Impact of Indoor Radon Exposure on Lung Cancer Incidence in Slovenia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
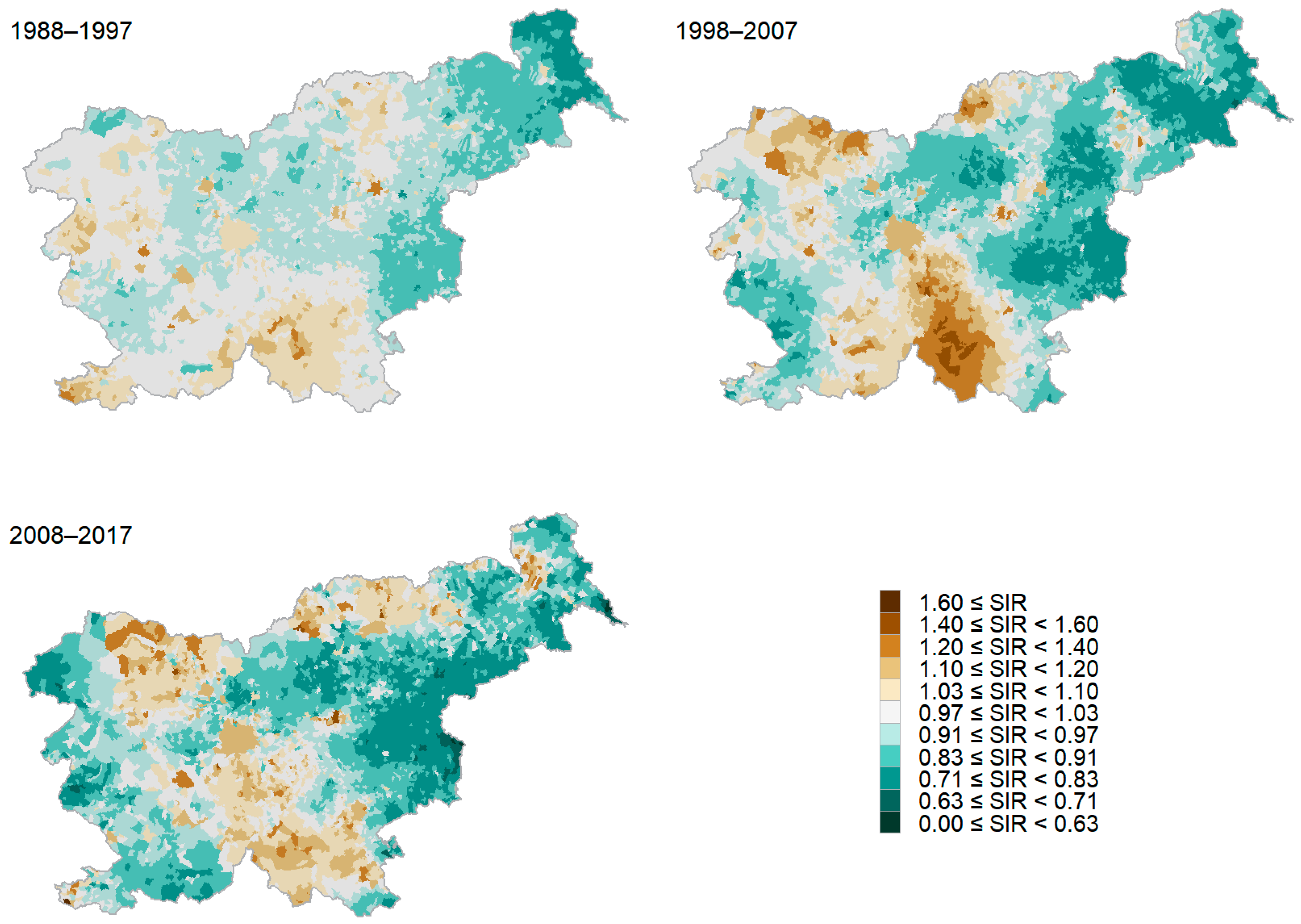
3.1. The Burden of Lung Cancer in Slovenia
3.2. The Burden of Lung Cancer in Slovenia and Indoor Radon Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 191–280. [Google Scholar] [CrossRef] [PubMed]
- Zadnik, V.; Žagar, T. SLORA: Slovenia and Cancer. Epidemiology and Cancer Registry. Istitute of Oncology Ljubljana. Available online: www.slora.si/en (accessed on 15 September 2023).
- World Health Organization. WHO Handbook on Indoor Radon: A Public Health Perspective, 1st ed.; Zeeb, H., Shannoun, F., Eds.; WHO Press: Genova, Switzerland, 2009; pp. 3–20, 41–56. [Google Scholar]
- Freedman, N.D.; Leitzmann, M.F.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Cigarette smoking and subsequent risk of lung cancer in men and women: Analysis of a prospective cohort study. Lancet Oncol. 2008, 9, 649–656. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Protection against Exposure Due to Radon Indoors and Gamma Radiation from Construction Materials: Methods of Prevention and Mitigation, 1st ed.; IAEA: Vienna, Austria, 2021. [Google Scholar]
- Grzywa-Celinska, A.; Krusinnski, A.; Mazur, J.; Szewczyk, K.; Kozak, K. Radon—The Element of Risk. The Impact of Radon Exposure on Human Health. Toxics 2020, 8, 120. [Google Scholar] [PubMed]
- Riudavets, M.; Garcia de Herreros, M.; Besse, B.; Mezquita, L. Radon and Lung Cancer: Current Trends and Future Perspectives. Cancers 2022, 14, 3142. [Google Scholar] [CrossRef] [PubMed]
- Eidy, M.; Tishkowski, K. Radon Toxicity. In Treasure Island; StatPearls: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Darby, S.; Hill, D.; Auvinen, A.; Barros-Dios, J.M.; Baysson, H.; Bochicchio, F.; Deo, H.; Falk, R.; Forastiere, F.; Hakama, M.; et al. Radon in homes and risk of lung cancer: Collaborative analysis of individual data from 13 European case-control studies. BMJ 2004, 330, 223. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2004; Volume 43. [Google Scholar]
- Pompe-Kirn, V. Spremljanje incidence pljučnega raka v vplivnem območju Rudnika urana Žirovski vrh. Poročevalec Skupščine Repub. Slov. 1990, 16, 65–68. [Google Scholar]
- Pompe-Kirn, V.; Ferligoj, A. Študija Regionalne Problematike raka v SR Sloveniji z Ozirom na Možnosti Primarnih in Sekundarnih Preventivnih Ukrepov: (Uporaba Multivariantnih Metod Razvrščanja v Skupine); Institute of Oncology Ljubljana: Ljubljana, Slovenia, 1984. [Google Scholar]
- Vaupotič, J. Indoor Radon in Slovenia. Nucl. Technol. Radiat. Prot. 2003, 2, 36–42. [Google Scholar] [CrossRef]
- Jovanovič, P. Sistematično Pregledovanje Delovnega in Bivalnega Okolja 2020; Center za fizikalne meritve: Ljubljana, Slovenia, 2020. [Google Scholar]
- Vaupotič, J.; Gregorič, A. Priprava Radonskega Zemljevida Slovenije na Ravni Naselij; Jožef Stefan Institute: Ljubljana, Slovenia, 2017. [Google Scholar]
- Vaupotič, J.; Kobal, I.; Križman, M.J. Background outdoor radon levels in Slovenia. Nukleonika 2010, 55, 579–582. [Google Scholar]
- dos Santos Silva, I. Cancer Epidemiology: Principles and Methods; IARC: Lyon, France, 1999. [Google Scholar]
- Morrisa, M.; Wheeler-Martinb, K.; Simpsonc, D.; Mooneyd, S.J.; Gelmane, A.; DiMaggiob, C. Bayesian hierarchical spatial models: Implementing the Besag York Mollié model in stan. Spat. Spatiotemporal Epidemiol. 2019, 31, 100301. [Google Scholar] [CrossRef]
- Besag, J. Spatial Interaction and the Statistical Analysis of Lattice Systems. J. Roy. Stat. Soc. Ser. B 1974, 36, 192–236. [Google Scholar] [CrossRef]
- Žagar, T.; Korat, S.; Zadnik, V. CanMapTool for Mapping Cancer Incidence Data (Prepared as a Report to the WASABY Project); Cancer Registry of Republic of Slovenia: Ljubljana, Slovenia, 2021. [Google Scholar]
- Laaksonen, M. Population Attributable Fraction (PAF) in Epidemiologic Follow-Up Studies. Academic Dissertation, University of Tampere, Helsinki, Finland, 18 June 2010. [Google Scholar]
- Greenland, S. Concepts and pitfalls in measuring and interpreting attributable fractions, prevented fractions, and causation probabilities. Ann. Epidemiol. 2015, 25, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Menzler, S.; Piller, G.; Gruson, M.; Schaffrath Rosario, A.; Wichmann, H.E.; Kreienbrock, L. Population attributable fraction for lung cancer due to residential radon in Switzerland and Germany. Health Phys. 2008, 95, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.; Hill, D.; Doll, R. Radon: A likely carcinogen at all exposures. Ann. Oncol. 2001, 12, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Radon. Available online: https://www.who.int/news-room/fact-sheets/detail/radon-and-health (accessed on 15 May 2023).
- Robertson, A.; Allen, J.; Laney, R.; Curnow, A. The Cellular and Molecular Carcinogenic Effects of Radon Exposure: A Review. Int. J. Mol. Sci. 2013, 14, 14024–14063. [Google Scholar] [CrossRef] [PubMed]
- Busby, C. Ionizing radiation and cancer: The failure of the risk model. Cancer Treat. Res. Commun. 2022, 31, 100565. [Google Scholar] [CrossRef]
- Pavia, M.; Bianco, A.; Pileggi, C.; Angelillo, I.F. Meta-analysis of residential exposure to radon gas and lung cancer. Bull. World Health Organ. 2013, 81, 732–738. [Google Scholar]
- Lubin, J.H.; Boice, J.D. Lung Cancer Risk from Residential Radon: Meta-analysis of Eight Epidemiologic Studies. J. Natl. Cancer Inst. 1991, 89, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, M.; Heinrich, J.; Wölke, G.; Schaffrath Rosario, A.; Gerken, M.; Wellmann, J.; Keller, G.; Kreienbrock, L.; Wichmann, H.E. Residential Radon and Risk of Lung Cancer in Eastern Germany. Epidemiology 2003, 14, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Žagar, T.; Zadnik, V.; Primic-Žakelj, M. Local standardized incidence ratio estimates and comparison with other mapping methods for small geographical areas using Slovenian breast cancer data. J. Appl. Stat. 2011, 38, 2751–2761. [Google Scholar] [CrossRef]
- Koprivnikar, H.; Zupanič, T. Tobaku Pripisljiva Umrljivost v Sloveniji 1997–2019; Nacionalni inštitut za javno zdravje: Ljubljana, Slovenija, 2021. [Google Scholar]
- Reddy, R.; Conde, C.; Peterson, C.; Nugent, K. Residential radon exposure and cancer. Oncol. Rev. 2022, 16, 558. [Google Scholar] [CrossRef]
- Puskin, J.S. Smoking as a confounder in ecologic correlations of cancer mortality rates with average county radon levels. Health Phys. 2003, 84, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, H.E. Krebsprävention durch Radon? Hier irrt der Physiker. Dtsch. Arztebl. 1999, 96, C-2213. [Google Scholar]
- Gray, A.; Read, S.; McGale, P.; Darby, S. Lung cancer deaths from indoor radon and the cost effectiveness and potential of policies to reduce them. BMJ 2009, 338, a3110. [Google Scholar] [CrossRef] [PubMed]
- Lokar, K.; Žagar, T.; Zadnik, V. Estimation of the Ecological Fallacy in the Geographical Analysis of the Association of Socio-Economic Deprivation and Cancer Incidence. Int. J. Environ. Res. Public. Health 2019, 16, 296. [Google Scholar] [CrossRef] [PubMed]
- The World Factbook 2016–17. Available online: https://www.cia.gov/the-world-factbook/field/gini-index-coefficient-distribution-of-family-income/country-comparison/ (accessed on 3 January 2019).

| 10-Year Period | Incidence | Average Annual Incidence | Average Annual Population | % of Population Living in Areas with a Moderate or High Risk of Indoor Radon Exposure |
|---|---|---|---|---|
| 1978–1987 | 7201 | 720 | 1,891,864 | 15.1% * |
| 1988–1997 | 9426 | 943 | 1,965,986 | 15.1% |
| 1998–2007 | 11,324 | 1132 | 1,978,897 | 15.5% |
| 2008–2017 | 13,304 | 1330 | 2,052,376 | 15.9% |
| 1978–2017 | 41,255 | 1031 | 1,972,281 | 15.4% |
| Settlements | Municipalities | ||||
|---|---|---|---|---|---|
| Incidence | PAF | Attributable Incidence | PAF | Attributable Incidence | |
| 2008−2017 | 13,304 | 4.3% | 572 | 6.5% | 865 |
| 1998−2007 | 11,324 | 5.6% | 634 | 6.2% | 702 |
| 1988−1997 | 9426 | 1.2% | 113 | 2.8% | 264 |
| 1978−1987 | 7201 | / * | / * | 6.2% | 447 |
| Males | Females | Males and Females | ||
|---|---|---|---|---|
| 2008–2017 | ||||
| Risk of radon exposure | low | 0.99 [0.97–1.01] | 1.00 [0.97–1.03] | 0.99 [0.97–1.01] |
| moderate or high | 1.05 [1.00–1.13] | 1.01 [0.94–1.09] | 1.05 [1.00–1.09] | |
| 1998–2007 | ||||
| Risk of radon exposure | low | 0.98 [0.96–1.01] | 1.00 [0.96–1.04] | 0.99 [0.97–1.01] |
| moderate or high | 1.08 [1.03–1.14] | 0.98 [0.89–1.09] | 1.07 [1.02–1.12] | |
| 1988–1997 | ||||
| Risk of radon exposure | low | 0.98 [0.96–1.01] | 1.01 [0.95–1.06] | 0.99 [0.97–1.01] |
| moderate or high | 1.09 [1.03–1.15] | 0.97 [0.85–1.10] | 1.07 [1.02–1.12] | |
| 1978–1987 | ||||
| Risk of radon exposure | low | 0.98 [0.95–1.01] | 1.00 [0.94–1.07] | 0.98 [0.96–1.01] |
| moderate or high | 1.07 [1.01–1.13] | 0.99 [0.86–1.12] | 1.05 [1.00–1.11] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birk, M.; Žagar, T.; Tomšič, S.; Lokar, K.; Mihor, A.; Bric, N.; Mlakar, M.; Zadnik, V. Impact of Indoor Radon Exposure on Lung Cancer Incidence in Slovenia. Cancers 2024, 16, 1445. https://doi.org/10.3390/cancers16081445
Birk M, Žagar T, Tomšič S, Lokar K, Mihor A, Bric N, Mlakar M, Zadnik V. Impact of Indoor Radon Exposure on Lung Cancer Incidence in Slovenia. Cancers. 2024; 16(8):1445. https://doi.org/10.3390/cancers16081445
Chicago/Turabian StyleBirk, Mojca, Tina Žagar, Sonja Tomšič, Katarina Lokar, Ana Mihor, Nika Bric, Miran Mlakar, and Vesna Zadnik. 2024. "Impact of Indoor Radon Exposure on Lung Cancer Incidence in Slovenia" Cancers 16, no. 8: 1445. https://doi.org/10.3390/cancers16081445






