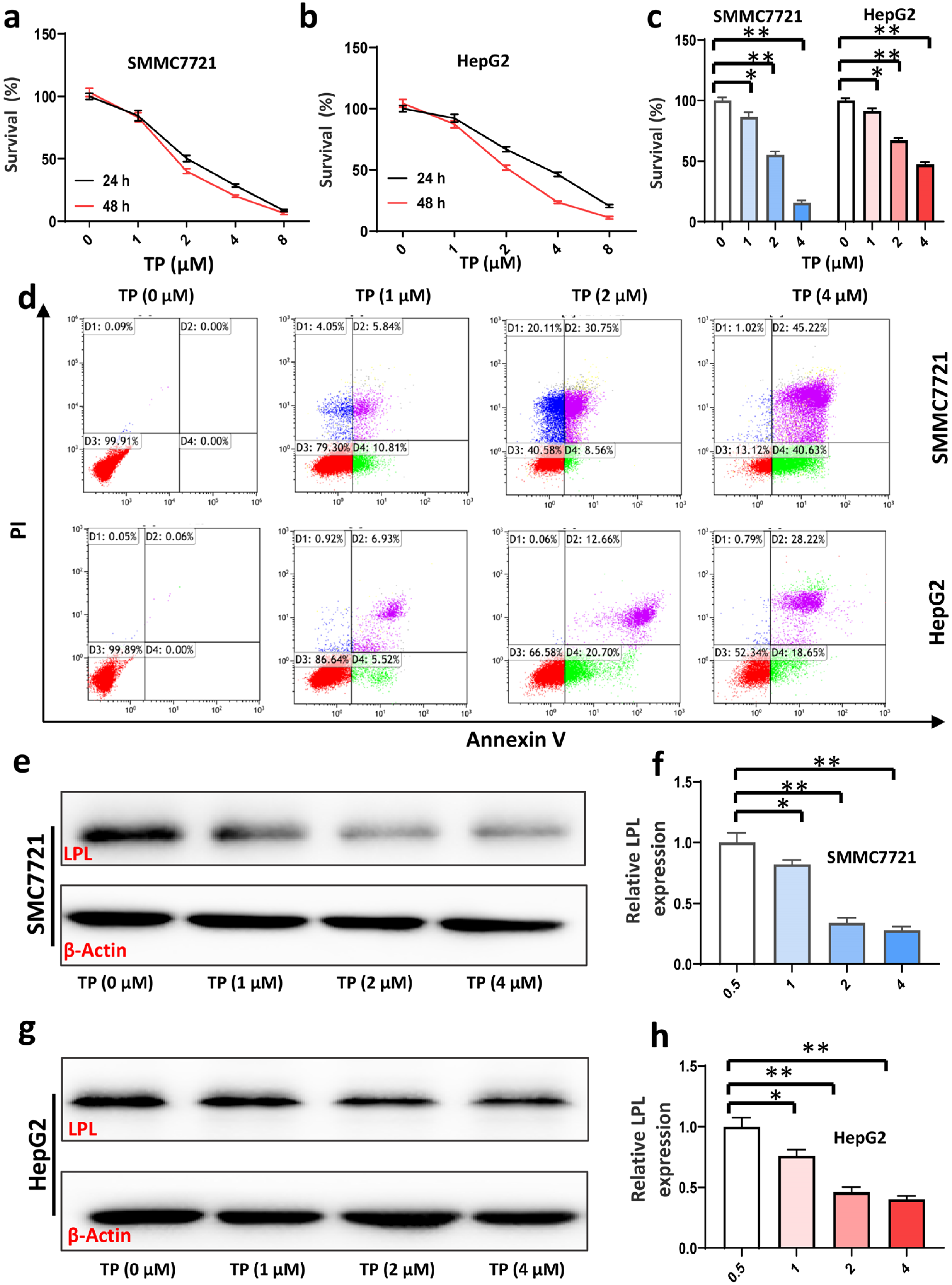
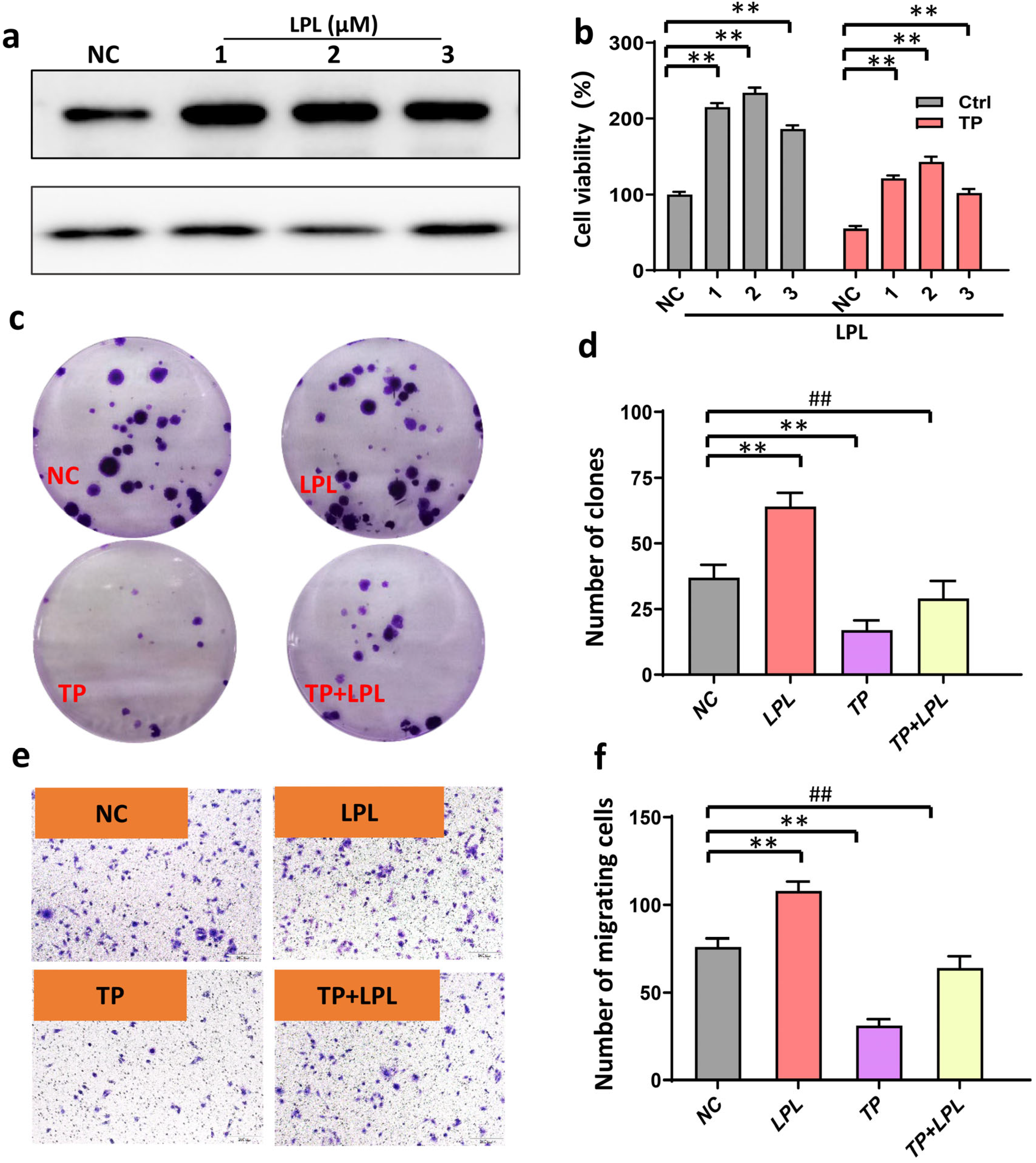
3.4. Triptolide Inhibits HCC Cell Viability and Proliferation, and Promotes HCC Cell Apoptosis by Suppressing Expression of LPL
3.5. Inhibition of LPL Decreased HCC Cell Proliferation and Invasion
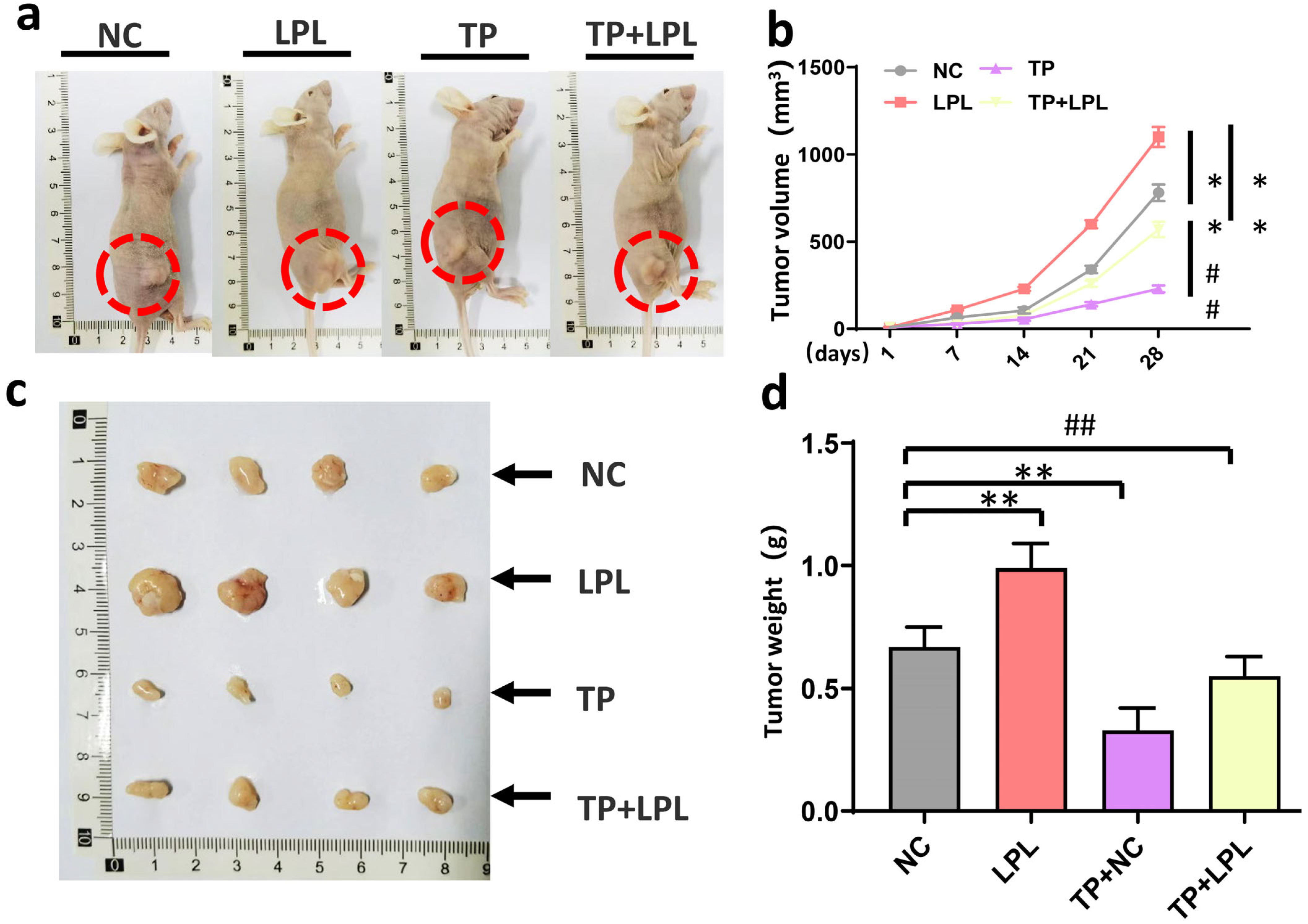
3.6. Triptolide Suppressed Xenograft Tumor Growth of HCC Cells In Vivo via Targeting LPL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Romano, A.; Angeli, P.; Piovesan, S.; Noventa, F.; Anastassopoulos, G.; Chemello, L.; Cavalletto, L.; Gambato, M.; Russo, F.P.; Burra, P.; et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: A prospective population study. J. Hepatol. 2018, 69, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.; Park, S.; Murad, M.H.; Barnard, A.; Prokop, L.; Adams, L.A.; Singh, S.; Loomba, R. Comparative Effectiveness of Entecavir Versus Tenofovir for Preventing Hepatocellular Carcinoma in Patients with Chronic Hepatitis B: A Systematic Review and Meta-Analysis. Hepatology 2021, 73, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Hellerbrand, C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: Mechanisms and implications. Gut 2010, 59, 1303–1307. [Google Scholar] [CrossRef]
- Cao, D.; Song, X.; Che, L.; Li, X.; Pilo, M.G.; Vidili, G.; Porcu, A.; Solinas, A.; Cigliano, A.; Pes, G.M.; et al. Both de novo synthetized and exogenous fatty acids support the growth of hepatocellular carcinoma cells. Liver Int. 2017, 37, 80–89. [Google Scholar] [CrossRef]
- Jiang, C.M.; Pu, C.W.; Hou, Y.H.; Chen, Z.; Alanazy, M.; Hebbard, L. Non alcoholic steatohepatitis a precursor for hepatocellular carcinoma development. World J. Gastroenterol. 2014, 20, 16464–16473. [Google Scholar] [CrossRef]
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef]
- Porzsolt, F.; Digel, W.; Jacobsen, H.; Mittnacht, S.; Kirchner, H.; Heimpel, H. Different antitumor mechanisms of interferon-alpha in the treatment of hairy cell leukemia and renal cell cancer. Cancer 1988, 61, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Loomis, A.K.; van der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; Mosseveld, M.; et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: Real-world study of 18 million patients in four European cohorts. BMC Med. 2019, 17, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Ahmed, F.; Mara, K.C.; Addissie, B.D.; Allen, A.M.; Gores, G.J.; Roberts, L.R. Diabetes Is Associated With Increased Risk of Hepatocellular Carcinoma in Patients With Cirrhosis From Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Liu, C.; Qin, M.; Zhang, X.; Xi, T.; Yuan, S.; Hao, H.; Xiong, J. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm. Sin. B 2022, 12, 558–580. [Google Scholar] [CrossRef] [PubMed]
- Pope, E.D., 3rd; Kimbrough, E.O.; Vemireddy, L.P.; Surapaneni, P.K.; Copland, J.A., 3rd; Mody, K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert. Opin. Ther. Targets 2019, 23, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, Y.; Xu, H.; Wang, X.; Zhang, Y.; Shang, R.; O’Farrell, M.; Roessler, S.; Sticht, C.; Stahl, A.; et al. Therapeutic efficacy of FASN inhibition in preclinical models of HCC. Hepatology 2022, 76, 951–966. [Google Scholar] [CrossRef]
- Koch, M.; DeKosky, S.T.; Goodman, M.; Sun, J.; Furtado, J.D.; Fitzpatrick, A.L.; Mackey, R.H.; Cai, T.; Lopez, O.L.; Kuller, L.H.; et al. Association of Apolipoprotein E in Lipoprotein Subspecies With Risk of Dementia. JAMA Netw. Open 2020, 3, e209250. [Google Scholar] [CrossRef]
- Cruz-Bautista, I.; Mehta, R.; Cabiedes, J.; García-Ulloa, C.; Guillen-Pineda, L.E.; Almeda-Valdés, P.; Cuevas-Ramos, D.; Aguilar-Salinas, C.A. Determinants of VLDL composition and apo B-containing particles in familial combined hyperlipidemia. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 438, 160–165. [Google Scholar] [CrossRef]
- Rozovski, U.; Grgurevic, S.; Bueso-Ramos, C.; Harris, D.M.; Li, P.; Liu, Z.; Wu, J.Y.; Jain, P.; Wierda, W.; Burger, J.; et al. Aberrant LPL Expression, Driven by STAT3, Mediates Free Fatty Acid Metabolism in CLL Cells. Mol. Cancer Res. 2015, 13, 944–953. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Court, W.A.; Dailey, R.G., Jr.; Gilmore, C.J.; Bryan, R.F. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J. Am. Chem. Soc. 1972, 94, 7194–7195. [Google Scholar] [CrossRef]
- Li, M.; Wang, G.; Yan, Y.; Jiang, M.; Wang, Z.; Zhang, Z.; Wu, X.; Zeng, H. Triptolide and l-ascorbate palmitate co-loaded micelles for combination therapy of rheumatoid arthritis and side effect attenuation. Drug Deliv. 2022, 29, 2751–2758. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liang, Z.; Zhao, F.; Liu, X.; Ma, N. Triptolide inhibits oxidative stress and inflammation via the microRNA-155-5p/brain-derived neurotrophic factor to reduce podocyte injury in mice with diabetic nephropathy. Bioengineered 2022, 13, 12275–12288. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, X.; Ke, X.; Dai, Y.; Shi, J. Triptolide Suppresses NF-kappaB-Mediated Inflammatory Responses and Activates Expression of Nrf2-Mediated Antioxidant Genes to Alleviate Caerulein-Induced Acute Pancreatitis. Int. J. Mol. Sci. 2022, 23, 1252. [Google Scholar] [CrossRef] [PubMed]
- Noel, P.; Von Hoff, D.D.; Saluja, A.K.; Velagapudi, M.; Borazanci, E.; Han, H. Triptolide and Its Derivatives as Cancer Therapies. Trends Pharmacol. Sci. 2019, 40, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Alsaied, O.A.; Sangwan, V.; Banerjee, S.; Krosch, T.C.; Chugh, R.; Saluja, A.; Vickers, S.M.; Jensen, E.H. Sorafenib and triptolide as combination therapy for hepatocellular carcinoma. Surgery 2014, 156, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, D.; Wang, C.; Zhao, S.; Liu, C. Triptolide Inhibits Invasion and Tumorigenesis of Hepatocellular Carcinoma MHCC-97H Cells Through NF-kappaB Signaling. Med. Sci. Monit. 2016, 22, 1827–1836. [Google Scholar] [CrossRef]
- Chang, W.; He, W.; Li, P.P.; Song, S.S.; Yuan, P.F.; Lu, J.T.; Wei, W. Protective effects of Celastrol on diethylnitrosamine-induced hepatocellular carcinoma in rats and its mechanisms. Eur. J. Pharmacol. 2016, 784, 173–180. [Google Scholar] [CrossRef]
- Stefan, N.; Haring, H.U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Cerne, D.; Zitnik, I.P.; Sok, M. Increased fatty acid synthase activity in non-small cell lung cancer tissue is a weaker predictor of shorter patient survival than increased lipoprotein lipase activity. Arch. Med. Res. 2010, 41, 405–409. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, H.; Wang, L.; Song, X.; Zhang, J.; Liu, W.; Ge, Y.; Sun, Y.; Yu, X.; Wang, Z.; et al. Tumor suppressor ZHX2 inhibits NAFLD-HCC progression via blocking LPL-mediated lipid uptake. Cell Death Differ. 2020, 27, 1693–1708. [Google Scholar] [CrossRef]
- Li, X.J.; Jiang, Z.Z.; Zhang, L.Y. Triptolide: Progress on research in pharmacodynamics and toxicology. J. Ethnopharmacol. 2014, 155, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, Y.; Liu, X.; Wu, X.; Huang, L.; Gao, W. Triptolide: Pharmacological spectrum, biosynthesis, chemical synthesis and derivatives. Theranostics 2021, 11, 7199–7221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.L.; Yang, Y.X.; Ding, J.; Li, Y.C.; Miao, Z.H. Triptolide: Structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat. Prod. Rep. 2012, 29, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, G.; Wang, R.; Wang, Y.; Wang, J.; Yang, M.; Gong, C.; Yuan, Y. gamma-Cyclodextrin metal-organic framework as a carrier to deliver triptolide for the treatment of hepatocellular carcinoma. Drug Deliv. Transl. Res. 2022, 12, 1096–1104. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Zhang, P.; Liu, Y.; Ran, W.; Cai, Y.; Wang, J.; Zhai, Y.; Wang, G.; Ding, Y.; et al. Injectable peptide hydrogel as intraperitoneal triptolide depot for the treatment of orthotopic hepatocellular carcinoma. Acta Pharm. Sin. B 2019, 9, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, S.; Wu, M.; Zhao, D.; Sun, H.; Yav, S.; Chen, Y.; Zhang, Z.; Yang, M.; Dong, Y.; et al. The prognostic role of the AST/ALT ratio in hepatocellular carcinoma patients receiving thermal ablation combined with simultaneous TACE. BMC Gastroenterol. 2023, 23, 80–87. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Zavadskiy, S.P.; Terentiev, A.A. Proteomic Profiling and Artificial Intelligence for Hepatocellular Carcinoma Translational Medicine. Biomedicines 2021, 9, 159. [Google Scholar] [CrossRef]
- Lin, H.; Patel, S.; Affleck, V.S.; Wilson, I.; Turnbull, D.M.; Joshi, A.R.; Maxwell, R.; Stoll, E.A. Fatty acid oxidation is required for the respiration and proliferation of malignant glioma cells. Neuro Oncol. 2017, 19, 43–54. [Google Scholar] [CrossRef]
- Kuemmerle, N.B.; Rysman, E.; Lombardo, P.S.; Flanagan, A.J.; Lipe, B.C.; Wells, W.A.; Pettus, J.R.; Froehlich, H.M.; Memoli, V.A.; Morganelli, P.M.; et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol. Cancer Ther. 2011, 10, 427–436. [Google Scholar] [CrossRef]
- Yang, H.; Deng, Q.; Ni, T.; Liu, Y.; Lu, L.; Dai, H.; Wang, H.; Yang, W. Targeted Inhibition of LPL/FABP4/CPT1 fatty acid metabolic axis can effectively prevent the progression of nonalcoholic steatohepatitis to liver cancer. Int. J. Biol. Sci. 2021, 17, 4207–4222. [Google Scholar] [CrossRef]
- Krstic, J.; Reinisch, I.; Schupp, M.; Schulz, T.J.; Prokesch, A. p53 Functions in Adipose Tissue Metabolism and Homeostasis. Int. J. Mol. Sci. 2018, 19, 2622. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.L.; Li, K.; Yang, Z.; Chen, B.; Zhang, T. Dulcitol suppresses proliferation and migration of hepatocellular carcinoma via regulating SIRT1/p53 pathway. Phytomedicine 2020, 66, 153112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hurst, D.R.; Qu, X.; Lu, L.G.; Wu, C.Z.; Li, Y.Y.; Li, Y. Re-expression of DIRAS3 and p53 induces apoptosis and impaired autophagy in head and neck squamous cell carcinoma. Mil. Med. Res. 2020, 7, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, Z.; Mo, Z.; Zou, B.; Yang, Y.; Sun, R.; Ma, W.; Yu, M.; Zhang, S.; Yu, Z. Synergetic delivery of triptolide and Ce6 with light-activatable liposomes for efficient hepatocellular carcinoma therapy. Acta Pharm. Sin. B 2021, 11, 2004–2015. [Google Scholar] [CrossRef]
- Li, Z.; Yang, G.; Han, L.; Wang, R.; Gong, C.; Yuan, Y. Sorafenib and triptolide loaded cancer cell-platelet hybrid membrane-camouflaged liquid crystalline lipid nanoparticles for the treatment of hepatocellular carcinoma. J. Nanobiotechnol. 2021, 19, 360–371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.; Wang, J.; You, Y.; Wang, H.; Xu, S.; Vulcano, S.; Xu, C.; Shen, C.; Li, Z.; Wang, J. Triptolide Reduces Neoplastic Progression in Hepatocellular Carcinoma by Downregulating the Lipid Lipase Signaling Pathway. Cancers 2024, 16, 550. https://doi.org/10.3390/cancers16030550
Chang W, Wang J, You Y, Wang H, Xu S, Vulcano S, Xu C, Shen C, Li Z, Wang J. Triptolide Reduces Neoplastic Progression in Hepatocellular Carcinoma by Downregulating the Lipid Lipase Signaling Pathway. Cancers. 2024; 16(3):550. https://doi.org/10.3390/cancers16030550
Chicago/Turabian StyleChang, Wei, Jingjing Wang, Yuanqi You, Hongqian Wang, Shendong Xu, Stephen Vulcano, Changlu Xu, Chenlin Shen, Zhi Li, and Jie Wang. 2024. "Triptolide Reduces Neoplastic Progression in Hepatocellular Carcinoma by Downregulating the Lipid Lipase Signaling Pathway" Cancers 16, no. 3: 550. https://doi.org/10.3390/cancers16030550
APA StyleChang, W., Wang, J., You, Y., Wang, H., Xu, S., Vulcano, S., Xu, C., Shen, C., Li, Z., & Wang, J. (2024). Triptolide Reduces Neoplastic Progression in Hepatocellular Carcinoma by Downregulating the Lipid Lipase Signaling Pathway. Cancers, 16(3), 550. https://doi.org/10.3390/cancers16030550







