A Potential Prognostic Gene Signature Associated with p53-Dependent NTRK1 Activation and Increased Survival of Neuroblastoma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Lines and Transfections
2.2. In Vivo Studies with 9464D and 262226 Cells
2.3. RNA Extraction and cDNA Preparation
2.4. RNA Extraction from Murine Tumours and cDNA Preparation
2.5. Real-Time qPCR
2.6. RNA Extraction from Cell Lines and Sequencing
2.7. RNA-Seq Data Analysis of Cell Lines
2.8. Datasets
2.9. Data Pre-Processing
2.10. Training the Model
2.11. Statistical Analysis
3. Results
3.1. Filtering Strategy to Identify Differentially Expressed Genes Associated with NTRK1-PTPN6-TP53 Activation
3.2. Development of a Prognostic Model
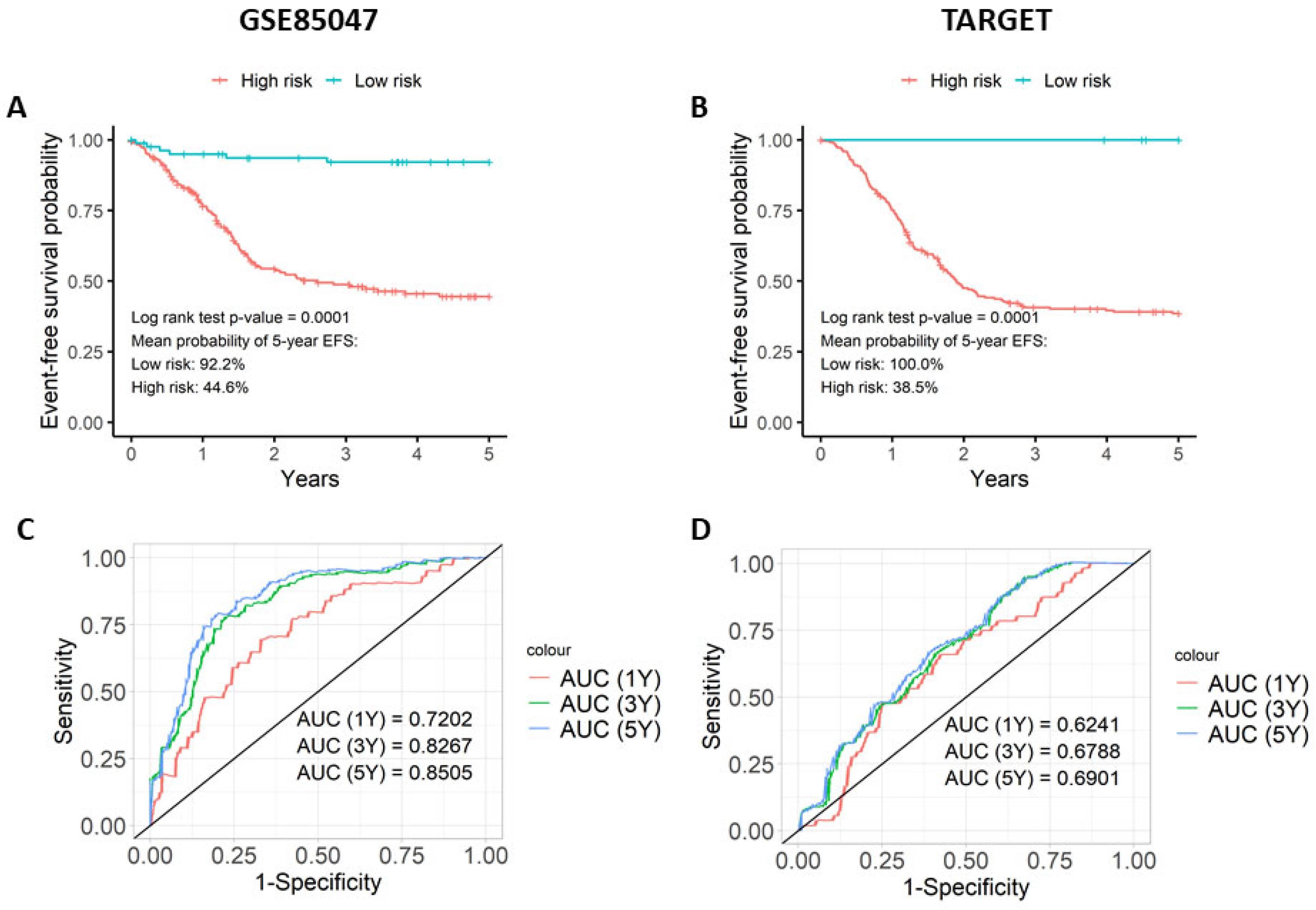
3.3. The Prognostic Score Is Associated with Event-Free Survival (EFS)
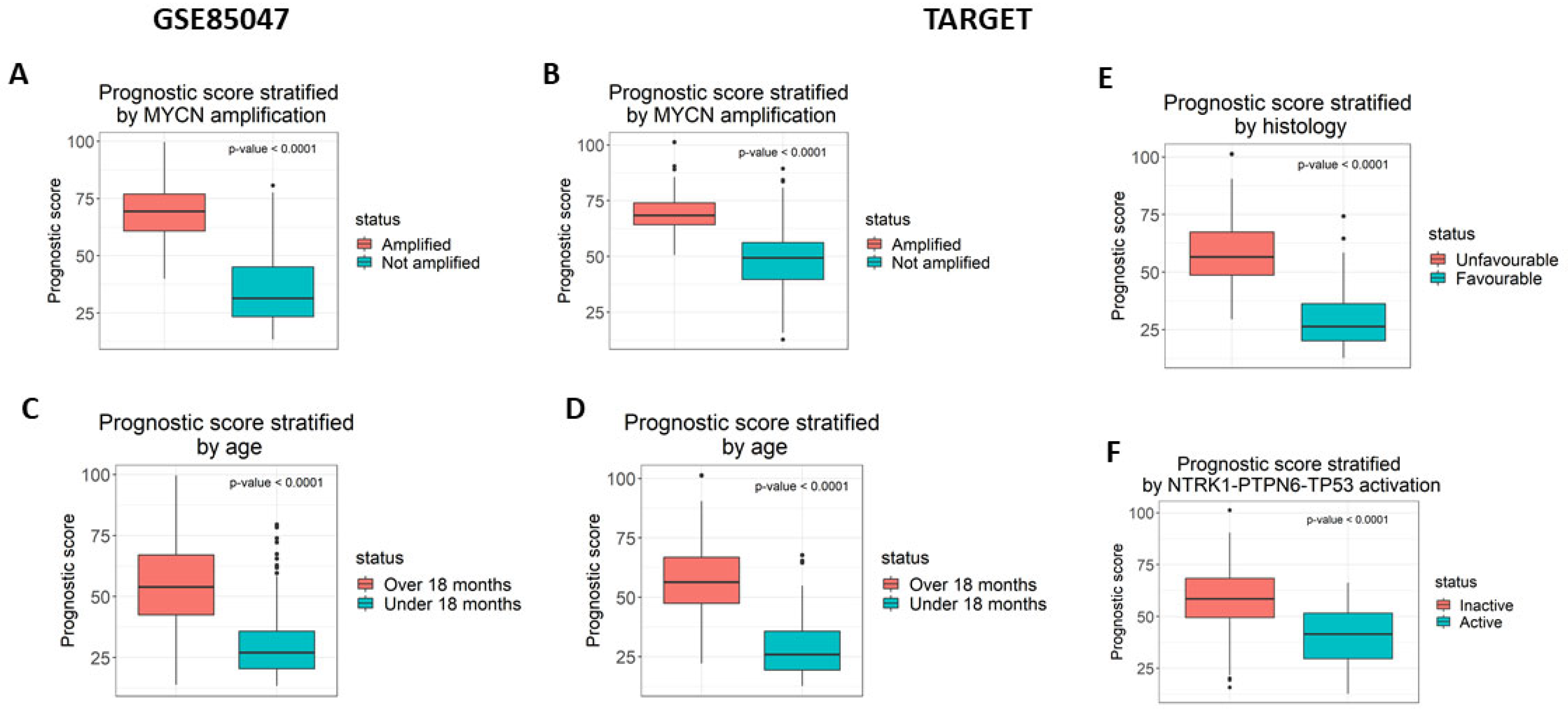
3.4. Analysis of the Prognostic Model
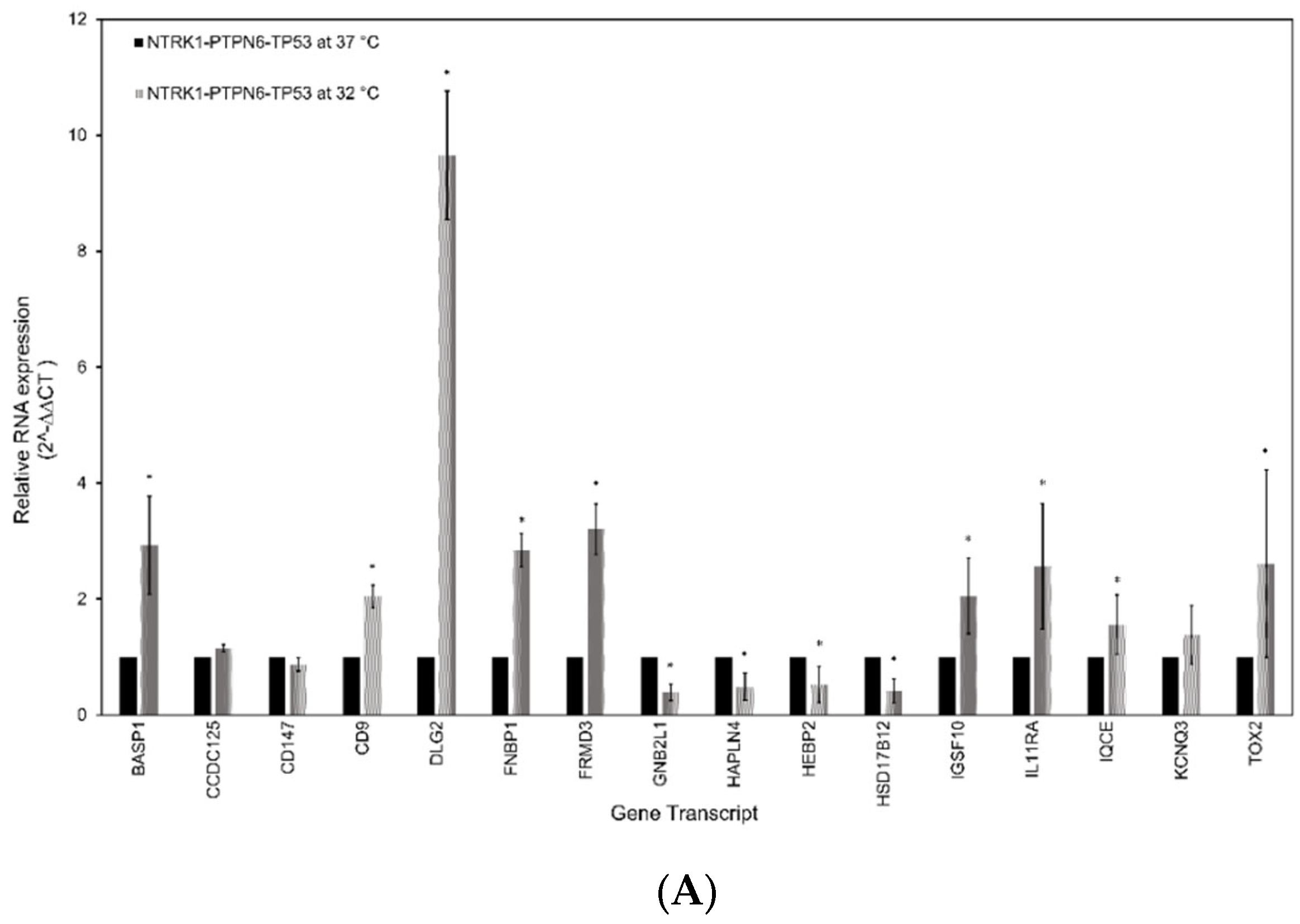
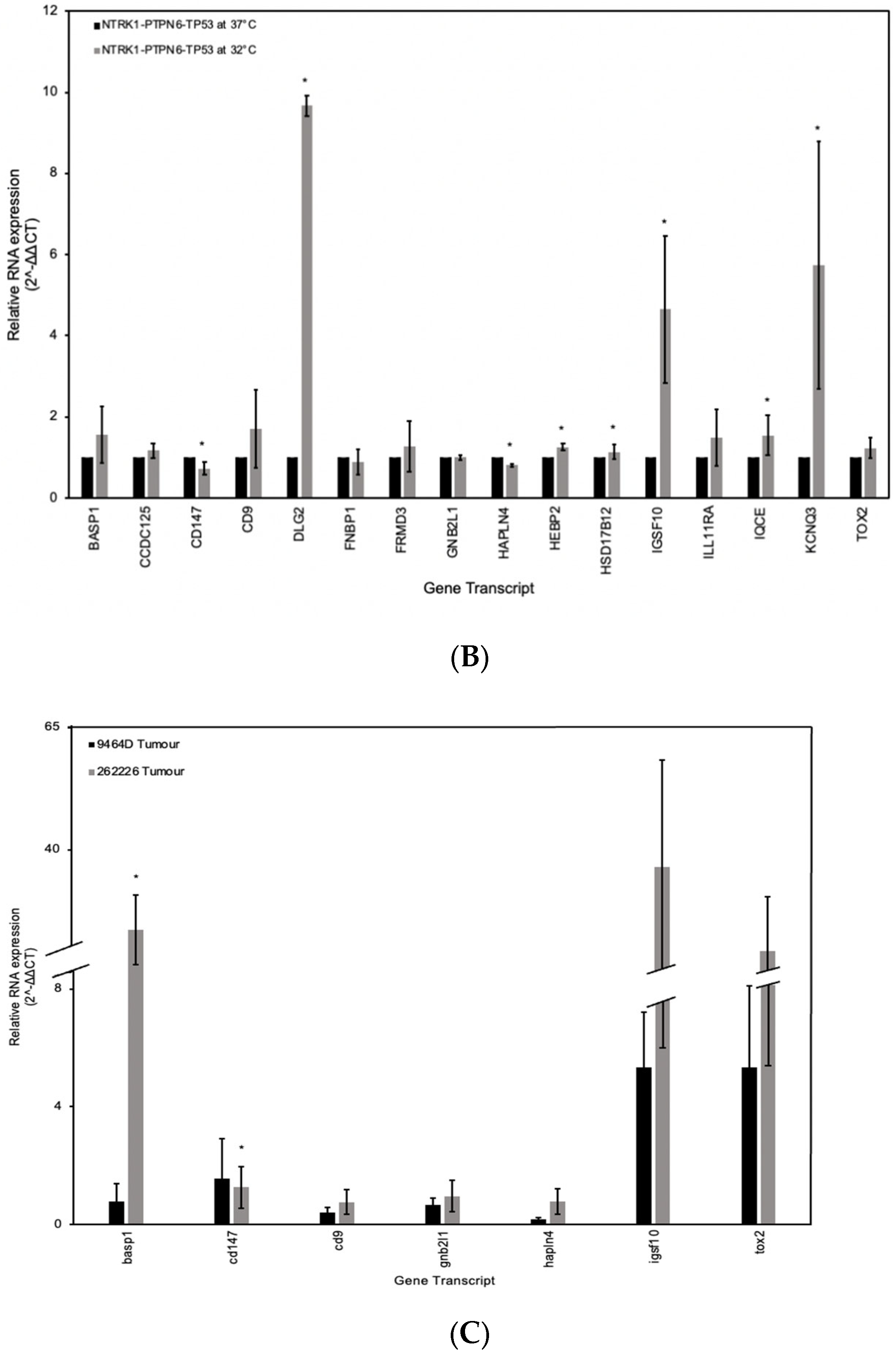
3.5. RT-qPCR Analysis of the Gene Signature in Neuroblastoma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institution Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irwin, M.S.; Park, J.R. Neuroblastoma: Paradigm for Precision Medicine. Pediatr. Clin. N. Am. 2015, 62, 225–256. [Google Scholar] [CrossRef]
- Chen, A.M.; Trout, A.T.; Towbin, A.J. A Review of Neuroblastoma Image-Defined Risk Factors on Magnetic Resonance Imaging. Pediatr. Radiol. 2018, 48, 1337–1347. [Google Scholar] [CrossRef]
- Braoudaki, M.; Hatziagapiou, K.; Zaravinos, A.; Lambrou, G.I. MYCN in Neuroblastoma: “Old Wine into New Wineskins”. Diseases 2021, 9, 78. [Google Scholar] [CrossRef]
- Schleiermacher, G.; Mosseri, V.; London, W.B.; Maris, J.M.; Brodeur, G.M.; Attiyeh, E.; Haber, M.; Khan, J.; Nakagawara, A.; Speleman, F.; et al. Segmental Chromosomal Alterations Have Prognostic Impact in Neuroblastoma: A Report from the INRG Project. Br. J. Cancer 2012, 107, 1418–1422. [Google Scholar] [CrossRef]
- Li, F.; Zhang, W.; Hu, H.; Zhang, Y.; Li, J.; Huang, D. Factors of Recurrence After Complete Response in Children with Neuroblastoma: A 16-Year Retrospective Study of 179 Cases. Cancer Manag. Res. 2022, 14, 107–122. [Google Scholar] [CrossRef]
- Shimada, H.; Ikegaki, N. Genetic and Histopathological Heterogeneity of Neuroblastoma and Precision Therapeutic Approaches for Extremely Unfavorable Histology Subgroups. Biomolecules 2022, 12, 79. [Google Scholar] [CrossRef]
- Bothwell, M. Recent Advances in Understanding Neurotrophin Signaling. F1000Research 2016, 5, 1885. [Google Scholar] [CrossRef] [PubMed]
- Light, J.E.; Koyama, H.; Minturn, J.E.; Ho, R.; Simpson, A.M.; Iyer, R.; Mangino, J.L.; Kolla, V.; London, W.B.; Brodeur, G.M. Clinical Significance of NTRK Family Gene Expression in Neuroblastomas. Pediatr. Blood Cancer 2012, 59, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Marsh, H.N.; Dubreuil, C.I.; Quevedo, C.; Lee, A.; Majdan, M.; Walsh, G.S.; Hausdorff, S.; Said, F.A.; Zoueva, O.; Kozlowski, M.; et al. SHP-1 Negatively Regulates Neuronal Survival by Functioning as a TrkA Phosphatase. J. Cell Biol. 2003, 163, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Montano, X. Repression of SHP-1 Expression by P53 Leads to TrkA Tyrosine Phosphorylation and Suppression of Breast Cancer Cell Proliferation. Oncogene 2009, 28, 3787–3800. [Google Scholar] [CrossRef]
- Youssef, G.; Gillett, C.; Rampling, D.; Chagtai, T.; Virasami, A.; Barton, J.; Edwards, D.; Sebire, N.; Anderson, J.; Montano, X. The Presence of Y674/Y675 Phosphorylated NTRK1 via TP53 Repression of PTPN6 Expression as a Potential Prognostic Marker in Neuroblastoma. Hum. Pathol. 2019, 86, 182–192. [Google Scholar] [CrossRef]
- Bergsneider, B.; Bailey, E.; Ahmed, Y.; Gogineni, N.; Huntley, D.; Montano, X. Analysis of SARS-CoV-2 Infection Associated Cell Entry Proteins ACE2, CD147, PPIA, and PPIB in Datasets from Non SARS-CoV-2 Infected Neuroblastoma Patients, as Potential Prognostic and Infection Biomarkers in Neuroblastoma. Biochem. Biophys. Rep. 2021, 27, 101081. [Google Scholar] [CrossRef]
- de la Cruz Concepción, B.; Bartolo-García, L.D.; Tizapa-Méndez, M.D.; Martínez-Vélez, M.; Valerio-Diego, J.J.; Illades-Aguiar, B.; Salmerón-Bárcenas, E.G.; Ortiz-Ortiz, J.; Torres-Rojas, F.I.; Mendoza-Catalán, M.Á.; et al. EMMPRIN Is an Emerging Protein Capable of Regulating Cancer Hallmarks. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6700–6724. [Google Scholar] [CrossRef]
- Grass, G.D.; Toole, B.P. How, with Whom and When: An Overview of CD147-Mediated Regulatory Networks Influencing Matrix Metalloproteinase Activity. Biosci. Rep. 2015, 36, e00283. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, Y. RACK1 Affects Glioma Cell Growth and Differentiation through the CNTN2-Mediated RTK/Ras/MAPK Pathway. Int. J. Mol. Med. 2016, 37, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Duff, D.; Long, A. Roles for RACK1 in Cancer Cell Migration and Invasion. Cell Signal. 2017, 35, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Hartl, M.; Schneider, R. A Unique Family of Neuronal Signaling Proteins Implicated in Oncogenesis and Tumor Suppression. Front. Oncol. 2019, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tian, Z.; Song, X.; Zhang, J. Membrane Tension Sensing Molecule-FNBP1 Is a Prognostic Biomarker Related to Immune Infiltration in BRCA, LUAD and STAD. BMC Immunol. 2022, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, B.J.; Freire, F.; Carvalho, A.L.; Aveiro, S.S.; Charbonnier, P.; Moulis, J.-M.; Delgado, L.; Ferreira, G.C.; Rodrigues, J.E.; Poussin-Courmontagne, P.; et al. The SOUL Family of Heme-Binding Proteins: Structure and Function 15 Years Later. Coord. Chem. Rev. 2021, 448, 214189. [Google Scholar] [CrossRef]
- Fabian, J.; Opitz, D.; Althoff, K.; Lodrini, M.; Hero, B.; Volland, R.; Beckers, A.; de Preter, K.; Decock, A.; Patil, N.; et al. MYCN and HDAC5 Transcriptionally Repress CD9 to Trigger Invasion and Metastasis in Neuroblastoma. Oncotarget 2016, 7, 66344–66359. [Google Scholar] [CrossRef] [PubMed]
- Keane, S.; Améen, S.; Lindlöf, A.; Ejeskär, K. Low DLG2 Gene Expression, a Link between 11q-Deleted and MYCN-Amplified Neuroblastoma, Causes Forced Cell Cycle Progression, and Predicts Poor Patient Survival. Cell Commun. Signal. 2020, 18, 65. [Google Scholar] [CrossRef]
- Delahanty, R.J.; Zhang, Y.; Bichell, T.J.; Shen, W.; Verdier, K.; Macdonald, R.L.; Xu, L.; Boyd, K.; Williams, J.; Kang, J.-Q. Beyond Epilepsy and Autism: Disruption of GABRB3 Causes Ocular Hypopigmentation. Cell Rep. 2016, 17, 3115–3124. [Google Scholar] [CrossRef]
- Zhai, T.; Muhanhali, D.; Jia, X.; Wu, Z.; Cai, Z.; Ling, Y. Identification of Gene Co-Expression Modules and Hub Genes Associated with Lymph Node Metastasis of Papillary Thyroid Cancer. Endocrine 2019, 66, 573–584. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, Q.; Yin, S.; Ding, X.; Wang, H. Genome-Wide Expression Profiling and Bioinformatics Analysis of Deregulated Genes in Human Gastric Cancer Tissue after Gastroscopy. Asia-Pac. J. Clin. Oncol. 2018, 14, e29–e36. [Google Scholar] [CrossRef]
- Zhang, X.; Groen, K.; Morten, B.C.; Steffens Reinhardt, L.; Campbell, H.G.; Braithwaite, A.W.; Bourdon, J.; Avery-Kiejda, K.A. Effect of P53 and Its N-terminally Truncated Isoform, Δ40p53, on Breast Cancer Migration and Invasion. Mol. Oncol. 2022, 16, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Hong, F.; Wang, H.; Zhang, X.; Chen, J. An Eight-MRNA Signature Outperforms the LncRNA-Based Signature in Predicting Prognosis of Patients with Glioblastoma. BMC Med. Genet. 2020, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Liao, X.; Huang, Y.; Liang, L.; Jiang, Y.; Pang, Y.; Qi, G. Identification of Prognostic Markers of Lung Cancer through Bioinformatics Analysis and in Vitro Experiments. Int. J. Oncol. 2020, 56, 193–205. [Google Scholar] [CrossRef]
- Veldman, J.; Rodrigues Plaça, J.; Chong, L.; Terpstra, M.M.; Mastik, M.; van Kempen, L.C.; Kok, K.; Aoki, T.; Steidl, C.; van den Berg, A.; et al. CD4+ T Cells in Classical Hodgkin Lymphoma Express Exhaustion Associated Transcription Factors TOX and TOX2. Oncoimmunology 2022, 11, 2033433. [Google Scholar] [CrossRef]
- van der Burg, S.H. IL11: A Specific Repressor of Tumor-Specific CD4+ T Cells. Cancer Immunol. Res. 2021, 9, 724. [Google Scholar] [CrossRef]
- Tsachaki, M.; Strauss, P.; Dunkel, A.; Navrátilová, H.; Mladenovic, N.; Odermatt, A. Impact of 17β-HSD12, the 3-Ketoacyl-CoA Reductase of Long-Chain Fatty Acid Synthesis, on Breast Cancer Cell Proliferation and Migration. Cell Mol. Life Sci. 2020, 77, 1153–1175. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, K.M.; Lee, S.Y.; Jung, J. Estrogen Aggravates Tumor Growth in a Diffuse Gastric Cancer Xenograft Model. Pathol. Oncol. Res. 2021, 27, 622733. [Google Scholar] [CrossRef]
- Haase, D.; Meister, M.; Muley, T.; Hess, J.; Teurich, S.; Schnabel, P.; Hartenstein, B.; Angel, P. FRMD3, a Novel Putative Tumour Suppressor in NSCLC. Oncogene 2007, 26, 4464–4468. [Google Scholar] [CrossRef]
- Chen, T.J.; Chou, C.L.; Tian, Y.F.; Yeh, C.F.; Chan, T.C.; He, H.L.; Li, W.S.; Tsai, H.H.; Li, C.F.; Lai, H.Y. High FRMD3 Expression Is Prognostic for Worse Survival in Rectal Cancer Patients Treated with CCRT. Int. J. Clin. Oncol. 2021, 26, 1689–1697. [Google Scholar] [CrossRef]
- Michalovitz, D.; Halevy, O.; Oren, M. Conditional Inhibition of Transformation and of Cell Proliferation by a Temperature-Sensitive Mutant of P53. Cell 1990, 62, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Frankish, A.; Diekhans, M.; Ferreira, A.M.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.M.; Sisu, C.; Wright, J.; Armstrong, J.; et al. GENCODE Reference Annotation for the Human and Mouse Genomes. Nucleic Acids Res. 2019, 47, D766–D773. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating High-Throughput Genomic Analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 30 September 2023).
- Therneau, T. A Package for Survival Analysis in R. R Package Version 3.5-7. 2023. Available online: https://CRAN.R-project.org/package=survival (accessed on 30 September 2023).
- Ishwaran, H.; Kogalur, U.B.; Blackstone, E.H.; Lauer, M.S. Random Survival Forests. Ann. Appl. Stat. 2008, 2, 841–860. [Google Scholar] [CrossRef]
- Ishwaran, H.; Kogalur, U.B. Fast Unified Random Forests for Survival, Regression, and Classification (RF-SRC). R Package Version 3.2.2. 2023. Available online: https://cran.r-project.org/package=randomForestSRC (accessed on 1 February 2022).
- Heagerty, P.J.; Saha-Chaudhuri, P. SurvivalROC: Time-Dependent ROC Curve Estimation from Censored Survival Data. R Package Version 1.0.3. 2013. Available online: https://cran.r-project.org/package=survivalROC (accessed on 13 September 2023).
- Constantinescu, R.; Constantinescu, A.T.; Reichmann, H.; Janetzky, B. Neuronal Differentiation and Long-Term Culture of the Human Neuroblastoma Line SH-SY5Y. J. Neural Transm. Suppl. 2007, 72, 17–28. [Google Scholar] [CrossRef]
- Campos Cogo, S.; Gradowski Farias da Costa do Nascimento, T.; de Almeida Brehm Pinhatti, F.; de França Junior, N.; Santos Rodrigues, B.; Cavalli, L.R.; Elifio-Esposito, S. An Overview of Neuroblastoma Cell Lineage Phenotypes and in vitro Models. Exp. Biol. Med. 2020, 245, 1637–1647. [Google Scholar] [CrossRef]
- Mitsumoto, Y.; Oka, S.; Sakuma, H.; Inazawa, J.; Kawasaki, T. Cloning and Chromosomal Mapping of Human Glucuronyltransferase Involved in Biosynthesis of the HNK-1 Carbohydrate Epitope. Genomics 2000, 65, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Tonoyama, Y.; Oka, S. Distinct Transport and Intracellular Activities of Two GlcAT-P Isoforms. J. Biol. Chem. 2009, 284, 9247–9256. [Google Scholar] [CrossRef] [PubMed]
- Schlitter, A.M.; Dorneburg, C.; Barth, T.F.E.; Wahl, J.; Schulte, J.H.; Brüderlein, S.; Debatin, K.M.; Beltinger, C. CD57high Neuroblastoma Cells Have Aggressive Attributes Ex Situ and an Undifferentiated Phenotype in Patients. PLoS ONE 2012, 7, e42025. [Google Scholar] [CrossRef] [PubMed]
- Regan, P.L.; Jacobs, J.; Wang, G.; Torres, J.; Edo, R.; Friedmann, J.; Tang, X.X. Hsp90 Inhibition Increases p53 Expression and Destabilizes MYCN and MYC in Neuroblastoma. Int. J. Oncol. 2011, 38, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ronca, F.; Yee, K.S.Y.; Yu, V.C. Retinoic Acid Confers Resistance to p53-dependent Apoptosis in SH-SY5Y Neuroblastoma Cells by Modulating Nuclear Import of p53. J. Biol. Chem. 1999, 274, 18128–18134. [Google Scholar] [CrossRef] [PubMed]
- Voeller, J.; Erbe, A.K.; Slowinski, J.; Rasmussen, K.; Carlson, P.M.; Hoefges, A.; VandenHeuvel, S.; Stuckwisch, A.; Wang, X.; Gillies, S.D.; et al. Combined Innate and Adaptive Immunotherapy Overcomes Resistance of Immunologically Cold Syngeneic Murine Neuroblastoma to Checkpoint Inhibition. J. Immunother. Cancer. 2019, 7, 344. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.; Luther, W.; Bhatnagar, N.; Jamin, Y.; Poon, E.; Sanda, T.; Pei, D.; Sharma, B.; Vetharoy, W.R.; Hallsworth, A.; et al. The ALKF1174L Mutation Potentiates the Oncogenic Activity of MYCN in Neuroblastoma. Cancer Cell 2012, 22, 117–130. [Google Scholar] [CrossRef]
- Olivares-Illana, V.; Råhraeus, R. p53 Isoforms Gain Functions. Oncogene 2010, 29, 5113–5119. [Google Scholar] [CrossRef]
- Bourdon, J.C.; Fernandes, K.; Murray-Zmijewski, F.; Liu, G.; Diot, A.; Xirodimas, D.P.; Saville, M.K.; Lane, D.P. p53 Isoforms can Regulate p53 Transcriptional Activity. Genes Dev. 2005, 19, 2122–2137. [Google Scholar] [CrossRef]
- Khoury, M.P.; Bourdon, J.C. p53 Isoforms: An Intracellular Microprocessor? Genes Cancer 2011, 2, 453–465. [Google Scholar] [CrossRef]
- Breschi, A.; Gingeras, T.R.; Guigó, R. Comparative Transcriptomics in Human and Mouse. Nat. Rev. Genet 2017, 18, 425–440. [Google Scholar] [CrossRef]
- Zimmerman, S.M.; Fropf, R.; Kulasekara, B.R.; Griswold, M.; Appelbe, O.; Bahrami, A.; Boykin, R.; Buhr, D.L.; Fuhrman, K.; Hoang, M.L.; et al. Spatially Resolved Whole Transcriptome Profiling in Human and Mouse Tissue using Digital Spatial Profiling. Genome Res. 2022, 32, 1892–1905. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, L.; Liu, Z.; Bush, B.D.; Hartman, Y.; Zhou, T.; Rosenthal, E.L. CD147 and AGR2 Expression Promote Cellular Proliferation and Metastasis of Head and Neck Squamous Cell Carcinoma. Exp. Cell Res. 2012, 318, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cui, L.; Zhang, Y.; Chen, L.; Wang, Y.; Fan, Y.; Lei, T.; Gu, F.; Lang, R.; Pringle, G.A.; et al. Expression of HAb18G Is Associated with Tumor Progression and Prognosis of Breast Carcinoma. Breast Cancer Res. Treat. 2010, 124, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Givant-Horwitz, V.; Lazarovici, P.; Risberg, B.; Nesland, J.M.; Trope, C.G.; Schaefer, E.; Reich, R. Matrix Metalloproteinases (MMP), EMMPRIN (Extracellular Matrix Metalloproteinase Inducer) and Mitogen-Activated Protein Kinases (MAPK): Co-Expression in Metastatic Serous Ovarian Carcinoma. Clin. Exp. Metastasis 2003, 20, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Marimpietri, D.; Petretto, A.; Raffaghello, L.; Pezzolo, A.; Gagliani, C.; Tacchetti, C.; Mauri, P.; Melioli, G.; Pistoia, V. Proteome Profiling of Neuroblastoma-Derived Exosomes Reveal the Expression of Proteins Potentially Involved in Tumor Progression. PLoS ONE 2013, 8, e75054. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Higashi, Y.; Kawai, K.; Su, J.; Zeng, W.; Chen, X.; Kanekura, T. CD147-Targeted SiRNA in A375 Malignant Melanoma Cells Induces the Phosphorylation of EGFR and Downregulates Cdc25C and MEK Phosphorylation. Oncol. Lett. 2016, 11, 2424–2428. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, L.; Kuang, Y.; Su, J.; Luo, Z.; Wang, Y.; Li, J.; Zhang, J.; Chen, W.; Li, F.; et al. Downregulation of CD147 Induces Malignant Melanoma Cell Apoptosis via the Regulation of IGFBP2 Expression. Int. J. Oncol. 2018, 53, 2397–2408. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-Spike Protein Interaction in COVID-19: Get the Ball Rolling with a Novel Receptor and Therapeutic Target. Sci. Total Environ. 2022, 808, 152072. [Google Scholar] [CrossRef]
- Qin, J.; Yang, Y.; Gao, S.; Liu, Y.; Yu, F.; Zhou, Y.; Lyu, R.; Liu, M.; Liu, X.; Li, D.; et al. Deregulated ALG-2/HEBP2 Axis Alters Microtubule Dynamics and Mitotic Spindle Behavior to Stimulate Cancer Development. J. Cell Physiol. 2017, 232, 3067–3076. [Google Scholar] [CrossRef]
- Pozzi, A.; Popescu, V.; Yang, S.; Mei, S.; Shi, M.; Puolitaival, S.M.; Caprioli, R.M.; Capdevila, J.H. The Anti-Tumorigenic Properties of Peroxisomal Proliferator-Activated Receptor Alpha Are Arachidonic Acid Epoxygenase-Mediated. J. Biol. Chem. 2010, 285, 12840–12850. [Google Scholar] [CrossRef] [PubMed]
- Kemiläinen, H.; Huhtinen, K.; Auranen, A.; Carpén, O.; Strauss, L.; Poutanen, M. The Expression of HSD17B12 Is Associated with COX-2 Expression and Is Increased in High-Grade Epithelial Ovarian Cancer. OCL 2018, 94, 233–242. [Google Scholar] [CrossRef]
- Lu, F.; Zhang, C.; Wu, W.-J.; Wu, Y.-M. RACK1 Downregulation Suppresses Migration and Proliferation of Neuroblastoma Cell Lines. Oncol. Rep. 2012, 27, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Golubovskaya, V.M.; Conroy, J.; Liu, S.; Wang, D.; Liu, B.; Cance, W.G. FAK Inhibition with Small Molecule Inhibitor Y15 Decreases Viability, Clonogenicity, and Cell Attachment in Thyroid Cancer Cell Lines and Synergizes with Targeted Therapeutics. Oncotarget 2014, 5, 7945–7959. [Google Scholar] [CrossRef]
- Araya, N.; Arimura, H.; Kawahara, K.-I.; Yagishita, N.; Ishida, J.; Fujii, R.; Aratani, S.; Fujita, H.; Sato, T.; Yamano, Y.; et al. Role of Kenae/CCDC125 in Cell Motility through the Deregulation of RhoGTPase. Int. J. Mol. Med. 2009, 24, 605–611. [Google Scholar] [CrossRef]
- Tanaka, M.; DeLorey, T.M.; Delgado-Escueta, A.V.; Olsen, R.W. GABRB3, Epilepsy, and Neurodevelopment. Epilepsia 2010, 51, 77. [Google Scholar] [CrossRef]
- Edamatsu, M.; Miyano, R.; Fujikawa, A.; Fujii, F.; Hori, T.; Sakaba, T.; Oohashi, T. Hapln4/Bral2 Is a Selective Regulator for Formation and Transmission of GABAergic Synapses between Purkinje and Deep Cerebellar Nuclei Neurons. J. Neurochem. 2018, 147, 748–763. [Google Scholar] [CrossRef]
- Belali, T.; Wodi, C.; Clark, B.; Cheung, M.K.; Craig, T.J.; Wheway, G.; Wagner, N.; Wagner, K.D.; Roberts, S.; Porazinski, S.; et al. WT1 Activates Transcription of the Splice Factor Kinase SRPK1 Gene in PC3 and K562 Cancer Cells in the Absence of Corepressor BASP1. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194642. [Google Scholar] [CrossRef]
- Pusapati, G.V.; Hughes, C.E.; Dorn, K.V.; Zhang, D.; Sugianto, P.; Aravind, L.; Rohatgi, R. EFCAB7 and IQCE Regulate Hedgehog Signaling by Tethering the EVC-EVC2 Complex to the Base of Primary Cilia. Dev. Cell 2014, 28, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Cuzcano, A.; Etard, C.; Delvallée, C.; Stoetzel, C.; Schaefer, E.; Scheidecker, S.; Geoffroy, V.; Schneider, A.; Studer, F.; Mattioli, F.; et al. Novel IQCE Variations Confirm Its Role in Postaxial Polydactyly and Cause Ciliary Defect Phenotype in Zebrafish. Hum. Mutat. 2020, 41, 240–254. [Google Scholar] [CrossRef]
- Umair, M.; Shah, K.; Alhaddad, B.; Haack, T.B.; Graf, E.; Strom, T.M.; Meitinger, T.; Ahmad, W. Exome Sequencing Revealed a Splice Site Variant in the IQCE Gene Underlying Post-Axial Polydactyly Type A Restricted to Lower Limb. Eur. J. Hum. Genet 2017, 25, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Springer, K.; Varghese, N.; Tzingounis, A.V. Flexible Stoichiometry: Implications for KCNQ2- and KCNQ3-Associated Neurodevelopmental Disorders. Dev. Neurosci. 2021, 43, 191–200. [Google Scholar] [CrossRef]
- Kanaumi, T.; Takashima, S.; Iwasaki, H.; Itoh, M.; Mitsudome, A.; Hirose, S. Developmental Changes in KCNQ2 and KCNQ3 Expression in Human Brain: Possible Contribution to the Age-Dependent Etiology of Benign Familial Neonatal Convulsions. Brain Dev. 2008, 30, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Huang, S.; Li, L.; Huang, D.; Yan, Y.; Du, X.; Zhang, H. Suppression of KV7/KCNQ Potassium Channel Enhances Neuronal Differentiation of PC12 Cells. Neuroscience 2016, 333, 356–367. [Google Scholar] [CrossRef]
- Suman, P.; Mishra, S.; Chander, H. High Formin Binding Protein 17 (FBP17) Expression Indicates Poor Differentiation and Invasiveness of Ductal Carcinomas. Sci. Rep. 2020, 10, 11543. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Z.; Liu, H.; Yao, S.; Zhao, D. Weighted Gene Co-Expression Network Analysis Identified a Novel Thirteen-Gene Signature Associated With Progression, Prognosis, and Immune Microenvironment of Colon Adenocarcinoma Patients. Front. Genet 2021, 12, 657658. [Google Scholar] [CrossRef]
- Fideles, S.O.M.; Ortiz, A.C.; Assis, A.F.; Duarte, M.J.; Oliveira, F.S.; Passos, G.A.; Beloti, M.M.; Rosa, A.L. Effect of Cell Source and Osteoblast Differentiation on Gene Expression Profiles of Mesenchymal Stem Cells Derived from Bone Marrow or Adipose Tissue. J. Cell Biochem. 2019, 120, 11842–11852. [Google Scholar] [CrossRef]
- Balakrishnan, L.; Soman, S.; Patil, Y.B.; Advani, J.; Thomas, J.K.; Desai, D.V.; Kulkarni-Kale, U.; Harsha, H.C.; Prasad, T.S.K.; Raju, R.; et al. IL-11/IL11RA Receptor Mediated Signaling: A Web Accessible Knowledgebase. Cell Commun. Adhes. 2013, 20, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Marino, N.; Collins, J.W.; Shen, C.; Caplen, N.J.; Merchant, A.S.; Gökmen-Polar, Y.; Goswami, C.P.; Hoshino, T.; Qian, Y.; Sledge, G.W.; et al. Identification and Validation of Genes with Expression Patterns Inverse to Multiple Metastasis Suppressor Genes in Breast Cancer Cell Lines. Clin. Exp. Metastasis. 2014, 31, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Lokau, J.; Kespohl, B.; Kirschke, S.; Garbers, C. The Role of Proteolysis in Interleukin-11 Signaling. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119135. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Furuta, H.; Matsuo, K.; Tominaga, K.; Takahashi, T.; Nakanishi, O. The Cellular and Molecular Mechanisms of Bone Invasion by Oral Squamous Cell Carcinoma. Oral Dis. 2011, 17, 462–468. [Google Scholar] [CrossRef]
- Zhou, J.; Toh, S.H.M.; Tan, T.K.; Balan, K.; Lim, J.Q.; Tan, T.Z.; Xiong, S.; Jia, Y.; Ng, S.B.; Peng, Y.; et al. Super-Enhancer-Driven TOX2 Mediates Oncogenesis in Natural Killer/T Cell Lymphoma. Mol. Cancer 2023, 22, 69. [Google Scholar] [CrossRef]
- Saleh, R.; Taha, R.Z.; Toor, S.M.; Sasidharan Nair, V.; Murshed, K.; Khawar, M.; Al-Dhaheri, M.; Petkar, M.A.; Abu Nada, M.; Elkord, E. Expression of Immune Checkpoints and T Cell Exhaustion Markers in Early and Advanced Stages of Colorectal Cancer. Cancer Immunol. Immunother. 2020, 69, 1989–1999. [Google Scholar] [CrossRef]
- Vong, Q.P.; Leung, W.H.; Houston, J.; Li, Y.; Rooney, B.; Holladay, M.; Oostendorp, R.A.J.; Leung, W. TOX2 Regulates Human Natural Killer Cell Development by Controlling T-BET Expression. Blood 2014, 124, 3905–3913. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Currie, D.; Wong, N.; Zane, I.; Rix, T.; Vardakastanis, M.; Claxton, A.; Ong, K.K.V.; Macmorland, W.; Poivet, A.; Brooks, A.; et al. A Potential Prognostic Gene Signature Associated with p53-Dependent NTRK1 Activation and Increased Survival of Neuroblastoma Patients. Cancers 2024, 16, 722. https://doi.org/10.3390/cancers16040722
Currie D, Wong N, Zane I, Rix T, Vardakastanis M, Claxton A, Ong KKV, Macmorland W, Poivet A, Brooks A, et al. A Potential Prognostic Gene Signature Associated with p53-Dependent NTRK1 Activation and Increased Survival of Neuroblastoma Patients. Cancers. 2024; 16(4):722. https://doi.org/10.3390/cancers16040722
Chicago/Turabian StyleCurrie, David, Nicole Wong, Isabelle Zane, Tom Rix, Marios Vardakastanis, Amelia Claxton, Karine K. V. Ong, William Macmorland, Arthur Poivet, Anthony Brooks, and et al. 2024. "A Potential Prognostic Gene Signature Associated with p53-Dependent NTRK1 Activation and Increased Survival of Neuroblastoma Patients" Cancers 16, no. 4: 722. https://doi.org/10.3390/cancers16040722




