Hypoxic Signaling Pathways in Carotid Body Tumors
Abstract
Simple Summary
Abstract
1. Introduction
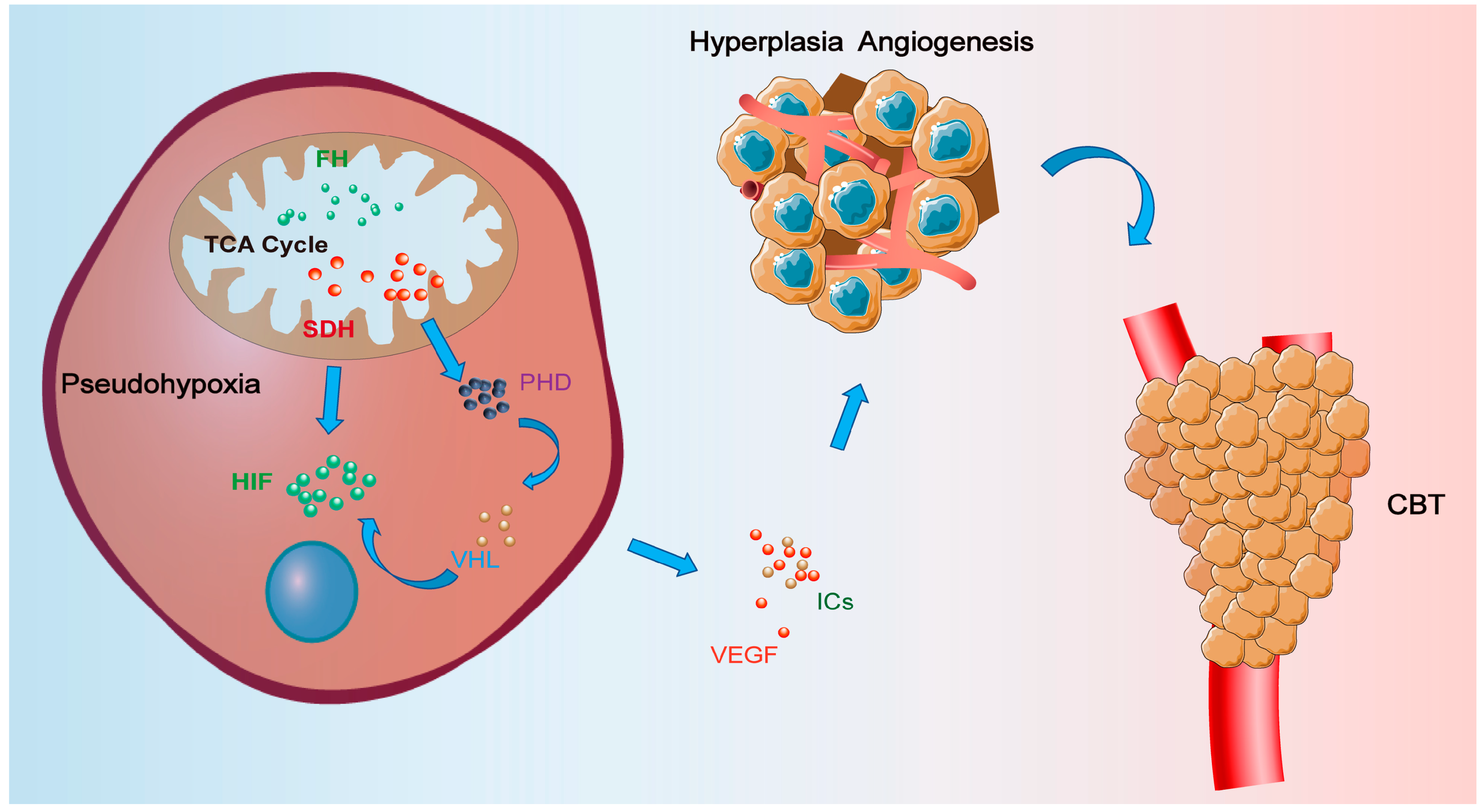
2. Anatomic and Physiologic Basis of Hypoxic Signaling in the Carotid Body (CB)
3. Hypoxic Signaling and Related Functions in CBTs
3.1. The Succinate Dehydrogenase (SDH) Signaling Pathway
3.2. The Hypoxia-Inducible Factor (HIF) Signaling Pathway
| Genes | Localization | Species | Detection Methods | Reference |
|---|---|---|---|---|
| HIF1A | Type I cells, Type II cells | Rats | Immunohistochemistry | Roux JC et al., 2005 [51] |
| EPAS1 | Type I cells | Rats | Immunohistochemistry | Roux JC et al., 2005 [51] |
| Carotid body | Human | Immunohistochemistry | Celada L et al., 2022 [59] |
3.3. Vascular Endothelial Growth Factor (VEGF)
3.4. Functions of Inflammatory Cytokines (ICs) in Carotid Body Concerning Hypoxia
4. Treatment Based on Hypoxic Signaling Pathways in CBTs
5. Future Expectations of CBTs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amato, B.; Bianco, T.; Compagna, R.; Siano, M.; Esposito, G.; Buffone, G.; Serra, R.; de Franciscis, S. Surgical resection of carotid body paragangliomas: 10 years of experience. Am. J. Surg. 2014, 207, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Amato, B.; Serra, R.; Fappiano, F.; Rossi, R.; Danzi, M.; Milone, M.; Quarto, G.; Benassai, G.; Bianco, T.; Amato, M.; et al. Surgical complications of carotid body tumors surgery: A review. Int. Angiol. J. Int. Union Angiol. 2015, 34, 15–22. [Google Scholar]
- Bobadilla-Rosado, L.O.; Garcia-Alva, R.; Anaya-Ayala, J.E.; Peralta-Vazquez, C.; Hernandez-Sotelo, K.; Luna, L.; Cuen-Ojeda, C.; Hinojosa, C.A. Surgical Management of Bilateral Carotid Body Tumors. Ann. Vasc. Surg. 2019, 57, 187–193. [Google Scholar] [CrossRef]
- Gonzalez-Urquijo, M.; Castro-Varela, A.; Barrios-Ruiz, A.; Hinojosa-Gonzalez, D.E.; Salas, A.K.G.; Morales, E.A.; González-González, M.; Fabiani, M.A. Current trends in carotid body tumors: Comprehensive review. Head Neck 2022, 44, 2316–2332. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, X.; Zhen, Y.; Chen, J.; Zheng, X.; Ma, B.; Xu, R.; Kong, J.; Ye, Z.; Liu, P. Impact of preoperative transarterial embolization of carotid body tumor: A single center retrospective cohort experience. Int. J. Surg. 2018, 54, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Unlü, Y.; Becit, N.; Ceviz, M.; Koçak, H. Management of carotid body tumors and familial paragangliomas: Review of 30 years’ experience. Ann. Vasc. Surg. 2009, 23, 616–620. [Google Scholar] [CrossRef]
- Knight, T.T., Jr.; Gonzalez, J.A.; Rary, J.M.; Rush, D.S. Current concepts for the surgical management of carotid body tumor. Am. J. Surg. 2006, 191, 104–110. [Google Scholar] [CrossRef]
- Georgiadis, G.S.; Lazarides, M.K.; Tsalkidis, A.; Argyropoulou, P.; Giatromanolaki, A. Carotid body tumor in a 13-year-old child: Case report and review of the literature. J. Vasc. Surg. 2008, 47, 874–880. [Google Scholar] [CrossRef]
- Weir, E.K.; López-Barneo, J.; Buckler, K.J.; Archer, S.L. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 2005, 353, 2042–2055. [Google Scholar] [CrossRef]
- Dahan, A.; DeGoede, J.; Berkenbosch, A.; Olievier, I.C. The influence of oxygen on the ventilatory response to carbon dioxide in man. J. Physiol. 1990, 428, 485–499. [Google Scholar] [CrossRef]
- Dahan, A.; Taschner, P.E.; Jansen, J.C.; van der Mey, A.; Teppema, L.J.; Cornelisse, C.J. Carotid body tumors in humans caused by a mutation in the gene for succinate dehydrogenase D (SDHD). Adv. Exp. Med. Biol. 2004, 551, 71–76. [Google Scholar] [CrossRef]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Ortega-Sáenz, P.; Pardal, R.; Levitsky, K.; Villadiego, J.; Muñoz-Manchado, A.B.; Durán, R.; Bonilla-Henao, V.; Arias-Mayenco, I.; Sobrino, V.; Ordóñez, A.; et al. Cellular properties and chemosensory responses of the human carotid body. J. Physiol. 2013, 591, 6157–6173. [Google Scholar] [CrossRef]
- Crapo, R.O.; Jensen, R.L.; Hegewald, M.; Tashkin, D.P. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am. J. Respir. Crit. Care Med. 1999, 160, 1525–1531. [Google Scholar] [CrossRef]
- van den Berg, R. Imaging and management of head and neck paragangliomas. Eur. Radiol. 2005, 15, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. K+ channels in O2 sensing and postnatal development of carotid body glomus cell response to hypoxia. Respir. Physiol. Neurobiol. 2013, 185, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Teppema, L.J.; Nieuwenhuijs, D.; Sarton, E.; Romberg, R.; Olievier, C.N.; Ward, D.S.; Dahan, A. Antioxidants prevent depression of the acute hypoxic ventilatory response by subanaesthetic halothane in men. J. Physiol. 2002, 544, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Baysal, B.E.; Ferrell, R.E.; Willett-Brozick, J.E.; Lawrence, E.C.; Myssiorek, D.; Bosch, A.; van der Mey, A.; Taschner, P.E.; Rubinstein, W.S.; Myers, E.N.; et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000, 287, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Cardaci, S.; Zheng, L.; MacKay, G.; van den Broek, N.J.; MacKenzie, E.D.; Nixon, C.; Stevenson, D.; Tumanov, S.; Bulusu, V.; Kamphorst, J.J.; et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat. Cell Biol. 2015, 17, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Lussey-Lepoutre, C.; Hollinshead, K.E.; Ludwig, C.; Menara, M.; Morin, A.; Castro-Vega, L.J.; Parker, S.J.; Janin, M.; Martinelli, C.; Ottolenghi, C.; et al. Loss of succinate dehydrogenase activity results in dependency on pyruvate carboxylation for cellular anabolism. Nat. Commun. 2015, 6, 8784. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, A.; Moussallieh, F.M.; Sebag, F.; Brunaud, L.; Barlier, A.; Elbayed, K.; Bachellier, P.; Goichot, B.; Pacak, K.; Namer, I.J.; et al. A new specific succinate-glutamate metabolomic hallmark in SDHx-related paragangliomas. PLoS ONE 2013, 8, e80539. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.U.; Engelke, U.F.; Rodenburg, R.J.; Wevers, R.A.; Pacak, K.; Eisenhofer, G.; Qin, N.; Kusters, B.; Goudswaard, A.G.; Lenders, J.W.; et al. Genotype-specific abnormalities in mitochondrial function associate with distinct profiles of energy metabolism and catecholamine content in pheochromocytoma and paraganglioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 3787–3795. [Google Scholar] [CrossRef]
- Chandel, N.S.; McClintock, D.S.; Feliciano, C.E.; Wood, T.M.; Melendez, J.A.; Rodriguez, A.M.; Schumacker, P.T. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: A mechanism of O2 sensing. J. Biol. Chem. 2000, 275, 25130–25138. [Google Scholar] [CrossRef]
- Guzy, R.D.; Sharma, B.; Bell, E.; Chandel, N.S.; Schumacker, P.T. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol. Cell. Biol. 2008, 28, 718–731. [Google Scholar] [CrossRef]
- Lin, E.P.; Chin, B.B.; Fishbein, L.; Moritani, T.; Montoya, S.P.; Ellika, S.; Newlands, S. Head and Neck Paragangliomas: An Update on the Molecular Classification, State-of-the-Art Imaging, and Management Recommendations. Radiol. Imaging Cancer 2022, 4, e210088. [Google Scholar] [CrossRef]
- Dahia, P.L.; Ross, K.N.; Wright, M.E.; Hayashida, C.Y.; Santagata, S.; Barontini, M.; Kung, A.L.; Sanso, G.; Powers, J.F.; Tischler, A.S.; et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005, 1, e8. [Google Scholar] [CrossRef] [PubMed]
- Buffet, A.; Burnichon, N.; Favier, J.; Gimenez-Roqueplo, A.P. An overview of 20 years of genetic studies in pheochromocytoma and paraganglioma. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101416. [Google Scholar] [CrossRef]
- Clark, G.R.; Sciacovelli, M.; Gaude, E.; Walsh, D.M.; Kirby, G.; Simpson, M.A.; Trembath, R.C.; Berg, J.N.; Woodward, E.R.; Kinning, E.; et al. Germline FH mutations presenting with pheochromocytoma. J. Clin. Endocrinol. Metab. 2014, 99, E2046–E2050. [Google Scholar] [CrossRef]
- Gill, A.J. Succinate dehydrogenase (SDH) and mitochondrial driven neoplasia. Pathology 2012, 44, 285–292. [Google Scholar] [CrossRef]
- Tennant, D.A.; Durán, R.V.; Gottlieb, E. Targeting metabolic transformation for cancer therapy. Nat. Rev. Cancer 2010, 10, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.J. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology 2018, 72, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Piruat, J.I.; Pintado, C.O.; Ortega-Sáenz, P.; Roche, M.; López-Barneo, J. The mitochondrial SDHD gene is required for early embryogenesis, and its partial deficiency results in persistent carotid body glomus cell activation with full responsiveness to hypoxia. Mol. Cell. Biol. 2004, 24, 10933–10940. [Google Scholar] [CrossRef] [PubMed]
- van der Tuin, K.; Mensenkamp, A.R.; Tops, C.M.J.; Corssmit, E.P.M.; Dinjens, W.N.; van de Horst-Schrivers, A.N.A.; Jansen, J.C.; de Jong, M.M.; Kunst, H.P.M.; Kusters, B.; et al. Clinical Aspects of SDHA-Related Pheochromocytoma and Paraganglioma: A Nationwide Study. J. Clin. Endocrinol. Metab. 2018, 103, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Bausch, B.; Schiavi, F.; Ni, Y.; Welander, J.; Patocs, A.; Ngeow, J.; Wellner, U.; Malinoc, A.; Taschin, E.; Barbon, G.; et al. Clinical Characterization of the Pheochromocytoma and Paraganglioma Susceptibility Genes SDHA, TMEM127, MAX, and SDHAF2 for Gene-Informed Prevention. JAMA Oncol. 2017, 3, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Benn, D.E.; Zhu, Y.; Andrews, K.A.; Wilding, M.; Duncan, E.L.; Dwight, T.; Tothill, R.W.; Burgess, J.; Crook, A.; Gill, A.J.; et al. Bayesian approach to determining penetrance of pathogenic SDH variants. J. Med. Genet. 2018, 55, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.A.; Ascher, D.B.; Pires, D.E.V.; Barnes, D.R.; Vialard, L.; Casey, R.T.; Bradshaw, N.; Adlard, J.; Aylwin, S.; Brennan, P.; et al. Tumour risks and genotype-phenotype correlations associated with germline variants in succinate dehydrogenase subunit genes SDHB, SDHC and SDHD. J. Med. Genet. 2018, 55, 384–394. [Google Scholar] [CrossRef]
- Ackrell, B.A. Cytopathies involving mitochondrial complex II. Mol. Asp. Med. 2002, 23, 369–384. [Google Scholar] [CrossRef]
- Rustin, P.; Munnich, A.; Rötig, A. Succinate dehydrogenase and human diseases: New insights into a well-known enzyme. Eur. J. Hum. Genet. EJHG 2002, 10, 289–291. [Google Scholar] [CrossRef]
- Rustin, P.; Rötig, A. Inborn errors of complex II--unusual human mitochondrial diseases. Biochim. Biophys. Acta 2002, 1553, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Roqueplo, A.P.; Favier, J.; Rustin, P.; Mourad, J.J.; Plouin, P.F.; Corvol, P.; Rötig, A.; Jeunemaitre, X. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am. J. Hum. Genet. 2001, 69, 1186–1197. [Google Scholar] [CrossRef]
- Cass, N.D.; Schopper, M.A.; Lubin, J.A.; Fishbein, L.; Gubbels, S.P. The Changing Paradigm of Head and Neck Paragangliomas: What Every Otolaryngologist Needs to Know. Ann. Otol. Rhinol. Laryngol. 2020, 129, 1135–1143. [Google Scholar] [CrossRef]
- Adam, M.P.; Feldman, J.; Mirzaa, G.M.; Pagon, R.A.; Wallace, S.E.; Bean, L.J.H.; Gripp, K.W.; Amemiya, A. GeneReviews®; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Galan, S.R.; Kann, P.H. Genetics and molecular pathogenesis of pheochromocytoma and paraganglioma. Clin. Endocrinol. 2013, 78, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. The Genomics and Genetics of Oxygen Homeostasis. Annu. Rev. Genom. Hum. Genet. 2020, 21, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Ponka, P.; Prchal, J.T. Hypoxia. 5. Hypoxia and hematopoiesis. Am. J. Physiol. Cell Physiol. 2011, 300, C1215–C1222. [Google Scholar] [CrossRef] [PubMed]
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1α and HIF2α: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 2011, 12, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, P.J. Oxygen sensing and hypoxia signalling pathways in animals: The implications of physiology for cancer. J. Physiol. 2013, 591, 2027–2042. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Nobre, A.R.; Entenberg, D.; Wang, Y.; Condeelis, J.; Aguirre-Ghiso, J.A. The Different Routes to Metastasis via Hypoxia-Regulated Programs. Trends Cell Biol. 2018, 28, 941–956. [Google Scholar] [CrossRef]
- Roux, J.C.; Brismar, H.; Aperia, A.; Lagercrantz, H. Developmental changes in HIF transcription factor in carotid body: Relevance for O2 sensing by chemoreceptors. Pediatr. Res. 2005, 58, 53–57. [Google Scholar] [CrossRef]
- Paredes, F.; Williams, H.C.; San Martin, A. Metabolic adaptation in hypoxia and cancer. Cancer Lett. 2021, 502, 133–142. [Google Scholar] [CrossRef]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef]
- Bishop, T.; Talbot, N.P.; Turner, P.J.; Nicholls, L.G.; Pascual, A.; Hodson, E.J.; Douglas, G.; Fielding, J.W.; Smith, T.G.; Demetriades, M.; et al. Carotid body hyperplasia and enhanced ventilatory responses to hypoxia in mice with heterozygous deficiency of PHD2. J. Physiol. 2013, 591, 3565–3577. [Google Scholar] [CrossRef]
- Kamihara, J.; Hamilton, K.V.; Pollard, J.A.; Clinton, C.M.; Madden, J.A.; Lin, J.; Imamovic, A.; Wall, C.B.; Wassner, A.J.; Weil, B.R.; et al. Belzutifan, a Potent HIF2α Inhibitor, in the Pacak-Zhuang Syndrome. N. Engl. J. Med. 2021, 385, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cox, S.R.; Morita, T.; Kourembanas, S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 1995, 77, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69 (Suppl. S3), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Celada, L.; Cubiella, T.; San-Juan-Guardado, J.; San José Martínez, A.; Valdés, N.; Jiménez-Fonseca, P.; Díaz, I.; Enguita, J.M.; Astudillo, A.; Álvarez-González, E.; et al. Differential HIF2α Protein Expression in Human Carotid Body and Adrenal Medulla under Physiologic and Tumorigenic Conditions. Cancers 2022, 14, 2986. [Google Scholar] [CrossRef]
- Cheng, X.; Prange-Barczynska, M.; Fielding, J.W.; Zhang, M.; Burrell, A.L.; Lima, J.D.; Eckardt, L.; Argles, I.; Pugh, C.W.; Buckler, K.J.; et al. Marked and rapid effects of pharmacological HIF-2α antagonism on hypoxic ventilatory control. J. Clin. Investig. 2020, 130, 2237–2251. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Lonergan, K.M.; Iliopoulos, O.; Ohh, M.; Kamura, T.; Conaway, R.C.; Conaway, J.W.; Kaelin, W.G., Jr. Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul2. Mol. Cell. Biol. 1998, 18, 732–741. [Google Scholar] [CrossRef]
- Gnarra, J.R.; Zhou, S.; Merrill, M.J.; Wagner, J.R.; Krumm, A.; Papavassiliou, E.; Oldfield, E.H.; Klausner, R.D.; Linehan, W.M. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc. Natl. Acad. Sci. USA 1996, 93, 10589–10594. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Kaelin, W.G., Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat. Med. 2020, 26, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Mahase, S.; Rattenni, R.N.; Wesseling, P.; Leenders, W.; Baldotto, C.; Jain, R.; Zagzag, D. Hypoxia-Mediated Mechanisms Associated with Antiangiogenic Treatment Resistance in Glioblastomas. Am. J. Pathol. 2017, 187, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.; Bursill, C.A. The Role of CC-Chemokines in the Regulation of Angiogenesis. Int. J. Mol. Sci. 2016, 17, 1856. [Google Scholar] [CrossRef]
- Dewangan, J.; Kaushik, S.; Rath, S.K.; Balapure, A.K. Centchroman regulates breast cancer angiogenesis via inhibition of HIF-1α/VEGFR2 signalling axis. Life Sci. 2018, 193, 9–19. [Google Scholar] [CrossRef]
- Ahmed, R.; Ornstein, M.C. Targeting HIF-2 Alpha in Renal Cell Carcinoma. Curr. Treat. Options Oncol. 2023, 24, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.Y.; Tipoe, G.L.; Liong, E.C.; Fung, M.L. Differential expressions and roles of hypoxia-inducible factor-1alpha, -2alpha and -3alpha in the rat carotid body during chronic and intermittent hypoxia. Histol. Histopathol. 2008, 23, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dinger, B.; Jyung, R.; Stensaas, L.; Fidone, S. Altered expression of vascular endothelial growth factor and FLK-1 receptor in chronically hypoxic rat carotid body. Adv. Exp. Med. Biol. 2003, 536, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, C.; Antosiewicz, J.; Walski, M.; Petruccelli, G.; Verratti, V.; Bianchi, G.; Pokorski, M. Physiological carotid body denervation during aging. Adv. Exp. Med. Biol. 2009, 648, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, B.Y.; Cui, L.Y.; Wang, B.L.; Liu, C.X.; Chen, P.F.; Guo, M.N.; Dong, L.X.; Li, S. Carotid body inflammation and carotid sinus nerve afferent activity after intermittent hypoxia exposure of various frequencies in rabbits. Chin. J. Tuberc. Respir. Dis. 2008, 31, 670–674. [Google Scholar]
- Belzunegui, S.; Izal-Azcárate, A.; San Sebastián, W.; Garrido-Gil, P.; Vázquez-Claverie, M.; López, B.; Marcilla, I.; Luquin, M.R. Striatal carotid body graft promotes differentiation of neural progenitor cells into neurons in the olfactory bulb of adult hemiparkisonian rats. Brain Res. 2008, 1217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Felix, A.S.; Rocha, V.N.; Nascimento, A.L.; de Carvalho, J.J. Carotid body remodelling in l-NAME-induced hypertension in the rat. J. Comp. Pathol. 2012, 146, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zara, S.; Pokorski, M.; Cataldi, A.; Porzionato, A.; De Caro, R.; Antosiewicz, J.; Di Giulio, C. Development and aging are oxygen-dependent and correlate with VEGF and NOS along life span. Adv. Exp. Med. Biol. 2013, 756, 223–228. [Google Scholar] [CrossRef]
- Salman, S.; Vollmer, C.; McClelland, G.B.; Nurse, C.A. Characterization of ectonucleotidase expression in the rat carotid body: Regulation by chronic hypoxia. Am. J. Physiology. Cell Physiol. 2017, 313, C274–C284. [Google Scholar] [CrossRef]
- Porzionato, A.; Macchi, V.; Parenti, A.; De Caro, R. Trophic factors in the carotid body. Int. Rev. Cell Mol. Biol. 2008, 269, 1–58. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J.; Kumar, G.K.; Nanduri, J.; Di Giulio, C.; Lahiri, S. Long-term regulation of carotid body function: Acclimatization and adaptation--invited article. Adv. Exp. Med. Biol. 2009, 648, 307–317. [Google Scholar] [CrossRef]
- Del Rio, R.; Muñoz, C.; Arias, P.; Court, F.A.; Moya, E.A.; Iturriaga, R. Chronic intermittent hypoxia-induced vascular enlargement and VEGF upregulation in the rat carotid body is not prevented by antioxidant treatment. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L702–L711. [Google Scholar] [CrossRef]
- Zara, S.; Porzionato, A.; De Colli, M.; Macchi, V.; Cataldi, A.; De Caro, R.; Di Giulio, C. Human carotid body neuroglobin, vascular endothelial growth factor and inducible nitric oxide synthase expression in heroin addiction. Histol. Histopathol. 2013, 28, 903–911. [Google Scholar] [CrossRef]
- Porzionato, A.; Macchi, V.; De Caro, R.; Di Giulio, C. Inflammatory and immunomodulatory mechanisms in the carotid body. Respir. Physiol. Neurobiol. 2013, 187, 31–40. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Tortorella, C.; Macchi, V.; De Caro, R.; Porzionato, A. Growth Factors in the Carotid Body-An Update. Int. J. Mol. Sci. 2020, 21, 7267. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, L.; Stensaas, L.; Dinger, B.; Fidone, S. Adaptation to chronic hypoxia involves immune cell invasion and increased expression of inflammatory cytokines in rat carotid body. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L158–L166. [Google Scholar] [CrossRef] [PubMed]
- Kåhlin, J.; Mkrtchian, S.; Ebberyd, A.; Hammarstedt-Nordenvall, L.; Nordlander, B.; Yoshitake, T.; Kehr, J.; Prabhakar, N.; Poellinger, L.; Fagerlund, M.J.; et al. The human carotid body releases acetylcholine, ATP and cytokines during hypoxia. Exp. Physiol. 2014, 99, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, R.; Moya, E.A.; Iturriaga, R. Contribution of inflammation on carotid body chemosensory potentiation induced by intermittent hypoxia. Adv. Exp. Med. Biol. 2012, 758, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Liu, L.; Fan, J.; He, S.; Li, R.; Peng, Z.W.; Wang, B.R. Interleukin-1β promotes the neurogenesis of carotid bodies by stimulating the activation of ERK1/2. Respir. Physiol. Neurobiol. 2015, 219, 78–84. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, B.; Shu, H.F.; Zhang, X.Y.; Wang, X.; Kuang, F.; Liu, L.; Peng, Z.W.; Wu, R.; Zhou, Z.; et al. Interleukin-6 increases intracellular Ca2+ concentration and induces catecholamine secretion in rat carotid body glomus cells. J. Neurosci. Res. 2009, 87, 2757–2762. [Google Scholar] [CrossRef]
- Valero, C.; Ganly, I. Paragangliomas of the head and neck. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2022, 51, 897–903. [Google Scholar] [CrossRef]
- Rajendran, J.G.; Hendrickson, K.R.; Spence, A.M.; Muzi, M.; Krohn, K.A.; Mankoff, D.A. Hypoxia imaging-directed radiation treatment planning. Eur. J. Nucl. Med. Mol. Imaging 2006, 33 (Suppl. S1), 44–53. [Google Scholar] [CrossRef]
- Toyonaga, T.; Hirata, K.; Shiga, T.; Nagara, T. Players of ‘hypoxia orchestra’—What is the role of FMISO? Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1679–1681. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, W.; Qiu, P.; Zhang, X.; Qin, J.; Cai, Y.; Lu, X. MnO2 doped graphene nanosheets for carotid body tumor combination therapy. Nanoscale Adv. 2022, 4, 4304–4313. [Google Scholar] [CrossRef] [PubMed]
- Joshua, A.M.; Ezzat, S.; Asa, S.L.; Evans, A.; Broom, R.; Freeman, M.; Knox, J.J. Rationale and evidence for sunitinib in the treatment of malignant paraganglioma/pheochromocytoma. J. Clin. Endocrinol. Metab. 2009, 94, 5–9. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, G.M.; Ezzat, S.; Joshua, A.M.; Bourdeau, I.; Leibowitz-Amit, R.; Olney, H.J.; Krzyzanowska, M.; Reuther, D.; Chin, S.; Wang, L.; et al. A phase 2 trial of sunitinib in patients with progressive paraganglioma or pheochromocytoma: The SNIPP trial. Br. J. Cancer 2019, 120, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Crona, J.; Beuschlein, F.; Grossman, A.B.; Pacak, K.; Nölting, S. Targeted Therapies in Pheochromocytoma and Paraganglioma. J. Clin. Endocrinol. Metab. 2022, 107, 2963–2972. [Google Scholar] [CrossRef]
- Fallah, J.; Brave, M.H.; Weinstock, C.; Mehta, G.U.; Bradford, D.; Gittleman, H.; Bloomquist, E.W.; Charlab, R.; Hamed, S.S.; Miller, C.P.; et al. FDA Approval Summary: Belzutifan for von Hippel-Lindau Disease-Associated Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 4843–4848. [Google Scholar] [CrossRef]
- Richter, S.; Garrett, T.J.; Bechmann, N.; Clifton-Bligh, R.J.; Ghayee, H.K. Metabolomics in paraganglioma: Applications and perspectives from genetics to therapy. Endocr. Relat. Cancer 2023, 30, e220376. [Google Scholar] [CrossRef]
| Gene | Chromosome | Frequency * | Proportion of Attributed Hereditary Paraganglioma | Inheritance Pattern |
|---|---|---|---|---|
| SDHA | 5p15 | <1% | 0.6–3% | Autosomal dominant |
| SDHB | 1p36.1 | 5% | 22–38% 12–20% CBT | Autosomal dominant |
| SDHC | 1q21 | 1% | 4–8% | Autosomal dominant |
| SDHD | 11q23 | 5% | 30% 40–50% CBT | Autosomal dominant paternal inheritance |
| SDHAF2 | 11q13.1 | <1% | Unknown | Autosomal dominant paternal inheritance |
| Genes | Localization | Species | Detection Methods | Reference |
|---|---|---|---|---|
| VEGF | Type I cells | Rat | Immunohistochemistry | Lam et al., 2008 [71] |
| Type I cells | Rat | Immunohistochemistry | Chen et al., 2003 [72] | |
| Carotid body | Rat | Immunohistochemistry | Di Giulio et al., 2009 [73] | |
| Carotid body | Rabbit | ELISA | Feng et al., 2008 [74] | |
| Type I cells | Rat | Double immunofluorescence | Belzunegui et al., 2008 [75] | |
| Type I cells | Rat | Immunohistochemistry | Felix et al., 2012 [76] | |
| Carotid body | Human | Immunohistochemistry | Zara et al., 2013 [77] | |
| Carotid body | Rat | qRT-PCR | Salman et al., 2017 [78] | |
| Flk-1 | Type I cells | Rat | Immunohistochemistry | Chen et al., 2003 [72] |
| Genes | Localization | Species | Detection Methods | Reference |
|---|---|---|---|---|
| IL1B | Type I cells | Rat | Immunohistochemistry | Del Rio et al., 2012 [87] |
| Carotid body | Human | ELISA | Kåhlin et al., 2014 [86] | |
| IL6 | Type I cells | Rat | Immunohistochemistry | Del Rio et al., 2012 [87] |
| Carotid body | Human | ELISA | Kåhlin et al., 2014 [86] | |
| Carotid body | Human | ELISA | Kåhlin et al., 2014 [86] | |
| TNFA | Type I cells | Rat | Immunohistochemistry | Del Rio et al., 2012 [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, K.; Yuan, W.; Hou, C.; Wang, Z.; Yu, J.; Wang, T. Hypoxic Signaling Pathways in Carotid Body Tumors. Cancers 2024, 16, 584. https://doi.org/10.3390/cancers16030584
Cao K, Yuan W, Hou C, Wang Z, Yu J, Wang T. Hypoxic Signaling Pathways in Carotid Body Tumors. Cancers. 2024; 16(3):584. https://doi.org/10.3390/cancers16030584
Chicago/Turabian StyleCao, Kangxi, Wanzhong Yuan, Chaofan Hou, Zhongzheng Wang, Jiazhi Yu, and Tao Wang. 2024. "Hypoxic Signaling Pathways in Carotid Body Tumors" Cancers 16, no. 3: 584. https://doi.org/10.3390/cancers16030584
APA StyleCao, K., Yuan, W., Hou, C., Wang, Z., Yu, J., & Wang, T. (2024). Hypoxic Signaling Pathways in Carotid Body Tumors. Cancers, 16(3), 584. https://doi.org/10.3390/cancers16030584






