Simple Summary
Cancer is implicated in multiple pathways that increase thrombogenicity, and lung cancer patients have a 20% higher risk of venous thromboembolism in comparison to the general population. Venous thromboembolic disease (VTE) in cancer patients, which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), can lead to the delay of cancer treatment and, thus, result in increased mortality, morbidity, and burden on healthcare resources. Factors contributing to thrombotic burden are related to cancer, patients, treatment, and laboratory findings. Thromboprophylaxis during active lung cancer treatment with adequate anticoagulation might improve outcomes. Thromboprophylaxis with low molecular weight heparin (LMWH) is the standard of care, but due to the vast heterogeneity of lung cancer patients, there is no consensus on the optimal dose and duration of the treatment.
Abstract
Background: The aim of this study was to record and assess the efficacy and safety ofthromboprophylaxis with an intermediate dose of Tinzaparin in lung cancer patients with high thrombotic risk. Methods: This was a non-interventional, single-arm, prospective cohort study of lung cancer patients who received thromboprophylaxis with Tinzaparin 10.000 Anti-Xa IU in 0.5 mL, OD, used in current clinical practice. Enrolled ambulatory patients signed informed consent. Anti-Xa levels were tested. Results: In total, 140 patients were included in the study, of which 81.4% were males. The histology of the tumor was mainly adenocarcinoma. Lung cancer patients with high thrombotic risk based on tumor, patient, treatment, and laboratory-related factors were enrolled. Only one patient experienced a thrombotic event (0.7%), and 10 patients had bleeding events (7.1%), including only one major event. Anti-Xa levels measured at 10 days and 3 months did not differ significantly between patients who developed hemorrhagic events and those who did not (p = 0.26 and p = 0.32, respectively). Conclusion: Thromboprophylaxis with an intermediate Tinzaparin dose in high thrombotic-risk lung cancer patients is a safe and effective choice for the prevention of VTE.
1. Introduction
Venous thromboembolic disease (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication of malignancy. The relationship between cancer and thrombosis was first identified more than a century ago by Trousseau, and it is now estimated that up to 20% of patients with cancer develop VTE [1,2,3]. VTE in cancer patients is associated with a multitude of adverse outcomes, including increased morbidity and mortality, the postponement of therapy, the need for long-term anticoagulation with the potential for bleeding complications, and high rates of recurrent VTE [4,5]. In addition, it leads to a significant increase in healthcare resource utilization. In one study of cancer patients, the adjusted mean all-cause additional healthcare costs of VTE were USD 30,538 per patient [6]. The risk varies with the type of cancer, as well as the stage of the disease. Other risk factors such as age, gender, bed rest, venous catheters, surgery, chemotherapy with or without adjuvant hormone therapy, radiation therapy, and infections also increase the risk of thrombosis in cancer patients. Cancer-associated thrombosis involves a complex interplay between direct cancer cell-mediated pathways and indirect host cell-mediated mechanisms. Direct mechanisms include the expression or secretion by cancer cells of factors implicated in both primary and secondary hemostasis pathways. Some of the key mediators in the activation of the coagulation cascade include tissue factor (TF), Phosphatidyl serine (PS), the Cancer procoagulant (CP), and cancer microparticles. Furthermore, cancer cells promote platelet activation and aggregation through the expression of Podoplanin (PDPN) and the secretion of platelet agonists such as ADP and thrombin Plasminogen activation inhibitor-1 (PAI-1). Indirect mechanisms include The immune-mediated secretion of cytokines, which promote platelet activation and endothelial inflammation, as well as neutrophil extracellular traps (NETs), which serve as scaffolds to entrap platelets and red blood cells and further promote platelet activation [7].
Lung cancer belongs to the group of malignancies with the highest incidence rates of VTE [2,8,9,10]. Retrospective studies associate adenocarcinoma histology with The increased risk of VTE [11,12]. Blom et al. examined the thrombotic risk in 537 patients with NSCLC and found that it was 20 times higher compared to the general population [standardized morbidity ratio: 20.0 (95% Confidence Interval (CI), 14.6–27.4)]. The risk was three times higher in patients with adenocarcinoma compared to squamous cell carcinoma (incidence 66.7 vs. 21.2 per 1000 person-years) [13]. In recent years, VTE in lung cancer patients has received increasing attention. In the retrospective analysis of a lung cancer cohort of 6732 patients (control group 17,284 patients), VTE occurred in 13.9% of patients in the lung cancer cohort and 1.4% in the control cohort [14]. Among the prothrombotic mechanisms of cancer described above, increased levels of leukocytes, NETs, tissue factor-positive (TF+) microvesicles (MVs), and endothelial cell activation exerted an important role in lung cancer patients [15,16].
Systemic anticancer treatment further increases the risk for VTE in these patients. Specifically, chemotherapy is estimated to account for a 4-7-fold increase [17]. Among lung cancer patients receiving chemotherapy, the majority of VTE events occur within 6 months of starting chemotherapy [18]. In the CANTARISK study, out of 1980 patients with lung cancer treated in the pre-immunotherapy era, the 6-month incidence of VTE was 6.1% [19]. Immunotherapy, which has become the standard of care for advanced disease, has also been associated with an increased risk of VTE. In a study of 1686 patients with cancer who received immunotherapy, the 6-month incidence of VTE was 7.1% [20]. In another report of 522 immunotherapy-treated patients with lung cancer, the incidence of VTE occurred at 30.3% [21]. Furthermore, VTE in immunotherapy patients was associated with worse survival, but this association was not statistically significant when adjusting for age and metastasis [HR = 1.215, (95% CI 0.94 to 1.55) p-value = 0.121] [21]. The mechanism underlying the increased likelihood of venous thromboembolic events among immunotherapy-treated patients is not clear. Two of the mechanisms proposed by Goel et al. are cancer-mediated T-cell activation, leading to subsequent monocyte activation and tissue factor release, and immune-mediated vasculitis, resulting in endothelial damage [22].
In several clinical scenarios of high thromboembolic risk, low molecular weight heparins (LMWH) are safe and effective at preventing VTE [23,24,25,26,27,28]. Current ESMO and ASCO guidelines suggest considering thromboprophylaxis with either Direct Oral Anticoagulants (DOACs) or with low molecular weight heparin (LMWH) in ambulatory high-risk patients [29]. In clinical practice, the main factors affecting physicians’ decisions to use thromboprophylaxis in cancer patients are the Eastern European Cooperative Oncology (ECOG) group score, cancer type, advanced stage, malignancy, chemotherapy, co-morbidities and history of thrombosis [30].
The optimal dosage of LMWH treatment for thromboprophylaxis is not well established. There are data from studies using higher than usual doses for prophylaxis or using full therapeutic doses. For Tinzaparin, a prophylactic dose is considered a dose of 4500 anti-Factor Xa IU/mL, the intermediate dose is 10,000 anti-Factor Xa IU/mL, and the recommended dose is 175 anti-Xa IU/kg of body weight, administered subcutaneously once daily. Intermediate doses were used in the study by Pelzer in 2015, and therapeutic doses in the study by Maraveyas in 2012. Two studies initially administered a therapeutic dose of LMWH followed by intermediate doses (Klerk 2005, van Doormaal 2011) [31,32,33,34]. Regarding the optimal duration of treatment, a systematic review and meta-analysis published in 2020 showed that the extension of treatment beyond six months did not lead to superior efficacy but increased toxicity [27].
Based on the above evidence, we performed a prospective study of Tinzaparin thromboprophylaxis for lung cancer patients at high risk for thrombosis to obtain and evaluate data on efficacy, safety, and patient compliance. The primary objective of this study was to assess the frequency of all venous thromboembolism (VTE) events during the six-month treatment period to assess the frequency of major and minor bleeding events. Secondary objectives were to assess patient compliance and to assess the frequency of bleeding events in relation to anti-Xa levels and to compare the frequency of events between patients receiving and not receiving immune checkpoint inhibitors (ICIs).
2. Materials and Methods
This was a non-interventional, single-arm, prospective cohort study of consecutive lung cancer patients who received thromboprophylaxis with Tinzaparin, conducted at the Oncology Unit of the Third Department of Internal Medicine, “Sotiria” General Hospital for Chest Diseases between June 2021 and June 2022. The study protocol was approved by the Local Ethics Committee Review Board. All patients included in the study provided written informed consent. Ambulatory patients with histologically or cytologically confirmed lung cancer who fulfilled the following additional criteria were eligible for study inclusion: patients who were either receiving or were expecting to receive thromboprophylaxis based on current clinical practice; aged over 18 years; and life-expectancy over 6 months at the time of study inclusion. Patients were evaluated for study inclusion at the time of 1st or 2nd lung cancer treatment administration. Thromboprophylaxis was administered by the prescribing doctor based on the common local clinical practice at the time in patients with at least two of the following risk factors:
- Time since cancer diagnosis < 6 months.
- Metastatic cancer or high burden of disease (stage ≥ ΙΙΙB).
- Platinum-based chemotherapy.
- Antiangiogenesis therapy.
- Immunotherapy.
- Platelets > 350.000/μL.
- Hemoglobin < 10 g/dL.
- White blood cell count > 11,000/μL.
- Obesity (BMI > 35).
- Blood transfusion or use of hematopoietic factors.
- Recent hospitalization.
- Reduced mobility.
- History of deep venous thrombosis (DVT).
- Congenital thrombophilia (i.e., Factor V Leiden thrombophilia, prothrombin G20210A, Antithrombin III insufficiency, Protein C or protein S insufficiency, etc.).
- At least two of the following vascular risk factors: a history of peripheral arterial disease (PAN), cerebrovascular accident (CVA), coronary artery disease (CAD), hypertension, dyslipidemia, or diabetes mellitus.
- Atrial fibrillation.
Patients with at least 2 of these factors received an intermediate dose of Tinzaparin (10.000 anti-XaIU OD) daily during cancer treatment and a maximum of 6 months. The dose and duration of therapy were selected based on previous research showing no superior efficacy from increased dose or duration [24]. The following information was collected for each patient: the date of thromboprophylaxis onset, histology, anticancer treatment (type, agents, and line), risk factors for thrombotic events as described above, thromboembolic events (type and date), hemorrhagic events (date and type), date of tinzaparin discontinuation, date of disease progression and date of death (when applicable). Anti-Xa levels were measured 10 days and 3 months after the onset of Tinzaparin prophylaxis. Additionally, patients with adenocarcinoma were assessed for mutations in 58 genes through Next Generation Sequencing (NGS), as per standard local clinical practice. Specific investigations to detect DVT and PE were performed as requested based on the clinical suspicion of VTE on subsequent patient evaluations. Cases of incidentally diagnosed VTE during routine follow-up imaging were included.
Major bleeding was defined per the ISTH criteria [35] as follows:
- Fatal bleeding;
- Symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial, or intramuscular with compartment syndrome;
- Bleeding causes a fall in hemoglobin level of 2 g/dL or more, leading to the transfusion of two or more units of whole blood or red blood cells.
Statistical analysis was performed using R version 4.2.1. Patient demographics and disease characteristics were analyzed using descriptive statistics. The Pearson chi-squared test was used to assess differences in categorical variables. The Wilcoxon Rank Sum test was used for continuous variables. To account for the lack of a comparative group in the study, as an indirect measure of efficacy comparison, the expected rates of VTE and hemorrhagic events with thromboprophylaxis administered were calculated based on the reports of VTE incidence in the CANTARISK study, which was a global, real-world study of 1980 patients with lung cancer followed-up for 6 months [19]. The risk ratio (RR) was calculated as the ratio of the probability of a thrombotic event in the Tinzaparin group to the expected probability if prophylaxis was not administered, using the Cochran–Mantel–Haenszel test. All hypothesis testing was conducted at a two-sided significance level of α = 0.05.
3. Results
3.1. Population
In total, 140 patients were included in the analysis. The follow-up period was 6 months. The demographic characteristics of patients are summarized in Table 1. The majority of included patients were males (81.4%), and the median age was 66 (range 46–92). The most prevalent histologic subtype was adenocarcinoma (N = 73, 52%), followed by squamous cell carcinoma (N = 38, 27%), small-cell lung cancer (SCLC) (N = 20, 14%) and others (N = 9, 6.4%). The majority of patients (N = 90, 64%) were on first-line anticancer treatment at study inclusion. All patients but one had at least two risk factors for VTE at the baseline (as per protocol), with a median number of four (range 1–8). The most common risk factors at the baseline were the high burden of disease (stage > IIIB), cancer diagnosis within 6 months before treatment onset, platinum-based chemotherapy, and immunotherapy. Sixteen (11%) patients had a history of DVT (Table 2).

Table 1.
Demographic characteristics of lung cancer patients with high thrombotic risk who were enrolled in the study.

Table 2.
Break-down of risk factors for venous thromboembolism at baseline.
3.2. Tinzaparin Treatment
In total, 135 (96%) patients received intermediate doses of Tinzaparin as per the protocol, while 5 (3.6%) received therapeutic doses. The median time to Tinzaparin treatment discontinuation was 157 days [interquartile range (IQR), 85, 183]. Regarding the reasons for discontinuation, 65 (46.4%) patients completed 6 months of Tinzaparin prophylaxis, 17 (12%) completed the anticancer treatment and hence were no longer eligible for thromboprophylaxis, 24 (17%) died, 5 (3.6%) changed lines of treatment following PD and stopped fulfilling the minimum criteria for thromboprophylaxis, 18 (12.7%) had an adverse event (AE) that prompted the physician to discontinue treatment, 8 (5.7%) discontinued because of the physician’s decision for a reason other than AE, and lastly, 3 (2.1%) patients discontinued of their own will (Table 3).

Table 3.
Characteristics of tinzaparin treatment for lung cancer patients with high thrombotic risk enrolled in the study.
3.3. Efficacy and Safety of Tinzaparin Thromboprophylaxis
Out of 140 patients, only 1 (0.7%) developed radiologically confirmed DVT. This patient subsequently developed PE despite switching to a therapeutic dose of Tinzaparin following the DVT diagnosis.
Based on historical data from the CANTARISK study, the expected incidence of VTE in a similar population not treated with Tinzaparin would be 6.1% or 8 in 140 patients. Using the Mantel–Haenszel method, the relative risk (RR) for thromboembolism between these groups was 0.13 (95% CI, 0.02; 0.99, p = 0.048).
By the end of follow-up, 10 patients (7.1%) had developed hemorrhagic events, 9 had minor events, while 1 patient had a major event; specifically, they experienced the hemorrhagic turnover of brain metastasis. The total number of minor hemorrhagic events was 12: 9 episodes of hemoptysis, 2 episodes of rhinorrhagia, and 1 episode of bloody stools. Anti-Xa levels measured at 10 days and at 3 months did not differ significantly between patients who developed hemorrhagic events and those who did not (p = 0.26 and p = 0.32, respectively) (Table 4).

Table 4.
Adverse events that occurred during the treatment period with tinzaparin.
Thromboembolic and treatment-adverse events are summarized in Table 4. A swimmer’s plot demonstrating the timing of all events (thromboembolic, hemorrhagic, and other adverse events) is presented in Figure 1. Treatment-adverse effects included acute renal failure, allergic reaction, hematologic toxicity, anemia, and thrombocytopenia. The respective frequencies were 1 (0.7%), 2 (1.4%), 2 (1.4%), 1 (0.7%), and 3 (2.1%).
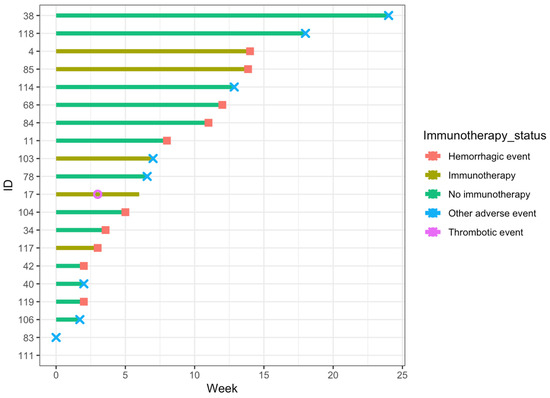
Figure 1.
Swimmers plot for patients who developed any event during Tinzaparin thromboprophylaxis. The x-axis represents the time in weeks since the onset of Tinzaparin thromboprophylaxis, and the y-axis represents the unique identification number assigned to each patient.
Regarding patients with a history of VTE prior to the onset of Tinzaparin thromboprophylaxis, no patients had thromboembolic or hemorrhagic events in the 6 months of observation.
3.4. Subgroup Analysis—ICIs vs. non-ICIs
Of the 140 patients, 62 (44.3%) received immunotherapy (IO). In the subgroup comparison of IO vs. non-IO-treated patients, patients who received IO had a lower rate of AE’s [4 (6.5%) vs. 14 (18%), p = 0.06, q = 0.3]. Furthermore, patients on IO, compared to the rest of the study population, were less likely to have a history of DVT at the baseline (4.8% vs. 17%). Still, the only patient who developed DVT during the six months of observation was in the IO group.
4. Discussion
In our cohort, the incidence of thromboembolic events was 0.7%. To make up for the lack of a comparative arm, we employed historical data to estimate the expected incidence of thromboembolic events in a cohort of an equivalent size, assuming that they did not receive thromboprophylaxis [16]. In this indirect comparison, we estimated a lower risk for venous thromboembolism (VTE) in patients treated with Tinzaparin (RR = 0.13, p = 0.048); however, the 95% CI was wide (0.02; 0.99), suggesting imprecision. Furthermore, we should acknowledge the inherent limitations associated with this methodology. Firstly, the historical cohort comprised a global population, potentially differing from our single-institution study group. Secondly, the historical data were applied to patients treated during the years 2011–2012. Since then, significant changes have occurred in standard treatment practices, with the advent of immunotherapy being the most notable advancement. Lastly, there have been observed temporal changes in thrombotic risk among cancer patients, with an increase in VTE incidence among cancer patients in recent years, and the introduction of immunotherapy may partly account for this increase. These changes in VTE incidence and current treatment practice may have led to an underestimation of the relative efficacy of Tinzaparin thromboprophylaxis. The limitations mentioned above emphasize the need for cautious interpretation and larger-scale research.
Regarding the safety of Tinzaparin thromboprophylaxis, the rate of hemorrhagic events was 7.1%, which was on the lower end of what has been reported in similar research with a focus on Tinzaparin use in cancer patients for the long-term treatment of VTE [3]. Anti-Xa levels did not differ significantly between patients with hemorrhagic events, which is in accordance with previously published research and endorses how monitoring in patients receiving LMWH prophylaxis is not necessary. Furthermore, they did not differ at 10 days and 3 months, suggesting that the daily intermediate dose of Tinzaparin suffices to achieve stable concentrations. This aligns with recent data suggesting that the use of intermediate doses of Tinzaparin may be more effective than prophylactic doses without safety concerns [36].
The anti-Xa levels at day 10 and 3 months in the IO group were numerically lower than the non-IO treated group [Median (IQR); 0.46 (0.30, 0.59) vs. 0.52 (0.33, 0.65) at day 10 and 0.37 (0.22, 0.60) vs. 0.52 (0.32, 0.75) at 3 months]. Although the study was not adequately powered to detect statistical differences in the rate of events between these subgroups, given the probable association of higher anti-Xa levels with a decrease in the likelihood of thrombotic events and the increase in the likelihood of hemorrhagic events [23], it is plausible that a difference in anti-Xa levels might have contributed to the numerically higher rate of thrombotic events and lower rate of hemorrhagic events in the IO group. More research is needed to elucidate how ICIs affect the anti-Xa levels to assess whether dose adjustment is needed in IO-treated patients.
Finally, although prophylactic treatment required daily injections for 6 months, patient compliance was high, with only three (2.1%) patients opting for early thromboprophylaxis discontinuation.
It is important to acknowledge the limitations of this study. Firstly, since it is non-comparative, it cannot lead to conclusions about the superiority or inferiority of prophylactic Tinzaparin treatment compared to no treatment. Furthermore, the small number of events limits the ability for hypothesis testing and exploratory biomarker analysis. Also, as this was a non-interventional study, anti-Xa could not be measured by our laboratory as we could not perform any interventions on the patients. This is why the measurement was performed externally, and the patients presented an examination on their next scheduled treatment. Finally, the single-center design limits the generalizability of results.
5. Conclusions
During the six months of observation, the frequency of VTE was lower than expected based on historical data had prophylaxis not been administered. Furthermore, the frequency of bleeding events was low, with only one major event. This indicates that thromboprophylaxis with intermediate doses of Tinzaparin in patients with lung cancer may be an effective and safe strategy. However, larger-scale comparative research is needed to establish the efficacy, safety, and impact on the survival of outpatient Tinzaparin thromboprophylaxis for patients with cancer receiving systemic treatment, especially for immunotherapy-treated patients where data are more immature.
Author Contributions
Conceptualization, M.K. and D.T.S.; methodology, M.K. and E.D.; validation D.T.S. and M.K.; formal analysis, I.A.V. and M.E.L.; investigation, M.K., F.S. and M.G.; resources, N.S. and E.D.; data curation, M.K.; writing—original draft preparation, M.E.L.; writing—review and editing, M.K.; visualization, M.E.L.; supervision, N.S.; project administration, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Sotiria Hospital of Chest Diseases; protocol code 4711; date of approval 12 February 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, A.Y.Y. Management of thrombosis in cancer: Primary prevention and secondary prophylaxis. Br. J. Haematol. 2005, 128, 291–302. [Google Scholar] [CrossRef]
- Levitan, N.; Dowlati, A.; Remick, S.C.; Tahsildar, H.I.; Sivinski, L.D.; Beyth, R.; Rimm, A.A. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine 1999, 78, 285–291. [Google Scholar] [CrossRef]
- Trousseau, A. Phlegmasia alba dolens. Clin. Medicule Hotel-Dieu Paris 1865, 3, 94. [Google Scholar]
- Prandoni, P.; Lensing, A.W.A.; Piccioli, A.; Bernardi, E.; Simioni, P.; Girolami, B.; Marchiori, A.; Sabbion, P.; Prins, M.H.; Noventa, F.; et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002, 100, 3484–3488. [Google Scholar] [CrossRef] [PubMed]
- Gervaso, L.; Dave, H.; Khorana, A.A. Venous and Arterial Thromboembolism in Patients With Cancer: JACC: CardioOncology State-of-the-Art Review. JACC Cardio Oncol. 2021, 3, 173–190. [Google Scholar] [CrossRef]
- Abdol Razak, N.B.; Jones, G.; Bhandari, M.; Berndt, M.C.; Metharom, P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef]
- Khorana, A.A.; Dalal, M.R.; Lin, J.; Connolly, G.C. Health care costs associated with venous thromboembolism in selected high-risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. Clin. Outcomes Res. CEOR 2013, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Shinagare, A.B.; Guo, M.; Hatabu, H.; Krajewski, K.M.; Andriole, K.; Abbeele, A.D.V.D.; DiPiro, P.J.; Nishino, M. Incidence of pulmonary embolism in oncologic outpatients at a tertiary cancer center. Cancer 2011, 117, 3860–3866. [Google Scholar] [CrossRef]
- Sallah, S.; Wan, J.Y.; Nguyen, N.P. Venous thrombosis in patients with solid tumors: Determination of frequency and characteristics. Thromb. Haemost. 2002, 87, 575–579. [Google Scholar] [PubMed]
- Noble, S.; Pasi, J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br. J. Cancer 2010, 102 (Suppl. S1), S2–S9. [Google Scholar] [CrossRef]
- Sack, G.H.; Levin, J.; Bell, W.R. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: Clinical, pathophysiologic, and therapeutic features. Medicine 1977, 56, 1–37. [Google Scholar] [CrossRef]
- Tagalakis, V.; Levi, D.; Agulnik, J.S.; Cohen, V.; Kasymjanova, G.; Small, D. High risk of deep vein thrombosis in patients with non-small cell lung cancer: A cohort study of 493 patients. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2007, 2, 729–734. [Google Scholar] [CrossRef]
- Blom, J.W.; Osanto, S.; Rosendaal, F.R. The risk of a venous thrombotic event in lung cancer patients: Higher risk for adenocarcinoma than squamous cell carcinoma. J. Thromb. Haemost. JTH 2004, 2, 1760–1765. [Google Scholar] [CrossRef]
- Connolly, G.C.; Dalal, M.; Lin, J.; Khorana, A.A. Incidence and predictors of venous thromboembolism (VTE) among ambulatory patients with lung cancer. Lung Cancer Amst. Neth. 2012, 78, 253–258. [Google Scholar] [CrossRef]
- Charpidou, A.; Gerotziafas, G.; Popat, S.; Araujo, A.; Scherpereel, A.; Kopp, H.G.; Bironzo, P.; Massard, G.; Jiménez, D.; Falanga, A.; et al. Lung Cancer Related Thrombosis (LCART): Focus on Immune Checkpoint Blockade. Cancers 2024, 16, 450. [Google Scholar] [CrossRef] [PubMed]
- Syrigos, K.; Grapsa, D.; Sangare, R.; Evmorfiadis, I.; Larsen, A.K.; Van Dreden, P.; Boura, P.; Charpidou, A.; Kotteas, E.; Sergentanis, T.N.; et al. Prospective Assessment of Clinical Risk Factors and Biomarkers of Hypercoagulability for the Identification of Patients with Lung Adenocarcinoma at Risk for Cancer-Associated Thrombosis: The Observational ROADMAP-CAT Study. Oncologist 2018, 23, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Mukai, M.; Oka, T. Mechanism and management of cancer-associated thrombosis. J. Cardiol. 2018, 72, 89–93. [Google Scholar] [CrossRef]
- Huang, H.; Korn, J.R.; Mallick, R.; Friedman, M.; Nichols, C.; Menzin, J. Incidence of venous thromboembolism among chemotherapy-treated patients with lung cancer and its association with mortality: A retrospective database study. J. Thromb. Thrombolysis 2012, 34, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Poniewierski, M.S.; Culakova, E.; Lyman, G.H.; Khorana, A.A.; Pabinger, I.; Agnelli, G.; Liebman, H.A.; Vicaut, E.; Meyer, G.; et al. Predictors of Venous Thromboembolism and Early Mortality in Lung Cancer: Results from a Global Prospective Study (CANTARISK). Oncologist 2018, 23, 247–255. [Google Scholar] [CrossRef]
- Roopkumar, J.; Swaidani, S.; Kim, A.S.; Thapa, B.; Gervaso, L.; Hobbs, B.P.; Wei, W.; Alban, T.J.; Funchain, P.; Kundu, S.; et al. Increased Incidence of Venous Thromboembolism with Cancer Immunotherapy. Med 2021, 2, 423–434. [Google Scholar] [CrossRef]
- Roopkumar, J.; Kim, A.S.; Bicky, T.; Hobbs, B.P.; Khorana, A.A. Venous Thromboembolism in Cancer Patients Receiving Immunotherapy. Blood 2018, 132, 2510. [Google Scholar] [CrossRef]
- Goel, A.; Khorana, A.; Kartika, T.; Gowda, S.; Tao, D.L.; Thawani, R.; Shatzel, J.J. Assessing the risk of thromboembolism in cancer patients receiving immunotherapy. Eur. J. Haematol. 2022, 108, 271–277. [Google Scholar] [CrossRef]
- Bick, R.L. Proficient and cost-effective approaches for the prevention and treatment of venous thrombosis and thromboembolism. Drugs 2000, 60, 575–595. [Google Scholar] [CrossRef]
- Dalteparin Thromboprophylaxis in Cancer Patients at High Risk for Venous Thromboembolism: A Randomized Trial—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28139259/ (accessed on 10 January 2024).
- Collins, R.; Scrimgeour, A.; Yusuf, S.; Peto, R. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N. Engl. J. Med. 1988, 318, 1162–1173. [Google Scholar] [CrossRef]
- Halkin, H.; Goldberg, J.; Modan, M.; Modan, B. Reduction of mortality in general medical in-patients by low-dose heparin prophylaxis. Ann. Intern. Med. 1982, 96, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Sevitt, S.; Gallagher, N.G. Prevention of venous thrombosis and pulmonary embolism in injured patients. A trial of anticoagulant prophylaxis with phenindione in middle-aged and elderly patients with fractured necks of femur. Lancet Lond. Engl. 1959, 2, 981–989. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Porreca, E.; Candeloro, M.; Valeriani, E.; Di Nisio, M. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst. Rev. 2020, 2020, CD008500. [Google Scholar] [CrossRef]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Gates, L.E.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Scotté, F.; Elalamy, I.; Mayeur, D.; Meyer, G. Physicians’ decision about long-term thromboprophylaxis in cancer outpatients: CAT AXIS, a case vignette study on clinical practice in France. Support Care Cancer Off. J. Multinatl. Assoc. Support Care Cancer 2018, 26, 2049–2056. [Google Scholar] [CrossRef]
- Maraveyas, A.; Waters, J.; Roy, R.; Fyfe, D.; Propper, D.; Lofts, F.; Sgouros, J.; Gardiner, E.; Wedgwood, K.; Ettelaie, C.; et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur. J. Cancer Oxf. Engl. 1990 2012, 48, 1283–1292. [Google Scholar] [CrossRef]
- Pelzer, U.; Opitz, B.; Deutschinoff, G.; Stauch, M.; Reitzig, P.C.; Hahnfeld, S.; Müller, L.; Grunewald, M.; Stieler, J.M.; Sinn, M.; et al. Efficacy of Prophylactic Low-Molecular Weight Heparin for Ambulatory Patients with Advanced Pancreatic Cancer: Outcomes From the CONKO-004 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Klerk, C.P.; Smorenburg, S.M.; Otten, H.-M.; Lensing, A.W.; Prins, M.H.; Piovella, F.; Prandoni, P.; Bos, M.M.; Richel, D.J.; van Tienhoven, G.; et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- van Doormaal, F.F.; Di Nisio, M.; Otten, H.M.; Richel, D.J.; Prins, M.; Buller, H.R. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, A.; Ardavanis, A.; Papandreou, C.; Koumakis, G.; Papatsimpas, G.; Papakotoulas, P.; Tsoukalas, N.; Andreadis, C.; Samelis, G.; Papakostas, P.; et al. Prophylaxis of cancer-associated venous thromboembolism with low-molecular-weight heparin-tinzaparin: Real world evidence. Oncol. Lett. 2022, 23, 115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).