Application of PET/MRI in Gynecologic Malignancies
Simple Summary
Abstract
1. Introduction
2. PET/MRI
2.1. Oncologic PET Tracers and Patient Preparation
2.2. Quantitative Imaging Biomarkers
2.3. Advantages of PET/MRI
| Biomarker | Description | Clinical Interpretation |
|---|---|---|
| PET Scan | ||
| SUV (Standardized Uptake Value) | Measure the uptake of the radioactive tracer in a specific region of interest (ROI) to assess the activity and metabolism of tissues SUV = Tracer concentration in ROI (kBq/mL)/Injected dose per body weight (kBq/g) | Inversely correlated with ADC [63,64,65,66,67,68,69,70,71,72,73] A higher SUV indicates higher metabolic activity in the ROI |
| SUVmean (Mean Standardized Uptake Value) | Calculating the average tracer uptake in the selected ROI A comprehensive assessment of the overall tracer uptake within the ROI, useful for areas with varying tracer uptake (e.g., tumors) | Monitoring treatment response: a decrease in SUV from baseline indicates metabolic response to treatment [37] Prognosis: Overall survival is better in metabolic responders compared with metabolic non-responders [37] |
| SUVmax (Maximum Standardized Uptake Value) | Indicating the highest level of tracer uptake within a defined ROI Notable inverse correlation with ADCmin [55,74] | Diagnosis and staging: distinguish malignant (higher SUVmax) and benign adnexal lesions [75] Treatment planning: Higher SUVmax values may indicate a more aggressive tumor [68] Monitoring treatment response: changes in SUVmax and especially the percent change value may have the potential to predict response to chemotherapy or chemoradiotherapy [36,38,76] Prognosis: changes in SUVmax predict the patient outcomes, disease recurrence, PFS [36,76,77] |
| MTV (metabolic tumor volume) | The metabolically active volume of the tumor (i.e., the portion of the tumor with a high SUV) | Staging: baseline MTV is a predictor of tumor characteristics such as MI and cervical stromal invasion, and lymph node metastasis; it is higher in cases with lymph node metastasis compared with those without such a metastasis Treatment planning: helps in determining the appropriate dosage and target volume for radiation treatment, ensuring that the radiation is delivered precisely to the areas containing tumor cells [78] Monitoring treatment response: the percentage of post-treatment changes in MTV is associated with the overall tumor response [35] Prognosis: the baseline MTV and the percentage of changes in MTV are predictive factors for OS, and PFS, recurrence [35,77,79] |
| TLG (Total Lesion Glycolysis) | provides a more comprehensive measure of tumor activity than SUVmax or SUVmean alone TLG = SUVmean × MTV | Staging: baseline TLG is a predictor of tumor characteristics, such as MI and cervical stromal invasion, and lymph node metastasis [77,80] Treatment planning: useful for radiation therapy planning by comprehensive assessment of the tumor burden [78] Monitoring treatment: change in TLG after treatment may have the potential to predict response to treatment [39,79] Prognosis: baseline TLG is prognostic factor of OS and PSF [39,77,78,79,81] |
| DWI | ||
| ADC (Apparent Diffusion Coefficient) | Provides valuable information about tissue microstructure and cellular integrity [63,64,65,66,67,68,69,70,71,72,73] Inversely correlated with SUV | Helpful in differentiating between benign and malignant lesions, assessing tumor aggressiveness, and monitoring treatment response |
| ADCmin (Minimum Apparent Diffusion Coefficient) | Represents the region with the most restricted diffusion or the highest tumor cellularity Notable inverse correlation with SUVmax [67,74] | Diagnosis and staging: malignant tumors and regions with high cellular density tend to have lower ADC values, while benign or necrotic regions have higher ADC values Monitoring treatment: a decrease in ADCmin values after therapy can indicate a positive treatment response [55] Prognosis: independent predictor of OS [55] |
3. Applications to Gynecologic Cancers
3.1. Cervical Cancer
3.2. Endometrial Cancer
3.3. Ovarian Cancer
3.4. Vaginal and Vulvar Cancers
4. PET/MR Considerations
4.1. Challenges
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Antoch, G.; Vogt, F.M.; Freudenberg, L.S.; Nazaradeh, F.; Goehde, S.C.; Barkhausen, J.; Dahmen, G.; Bockisch, A.; Debatin, J.F.; Ruehm, S.G. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA 2003, 290, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.; Townsend, D.W.; Brun, T.; Kinahan, P.E.; Charron, M.; Roddy, R.; Jerin, J.; Young, J.; Byars, L.; Nutt, R. A combined PET/CT scanner for clinical oncology. J. Nucl. Med. 2000, 41, 1369–1379. [Google Scholar] [PubMed]
- Bar-Shalom, R.; Yefremov, N.; Guralnik, L.; Gaitini, D.; Frenkel, A.; Kuten, A.; Altman, H.; Keidar, Z.; Israel, O. Clinical performance of PET/CT in evaluation of cancer: Additional value for diagnostic imaging and patient management. J. Nucl. Med. 2003, 44, 1200–1209. [Google Scholar] [PubMed]
- Delso, G.; Furst, S.; Jakoby, B.; Ladebeck, R.; Ganter, C.; Nekolla, S.G.; Schwaiger, M.; Ziegler, S.I. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J. Nucl. Med. 2011, 52, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Beiderwellen, K.; Grueneisen, J.; Ruhlmann, V.; Buderath, P.; Aktas, B.; Heusch, P.; Kraff, O.; Forsting, M.; Lauenstein, T.C.; Umutlu, L. [18F]FDG PET/MRI vs. PET/CT for whole-body staging in patients with recurrent malignancies of the female pelvis: Initial results. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Kirchner, J.; Sawicki, L.M.; Suntharalingam, S.; Grueneisen, J.; Ruhlmann, V.; Aktas, B.; Deuschl, C.; Herrmann, K.; Antoch, G.; Forsting, M.; et al. Whole-body staging of female patients with recurrent pelvic malignancies: Ultra-fast 18F-FDG PET/MRI compared to 18F-FDG PET/CT and CT. PLoS ONE 2017, 12, e0172553. [Google Scholar] [CrossRef]
- Sawicki, L.M.; Kirchner, J.; Grueneisen, J.; Ruhlmann, V.; Aktas, B.; Schaarschmidt, B.M.; Forsting, M.; Herrmann, K.; Antoch, G.; Umutlu, L. Comparison of 18F-FDG PET/MRI and MRI alone for whole-body staging and potential impact on therapeutic management of women with suspected recurrent pelvic cancer: A follow-up study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Makihara, N.; Ebina, Y.; Yamada, H.; Takahashi, S.; Sugimura, K. Value of fusion of PET and MRI in the detection of intra-pelvic recurrence of gynecological tumor: Comparison with 18F-FDG contrast-enhanced PET/CT and pelvic MRI. Ann. Nucl. Med. 2014, 28, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Grueneisen, J.; Schaarschmidt, B.M.; Heubner, M.; Suntharalingam, S.; Milk, I.; Kinner, S.; Heubner, A.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; et al. Implementation of FAST-PET/MRI for whole-body staging of female patients with recurrent pelvic malignancies: A comparison to PET/CT. Eur. J. Radiol. 2015, 84, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Grueneisen, J.; Beiderwellen, K.; Heusch, P.; Gratz, M.; Schulze-Hagen, A.; Heubner, M.; Kinner, S.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; et al. Simultaneous positron emission tomography/magnetic resonance imaging for whole-body staging in patients with recurrent gynecological malignancies of the pelvis: A comparison to whole-body magnetic resonance imaging alone. Investig. Radiol. 2014, 49, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, D.; Dufour, P.; Tordjeman-Rizzi, N.; Prolongeau, J.F.; Depret-Moser, S.; Monnier, J.C. Immunological aspects of ovarian function: Role of the cytokines. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 63, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.A.; Stein, J.A.; Pelc, N.J. How CT happened: The early development of medical computed tomography. J. Med. Imaging 2021, 8, 052110. [Google Scholar] [CrossRef] [PubMed]
- Kabasawa, H. MR Imaging in the 21st Century: Technical Innovation over the First Two Decades. Magn. Reson. Med. Sci. 2022, 21, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Townsend, D. History and future technical innovation in positron emission tomography. J. Med. Imaging 2017, 4, 011013. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, C.; Faria, S.; Devine, C.; Patnana, M.; Sagebiel, T.; Iyer, R.B.; Bhosale, P.R. [18F]-2-Fluoro-2-Deoxy-D-glucose-PET Assessment of Cervical Cancer. PET Clin. 2018, 13, 165–177. [Google Scholar] [CrossRef]
- Mahajan, A.; Sable, N.P.; Popat, P.B.; Bhargava, P.; Gangadhar, K.; Thakur, M.H.; Arya, S. Magnetic Resonance Imaging of Gynecological Malignancies: Role in Personalized Management. Semin. Ultrasound CT MR 2017, 38, 231–268. [Google Scholar] [CrossRef]
- Sala, E.; Rockall, A.G.; Freeman, S.J.; Mitchell, D.G.; Reinhold, C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: What the radiologist needs to know. Radiology 2013, 266, 717–740. [Google Scholar] [CrossRef] [PubMed]
- Kusmirek, J.; Robbins, J.; Allen, H.; Barroilhet, L.; Anderson, B.; Sadowski, E.A. PET/CT and MRI in the imaging assessment of cervical cancer. Abdom. Imaging 2015, 40, 2486–2511. [Google Scholar] [CrossRef] [PubMed]
- Monteil, J.; Maubon, A.; Leobon, S.; Roux, S.; Marin, B.; Renaudie, J.; Genet, D.; Fermeaux, V.; Aubard, Y.; Tubiana-Mathieu, N. Lymph node assessment with 18F-FDG-PET and MRI in uterine cervical cancer. Anticancer Res. 2011, 31, 3865–3871. [Google Scholar] [PubMed]
- Otero-García, M.M.; Mesa-Álvarez, A.; Nikolic, O.; Blanco-Lobato, P.; Basta-Nikolic, M.; de Llano-Ortega, R.M.; Paredes-Velázquez, L.; Nikolic, N.; Szewczyk-Bieda, M. Role of MRI in staging and follow-up of endometrial and cervical cancer: Pitfalls and mimickers. Insights Imaging 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, S.; Inubushi, M.; Okada, H. Physiological 18F-FDG uptake in the ovaries and uterus of healthy female volunteers. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.H.; Cheng, W.Y.; Cheng, X.; Dang, Y.H. Characteristics of physiological uptake of uterus and ovaries on 18F-fluorodeoxyglucose positron emission tomography. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2007, 29, 124–129. [Google Scholar] [PubMed]
- Lerman, H.; Metser, U.; Grisaru, D.; Fishman, A.; Lievshitz, G.; Even-Sapir, E. Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: Assessment by PET/CT. J. Nucl. Med. 2004, 45, 266–271. [Google Scholar] [PubMed]
- Reinhardt, M.J.; Ehritt-Braun, C.; Vogelgesang, D.; Ihling, C.; Högerle, S.; Mix, M.; Moser, E.; Krause, T.M. Metastatic lymph nodes in patients with cervical cancer: Detection with MR imaging and FDG PET. Radiology 2001, 218, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.C.; Sagebiel, T.; Balachandran, A.; Devine, C.; Lal, C.; Bhosale, P.R. Imaging in endometrial carcinoma. Indian J. Radiol. Imaging 2015, 25, 137–147. [Google Scholar] [CrossRef]
- Soper, J.T. Radiographic imaging in gynecologic oncology. Clin. Obstet. Gynecol. 2001, 44, 485–494. [Google Scholar] [CrossRef]
- Expert Panel on GYN and OB Imaging; Reinhold, C.; Ueno, Y.; Akin, E.A.; Bhosale, P.R.; Dudiak, K.M.; Jhingran, A.; Kang, S.K.; Kilcoyne, A.; Lakhman, Y.; et al. ACR Appropriateness Criteria(R) Pretreatment Evaluation and Follow-Up of Endometrial Cancer. J. Am. Coll. Radiol. 2020, 17, S472–S486. [Google Scholar] [CrossRef] [PubMed]
- Engbersen, M.P.; Van Driel, W.; Lambregts, D.; Lahaye, M. The role of CT, PET-CT, and MRI in ovarian cancer. Br. J. Radiol. 2021, 94, 20210117. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Liang, K.; Xiao, Z.; Yang, Q.; Yang, S. A meta-analysis on the diagnostic value of diffusion-weighted imaging on ovarian cancer. J. Buon 2019, 24, 2333–2340. [Google Scholar] [PubMed]
- Timmerman, D.; Planchamp, F.; Bourne, T.; Landolfo, C.; du Bois, A.; Chiva, L.; Cibula, D.; Concin, N.; Fischerova, D.; Froyman, W.; et al. ESGO/ISUOG/IOTA/ESGE Consensus Statement on pre-operative diagnosis of ovarian tumors. Int. J. Gynecol. Cancer 2021, 31, 961–982. [Google Scholar] [CrossRef] [PubMed]
- Vallius, T.; Hynninen, J.; Kemppainen, J.; Alves, V.; Auranen, K.; Matomaki, J.; Oksa, S.; Virtanen, J.; Grenman, S.; Auranen, A.; et al. 18F-FDG-PET/CT based total metabolic tumor volume change during neoadjuvant chemotherapy predicts outcome in advanced epithelial ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Kim, H.S.; Lee, J.Y.; Kang, W.J.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. Early Assessment of Response to Neoadjuvant Chemotherapy with 18F-FDG-PET/CT in Patients with Advanced-Stage Ovarian Cancer. Cancer Res. Treat. 2020, 52, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Avril, N.; Sassen, S.; Schmalfeldt, B.; Naehrig, J.; Rutke, S.; Weber, W.A.; Werner, M.; Graeff, H.; Schwaiger, M.; Kuhn, W. Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. J. Clin. Oncol. 2005, 23, 7445–7453. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Yamamoto, Y.; Kanenishi, K.; Ohno, M.; Hata, T.; Kushida, Y.; Haba, R.; Ohkawa, M. Monitoring the neoadjuvant therapy response in gynecological cancer patients using FDG PET. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Cho, A.; Lee, J.H.; Yun, M.; Lee, J.D.; Kim, Y.T.; Kang, W.J. The role of metabolic tumor volume and total lesion glycolysis on (1)(8)F-FDG PET/CT in the prognosis of epithelial ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1898–1906. [Google Scholar] [CrossRef]
- Martoni, A.A.; Fanti, S.; Zamagni, C.; Rosati, M.; De Iaco, P.; D’Errico Grigioni, A.; Castellucci, P.; Quercia, S.; Musto, A.; Ricci Maccarini, L.; et al. [18F]FDG-PET/CT monitoring early identifies advanced ovarian cancer patients who will benefit from prolonged neo-adjuvant chemotherapy. Q. J. Nucl. Med. Mol. Imaging 2011, 55, 81–90. [Google Scholar]
- Sohaib, S.A.; Reznek, R.H. MR imaging in ovarian cancer. Cancer Imaging 2007, 7, S119–S129. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.; Tsui, B.Q.; Bahrami, S.; Masamed, R.; Memarzadeh, S.; Raman, S.S.; Patel, M.K. Gynecologic tumor board: A radiologist’s guide to vulvar and vaginal malignancies. Abdom. Radiol. 2021, 46, 5669–5686. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.E.; Dehdashti, F.; Gibb, R.K.; Mutch, D.G.; Rader, J.S.; Siegel, B.A.; Herzog, T.J. Prospective evaluation of positron emission tomography for the detection of groin node metastases from vulvar cancer. Gynecol. Oncol. 2002, 85, 179–184. [Google Scholar] [CrossRef]
- Veit-Haibach, P.; Kuhn, F.P.; Wiesinger, F.; Delso, G.; von Schulthess, G. PET-MR imaging using a tri-modality PET/CT-MR system with a dedicated shuttle in clinical routine. Magma 2013, 26, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.; Friedman, K.P.; Shah, S.N.; Chandarana, H. Practical guide for implementing hybrid PET/MR clinical service: Lessons learned from our experience. Abdom. Imaging 2015, 40, 1366–1373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vandenberghe, S.; Moskal, P.; Karp, J.S. State of the art in total body PET. EJNMMI Phys. 2020, 7, 35. [Google Scholar] [CrossRef]
- Musafargani, S.; Ghosh, K.K.; Mishra, S.; Mahalakshmi, P.; Padmanabhan, P.; Gulyas, B. PET/MRI: A frontier in era of complementary hybrid imaging. Eur. J. Hybrid. Imaging 2018, 2, 12. [Google Scholar] [CrossRef]
- Sadowski, E.A.; Lees, B.; McMillian, A.B.; Kusmirek, J.E.; Cho, S.Y.; Barroilhet, L.M. Distribution of prostate specific membrane antigen (PSMA) on PET-MRI in patients with and without ovarian cancer. Abdom. Radiol. 2023, 48, 3643–3652. [Google Scholar] [CrossRef]
- Xi, Y.; Sun, L.; Che, X.; Huang, X.; Liu, H.; Wang, Q.; Meng, H.; Miao, Y.; Qu, Q.; Hai, W.; et al. A comparative study of [(68)Ga]Ga-FAPI-04 PET/MR and [18F]FDG PET/CT in the diagnostic accuracy and resectability prediction of ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2885–2898. [Google Scholar] [CrossRef]
- Zhang, X.; Song, W.; Qin, C.; Song, Y.; Liu, F.; Hu, F.; Lan, X. Uterine Uptake of 68Ga-FAPI-04 in Uterine Pathology and Physiology. Clin. Nucl. Med. 2022, 47, 7–13. [Google Scholar] [CrossRef]
- Ahangari, S.; Littrup Andersen, F.; Liv Hansen, N.; Jakobi Nøttrup, T.; Berthelsen, A.K.; Folsted Kallehauge, J.; Richter Vogelius, I.; Kjaer, A.; Espe Hansen, A.; Fischer, B.M. Multi-parametric PET/MRI for enhanced tumor characterization of patients with cervical cancer. Eur. J. Hybrid. Imaging 2022, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Narva, S.I.; Seppänen, M.P.; Raiko, J.R.H.; Forsback, S.J.; Orte, K.J.; Virtanen, J.M.; Hynninen, J.; Hietanen, S. Imaging of Tumor Hypoxia With 18F-EF5 PET/MRI in Cervical Cancer. Clin. Nucl. Med. 2021, 46, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Maucherat, B.; Movassaghi, R.; Fleury, V.; Le Thiec, M.; Geffroy, D.; Labbe-Devilliers, C.; Rousseau, C. Is Glucagon Administration Compatible With FDG PET/MRI? Clin. Nucl. Med. 2022, 47, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Taylor, M.B.; Carrington, B.M.; Bonington, S.C.; Swindell, R. The value of hyoscine butylbromide in pelvic MRI. Clin. Radiol. 2007, 62, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.L.; Yen, R.F.; Chen, C.A.; Cheng, W.F.; Chen, B.B.; Chang, Y.H.; Cheng, M.F.; Shih, T.T. PET/MRI in Cervical Cancer: Associations Between Imaging Biomarkers and Tumor Stage, Disease Progression, and Overall Survival. J. Magn. Reson. Imaging 2021, 53, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.; Narva, S.; Rinta-Kiikka, I.; Hietanen, S.; Hynninen, J.; Virtanen, J. Diagnostic efficiency of whole-body 18F-FDG PET/MRI, MRI alone, and SUV and ADC values in staging of primary uterine cervical cancer. Cancer Imaging 2021, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.C.; Seghers, V.; Paldino, M.J.; Dodge, C.; Krishnamurthy, R.; Krishnamurthy, R.; Rohren, E.M. Assessment of Sequential PET/MRI in Comparison With PET/CT of Pediatric Lymphoma: A Prospective Study. AJR Am. J. Roentgenol. 2016, 206, 623–631. [Google Scholar] [CrossRef]
- Bian, L.H.; Wang, M.; Gong, J.; Liu, H.H.; Wang, N.; Wen, N.; Fan, W.S.; Xu, B.X.; Wang, M.Y.; Ye, M.X.; et al. Comparison of integrated PET/MRI with PET/CT in evaluation of endometrial cancer: A retrospective analysis of 81 cases. PeerJ 2019, 7, e7081. [Google Scholar] [CrossRef] [PubMed]
- Donati, O.F.; Hany, T.F.; Reiner, C.S.; von Schulthess, G.K.; Marincek, B.; Seifert, B.; Weishaupt, D. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: Comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J. Nucl. Med. 2010, 51, 692–699. [Google Scholar] [CrossRef]
- Beiderwellen, K.; Gomez, B.; Buchbender, C.; Hartung, V.; Poeppel, T.D.; Nensa, F.; Kuehl, H.; Bockisch, A.; Lauenstein, T.C. Depiction and characterization of liver lesions in whole body [(1)(8)F]-FDG PET/MRI. Eur. J. Radiol. 2013, 82, e669–e675. [Google Scholar] [CrossRef]
- Gardner, A.B.; Charo, L.M.; Mann, A.K.; Kapp, D.S.; Eskander, R.N.; Chan, J.K. Ovarian, uterine, and cervical cancer patients with distant metastases at diagnosis: Most common locations and outcomes. Clin. Exp. Metastasis 2020, 37, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Niekel, M.C.; Bipat, S.; Stoker, J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: A meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 2010, 257, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Floberg, J.M.; Fowler, K.J.; Fuser, D.; DeWees, T.A.; Dehdashti, F.; Siegel, B.A.; Wahl, R.L.; Schwarz, J.K.; Grigsby, P.W. Spatial relationship of 2-deoxy-2-[18F]-fluoro-D-glucose positron emission tomography and magnetic resonance diffusion imaging metrics in cervical cancer. EJNMMI Res. 2018, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Grueneisen, J.; Schaarschmidt, B.M.; Heubner, M.; Aktas, B.; Kinner, S.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; Umutlu, L. Integrated PET/MRI for whole-body staging of patients with primary cervical cancer: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Brandmaier, P.; Purz, S.; Bremicker, K.; Hockel, M.; Barthel, H.; Kluge, R.; Kahn, T.; Sabri, O.; Stumpp, P. Simultaneous [18F]FDG-PET/MRI: Correlation of Apparent Diffusion Coefficient (ADC) and Standardized Uptake Value (SUV) in Primary and Recurrent Cervical Cancer. PLoS ONE 2015, 10, e0141684. [Google Scholar] [CrossRef] [PubMed]
- Grueneisen, J.; Beiderwellen, K.; Heusch, P.; Buderath, P.; Aktas, B.; Gratz, M.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; Umutlu, L. Correlation of standardized uptake value and apparent diffusion coefficient in integrated whole-body PET/MRI of primary and recurrent cervical cancer. PLoS ONE 2014, 9, e96751. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.L.; Yen, R.F.; Chen, C.A.; Chen, B.B.; Wei, S.Y.; Chang, W.C.; Sheu, B.C.; Cheng, W.F.; Tseng, Y.H.; Chen, X.J.; et al. Standardized uptake value and apparent diffusion coefficient of endometrial cancer evaluated with integrated whole-body PET/MR: Correlation with pathological prognostic factors. J. Magn. Reson. Imaging 2015, 42, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Joja, I.; Fukushima, C.; Haruma, T.; Hayashi, C.; Kusumoto, T.; Seki, N.; Hongo, A.; Hiramatsu, Y. The preoperative SUVmax is superior to ADCmin of the primary tumour as a predictor of disease recurrence and survival in patients with endometrial cancer. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lee, E.Y.; Lai, V.; Chan, Q. Correlation between tissue metabolism and cellularity assessed by standardized uptake value and apparent diffusion coefficient in peritoneal metastasis. J. Magn. Reson. Imaging 2014, 40, 99–105. [Google Scholar] [CrossRef]
- Er, H.C.; Erden, A.; Kucuk, N.O.; Gecim, E. Correlation of minimum apparent diffusion coefficient with maximum standardized uptake on fluorodeoxyglucose PET-CT in patients with rectal adenocarcinoma. Diagn. Interv. Radiol. 2014, 20, 105–109. [Google Scholar] [CrossRef]
- Rakheja, R.; Chandarana, H.; DeMello, L.; Jackson, K.; Geppert, C.; Faul, D.; Glielmi, C.; Friedman, K.P. Correlation between standardized uptake value and apparent diffusion coefficient of neoplastic lesions evaluated with whole-body simultaneous hybrid PET/MRI. AJR Am. J. Roentgenol. 2013, 201, 1115–1119. [Google Scholar] [CrossRef]
- Olsen, J.R.; Esthappan, J.; DeWees, T.; Narra, V.R.; Dehdashti, F.; Siegel, B.A.; Schwarz, J.K.; Grigsby, P.W. Tumor volume and subvolume concordance between FDG-PET/CT and diffusion-weighted MRI for squamous cell carcinoma of the cervix. J. Magn. Reson. Imaging 2013, 37, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Schob, S.; Höhn, A.K.; Bremicker, K.; Exner, M.; Stumpp, P.; Purz, S. Parameters of simultaneous 18F-FDG-PET/MRI predict tumor stage and several histopathological features in uterine cervical cancer. Oncotarget 2017, 8, 28285–28296. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Wang, N.; Bian, L.; Wang, M.; Ye, M.; Wen, N.; Fu, M.; Fan, W.; Meng, Y. Cervical cancer evaluated with integrated 18F-FDG PET/MR. Oncol. Lett. 2019, 18, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.A.; Kubik-Huch, R.A.; Hauser, N.; Freiwald-Chilla, B.; von Schulthess, G.; Froehlich, J.M.; Veit-Haibach, P. PET/MRI and PET/CT in advanced gynaecological tumours: Initial experience and comparison. Eur. Radiol. 2015, 25, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E.A.; Siegel, B.A.; Dehdashti, F.; Grigsby, P.W. Pelvic lymph node F-18 fluorodeoxyglucose uptake as a prognostic biomarker in newly diagnosed patients with locally advanced cervical cancer. Cancer 2010, 116, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Yanarateş, A.; Budak, E.; Budak, A.; Inan, A.H.; Kanmaz, A.G.; Oral, A.; Yazici, B. Clinical value of metabolic PET parameters of primary vulvar carcinoma. Rev. Esp. Med. Nucl. Imagen Mol. (Engl. Ed.) 2021, 40, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Im, H.J.; Bradshaw, T.; Solaiyappan, M.; Cho, S.Y. Current Methods to Define Metabolic Tumor Volume in Positron Emission Tomography: Which One is Better? Nucl. Med. Mol. Imaging 2018, 52, 5–15. [Google Scholar] [CrossRef]
- Yoo, J.; Choi, J.Y.; Moon, S.H.; Bae, D.S.; Park, S.B.; Choe, Y.S.; Lee, K.H.; Kim, B.T. Prognostic significance of volume-based metabolic parameters in uterine cervical cancer determined using 18F-fluorodeoxyglucose positron emission tomography. Int. J. Gynecol. Cancer 2012, 22, 1226–1233. [Google Scholar] [CrossRef]
- Vural Topuz, Ö.; Aksu, A.; Erinç, S.R.; Tokgözoğlu, N.; Tamam, M. The Evaluation of Preoperative 18F-FDG PET/CT in Patients with Endometrial Cancer and the Correlation Between PET Parameters and Postoperative Pathology Results. Mol. Imaging Radionucl. Ther. 2022, 31, 16–22. [Google Scholar] [CrossRef]
- De Cuypere, M.; Lovinfosse, P.; Gennigens, C.; Hermesse, J.; Rovira, R.; Duch, J.; Goffin, F.; Hustinx, R.; Kridelka, F. Tumor total lesion glycolysis and number of positive pelvic lymph nodes on pretreatment positron emission tomography/computed tomography (PET/CT) predict survival in patients with locally advanced cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Mirpour, S.; Mhlanga, J.C.; Logeswaran, P.; Russo, G.; Mercier, G.; Subramaniam, R.M. The role of PET/CT in the management of cervical cancer. AJR Am. J. Roentgenol. 2013, 201, W192–W205. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Atri, M. 2018 FIGO Staging System for Uterine Cervical Cancer: Enter Cross-sectional Imaging. Radiology 2019, 292, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Deguchi, M.; Ebina, Y.; Yamada, H.; Takahashi, S.; Sugimura, K. Fusion of PET and MRI for staging of uterine cervical cancer: Comparison with contrast-enhanced 18F-FDG PET/CT and pelvic MRI. Clin. Imaging 2014, 38, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Choi, H.J.; Park, S.Y.; Lee, H.Y.; Seo, S.S.; Yoo, C.W.; Jung, D.C.; Kang, S.; Cho, K.S. Additional value of MR/PET fusion compared with PET/CT in the detection of lymph node metastases in cervical cancer patients. Eur. J. Cancer 2009, 45, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Grigsby, P.W. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced cervical cancer treated by radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.T.; Wale, D.J.; Viglianti, B.L.; Townsend, D.M.; Manganaro, M.S.; Gross, M.D.; Wong, K.K.; Rubello, D. The impact of infection and inflammation in oncologic 18F-FDG PET/CT imaging. Biomed. Pharmacother. 2019, 117, 109168. [Google Scholar] [CrossRef] [PubMed]
- White, N.S.; McDonald, C.R.; Farid, N.; Kuperman, J.M.; Kesari, S.; Dale, A.M. Improved conspicuity and delineation of high-grade primary and metastatic brain tumors using “restriction spectrum imaging”: Quantitative comparison with high B-value DWI and ADC. AJNR Am. J. Neuroradiol. 2013, 34, 958–964, S951. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, J.; Gao, J.; Guo, L.; Zhou, H.; Hu, Y.; Zhu, C.; Li, Q.; Ma, X. Diagnostic role of 18F-FDG PET/MRI in patients with gynecological malignancies of the pelvis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0175401. [Google Scholar] [CrossRef]
- McGettigan, M.; Zulfiqar, M.; Shetty, A.S. Imaging of Vaginal and Vulvar Malignancy. Radiol. Clin. N. Am. 2023, 61, 651–670. [Google Scholar] [CrossRef]
- Schwarz, J.K.; Siegel, B.A.; Dehdashti, F.; Grigsby, P.W. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA 2007, 298, 2289–2295. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Tsujikawa, T.; Yamada, S.; Chino, Y.; Shinagawa, A.; Kurokawa, T.; Okazawa, H.; Yoshida, Y. FDG-PET/MRI with high-resolution DWI characterises the distinct phenotypes of endometrial cancer. Clin. Radiol. 2020, 75, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Creutzberg, C.L.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.A.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with endometrial carcinoma. Virchows Arch. 2021, 478, 153–190. [Google Scholar] [CrossRef] [PubMed]
- Network, N.C.C. Uterine Neoplasms, Version 2.2023, NCCN Clincal Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 8 January 2024).
- Gadducci, A.; Cosio, S.; Fanucchi, A.; Cristofani, R.; Genazzani, A.R. An intensive follow-up does not change survival of patients with clinical stage I endometrial cancer. Anticancer Res. 2000, 20, 1977–1984. [Google Scholar]
- Bollineni, V.R.; Ytre-Hauge, S.; Bollineni-Balabay, O.; Salvesen, H.B.; Haldorsen, I.S. High Diagnostic Value of 18F-FDG PET/CT in Endometrial Cancer: Systematic Review and Meta-Analysis of the Literature. J. Nucl. Med. 2016, 57, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Terai, Y.; Yamamoto, K.; Yamada, T.; Ohmichi, M. The diagnostic accuracy of fluorodeoxyglucose-positron emission tomography/computed tomography and sentinel node biopsy in the prediction of pelvic lymph node metastasis in patients with endometrial cancer: A retrospective observational study. Medicine 2018, 97, e12522. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Tsujikawa, T.; Yamada, S.; Okazawa, H.; Yoshida, Y. Diagnostic value of 18F-FDG PET/MRI for staging in patients with endometrial cancer. Cancer Imaging 2020, 20, 75. [Google Scholar] [CrossRef]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Takahashi, S.; Ebina, Y.; Miyahara, Y.; Yamada, H.; Sugimura, K. Value of fusion of PET and MRI for staging of endometrial cancer: Comparison with ¹⁸F-FDG contrast-enhanced PET/CT and dynamic contrast-enhanced pelvic MRI. Eur. J. Radiol. 2013, 82, 1672–1676. [Google Scholar] [CrossRef]
- Ironi, G.; Mapelli, P.; Bergamini, A.; Fallanca, F.; Candotti, G.; Gnasso, C.; Taccagni, G.L.; Sant’Angelo, M.; Scifo, P.; Bezzi, C.; et al. Hybrid PET/MRI in Staging Endometrial Cancer: Diagnostic and Predictive Value in a Prospective Cohort. Clin. Nucl. Med. 2022, 47, e221–e229. [Google Scholar] [CrossRef]
- Jónsdóttir, B.; Marcickiewicz, J.; Borgfeldt, C.; Bjurberg, M.; Dahm-Kähler, P.; Flöter-Rådestad, A.; Hellman, K.; Holmberg, E.; Kjølhede, P.; Rosenberg, P.; et al. Preoperative and intraoperative assessment of myometrial invasion in endometrial cancer-A Swedish Gynecologic Cancer Group (SweGCG) study. Acta Obstet. Gynecol. Scand. 2021, 100, 1526–1533. [Google Scholar] [CrossRef]
- Gordon, B.A.; Flanagan, F.L.; Dehdashti, F. Whole-body positron emission tomography: Normal variations, pitfalls, and technical considerations. AJR Am. J. Roentgenol. 1997, 169, 1675–1680. [Google Scholar] [CrossRef]
- Shreve, P.D.; Anzai, Y.; Wahl, R.L. Pitfalls in oncologic diagnosis with FDG PET imaging: Physiologic and benign variants. Radiographics 1999, 19, 61–77; quiz 150–151. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Ide, M.; Takagi, S.; Shohtsu, A. Intrauterine accumulation of F-18 FDG during menstruation. Clin. Nucl. Med. 1997, 22, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Chander, S.; Meltzer, C.C.; McCook, B.M. Physiologic uterine uptake of FDG during menstruation demonstrated with serial combined positron emission tomography and computed tomography. Clin. Nucl. Med. 2002, 27, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Kunz, G.; Leyendecker, G. Uterine peristaltic activity during the menstrual cycle: Characterization, regulation, function and dysfunction. Reprod. Biomed. Online 2002, 4 (Suppl. S3), 5–9. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Togashi, K.; Yamaoka, T.; Fujiwara, T.; Ueda, H.; Koyama, T.; Kobayashi, H.; Kagimura, T.; Fujii, S.; Konishi, J. Uterine peristalsis shown on cine MR imaging using ultrafast sequence. J. Magn. Reson. Imaging 2003, 18, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Togashi, K.; Yamaoka, T.; Nakai, A.; Kido, A.; Nishio, S.; Yamamoto, T.; Kitagaki, H.; Fujii, S. Kinematics of the uterus: Cine mode MR imaging. Radiographics 2004, 24, e19. [Google Scholar] [CrossRef]
- Fiaschetti, V.; Calabria, F.; Crusco, S.; Meschini, A.; Nucera, F.; Schillaci, O.; Simonetti, G. MR-PET fusion imaging in evaluating adnexal lesions: A preliminary study. Radiol. Med. 2011, 116, 1288–1302. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Yamada, S.; Kubota, K.; Ishiwata, K.; Tamahashi, N.; Ido, T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: High accumulation in macrophages and granulation tissues studied by microautoradiography. J. Nucl. Med. 1992, 33, 1972–1980. [Google Scholar]
- Expert Panel on GYN and OB Imaging; Kilcoyne, A.; Gottumukkala, R.V.; Kang, S.K.; Akin, E.A.; Hauck, C.; Hindman, N.M.; Huang, C.; Khanna, N.; Paspulati, R.; et al. ACR Appropriateness Criteria(R) Staging and Follow-up of Primary Vaginal Cancer. J. Am. Coll. Radiol. 2021, 18, S442–S455. [Google Scholar] [CrossRef]
- Preti, M.; Bucchi, L.; Micheletti, L.; Privitera, S.; Corazza, M.; Cosma, S.; Gallio, N.; Borghi, A.; Bevilacqua, F.; Benedetto, C. Four-decade trends in lymph node status of patients with vulvar squamous cell carcinoma in northern Italy. Sci. Rep. 2021, 11, 5661. [Google Scholar] [CrossRef] [PubMed]
- Rufini, V.; Garganese, G.; Ieria, F.P.; Pasciuto, T.; Fragomeni, S.M.; Gui, B.; Florit, A.; Inzani, F.; Zannoni, G.F.; Scambia, G.; et al. Diagnostic performance of preoperative [18F]FDG-PET/CT for lymph node staging in vulvar cancer: A large single-centre study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3303–3314. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Berchiolli, R.; Kirienko, M.; Rossi, A.; Glaudemans, A.; Slart, R.; Erba, P.A. PET/MRI in Infection and Inflammation. Semin. Nucl. Med. 2018, 48, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Disselhorst, J.A.; Bezrukov, I.; Kolb, A.; Parl, C.; Pichler, B.J. Principles of PET/MR Imaging. J. Nucl. Med. 2014, 55, 2S–10S. [Google Scholar] [CrossRef] [PubMed]
- Rausch, I.; Rischka, L.; Ladefoged, C.N.; Furtner, J.; Fenchel, M.; Hahn, A.; Lanzenberger, R.; Mayerhoefer, M.E.; Traub-Weidinger, T.; Beyer, T. PET/MRI for Oncologic Brain Imaging: A Comparison of Standard MR-Based Attenuation Corrections with a Model-Based Approach for the Siemens mMR PET/MR System. J. Nucl. Med. 2017, 58, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Catana, C. Attenuation correction for human PET/MRI studies. Phys. Med. Biol. 2020, 65, 23TR02. [Google Scholar] [CrossRef] [PubMed]
- Wallstén, E.; Axelsson, J.; Jonsson, J.; Karlsson, C.T.; Nyholm, T.; Larsson, A. Improved PET/MRI attenuation correction in the pelvic region using a statistical decomposition method on T2-weighted images. EJNMMI Phys. 2020, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moller, A.; Souvatzoglou, M.; Delso, G.; Bundschuh, R.A.; Chefd’hotel, C.; Ziegler, S.I.; Navab, N.; Schwaiger, M.; Nekolla, S.G. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: Evaluation with PET/CT data. J. Nucl. Med. 2009, 50, 520–526. [Google Scholar] [CrossRef]
- Hofmann, M.; Bezrukov, I.; Mantlik, F.; Aschoff, P.; Steinke, F.; Beyer, T.; Pichler, B.J.; Scholkopf, B. MRI-based attenuation correction for whole-body PET/MRI: Quantitative evaluation of segmentation- and atlas-based methods. J. Nucl. Med. 2011, 52, 1392–1399. [Google Scholar] [CrossRef]
- Paulus, D.H.; Quick, H.H.; Geppert, C.; Fenchel, M.; Zhan, Y.; Hermosillo, G.; Faul, D.; Boada, F.; Friedman, K.P.; Koesters, T. Whole-Body PET/MR Imaging: Quantitative Evaluation of a Novel Model-Based MR Attenuation Correction Method Including Bone. J. Nucl. Med. 2015, 56, 1061–1066. [Google Scholar] [CrossRef]
- Bailey, D.L.; Pichler, B.J.; Guckel, B.; Antoch, G.; Barthel, H.; Bhujwalla, Z.M.; Biskup, S.; Biswal, S.; Bitzer, M.; Boellaard, R.; et al. Combined PET/MRI: Global Warming-Summary Report of the 6th International Workshop on PET/MRI, March 27–29, 2017, Tubingen, Germany. Mol. Imaging Biol. 2018, 20, 4–20. [Google Scholar] [CrossRef]
- Schramm, G.; Ladefoged, C.N. Metal artifact correction strategies in MRI-based attenuation correction in PET/MRI. BJR Open 2019, 1, 20190033. [Google Scholar] [CrossRef]
- Lindemann, M.E.; Gratz, M.; Blumhagen, J.O.; Jakoby, B.; Quick, H.H. MR-based truncation correction using an advanced HUGE method to improve attenuation correction in PET/MR imaging of obese patients. Med. Phys. 2022, 49, 865–877. [Google Scholar] [CrossRef]
- Blumhagen, J.O.; Braun, H.; Ladebeck, R.; Fenchel, M.; Faul, D.; Scheffler, K.; Quick, H.H. Field of view extension and truncation correction for MR-based human attenuation correction in simultaneous MR/PET imaging. Med. Phys. 2014, 41, 022303. [Google Scholar] [CrossRef]
- Paulus, D.H.; Quick, H.H. Hybrid Positron Emission Tomography/Magnetic Resonance Imaging: Challenges, Methods, and State of the Art of Hardware Component Attenuation Correction. Investig. Radiol. 2016, 51, 624–634. [Google Scholar] [CrossRef]
- Quick, H.H. Integrated PET/MR. J. Magn. Reson. Imaging 2014, 39, 243–258. [Google Scholar] [CrossRef]
- Veit-Haibach, P.; Ahlström, H.; Boellaard, R.; Delgado Bolton, R.C.; Hesse, S.; Hope, T.; Huellner, M.W.; Iagaru, A.; Johnson, G.B.; Kjaer, A.; et al. International EANM-SNMMI-ISMRM consensus recommendation for PET/MRI in oncology. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3513–3537. [Google Scholar] [CrossRef]
- Eiber, M.; Martinez-Moller, A.; Souvatzoglou, M.; Holzapfel, K.; Pickhard, A.; Loffelbein, D.; Santi, I.; Rummeny, E.J.; Ziegler, S.; Schwaiger, M.; et al. Value of a Dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1691–1701. [Google Scholar] [CrossRef]
- Miles, K.A.; Voo, S.A.; Groves, A.M. Additional Clinical Value for PET/MRI in Oncology: Moving beyond Simple Diagnosis. J. Nucl. Med. 2018, 59, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.E.; Christensen, N.L.; Heil, B.G. Use of a magnetic field to increase the spatial resolution of positron emission tomography. Med. Phys. 1994, 21, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Herzog, H.; Weirich, C.; Tellmann, L.; Kaffanke, J.; Caldeira, L.; Kops, E.R.; Qaim, S.M.; Coenen, H.H.; Iida, H. Effects of magnetic fields of up to 9.4 T on resolution and contrast of PET images as measured with an MR-BrainPET. PLoS ONE 2014, 9, e95250. [Google Scholar] [CrossRef]
- Polycarpou, I.; Soultanidis, G.; Tsoumpas, C. Synergistic motion compensation strategies for positron emission tomography when acquired simultaneously with magnetic resonance imaging. Philos. Trans. A Math. Phys. Eng. Sci. 2021, 379, 20200207. [Google Scholar] [CrossRef]
- Manber, R.; Thielemans, K.; Hutton, B.F.; Barnes, A.; Ourselin, S.; Arridge, S.; O’Meara, C.; Wan, S.; Atkinson, D. Practical PET Respiratory Motion Correction in Clinical PET/MR. J. Nucl. Med. 2015, 56, 890–896. [Google Scholar] [CrossRef]
- Kolbitsch, C.; Neji, R.; Fenchel, M.; Schuh, A.; Mallia, A.; Marsden, P.; Schaeffter, T. Joint cardiac and respiratory motion estimation for motion-corrected cardiac PET-MR. Phys. Med. Biol. 2018, 64, 015007. [Google Scholar] [CrossRef]
- Küstner, T.; Schwartz, M.; Martirosian, P.; Gatidis, S.; Seith, F.; Gilliam, C.; Blu, T.; Fayad, H.; Visvikis, D.; Schick, F.; et al. MR-based respiratory and cardiac motion correction for PET imaging. Med. Image Anal. 2017, 42, 129–144. [Google Scholar] [CrossRef]
- Munoz, C.; Ellis, S.; Nekolla, S.G.; Kunze, K.P.; Vitadello, T.; Neji, R.; Botnar, R.M.; Schnabel, J.A.; Reader, A.J.; Prieto, C. MR-guided motion-corrected PET image reconstruction for cardiac PET-MR. J. Nucl. Med. 2021, 62, 1768–1774. [Google Scholar] [CrossRef]
- Fayad, H.; Schmidt, H.; Wuerslin, C.; Visvikis, D. Reconstruction-Incorporated Respiratory Motion Correction in Clinical Simultaneous PET/MR Imaging for Oncology Applications. J. Nucl. Med. 2015, 56, 884–889. [Google Scholar] [CrossRef]
- Munoz, C.; Neji, R.; Cruz, G.; Mallia, A.; Jeljeli, S.; Reader, A.J.; Botnar, R.M.; Prieto, C. Motion-corrected simultaneous cardiac positron emission tomography and coronary MR angiography with high acquisition efficiency. Magn. Reson. Med. 2018, 79, 339–350. [Google Scholar] [CrossRef]
- Wurslin, C.; Schmidt, H.; Martirosian, P.; Brendle, C.; Boss, A.; Schwenzer, N.F.; Stegger, L. Respiratory motion correction in oncologic PET using T1-weighted MR imaging on a simultaneous whole-body PET/MR system. J. Nucl. Med. 2013, 54, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Robson, P.M.; Trivieri, M.; Karakatsanis, N.A.; Padilla, M.; Abgral, R.; Dweck, M.R.; Kovacic, J.C.; Fayad, Z.A. Correction of respiratory and cardiac motion in cardiac PET/MR using MR-based motion modeling. Phys. Med. Biol. 2018, 63, 225011. [Google Scholar] [CrossRef] [PubMed]
- Petibon, Y.; Huang, C.; Ouyang, J.; Reese, T.G.; Li, Q.; Syrkina, A.; Chen, Y.-L.; El Fakhri, G. Relative role of motion and PSF compensation in whole-body oncologic PET-MR imaging. Med. Phys. 2014, 41, 042503. [Google Scholar] [CrossRef]
- Yang, J. AI applications for quantitative and qualitative PET in PET/MR-where do we stand? Eur. Radiol. 2023, 33, 7530–7531. [Google Scholar] [CrossRef]
- Balaji, V.; Song, T.A.; Malekzadeh, M.; Heidari, P.; Dutta, J. Artificial Intelligence for PET and SPECT Image Enhancement. J. Nucl. Med. 2024, 65, 4–12. [Google Scholar] [CrossRef]
- Zaharchuk, G.; Davidzon, G. Artificial Intelligence for Optimization and Interpretation of PET/CT and PET/MR Images. Semin. Nucl. Med. 2021, 51, 134–142. [Google Scholar] [CrossRef]
- Liu, J.; Malekzadeh, M.; Mirian, N.; Song, T.A.; Liu, C.; Dutta, J. Artificial Intelligence-Based Image Enhancement in PET Imaging: Noise Reduction and Resolution Enhancement. PET Clin. 2021, 16, 553–576. [Google Scholar] [CrossRef]


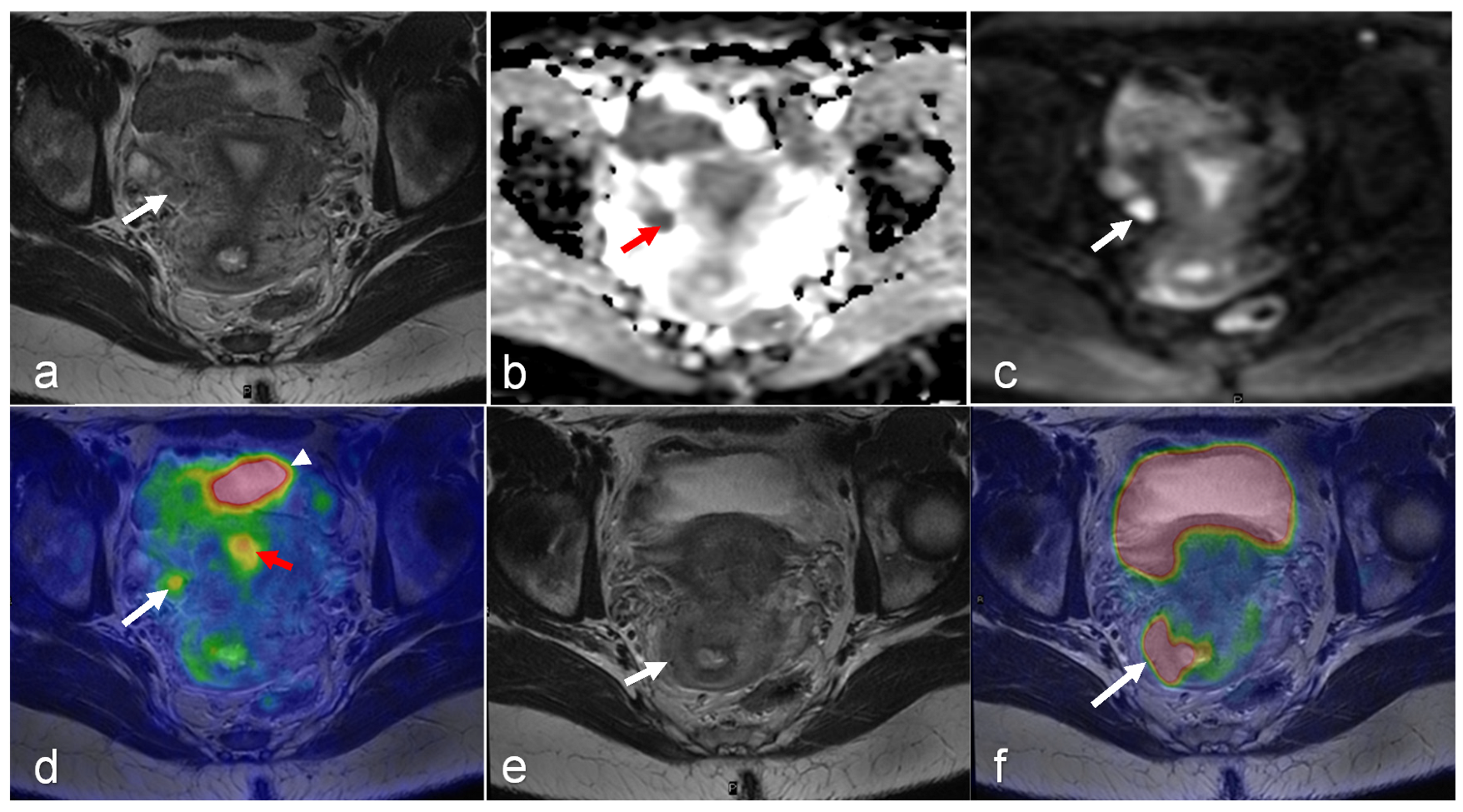

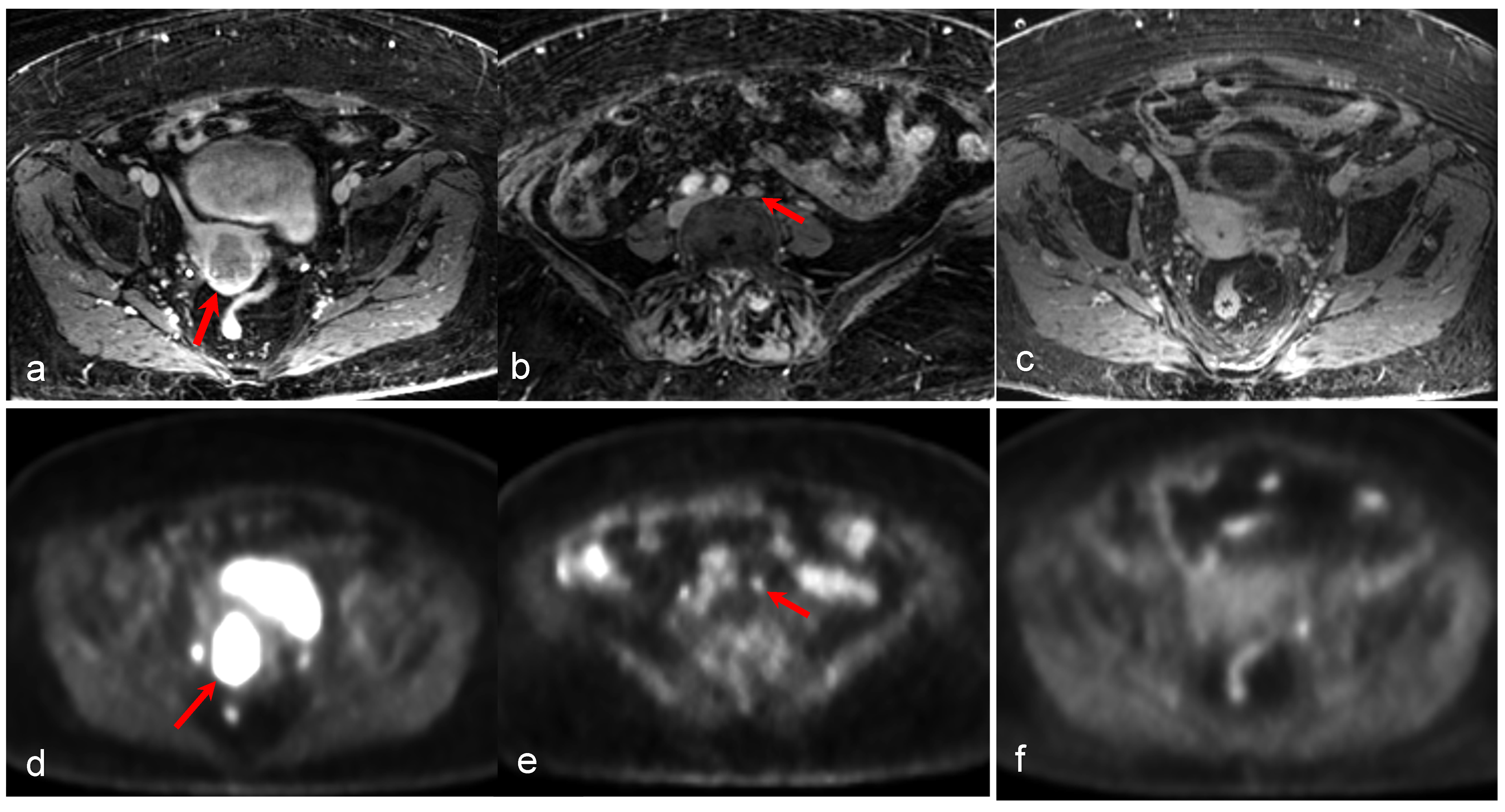

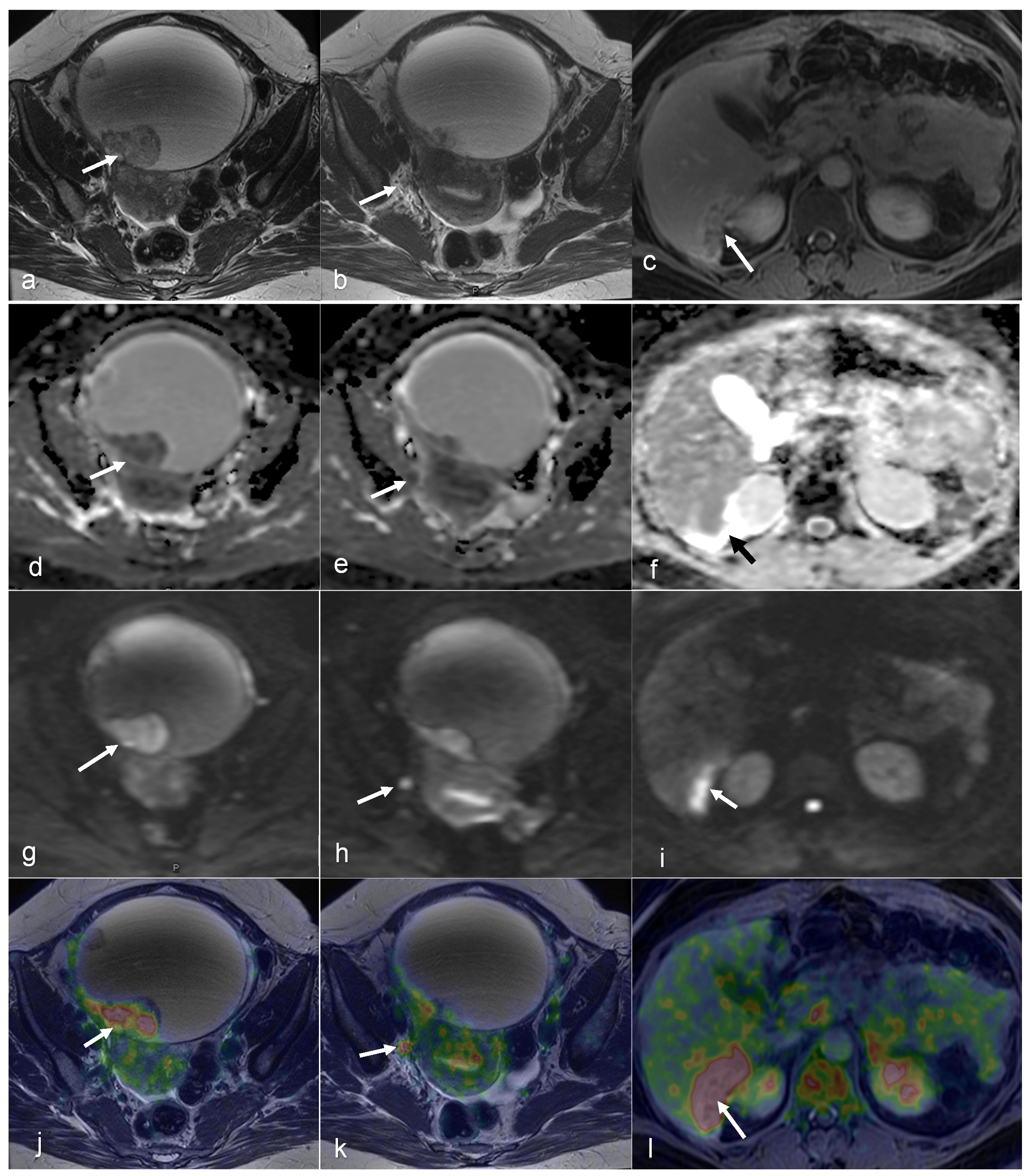
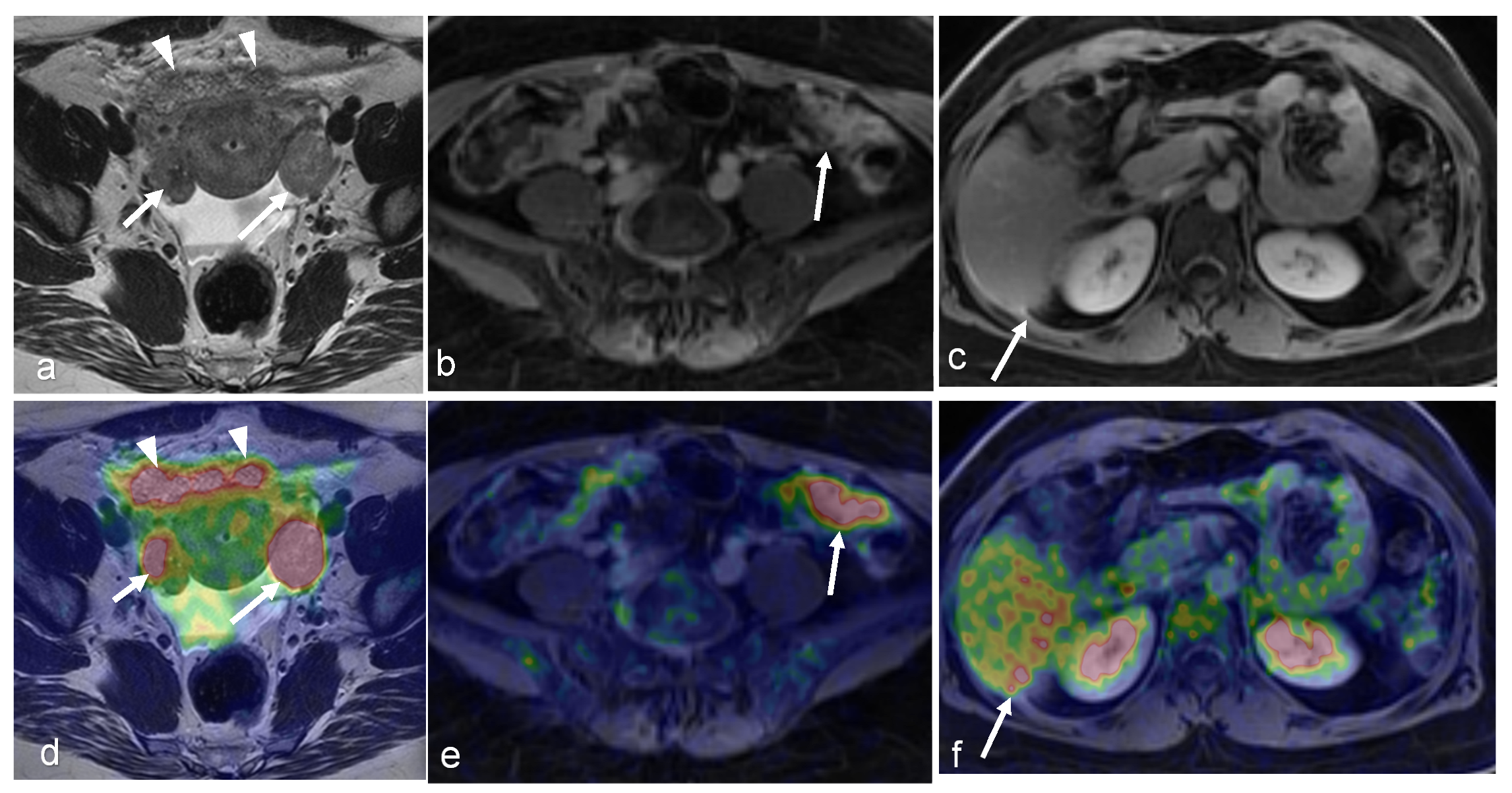

| Malignancy | CT | MRI | PET/CT | PET/MRI | |
|---|---|---|---|---|---|
| Cervical | Benefit(s) | Evaluation of regional lymph nodes, distal metastases, hydronephrosis [19] | High diagnostic accuracy for local staging and assessing primary tumor and pelvic lymph node metastasis, defining advanced disease Helpful in treatment planning, monitoring treatment response, and post-treatment surveillance to detect local recurrence [20,21,22,23] | Detection of primary tumor, assessment of tumor volume, lymph node, and distant metastases [23] Assessment of treatment response and tumor recurrence [19] | Excellent performance in the evaluation of stage, regional and distant nodal involvement, and metastatic disease Simultaneous soft tissue and metabolic assessment [19] |
| Pitfall(s) | Limited in assessment of cervical tumor invasion, parametrial invasion, and pelvic sidewall involvement Limited in evaluation of micro-metastatic disease in lymph nodes < 1 cm Cannot reliability detect reactive nodes versus metastatic nodes > 1 cm [19] | Limited in evaluation of micro-metastatic disease in lymph nodes [19] Cannot reliability detect reactive nodes versus metastatic nodes. Limited in differentiating between tumor recurrence and post-treatment inflammatory changes [24] | The physiological FDG uptake in the premenopausal endometrium adjacent to cervical cancer can be mistaken for endometrial tumor invasion [25,26,27] False positive FDG uptake during benign conditions (e.g., infection) and post-therapy changes can mimic malignancy [19] | Less sensitive for detection of pulmonary nodules compared with PET/CT [13] | |
| Endometrial | Benefit(s) | Routinely used in evaluation of patients to identify metastatic disease within the lungs and lymph nodes [24] | Accurate modality for local staging, tumor delineation, assessment of myometrial invasion and pelvic lymphadenopathy, defining advanced disease [27,28] Helpful in planning treatment, monitoring treatment response, and post-treatment surveillance [29] | Diagnostic tool for staging and surveillance of cancer Detecting positive pelvic and/or para-aortic lymphadenopathy and distant metastasis [29] | Staging of nodal and distant metastases during local staging Simultaneous soft tissue and metabolic assessment. [25] |
| Pitfall(s) | Limited in evaluation for local staging Difficult to assess the vaginal vault [24] Overestimating the central tumor volume due to the presence of tissue reaction and edema near the tumor–tissue interface [30] | Overestimating the tumor volume due to the presence of post treatment edema of the tumor [30] | Routine use is not recommended in preoperative staging in early stage disease as 45% of endometrial cancers are not FDG-avid [31] | Less sensitive for detection of pulmonary nodules compared with PET/CT | |
| Ovarian | Benefit(s) | Evaluates for metastatic disease and possible lymph node involvement. Useful for determining response to chemotherapy, can predict diaphragm and omental involvement [32] | Outperforms CT and PET/CT for detecting ovarian cancer [33] Helps differentiate between benign, malignant, and borderline masses by DCE-MRI and DWI [34] Useful for treatment planning in advanced ovarian cancer [32] | Evaluating possible metastatic extraperitoneal spread of the disease and metastatic lymph nodes [32] Detects recurrent disease [32] predicts treatment response after NAC [35,36,37,38,39,40] | Hybrid molecular and anatomic imaging provides high soft tissue contrast with lower radiation dose Detects lymph node metastases with high accuracy [32] |
| Pitfall(s) | Limited soft tissue evaluation and differentiation. Limited in evaluating local extent of disease | Limited sensitivity in detecting small peritoneal implants [41] | Lack of reliable differentiation between borderline and benign tumors according to ESGO/ISUOG/IOTA/ESGE Consensus Statement on pre-operative diagnosis of ovarian tumors. No clear cut-off value for maximum standardized uptake value for differentiation between benign and malignant ovarian tumors [32] Not recommended for primary detection of ovarian cancer [32] The physiologic FDG uptake in pre-menopausal ovaries can be mistaken with malignancy [25,26,27] | Less sensitive for detection of pulmonary nodules compared with PET/CT | |
| Vaginal/Vulvar | Benefit(s) | Helpful in determining disease extent and nodal/metastatic involvement [42] Useful for identifying distant metastases, including pulmonary and bony metastases in vulvar cancer [42] | The modality of choice for locoregional assessment, detection of primary and metastatic cancer, and treatment response The most sensitive modality for detecting pelvic lymph node involvement [42] | Useful for radiation therapy planning [43], assessing response to neoadjuvant chemotherapy and guide patient management Evaluation of nodal and distant metastatic involvement in staging of recurrent vaginal cancer [42] | Helpful in for detecting vulvar cancer recurrences and distant metastases [42] |
| Pitfall(s) | Difficulty in assessing lymph node involvement, especially in small or micro-metastatic nodes Inability to determine local tumor staging due to low soft tissue contrast [42] | Limited value in detecting lymph node metastases ≤ 5 mm and necrotic lymph nodes False-positive (e.g., inflammatory lymph node) [23] |
| Design | Description | Advantages | Disadvantages |
|---|---|---|---|
Tri-modality [44,45] | Separate PET/CT and MR systems Shared transport bed, compatible with both scanners, improves anatomical correspondence between PET and MRI | Relatively low cost Access to image data from three modalities (including CT-based attenuation correction of PET data) Flexibility to use the systems independently | Risk of misalignment due to patient motion or bowel motility Longer examination time compared to sequential and integrated systems |
Sequential [46] | PET and MR bores positioned in a serial fashion MR images acquired immediately after PET, within the same examination | Reduced dose of ionizing radiation by not conducting a CT Reduced total examination time Lower likelihood of image misalignment compared to tri-modality systems due to the shorter time between scans | Special shielding and additional space requirements due to systems proximity Lack of conventional CT-based attenuation correction Potential impact on the quality of reconstructed PET images due to MR-based attenuation correction |
Integrated [47] | Simultaneously acquiring PET and MR images by incorporating PET into the MR bore | True simultaneous PET and MRI acquisitions Improved image alignment between modalities Reduced dose of ionizing radiation Reduced total examination time | High purchase price compared to sequential systems Lack of CT-based attenuation correction The mutual negative impact of PET and MR hardware |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimi, S.; Lundström, E.; Batasin, S.J.; Hedlund, E.; Stålberg, K.; Ehman, E.C.; Sheth, V.R.; Iranpour, N.; Loubrie, S.; Schlein, A.; et al. Application of PET/MRI in Gynecologic Malignancies. Cancers 2024, 16, 1478. https://doi.org/10.3390/cancers16081478
Ebrahimi S, Lundström E, Batasin SJ, Hedlund E, Stålberg K, Ehman EC, Sheth VR, Iranpour N, Loubrie S, Schlein A, et al. Application of PET/MRI in Gynecologic Malignancies. Cancers. 2024; 16(8):1478. https://doi.org/10.3390/cancers16081478
Chicago/Turabian StyleEbrahimi, Sheida, Elin Lundström, Summer J. Batasin, Elisabeth Hedlund, Karin Stålberg, Eric C. Ehman, Vipul R. Sheth, Negaur Iranpour, Stephane Loubrie, Alexandra Schlein, and et al. 2024. "Application of PET/MRI in Gynecologic Malignancies" Cancers 16, no. 8: 1478. https://doi.org/10.3390/cancers16081478
APA StyleEbrahimi, S., Lundström, E., Batasin, S. J., Hedlund, E., Stålberg, K., Ehman, E. C., Sheth, V. R., Iranpour, N., Loubrie, S., Schlein, A., & Rakow-Penner, R. (2024). Application of PET/MRI in Gynecologic Malignancies. Cancers, 16(8), 1478. https://doi.org/10.3390/cancers16081478





