Safety and Outcomes of Inferior Vena Cava Filter Placement in Oncology Patients: A Single-Centre Experience
Abstract
:Simple Summary
Abstract
1. Introduction
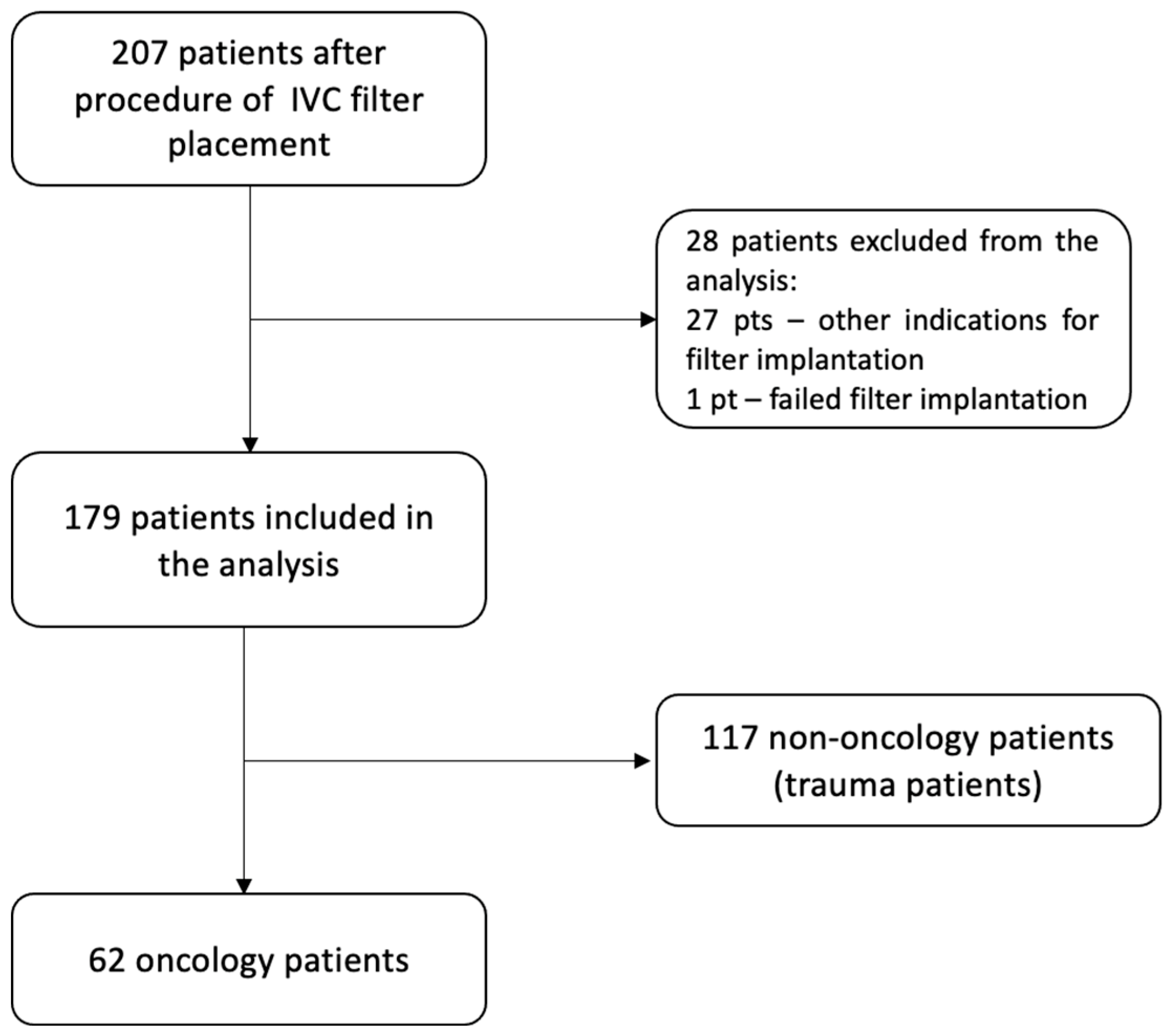
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Procedural Details
2.3. Statistics
3. Results
3.1. Patient Characteristics
3.2. Indications for Filter Placement
3.3. VTE Recurrence
3.4. Filter Retrieval and Complications
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heit, J.A.; O’Fallon, W.M.; Petterson, T.M.; Lohse, C.M.; Silverstein, M.D.; Mohr, D.N.; Melton, L.J., III. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: A population-based study. Arch. Intern. Med. 2002, 162, 1245–1248. [Google Scholar] [CrossRef]
- Blom, J.W.; Doggen, C.J.M.; Osanto, S.; Rosendaal, F.R. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005, 293, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, A.; Gąsecka, A.; Kurzyna, P.; Wrona, K.; Darocha, S.; Banaszkiewicz, M.; Zieliński, D.; Zajkowska, D.; Smyk, J.M.; Rymaszewska, D.; et al. Characteristics and Outcomes of Patients Consulted by a Multidisciplinary Pulmonary Embolism Response Team: 5-Year Experience. J. Clin. Med. 2022, 11, 3812. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, A.; Gąsecka, A.; Kurzyna, P.; Smyk, J.M.; Wasilewski, M.; Wolański, R.; Wrona, K.; Darocha, S.; Zieliński, D.; Grabowski, M.; et al. Cancer-associated thrombosis: Comparison of characteristics, treatment, and outcomes in oncologic and nononcologic patients followed by a pulmonary embolism response team. Pol. Arch. Intern. Med. 2023, 133, 16421. [Google Scholar] [CrossRef]
- Angel, L.F.; Tapson, V.; Galgon, R.E.; Restrepo, M.I.; Kaufman, J. Systematic review of the use of retrievable inferior vena cava filters. J. Vasc. Interv. Radiol. 2011, 22, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Kayali, F.; Olson, R.E. Twenty-one-year trends in the use of inferior vena cava filters. Arch. Intern. Med. 2004, 164, 1541–1545. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Decousus, H.; Leizorovicz, A.; Parent, F.; Page, Y.; Tardy, B.; Girard, P.; Laporte, S.; Faivre, R.; Charbonnier, B.; Barral, F.-G.; et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N. Engl. J. Med. 1998, 338, 409–416. [Google Scholar] [CrossRef]
- Decousus, H. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: The PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation 2005, 112, 416–422. [Google Scholar] [CrossRef]
- Schunn, C.; Schunn, G.B.; Hobbs, G.; Vona-Davis, L.C.; Waheed, U. Inferior vena cava filter placement in late-stage cancer. Vasc. Endovascular. Surg. 2006, 40, 287–294. [Google Scholar] [CrossRef]
- Levitan, N.; Dowlati, A.; Remick, S.C.; Tahsildar, H.I.; Sivinski, L.D.; Beyth, R.; Rimm, A.A. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine 1999, 78, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Barginear, M.F.; Lesser, M.; Akerman, M.L.; Strakhan, M.; Shapira, I.; Bradley, T.; Budman, D.R. Need for inferior vena cava filters in cancer patients: A surrogate marker for poor outcome. Clin. Appl. Thromb. Hemost. 2009, 15, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Matta, F.; Lawrence, F.R.; Hughes, M.J. Inferior Vena Cava Filters in Patients with Acute Pulmonary Embolism and Cancer. Am. J. Med. 2018, 131, 442.e9–442.e12. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, B.P.; Dougherty, M.J.; Calligaro, K.D. Inferior vena cava filters in malignant disease. J. Vasc. Surg. 2002, 36, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.J.; Jean, J.L.; Gupta, S.; Eapen, G.A.; Johnson, M.M.; Ahrar, K.; Madoff, D.C.; Morello, F.A.; Murthy, R.; Hicks, M.E. Use of inferior vena caval filters and survival in patients with malignancy. Cancer 2004, 101, 1902–1907. [Google Scholar] [CrossRef]
- Mansour, A.; Ismael, Y.; Abdel-Razeq, H. Inferior vena cava filters in patients with advanced-stage cancer. Hematol. Oncol. Stem Cell Ther. 2014, 7, 136–141. [Google Scholar] [CrossRef]
- Shaikh, S.S.; Kamath, S.D.; Ghosh, D.; Lewandowski, R.J.; McMahon, B.J. Safety and Outcomes of Permanent and Retrievable Inferior Vena Cava Filters in the Oncology Population. Int. J. Vasc. Med. 2020, 2020, 6582742. [Google Scholar] [CrossRef]
- Chau, Q.; Cantor, S.B.; Caramel, E.; Hicks, M.; Kurtin, D.; Grover, T.; Elting, L.S. Cost-effectiveness of the bird’s nest filter for preventing pulmonary embolism among patients with malignant brain tumors and deep venous thrombosis of the lower extremities. Support. Care Cancer 2003, 11, 795–799. [Google Scholar] [CrossRef]
- Mission, J.F.; Kerlan, R.K.; Tan, J.H.; Fang, M.C. Rates and predictors of plans for inferior vena cava filter retrieval in hospitalized patients. J. Gen. Intern. Med. 2010, 25, 321–325. [Google Scholar] [CrossRef]
- Dabbagh, O.; Nagam, N.; Chitima-Matsiga, R.; Bearelly, S.; Bearelly, D. Retrievable Inferior Vena Cava filters are not getting retrieved: Where is the gap? Thromb. Res. 2010, 126, 493–497. [Google Scholar] [CrossRef]
- Sarosiek, S.; Crowther, M.; Sloan, J.M. Indications, Complications, and Management of Inferior Vena Cava Filters: The Experience in 952 Patients at an Academic Hospital With a Level I Trauma Center. JAMA Intern. Med. 2013, 173, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.K.; Desai, K.R.; Salem, R.; Ryu, R.K.; Lewandowski, R.J. A Dedicated Inferior Vena Cava Filter Service Line: How to Optimize Your Practice. Semin. Intervent. Radiol. 2016, 33, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Eifler, A.C.; Lewandowski, R.J.; Gupta, R.; Karp, J.; Salem, R.; Lee, J.; Ryu, R.K. Optional or permanent: Clinical factors that optimize inferior vena cava filter utilization. J. Vasc. Interv. Radiol. 2013, 24, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, J.M.; Lewandowski, R.J.; Vogelzang, R.L.; Ryu, R.K. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: A review of the MAUDE database. J. Vasc. Interv. Radiol. 2014, 25, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Schutzer, R.; Ascher, E.; Hingorani, A.; Jacob, T.; Kallakuri, S. Preliminary results of the new 6F TrapEase inferior vena cava filter. Ann. Vasc. Surg. 2003, 17, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Kinney, T.B. Update on inferior vena cava filters. J. Vasc. Interv. Radiol. 2003, 14, 425–440. [Google Scholar] [CrossRef]
- Cho, K.J.; Greenfield, L.J.; Proctor, M.C.; Hausmann, L.A.; Bonn, J.; Dolmatch, B.L.; Eschelman, D.J.; Flick, P.A.; Kinney, T.B.; Marx, M.V.; et al. Evaluation of a new percutaneous stainless steel Greenfield filter. J. Vasc. Interv. Radiol. 1997, 8, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, H.; Perreault, P.; Otal, P.; Stockx, L.; Golzarian, J.; Oliva, V.; Reynaud, P.; Raat, F.; Szatmari, F.; Santoro, G.; et al. The 6-F nitinol TrapEase inferior vena cava filter: Results of a prospective multicenter trial. J. Vasc. Interv. Radiol. 2001, 12, 299–304. [Google Scholar] [CrossRef]
- Athanasoulis, C.A.; Kaufman, J.A.; Halpern, E.F.; Waltman, A.C.; Geller, S.C.; Fan, C.M. Inferior vena caval filters: Review of a 26-year single-center clinical experience. Radiology 2000, 216, 54–66. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.; Cogo, A.; Cuppini, S.; Villalta, S.; Carta, M.; Cattelan, A.M.; Polistena, P.; Bernardi, E.; Prins, M.H. The long-term clinical course of acute deep venous thrombosis. Ann. Intern. Med. 1996, 125, 1–7. [Google Scholar] [CrossRef]
- Hutten, B.A.; Prins, M.H.; Gent, M.; Ginsberg, J.; Tijssen, J.G.P.; Büller, H.R. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: A retrospective analysis. J. Clin. Oncol. 2000, 18, 3078–3083. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | All Patients (n = 179) | Oncology Patients (n = 62) | Non-Oncology Group (n = 117) | p Value |
|---|---|---|---|---|
| Median age (years) [mean ± SD] | 60.1 ± 15.2 | 67.8 ± 11.2 | 56.0 ± 15.4 | 0.13 |
| Male [n, %] | 121 (67.6%) | 37 (59.7%) | 84 (71.8%) | <0.001 |
| Recurrent VTE [n, %] | 33 (18.4%) | 19(30.7%) | 14 (12.0%) | 0.0004 |
| Manifestation of VTE | ||||
| DVT [n, %] | 66 (36.9%) | 15 (24.2%) | 51 (43.6%) | 0.031 |
| PE [n, %] | 49 (27.4%) | 22 (35.5%) | 27 (23.1%) | 0.090 |
| DVT and PE [n, %] | 64(35.8%) | 25 (40.3%) | 39 (33.3%) | 0.361 |
| Filter model | ||||
| Option™ Elite | 75 (41.8%) | 28 (45.1%) | 47 (40.2%) | 0.319 |
| OPTEASE™ | 49 (27.4%) | 12 (19.4%) | 37 (31.6%) | |
| Günther Tulip® | 20 (11.2%) | 9 (14.5%) | 11 (9.4%) | |
| Celect™ Platinum | 35 (19.6%) | 13 (21.0%) | 22 (18.8%) |
| Reason | Oncology Group | Non-Oncology Group | p Value |
|---|---|---|---|
| Recent/upcoming urgent procedure | 47 (75.8%) | 117 (100%) | <0.001 |
| Bleeding: | |||
| Pericardial | 3 (4.8%) | - | |
| Gastrointestinal | 4 (6.5%) | - | |
| Urinary | 5 (8.1%) | - | |
| Other source | 1 (1.6%) | - | |
| Failure of LMWH | 1 (1.6%) | - | |
| Other | 1 (1.6%) | - |
| Oncology Group | Non-Oncology Group | p | |
|---|---|---|---|
| Retrieval attempts [n, %] | 13/62 (21%) | 83/117 (71%) | <0.001 |
| Filters retrieved [n, %] | 9/13 (69%) | 63/83 (76%) | 0.731 |
| Time to retrieval (days) [median, IQR] | 77 (17–292) | 84 (49–146) | 0.764 |
| Abnormal findings during retrieval: | |||
| Thrombus at the top of the filter [n, %] | 2/13 (15.4%) | 33/83 (39.8%) | 0.124 |
| Apposition of the hook to the vessel wall [n, %] | 3/13 (23.1%) | 11/83 (13.3%) | 0.397 |
| Clotted filter [n, %] | 3/13 (23.1%) | 17/83 (20.5%) | 1.000 |
| Filters Retrieved (n) | Filters Non-Retrieved (n) | p Value | |
|---|---|---|---|
| Cancer stage | |||
| Locally limited cancer | 1 | 10 | 0.733 |
| Locally advanced cancer | 3 | 21 | |
| Disseminated cancer | 5 | 20 | |
| Lymphoma | 0 | 2 | |
| Localization of the neoplasm | |||
| Colorectal | 3 | 12 | |
| Kidney | 2 | 7 | |
| Gynecological | 1 | 6 | |
| Lung | 0 | 5 | |
| Urinary bladder | 1 | 4 | |
| Gastric | 0 | 4 | |
| Multiple neoplasm | 1 | 3 | |
| Sarcoma | 0 | 2 | |
| Lymphoma | 0 | 2 | |
| Breast | 0 | 2 | |
| Prostate | 1 | 1 | |
| Pancreas | 0 | 2 | |
| Other | 0 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzyna, P.; Banaszkiewicz, M.; Florczyk, M.; Kępski, J.; Piłka, M.; Kędzierski, P.; Mańczak, R.; Szwed, P.; Kasperowicz, K.; Wrona, K.; et al. Safety and Outcomes of Inferior Vena Cava Filter Placement in Oncology Patients: A Single-Centre Experience. Cancers 2024, 16, 1562. https://doi.org/10.3390/cancers16081562
Kurzyna P, Banaszkiewicz M, Florczyk M, Kępski J, Piłka M, Kędzierski P, Mańczak R, Szwed P, Kasperowicz K, Wrona K, et al. Safety and Outcomes of Inferior Vena Cava Filter Placement in Oncology Patients: A Single-Centre Experience. Cancers. 2024; 16(8):1562. https://doi.org/10.3390/cancers16081562
Chicago/Turabian StyleKurzyna, Paweł, Marta Banaszkiewicz, Michał Florczyk, Jarosław Kępski, Michał Piłka, Piotr Kędzierski, Rafał Mańczak, Piotr Szwed, Krzysztof Kasperowicz, Katarzyna Wrona, and et al. 2024. "Safety and Outcomes of Inferior Vena Cava Filter Placement in Oncology Patients: A Single-Centre Experience" Cancers 16, no. 8: 1562. https://doi.org/10.3390/cancers16081562
APA StyleKurzyna, P., Banaszkiewicz, M., Florczyk, M., Kępski, J., Piłka, M., Kędzierski, P., Mańczak, R., Szwed, P., Kasperowicz, K., Wrona, K., Doroszewski, G., Torbicki, A., Kurzyna, M., Szmit, S., & Darocha, S. (2024). Safety and Outcomes of Inferior Vena Cava Filter Placement in Oncology Patients: A Single-Centre Experience. Cancers, 16(8), 1562. https://doi.org/10.3390/cancers16081562








