An Accelerated Failure Time Model to Predict Cause-Specific Survival and Prognostic Factors of Lung and Bronchus Cancer Patients with at Least Bone or Brain Metastases: Development and Internal Validation Using a SEER-Based Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Included in the Study
2.2. Demographic and Clinical Variables
2.3. Study Outcome
2.4. Statistical Analysis
2.4.1. Model Development
2.4.2. Model Performance
2.4.3. Model Validation
3. Results
3.1. Description of the Study Cohorts
3.2. Proportional Hazard Test
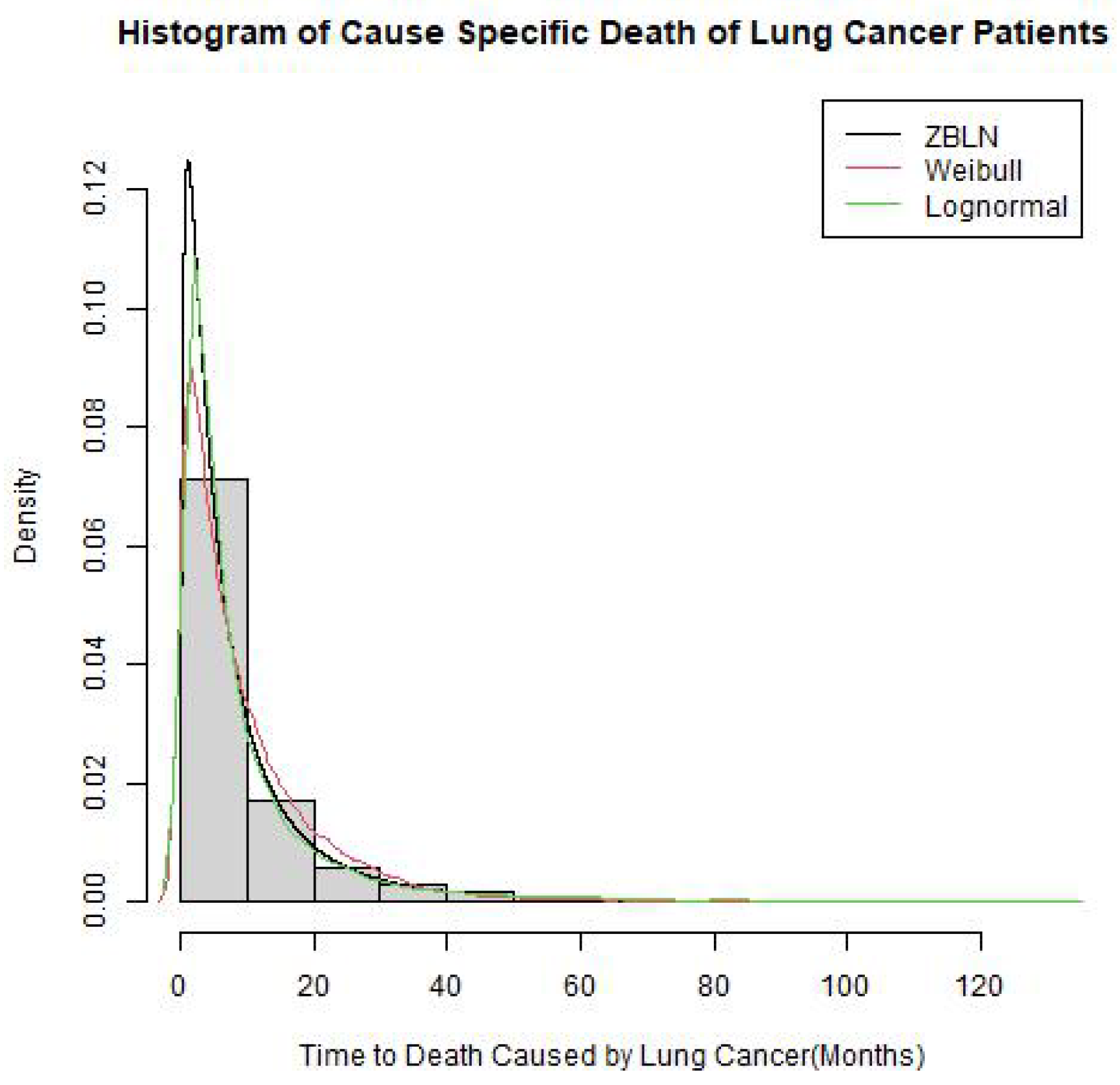
3.3. Baseline Distribution Selection
3.4. Survival Model Development
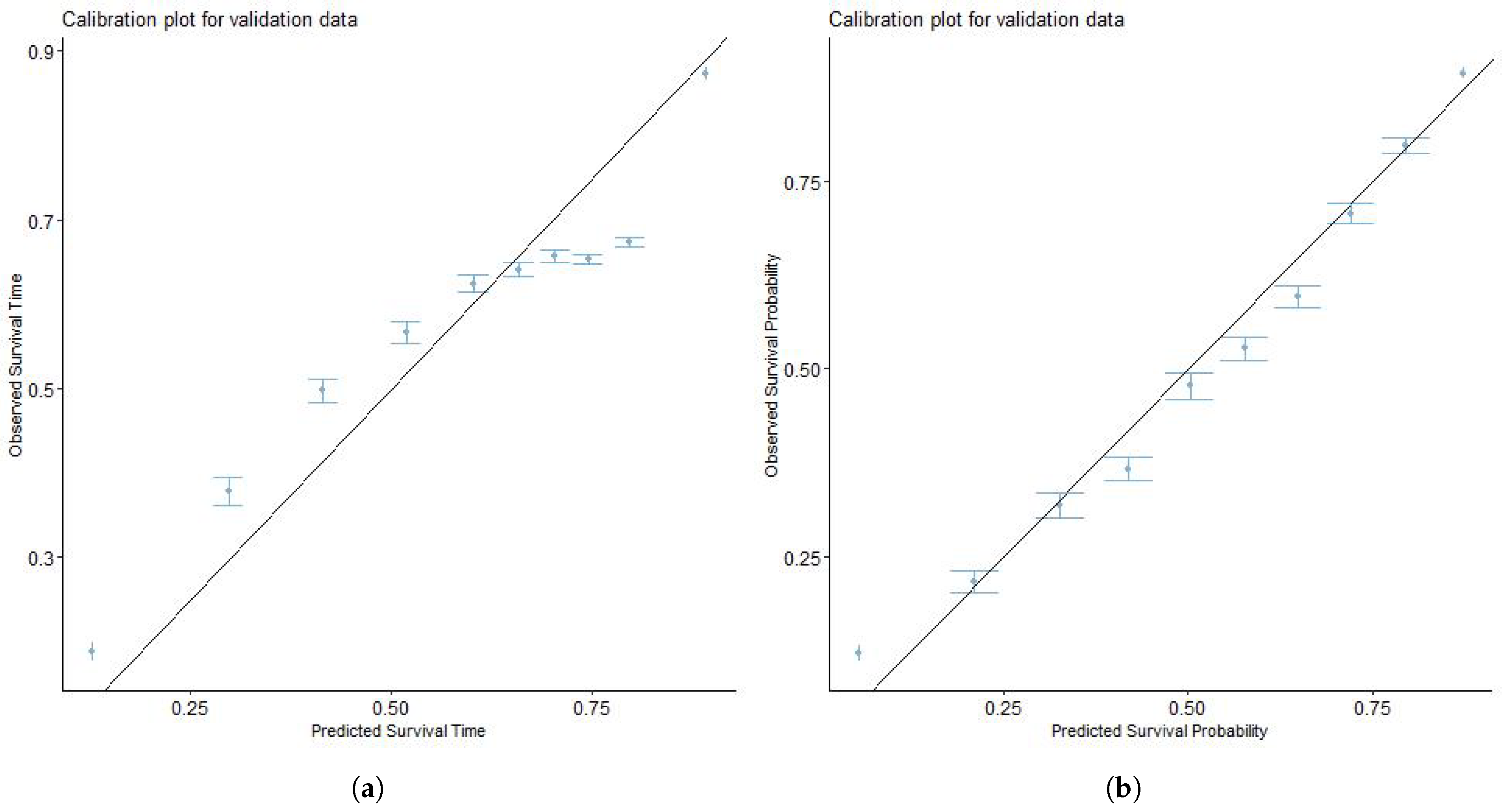
3.5. Model Validation
4. Discussion
5. Limitation of the Study
6. Further Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer: Lyon, France. Available online: https://gco.iarc.fr/today (accessed on 15 September 2020).
- National Cancer Institute Surveillance. Epidemiology, and End Results Program. Cancer Stat Facts: Common Cancer Sites. 2021. Available online: https://seer.cancer.gov/statfacts/html/common.html (accessed on 24 June 2023).
- de Sousa, V.M.L.; Carvalho, L. Heterogeneity in lung cancer. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2018, 85, 96–107. [Google Scholar] [CrossRef]
- Coleman, R.E. Skeletal complications of malignancy. Cancer 1997, 80 (Suppl. 8), 1588–1594. [Google Scholar] [CrossRef]
- Sugiura, H.; Yamada, K.; Sugiura, T.; Hida, T.; Mitsudomi, T. Predictors of survival in patients with bone metastasis of lung cancer. Clin. Orthop. Relat. Res. 2008, 466, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N. Gan to kagaku ryoho. Cancer Chemother. 2006, 33, 1049–1053. [Google Scholar]
- Johnston, A.D. Pathology of metastatic tumors in bone. Clin. Orthop. Relat. Res. 1970, 73, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Alomaish, H.; Ung, Y.; Wang, S.; Tyrrell, P.N.; Zahra, S.A.; Oikonomou, A. Survival analysis in lung cancer patients with interstitial lung disease. PLoS ONE 2021, 16, e0255375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Z.; Hu, X.; Zhang, L.; Liu, Y.; Wang, Y.; Guo, Y.; Zhang, T.; Li, W.; Li, B. Hyaluronic Acid Correlates With Bone Metastasis and Predicts Poor Prognosis in Small-Cell Lung Cancer Patients. Front. Endocrinol. 2022, 12, 785192. [Google Scholar] [CrossRef]
- Meng, C.; Wang, F.; Chen, M.; Shi, H.; Zhao, L.; Wang, P. Construction and Verification of Nomogram Model for Lung Adenocarcinoma With less 5 Bone-Only Metastases Basing on Hematology Markers. Front. Oncol. 2022, 12, 858634. [Google Scholar] [CrossRef]
- Freise, G.; Gabler, A.; Liebig, S. Bronchial carcinoma and long-term survival. Retrospective study of 433 patients who underwent resection. Thorax 1978, 33, 228–234. [Google Scholar] [CrossRef]
- Stanley, K.E. Prognostic factors for survival in patients with inoperable lung cancer. J. Natl. Cancer Inst. 1980, 65, 25–32. [Google Scholar]
- Wang, Z.; Hu, F.; Chang, R.; Yu, X.; Xu, C.; Liu, Y.; Wang, R.; Chen, H.; Liu, S.; Xia, D.; et al. Development and Validation of a Prognostic Model to Predict Overall Survival for Lung Adenocarcinoma: A Population-Based Study From the SEER Database and the Chinese Multicenter lung cancer Database. Technol. Cancer Res. Treat. 2022, 21, 15330338221133222. [Google Scholar] [CrossRef]
- Awodutire, P.O.; Olapade, A.K.; Kolawole, O.A.; Ilori, O.R. Assessing Survival Times of Breast Cancer Patients Using Type I Generalized Half Logistic Survival Model. J. Adv. Med. Med. Res. 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Awodutire, P.; Ilori, O.; Uwandu, C.; Akadiri, O. Pilot study of new statistical models for prognostic factors in short term survival of oral cancer. Afr. Health Sci. 2022, 22, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Baghestani, A.R.; Moghaddam, S.S.; Majd, H.A.; Akbari, M.E.; Nafissi, N.; Gohari, K. Survival Analysis of Patients with Breast Cancer using Weibull Parametric Model. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 8567–8571. [Google Scholar] [CrossRef]
- Nadarajah, S.; Cordeiro, G.; Ortega, E. The Zografos-Balakrishnan-G Family of Distributions: Mathematical Properties and Applications. Commun. Stat. Theory Methods 2015, 44, 186–215. [Google Scholar] [CrossRef]
- Shibu, D.S.; Nitin, S.L.; Irshad, M.R. A Study on Zografos-Balakrishnan Lognormal Distribution: Properties and Application to Cancer Dataset. Accepted: March 2022. REVSTAT-Statistical Journal. Available online: https://revstat.ine.pt/index.php/REVSTAT/article/view/436 (accessed on 30 October 2023).
- Wahed, A.S.; Luong, T.M.; Jeong, J.H. A new generalization of Weibull distribution with application to a breast cancer data set. Stat. Med. 2009, 28, 2077–2094. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.C.; Baglietto, L.; Manning, J.T.; McLean, C.; Hopper, J.L.; English, D.R.; Giles, G.G.; Severi, G. Second to fourth digit ratio (2D:4D), breast cancer risk factors, and breast cancer risk: A prospective cohort study. Br. J. Cancer 2012, 107, 1631–1636. [Google Scholar] [CrossRef]
- Gashu, C.; Tasfa, B.; Alemu, C.; Kassa, Y. Assessing survival time of outpatients with cervical cancer: At the university of Gondar referral hospital using the Bayesian approach. BMC Women’s Health 2023, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sonker, P.K.; Saroj, A.; Jain, A.; Bhattacharjee, A.; Saroj, R.K. Parametric survival analysis using R: Illustration with lung cancer data. Cancer Rep. 2020, 3, e1210. [Google Scholar] [CrossRef]
- Hosseini Teshnizi, S.; Taghi Ayatollahi, S.M. Comparison of Cox Regression and Parametric Models: Application for Assessment of Survival of Pediatric Cases of Acute Leukemia in Southern Iran. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 981–985. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, F.; Jiang, W.; Mao, R.; Zheng, H.; Qin, L.; Chen, C. Age at diagnosis is a heterogeneous factor for non-small cell lung cancer patients. J. Thorac. Dis. 2019, 11, 2251–2266. [Google Scholar] [CrossRef]
- Yang, L.; Fang, F.; Chan, J.; Chen, B.; Luo, W.; Zhu, Q.; Liu, D.; Li, W. Metastatic patterns and prognosis of young lung cancer patients: A population-based study by age. Ann. Transl. Med. 2021, 9, 1159. [Google Scholar] [CrossRef]
- Zou, J.; Guo, S.; Xiong, M.T.; Xu, Y.; Shao, J.; Tong, Z.; Zhang, P.; Pan, L.; Peng, A.; Li, X. Ageing as key factor for distant metastasis patterns and prognosis in patients with extensive-stage Small Cell Lung Cancer. J. Cancer 2021, 12, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.C.; Mercaldo, S.F.; Blume, J.D.; Wenzlaff, A.S.; Schwartz, A.G.; Chen, H.; Deppen, S.A.; Bush, W.S.; Crawford, D.C.; Chanock, S.J.; et al. Racial Disparities in Lung Cancer Survival: The Contribution of Stage, Treatment, and Ancestry. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Alpert, N.; Tran, B.V.; Liu, B.; Flores, R.; Taioli, E. Persistence of racial disparities in early-stage lung cancer treatment. J. Thorac. Cardiovasc. Surg. 2019, 157, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, I.S.; Stjepanovic, M.; Mitrovic, D. Distribution patterns of the metastases of the lung carcinoma in relation to histological type of the primary tumor: An autopsy study. Ann. Thorac. Med. 2017, 12, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Sagerup, C.M.; Småstuen, M.; Johannesen, T.B.; Helland, Å.; Brustugun, O.T. Sex-specific trends in lung cancer incidence and survival: A population study of 40,118 cases. Thorax 2011, 66, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ogunbiyi, J.O. Lung cancer at the University College Hospital, Ibadan, Nigeria. East Afr. Med. J. 1995, 72, 271–275. [Google Scholar] [PubMed]

| Variable | Description | Data Type | Data Format |
|---|---|---|---|
| CSLCD | Time to death caused by lung Ccancer | Continuous | 1,2,3,4,5,… |
| Age | Age of patients | Continuous | 1,2,3,4,5,… |
| Race | Race of patients | Categorical | 1,2,3,4 |
| Histology | Histology | Categorical | 1,2,3 |
| Treatment Modality | Treatments administered | Categorical | 1,2,3,4 |
| TISD | In situ malignant tumors | Continuous | 1,2,3,4,5,… |
| Histological Grade | Histological grade of tumor | Categorical | 1,2,3,4 |
| Gender | Gender of the patients | Categorical | 1,2 |
| Primary Site | Tumor primary location | Categorical | 1,2,3,4,5,6 |
| Censor | Censored value | Categorical | 0,1 |
| Metastases | Metastases | Categorical | 1,2,3 |
| Variable | Total Cohort | Training Cohort | Validation Cohort |
|---|---|---|---|
| Percentages | Percentages | Percentages | |
| Survival Time in Months | 5 (2, 12) * | 5 (2, 12) * | 5 (2, 12) * |
| Uncensored (3-year) | 13,298 (65%) | 9331 (65%) | 3967 (65%) |
| Uncensored (5-year) | 17,753 (87%) | 12,441 (87%) | 5312 (87%) |
| Age of Patients | 66 (11) ** | 66 (11) ** | 67 (10) ** |
| Race | |||
| American Indian | 108 (0.5%) | 76 (0.5%) | 32 (0.5%) |
| Asian | 1614 (7.9%) | 1148 (8.0%) | 466 (7.6%) |
| Black | 2274 (11%) | 1608 (11%) | 666 (11%) |
| White | 16,416 (80%) | 11,458 (80%) | 4958 (81%) |
| Sex | |||
| Male | 11,447 (56%) | 8042 (56%) | 3405 (56%) |
| Female | 8965 (44%) | 6248 (44%) | 2717 (44%) |
| Primary Site | |||
| Main Bronchus | 993 (4.9%) | 699 (4.9%) | 294 (4.8%) |
| Upper Lobe | 10,913 (53%) | 7610 (53%) | 3303 (54%) |
| Middle Lobe | 863 (4.2%) | 571 (4.0%) | 292 (4.8%) |
| Lower Lobe | 5370 (26%) | 3828 (27%) | 1542 (25%) |
| Lung NOS | 214 (1.0%) | 145 (1.0%) | 69 (1.1%) |
| Overlapping Lesion of Lung | 2059 (10%) | 1437 (10%) | 622 (10%) |
| Grade | |||
| Well-Differentiated | 872 (4.3%) | 616 (4.3%) | 256 (4.2%) |
| Moderately Differentiated | 4637 (23%) | 3284 (23%) | 1353 (22%) |
| Poorly Differentiated | 12,927 (63%) | 9011 (63%) | 3916 (64%) |
| Undifferentiated | 1976 (9.7%) | 1379 (9.7%) | 597 (9.8%) |
| TISP | 1 (1, 1) * | 1 (1, 1) * | 1 (1, 1) * |
| Metastasis Type | |||
| Bone Only | 9837 (48%) | 6863 (48%) | 2974 (49%) |
| Bone and Brain | 3408 (17%) | 2410 (17%) | 998 (16%) |
| Brain Only | 7167 (35%) | 5017 (35%) | 2150 (35%) |
| Treatment Modality | |||
| No Treatment | 5640 (28%) | 3975 (28%) | 1665 (27%) |
| Monotherapy | 862 (4.2%) | 602 (4.2%) | 260 (4.2%) |
| Bimodal Therapy | 12,146 (60%) | 8489 (59%) | 3657 (60%) |
| Trimodal Therapy | 1764 (8.6%) | 1224 (8.6%) | 540 (8.8%) |
| Histology | |||
| Epithelial Neoplasms | 5024 (25%) | 3477 (24%) | 1547 (25%) |
| Squamous Cell Neoplasms | 3428 (17%) | 2400 (17%) | 1028 (17%) |
| Adenomas and Adenocarcinomas | 11,188 (55%) | 7890 (55%) | 3298 (54%) |
| Others | 772 (3.8%) | 523 (3.7%) | 249 (4.1%) |
| 3-Year | 5-Year | |
|---|---|---|
| Variable | p-Value | p-Value |
| Age | <0.001 * | <0.001 * |
| Race | 0.002 * | 0.001 * |
| Gender | 0.102 | 0.003 |
| Tumor Primary Site | 0.002 * | 0.003 |
| Grade | <0.001 * | <0.001 * |
| TISP | 0.031 * | 0.067 |
| Metastases | <0.001 * | 0.001 |
| Treatment | <0.001 * | <0.001 |
| Histology | 0.087 | 0.249 |
| Model | -log-likelihood | AIC | BIC |
|---|---|---|---|
| Weibull | 46,402 | 92,808 | 92,823 |
| Log-normal | 45,805 | 91,630 | 91,615 |
| ZBLN | 45,786 | 91,578 | 91,568 |
| 3-Year | 5-Year | |||||||
|---|---|---|---|---|---|---|---|---|
| Model | -log-likelihood | AIC | BIC | -log-likelihood | AIC | BIC | ||
| Cox | 79,323 | 158,691 | 158,848 | 105,540 | 211,125 | 211,287 | ||
| Weibull | 33,305 | 64,854 | 65,035 | 41,531 | 83,066 | 83,070 | ||
| Log-normal | 31,717 | 63,483 | 63,665 | 39,814 | 79,632 | 79,636 | ||
| ZBLN | 31,658 | 63,322 | 63,495 | 39,692 | 79,390 | 79,396 |
| 3-Year | 5-Year | |||||
|---|---|---|---|---|---|---|
| Model | RMSE | C-Index | RMSE | C-Index | ||
| Cox | - * | 0.669 | - * | 0.643 | ||
| Weibull | 7.344 | 0.636 | 6.264 | 0.652 | ||
| Log-normal | 0.453 | 0.665 | 5.739 | 0.666 | ||
| ZBLN | 0.425 | 0.682 | 2.628 | 0.667 |
| 3-Year | 5-Year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Estimates | AF | p | Estimates | AF | p | |||||
| Age | −0.003 | 0.997 | 0.025 | −0.007 | 0.993 | 0.000 | |||||
| Race | |||||||||||
| American Indian (Ref) | - | - | - | - | - | - | |||||
| Asian | 0.414 | 1.513 | 0.015 | 0.263 | 1.300 | 0.053 | |||||
| Black | −0.047 | 0.954 | 0.781 | −0.062 | 0.940 | 0.646 | |||||
| White | 0.023 | 1.023 | 0.890 | −0.025 | 0.975 | 0.847 | |||||
| Gender | |||||||||||
| Male (Ref) | - | - | - | - | - | - | |||||
| Female | 0.066 | 1.069 | 0.005 | 0.137 | 1.146 | <0.001 | |||||
| Treatment Modality | |||||||||||
| No Treatment (Ref) | - | - | - | - | - | - | |||||
| Monotherapy | −0.347 | 0.707 | 0.000 | −0.117 | 0.889 | 0.022 | |||||
| Bimodal Therapy | 1.238 | 3.448 | 0.000 | 1.053 | 2.867 | 0.000 | |||||
| Trimodal Therapy | 1.237 | 3.447 | 0.000 | 1.109 | 3.032 | 0.000 | |||||
| Histology | |||||||||||
| Epithelial Neoplasms, NOS | - | - | - | - | - | - | |||||
| Squamous Cell Neoplasms | −0.132 | 0.876 | 0.001 | −0.297 | 0.742 | 0.000 | |||||
| Adenomas and Adenocarcinomas | 0.016 | 1.016 | 0.620 | −0.083 | 0.920 | 0.002 | |||||
| Others | −0.355 | 0.701 | 0.000 | −0.162 | 0.851 | 0.004 | |||||
| Primary Site | |||||||||||
| Main Bronchus (Ref) | - | - | - | - | - | - | |||||
| Upper Lobe | 0.183 | 1.201 | 0.001 | −0.197 | 0.821 | 0.002 | |||||
| Middle Lobe | −0.706 | 0.494 | 0.000 | −0.145 | 0.864 | 0.002 | |||||
| Lower Lobe | −0.012 | 0.988 | 0.833 | 0.215 | 1.240 | 0.000 | |||||
| Lung NOS | −0.823 | 0.439 | 0.000 | −1.175 | 0.308 | 0.000 | |||||
| Overlapping Lesion of the Lung | −0.027 | 0.974 | 0.680 | −0.456 | 0.633 | 0.000 | |||||
| Grade | |||||||||||
| Well-Differentiated (Ref) | - | - | - | - | - | - | |||||
| Moderately Differentiated | 0.164 | 1.179 | 0.008 | 0.141 | 1.152 | 0.005 | |||||
| Poorly Differentiated | −0.122 | 0.885 | 0.038 | −0.219 | 0.803 | 0.000 | |||||
| Undifferentiated | −0.345 | 0.708 | 0.000 | −0.440 | 0.643 | <0.001 | |||||
| TISP | 0.202 | 1.223 | 0.000 | 0.161 | 1.174 | 0.00 | |||||
| Metastases | |||||||||||
| Bone Only (Ref) | - | - | - | - | - | - | |||||
| Bone and Brain | −0.320 | 0.726 | 0.000 | −0.305 | 0.736 | 0.000 | |||||
| Brain Only | 0.007 | 1.007 | 0.805 | −0.079 | 0.924 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awodutire, P.O.; Kattan, M.W.; Ilori, O.S.; Ilori, O.R. An Accelerated Failure Time Model to Predict Cause-Specific Survival and Prognostic Factors of Lung and Bronchus Cancer Patients with at Least Bone or Brain Metastases: Development and Internal Validation Using a SEER-Based Study. Cancers 2024, 16, 668. https://doi.org/10.3390/cancers16030668
Awodutire PO, Kattan MW, Ilori OS, Ilori OR. An Accelerated Failure Time Model to Predict Cause-Specific Survival and Prognostic Factors of Lung and Bronchus Cancer Patients with at Least Bone or Brain Metastases: Development and Internal Validation Using a SEER-Based Study. Cancers. 2024; 16(3):668. https://doi.org/10.3390/cancers16030668
Chicago/Turabian StyleAwodutire, Phillip Oluwatobi, Michael W. Kattan, Oluwatosin Stephen Ilori, and Oluwatosin Ruth Ilori. 2024. "An Accelerated Failure Time Model to Predict Cause-Specific Survival and Prognostic Factors of Lung and Bronchus Cancer Patients with at Least Bone or Brain Metastases: Development and Internal Validation Using a SEER-Based Study" Cancers 16, no. 3: 668. https://doi.org/10.3390/cancers16030668
APA StyleAwodutire, P. O., Kattan, M. W., Ilori, O. S., & Ilori, O. R. (2024). An Accelerated Failure Time Model to Predict Cause-Specific Survival and Prognostic Factors of Lung and Bronchus Cancer Patients with at Least Bone or Brain Metastases: Development and Internal Validation Using a SEER-Based Study. Cancers, 16(3), 668. https://doi.org/10.3390/cancers16030668






