Prevalence of and Impact on the Outcome of Myosteatosis in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
3.1. Characteristics of Patients
3.1.1. Characteristics of Patients with Myosteatosis
3.1.2. Characteristics of Patients without Myosteatosis
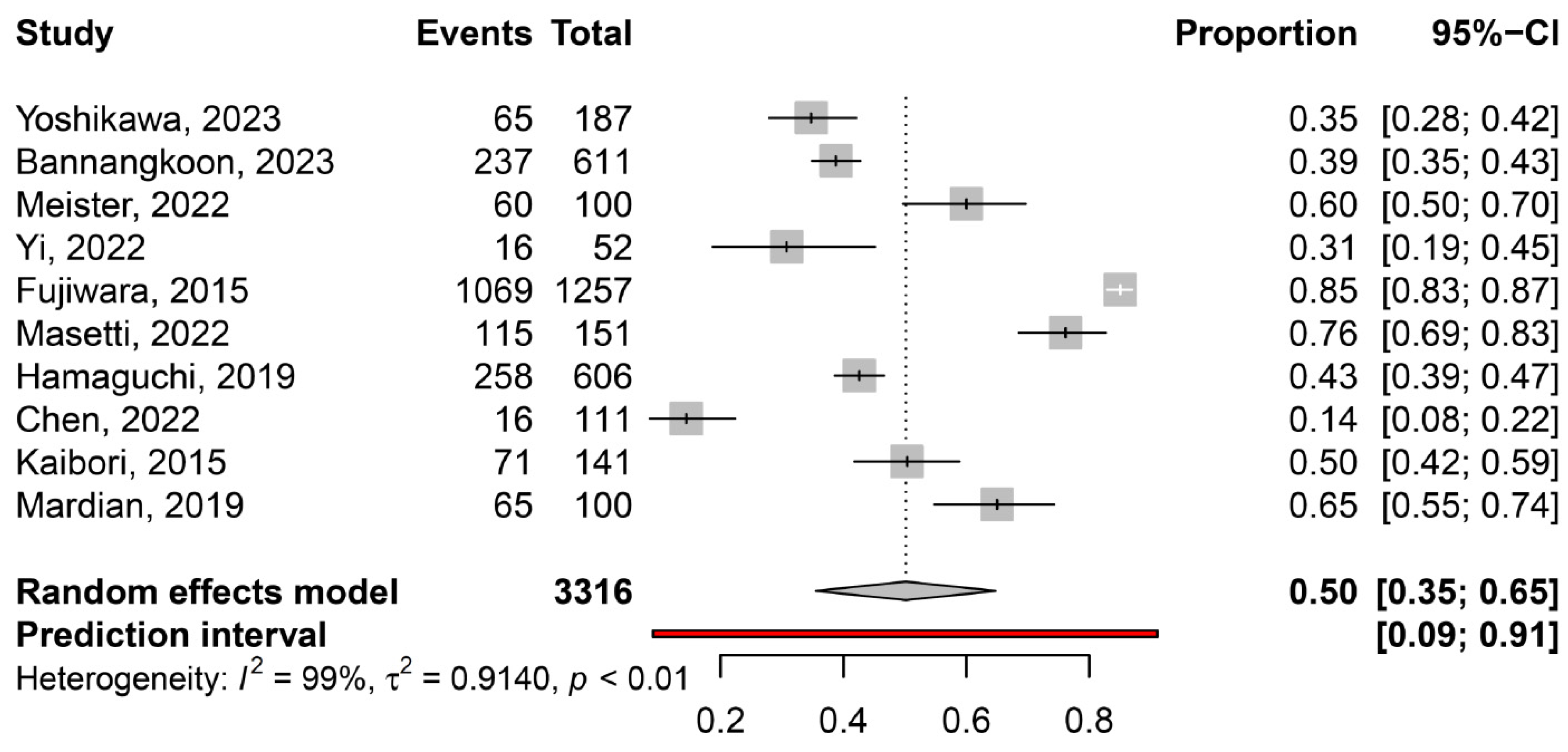
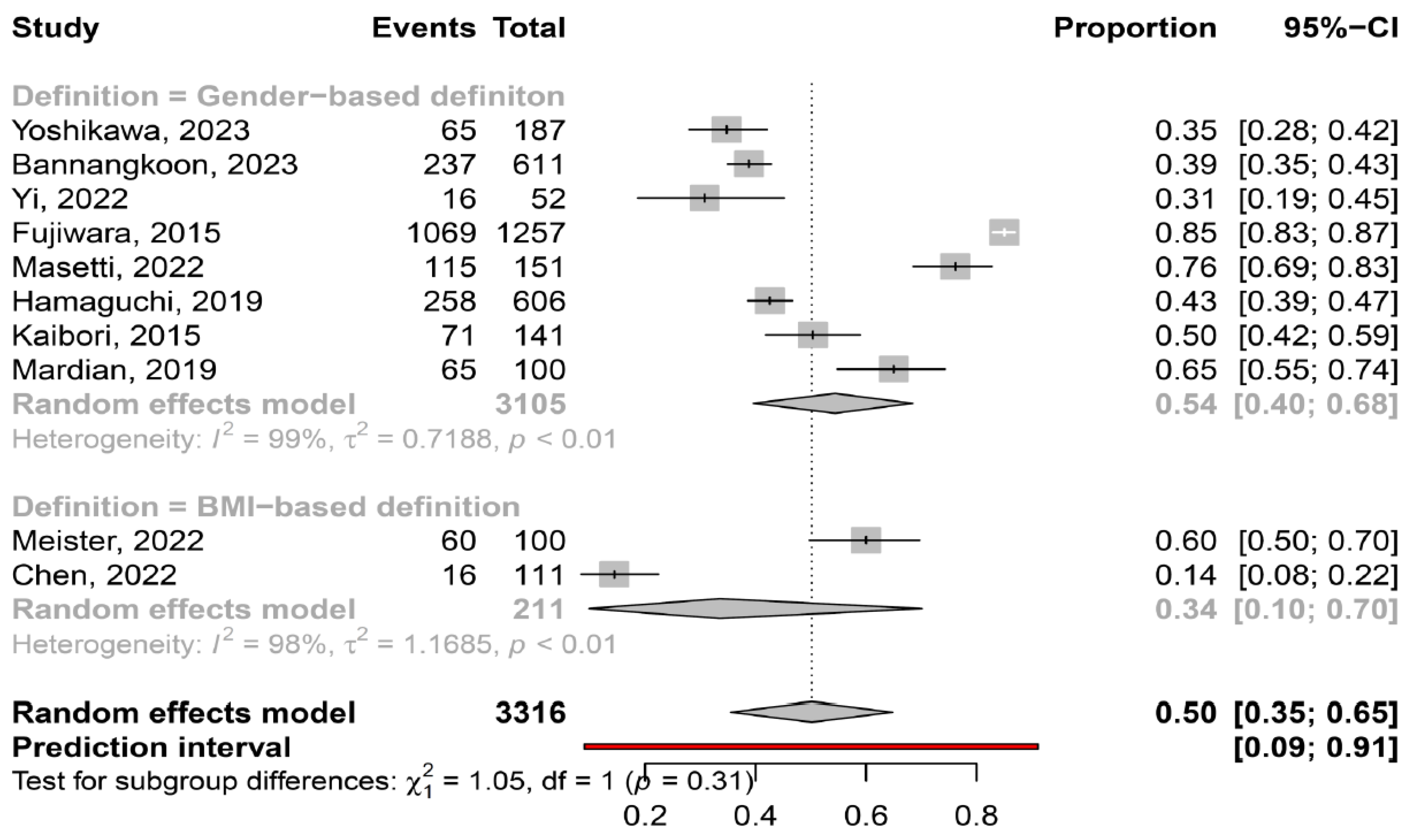
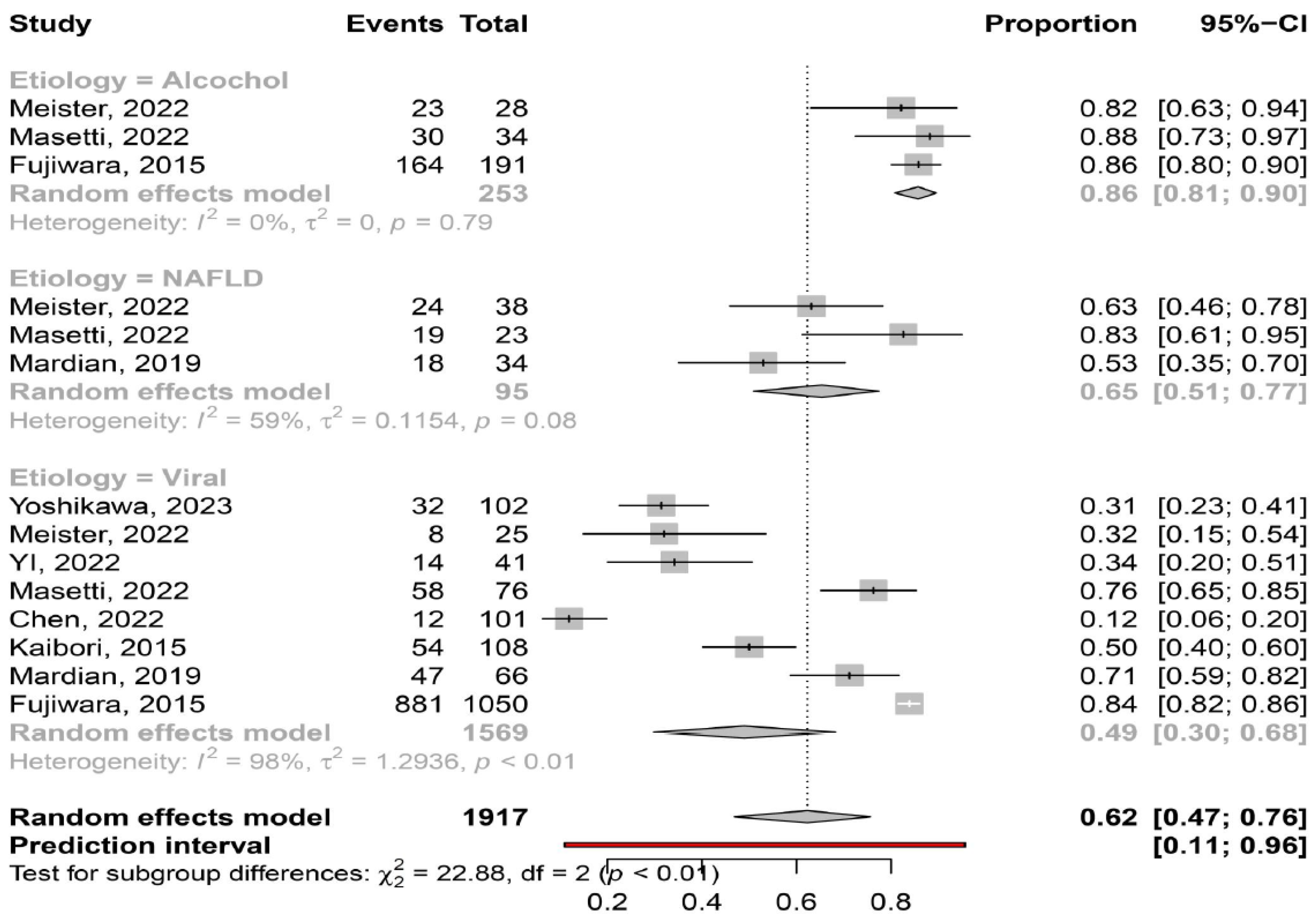
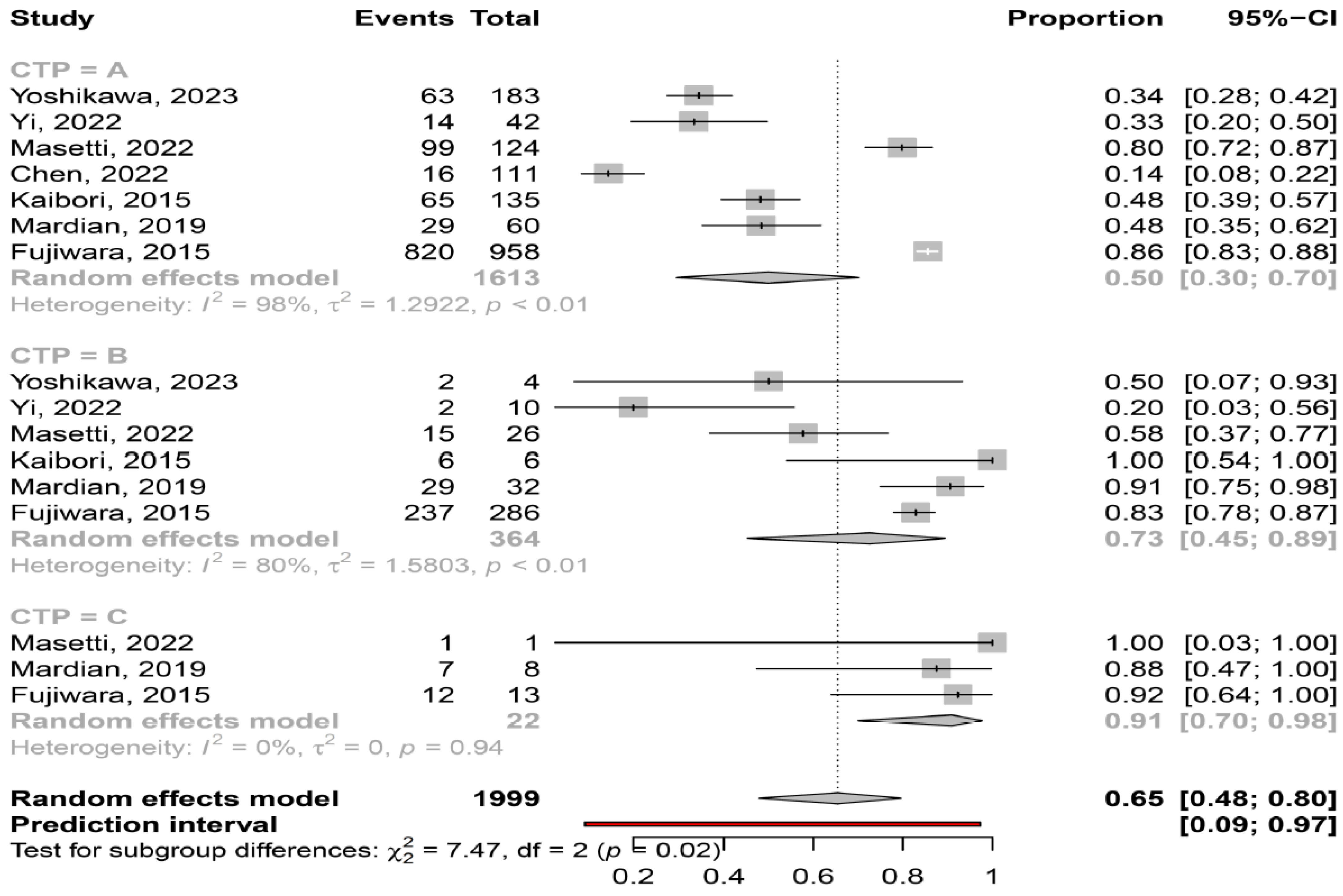
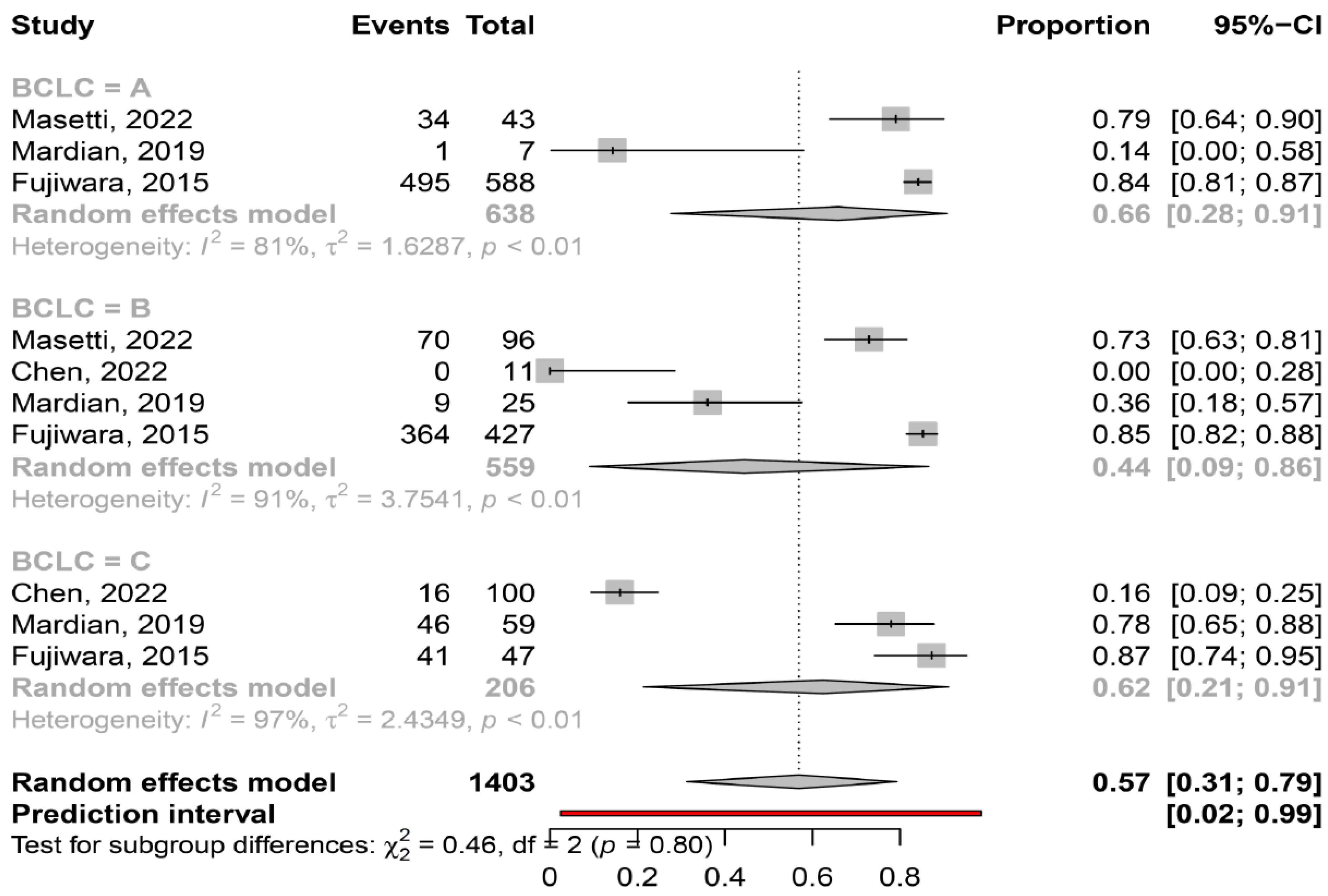
3.2. Prevalence of Myosteatosis in Total and in Specific Subgroups
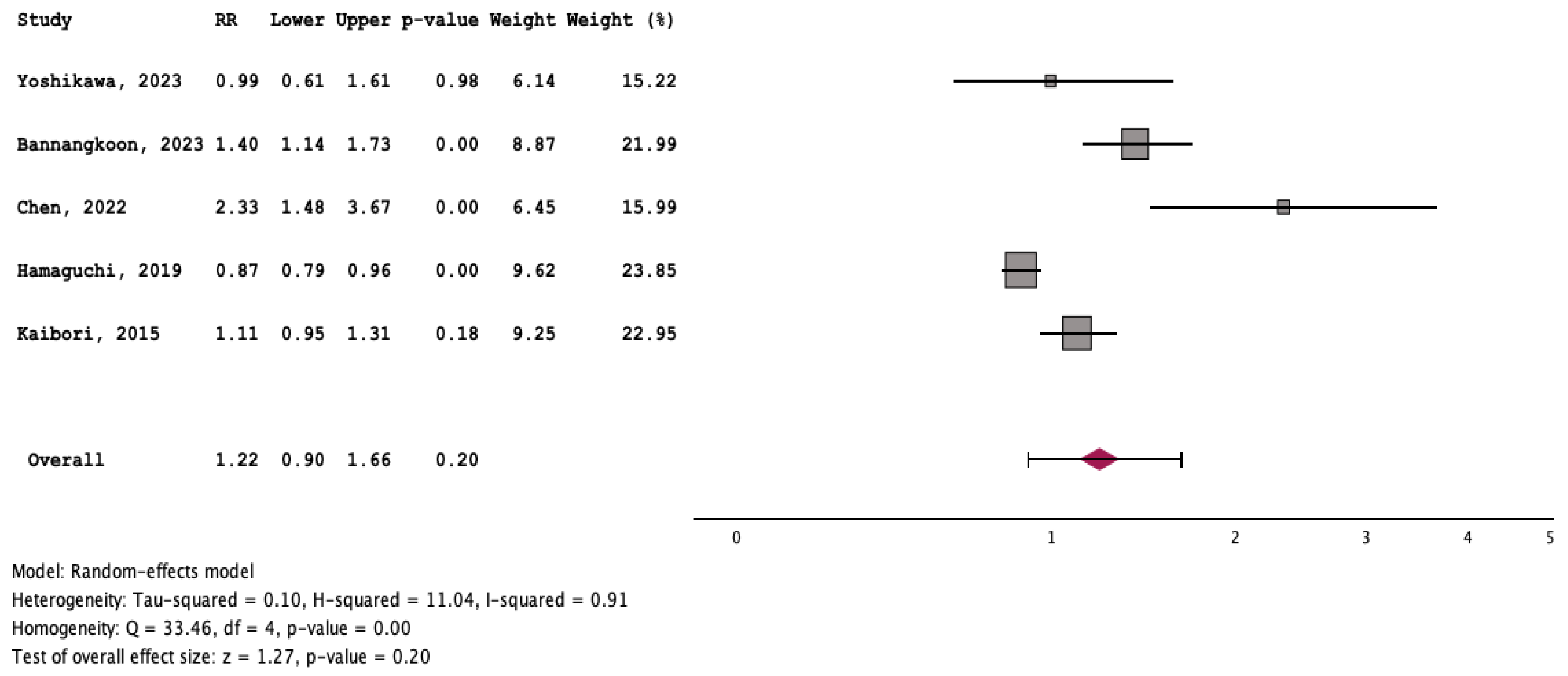
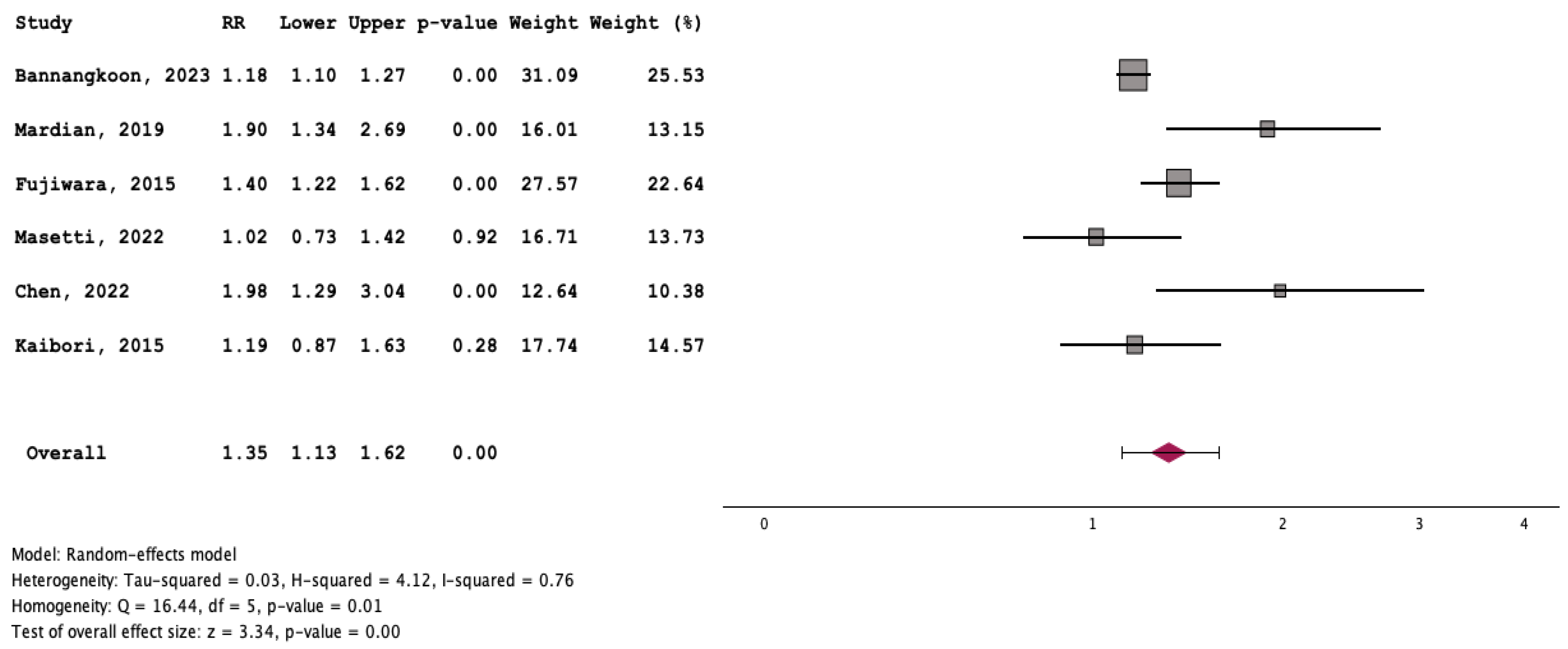
3.3. Outcome of Patients with and without Myosteatosis
3.4. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, D.W.; Ko, Y.; Ha, J.; Shin, Y.B.; Lee, J.; Sung, Y.S.; Kim, K.W. Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: A new paradigm beyond sarcopenia. Ageing Res. Rev. 2021, 70, 101398. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Malpica, L.; Williams, G.R. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar] [CrossRef] [PubMed]
- Stijnen, T.; Hamza, T.H.; Ozdemir, P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat. Med. 2010, 29, 3046–3067. [Google Scholar] [CrossRef] [PubMed]
- Clopper, C.J.; Pearson, E.S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrica 1934, 26, 404–413. [Google Scholar] [CrossRef]
- Hardy, R.J.; Thompson, S.G. A likelihood approach to meta-analysis with random effects. Stat. Med. 1996, 15, 619–629. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Int Hout, J.; Ioannidis, J.P.; Rovers, M.M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org/index.html (accessed on 25 January 2021).
- Yoshikawa, K.; Shimada, M.; Morine, Y.; Ikemoto, T.; Saito, Y.; Yamada, S.; Teraoku, H.; Takao, S. Clinical impact of myosteatosis measured by magnetic resonance imaging on long-term outcomes of hepatocellular carcinoma after radical hepatectomy. BMC Surg. 2023, 23, 281. [Google Scholar] [CrossRef]
- Bannangkoon, K.; Hongsakul, K.; Tubtawee, T.; Ina, N.; Chichareon, P. Association of myosteatosis with treatment response and survival in patients with hepatocellular carcinoma undergoing chemoembolization: A retrospective cohort study. Sci. Rep. 2023, 13, 3978. [Google Scholar] [CrossRef]
- Meister, F.A.; Lurje, G.; Verhoeven, S.; Wiltberger, G.; Heij, L.; Liu, W.J.; Jiang, D.; Bruners, P.; Lang, S.A.; Ulmer, T.F.; et al. The Role of Sarcopenia and Myosteatosis in Short- and Long-Term Outcomes Following Curative-Intent Surgery for Hepatocellular Carcinoma in a European Cohort. Cancers 2022, 14, 720. [Google Scholar] [CrossRef]
- Yi, X.; Fu, Y.; Long, Q.; Zhao, Y.; Li, S.; Zhou, C.; Lin, H.; Liu, X.; Liu, C.; Chen, C.; et al. Myosteatosis can Predict Unfavorable Outcomes in Advanced Hepatocellular Carcinoma Patients Treated With Hepatic Artery Infusion Chemotherapy and Anti-PD-1 Immunotherapy. Front. Oncol. 2022, 12, 892192. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef]
- Masetti, C.; Pugliese, N.; Lofino, L.; Colapietro, F.; Ceriani, R.; Lleo, A.; Poretti, D.; Pedicini, V.; De Nicola, S.; Torzilli, G.; et al. Myosteatosis Is Not Associated with Complications or Survival in HCC Patients Undergoing Trans Arterial Embolization. J. Clin. Med. 2022, 12, 262. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Kamo, N.; Seo, S.; Taura, K.; et al. Preoperative Visceral Adiposity and Muscularity Predict Poor Outcomes after Hepatectomy for Hepatocellular Carcinoma. Liver Cancer 2019, 8, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Liang, P.C.; Shih, T.T.; Liu, T.H.; Shen, Y.C.; Lu, L.C.; Lin, Z.Z.; Hsu, C.; Hsu, C.H.; Cheng, A.L.; et al. Sarcopenia and myosteatosis are associated with survival in patients receiving immunotherapy for advanced hepatocellular carcinoma. Eur. Radiol. 2023, 33, 512–522. [Google Scholar] [CrossRef]
- Kaibori, M.; Ishizaki, M.; Iida, H.; Matsui, K.; Sakaguchi, T.; Inoue, K.; Mizuta, T.; Ide, Y.; Iwasaka, J.; Kimura, Y.; et al. Effect of Intramuscular Adipose Tissue Content on Prognosis in Patients Undergoing Hepatocellular Carcinoma Resection. J. Gastrointest. Surg. 2015, 19, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Mardian, Y.; Yano, Y.; Ratnasari, N.; Choridah, L.; Wasityastuti, W.; Setyawan, N.H.; Hayashi, Y. Sarcopenia and intramuscular fat deposition are associated with poor survival in Indonesian patients with hepatocellular carcinoma: A retrospective study. BMC Gastroenterol. 2019, 19, 229. [Google Scholar] [CrossRef] [PubMed]
- Shirai, H.; Kaido, T.; Hamaguchi, Y.; Kobayashi, A.; Okumura, S.; Yao, S.; Yagi, S.; Kamo, N.; Taura, K.; Okajima, H.; et al. Preoperative Low Muscle Mass and Low Muscle Quality Negatively Impact on Pulmonary Function in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Liver Cancer 2018, 7, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kaido, T.; Hamaguchi, Y.; Okumura, S.; Taura, K.; Hatano, E.; Okajima, H.; Uemoto, S. Impact of postoperative changes in sarcopenic factors on outcomes after hepatectomy for hepatocellular carcinoma. J. Hepatobiliary Pancreat. Sci. 2016, 23, 57–64. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analyses. 2004. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 11 February 2024).
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- Okugawa, Y.; Kitajima, T.; Yamamoto, A.; Shimura, T.; Kawamura, M.; Fujiwara, T.; Mochiki, I.; Okita, Y.; Tsujiura, M.; Yokoe, T.; et al. Clinical Relevance of Myopenia and Myosteatosis in Colorectal Cancer. J. Clin. Med. 2022, 11, 2617. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Z.; Shen, Z.L.; Zhang, F.M.; Zhang, X.Z.; Chen, W.H.; Yan, X.L.; Zhuang, C.L.; Chen, X.L.; Yu, Z. Prognostic value of myosteatosis and sarcopenia for elderly patients with colorectal cancer: A large-scale double-center study. Surgery 2022, 172, 1185–1193. [Google Scholar] [CrossRef]
- Feng, S.; Mu, H.; Hou, R.; Liu, Y.; Zou, J.; Zhao, Z.; Zhu, Y. Prognostic value of myosteatosis in patients with lung cancer: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2022, 27, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Srpcic, M.; Jordan, T.; Popuri, K.; Sok, M. Sarcopenia and myosteatosis at presentation adversely affect survival after esophagectomy for esophageal cancer. Radiol. Oncol. 2020, 54, 237–246. [Google Scholar] [CrossRef]
- Wulan, S.N.; Raza, Q.; Prasmita, H.S.; Martati, E.; Maligan, J.M.; Mageshwari, U.; Fatima, I.; Plasqui, G. Energy Metabolism in Relation to Diet and Physical Activity: A South Asian Perspective. Nutrients 2021, 13, 3776. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Bae, S.J.; Lee, M.J.; Kim, E.H.; Park, H.; Kim, H.S. Association of Visceral Fat Obesity, Sarcopenia, and Myosteatosis with Non-Alcoholic Fatty Liver Disease without Obesity. Clin. Mol. Hepatol. 2023, 29, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Tsien, C.; Bhanji, R.A.; Dunichand-Hoedl, A.R.; Rider, E.; Motamedrad, M.; Mazurak, V.C.; Baracos, V.; Montano-Loza, A.J. Skeletal Muscle Pathological Fat Infiltration (Myosteatosis) Is Associated with Higher Mortality in Patients with Cirrhosis. Cells 2022, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Tsien, C.; Bhanji, R.A.; Dunichand-Hoedl, A.R.; Rider, E.; Motamedrad, M.; Mazurak, V.C.; Baracos, V.; Montano-Loza, A.J. Myosteatosis in Cirrhosis: A Review of Diagnosis, Pathophysiological Mechanisms and Potential Interventions. Cells 2022, 11, 1216. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, Y.; Zhu, L.; Yang, L.; Zheng, C. Association between sarcopenia and clinical outcomes in patients with hepatocellular carcinoma: An updated meta-analysis. Sci. Rep. 2023, 13, 934. [Google Scholar] [CrossRef]
- Cao, H.; Gong, Y.; Wang, Y. The prognostic impact of myosteatosis on overall survival in gynecological cancer patients: A meta-analysis and trial sequential analysis. Int. J. Cancer 2022, 151, 1997–2003. [Google Scholar] [CrossRef]
- Fang, T.; Gong, Y.; Wang, Y. Prognostic values of myosteatosis for overall survival in patients with gastric cancers: A meta-analysis with trial sequential analysis. Nutrition 2023, 105, 111866. [Google Scholar] [CrossRef]
- Long, J.; Zhang, X.; Mi, W.; Shi, J.; Ren, H.; Wang, Q. The predictive value of sarcopenia and myosteatosis in trans-arterial (chemo)-embolization treated HCC patients. Aging 2024, 16, 389–401. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, H.K.; Lee, M.J.; Bae, S.J.; Kim, K.W.; Choe, J. Association between type 2 diabetes and skeletal muscle quality assessed by abdominal computed tomography scan. Diabetes Metab. Res. Rev. 2022, 38, e3513. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Krishnaswami, S.; Resnick, H.; Kelley, D.E.; Haggerty, C.; Harris, T.B.; Schwartz, A.V.; Kritchevsky, S.; Newman, A.B. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003, 26, 372–379. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, J.; Zhu, H.; Zhang, Z.; Jiang, Y.; Zhang, X.; Wu, Y.; Lu, J.; Cun, H.; He, B. Low thigh muscle strength in relation to myosteatosis in patients with type 2 diabetes mellitus. Sci. Rep. 2023, 13, 1957. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, M.; Driever, D.; Ryan, É.J.; Elliott, J.A.; Finnegan, J.; McNamara, D.; Murphy, I.; Conlon, K.C.; Neary, P.C.; Kavanagh, D.O.; et al. Obesity, Sarcopenia and Myosteatosis: Impact on Clinical Outcomes in the Operative Management of Crohn’s Disease. Inflamm. Bowel Dis. 2023, izad225. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.K.; Lee, Y.R.; Song, J.E.; Kweon, Y.O.; Tak, W.Y.; Jang, S.Y.; Park, J.G.; Park, S.Y. Prognostic Impact of Myosteatosis on Mortality in Hospitalized Patients with COVID-19. Diagnostics 2022, 12, 2255. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Liu, H.; Zhu, L.; Wang, D.; Xie, F.; Shi, L.; Mei, J.; Jiang, X.; Zeng, Q.; Hu, P.; et al. Myosteatosis predicting risk of transition to severe COVID-19 infection. Clin. Nutr. 2022, 41, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Zoico, E.; Rossi, A.; Di Francesco, V.; Sepe, A.; Olioso, D.; Pizzini, F.; Fantin, F.; Bosello, O.; Cominacini, L.; Harris, T.B.; et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: Relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M. Interplay of adipokines and myokines in cancer pathophysiology: Emerging therapeutic implications. World J. Exp. Med. 2013, 3, 26–33. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiliou, A.; Lekakis, V.; Xynos, G.; Cholongitas, E. Prevalence of and Impact on the Outcome of Myosteatosis in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 952. https://doi.org/10.3390/cancers16050952
Kamiliou A, Lekakis V, Xynos G, Cholongitas E. Prevalence of and Impact on the Outcome of Myosteatosis in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(5):952. https://doi.org/10.3390/cancers16050952
Chicago/Turabian StyleKamiliou, Aikaterini, Vasileios Lekakis, George Xynos, and Evangelos Cholongitas. 2024. "Prevalence of and Impact on the Outcome of Myosteatosis in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis" Cancers 16, no. 5: 952. https://doi.org/10.3390/cancers16050952
APA StyleKamiliou, A., Lekakis, V., Xynos, G., & Cholongitas, E. (2024). Prevalence of and Impact on the Outcome of Myosteatosis in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers, 16(5), 952. https://doi.org/10.3390/cancers16050952