The Thermal Ablation with MRgFUS: From Physics to Oncological Applications
Simple Summary
Abstract
1. Introduction
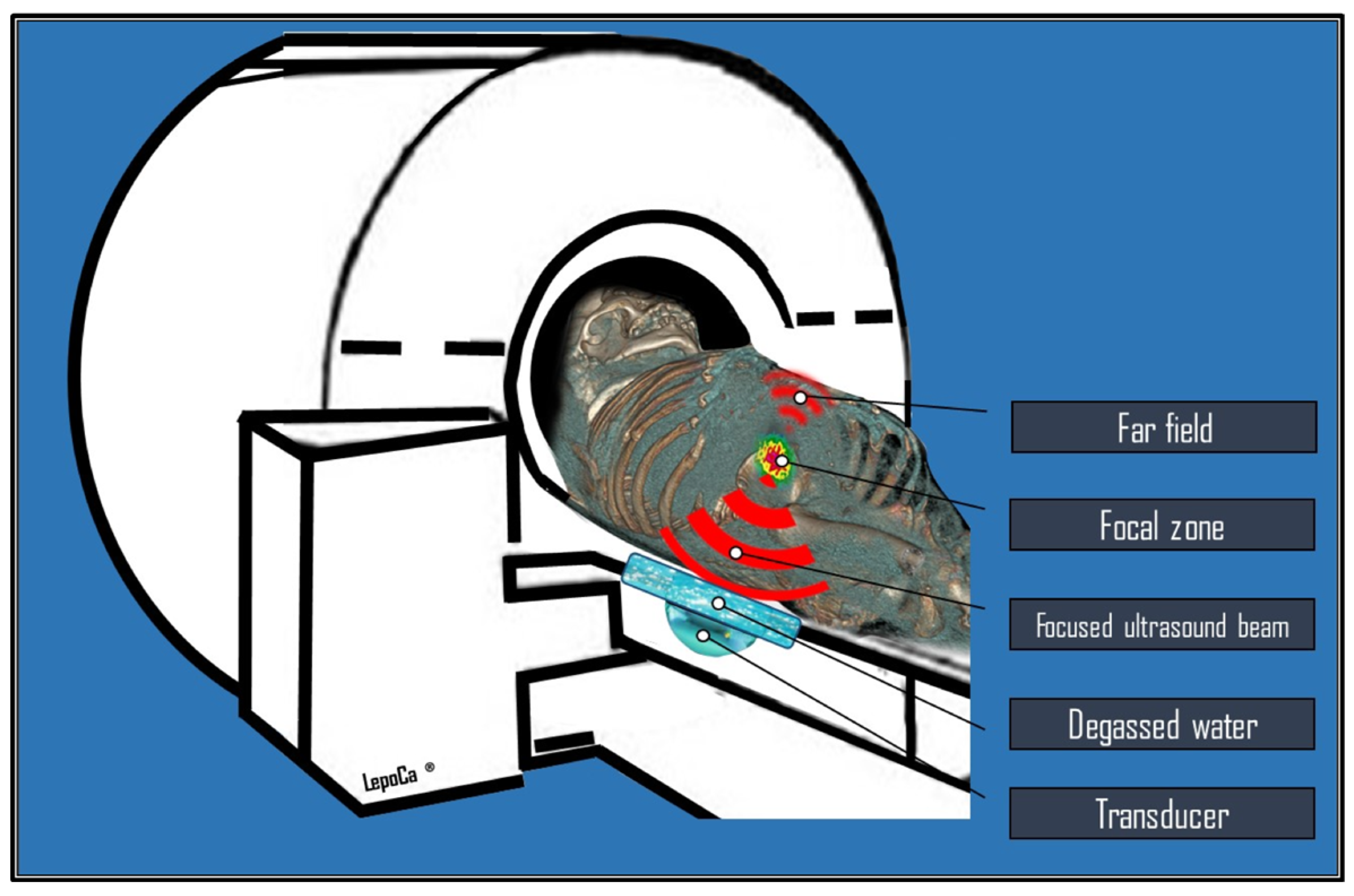
2. Physical Principles, Biological Effects, and Technical Notes
3. An Overview of Current and Prospective Applications in Oncology
3.1. Bone Metastases
3.2. Prostate Cancer
3.3. Breast Cancer
3.4. Abdominal Cancers
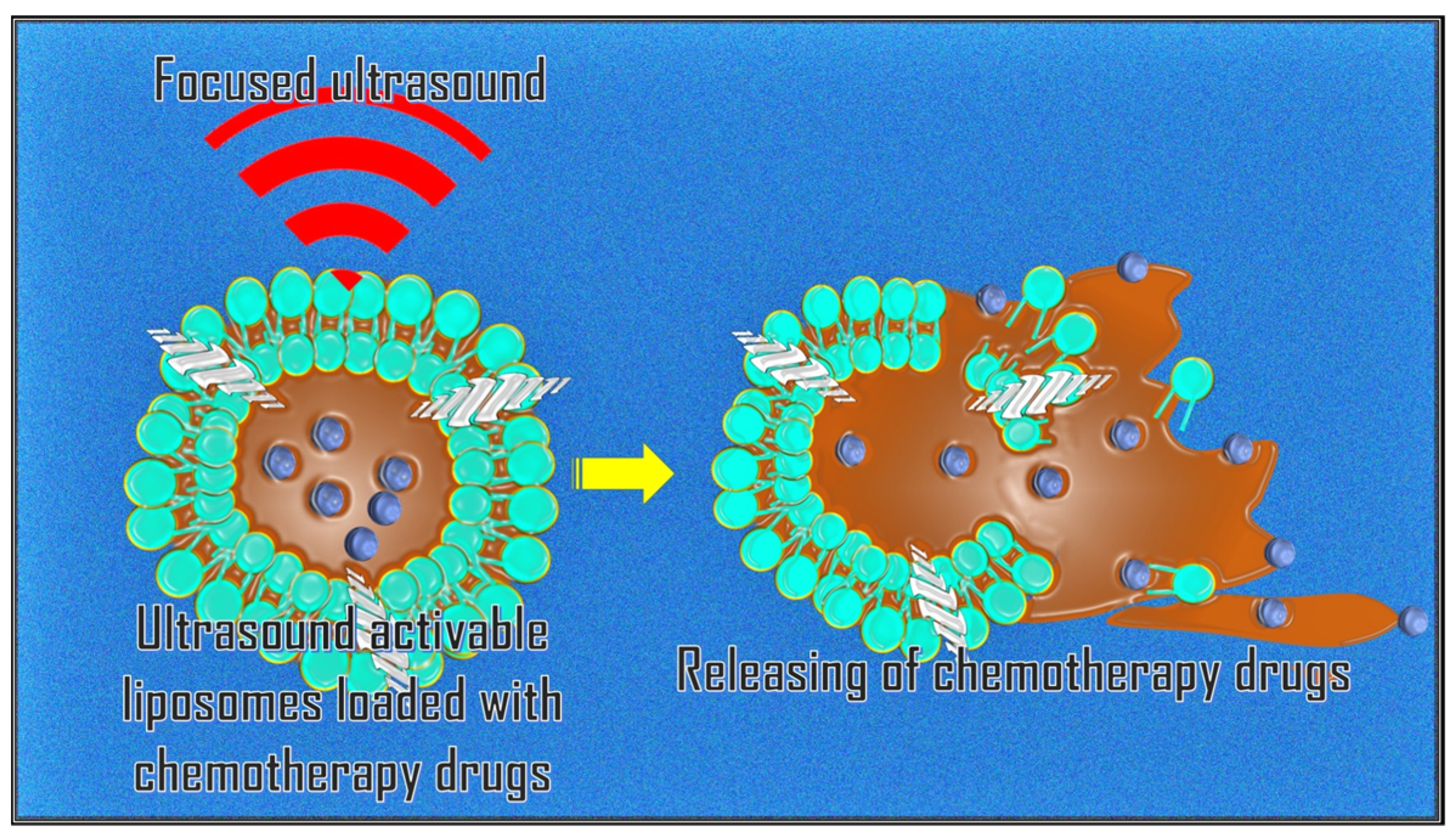
3.5. Targeted Drug Delivery
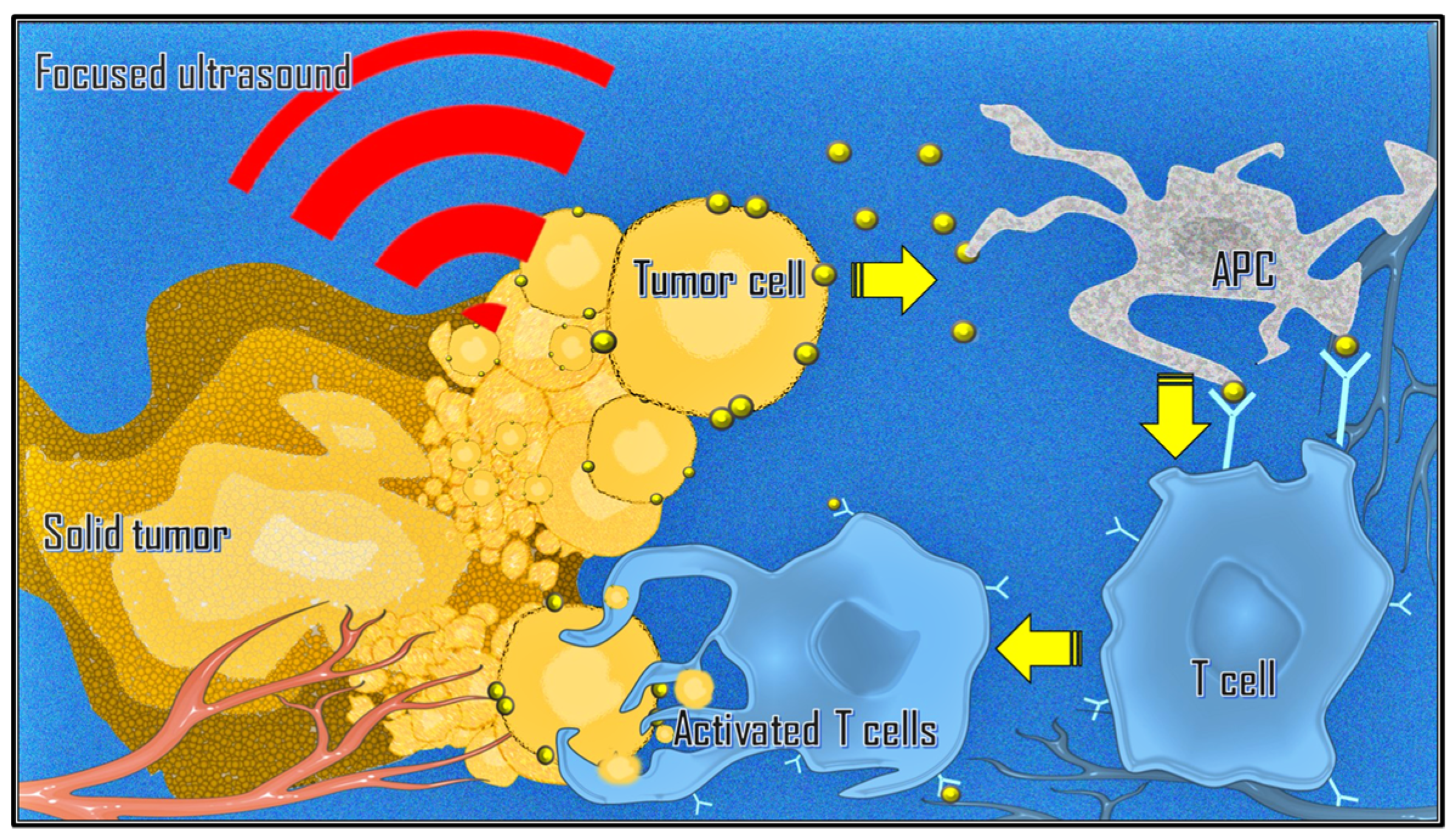
3.6. Immunological Effects
3.7. Neuro-Oncology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynn, J.; Zwemer, R.; Chick, A.; Miller, A. A new method for the generation and use of focused ultrasound in experimental biology. J. Gen. Physiol. 1942, 26, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Leporace, M.; Lancellotta, v.; Baccolini, V.; Calabria, F.; Castrovillari, F.; Filippiadis, D.; Tagliaferri, L.; Iezzi, R. Magnetic resonance-guided focused ultrasound versus percutaneous thermal ablation in local control of bone oligometastases: A systematic review and meta-analysis. Radiol. Med. 2024, 129, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Pediconi, F.; Napoli, A.; Di Mare, L.; Vasselli, F.; Catalano, C. MRgFUS: From diagnosis to therapy. Eur. J. Radiol. 2012, 81, S118–S120. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; He, J.; Gao, P.; Wang, Y.; Hui, H.; An, Y.; Tian, J. Correction to: Magnetic Particle Imaging-Guided Hyperthermia for Precise Treatment of Cancer: Review, Challenges, and Prospects. Mol. Imaging Biol. 2023, 25, 1151. [Google Scholar] [CrossRef]
- Haar, G.; Coussios, C. High intensity focused ultrasound: Physical principles and devices. Int. J. Hyperthermia 2007, 23, 89–104. [Google Scholar] [CrossRef]
- Bamber, J.; ter Haar, G.; Hill, C. Physical Principles of Medical Ultrasound; Wiley: London, UK, 2004. [Google Scholar]
- Hill, C. Optimum acoustic frequency for focused ultrasound surgery. Ultrasound Med. Biol. 1994, 20, 271–277. [Google Scholar] [CrossRef]
- Hynynen, K.; Watmough, D.; Mallard, J. The effects of some physical factors on the production of hyperthermia by ultrasound in neoplastic tissues. Radiat. Environ. Biophys. 1981, 19, 215–226. [Google Scholar] [CrossRef]
- Chu, K.; Dupuy, D. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Jenne, J.; Preusser, T.; Günther, M. High-intensity focused ultrasound: Principles, therapy guidance, simulations and applications. Z. Med. Phys. 2012, 22, 311–322. [Google Scholar] [CrossRef]
- Coussios, C.; Farny, C.; Haar, G.; Roy, R. Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU). Int. J. Hyperthermia 2007, 23, 105–120. [Google Scholar] [CrossRef]
- Zhou, Y. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Cline, H.; Schenck, J.; Hynynen, K.; Watkins, R.; Souza, S.; Jolesz, F. MR-guided focused ultrasound surgery. J. Comput. Assist. Tomogr. 1992, 16, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Connolly, D.; Peng, X.; Badran, B. A Comprehensive Review of Low-Intensity Focused Ultrasound Parameters and Applications in Neurologic and Psychiatric Disorders. Neuromodulation 2024, 3, S1094–S7159. [Google Scholar] [CrossRef] [PubMed]
- Mungur, R.; Zheng, J.; Wang, B.; Chen, X.; Zhan, R.; Tong, Y. Low-Intensity Focused Ultrasound Technique in Glioblastoma Multiforme Treatment. Front. Oncol. 2022, 12, 903059. [Google Scholar] [CrossRef]
- Napoli, A.; Anzidei, M.; Ciolina, F.; Marotta, E.; Cavallo, M.B.; Brachetti, G.; Di Mare, L.; Cartocci, G.; Boni, F.; Noce, V.; et al. MR-guided high-intensity focused ultrasound: Current status of an emerging technology. Cardiovasc. Intervent Radiol. 2013, 36, 1190–1203. [Google Scholar] [CrossRef]
- Payne, A.; Chopra, R.; Ellens, N.; Chen, L.; Ghanouni, P.; Sammet, S.; Diederich, C.; Ter Haar, G.; Parker, D.; Moonen, C.; et al. AAPM Task Group 241: A medical physicist’s guide to MRI-guided focused ultrasound body systems. Med. Phys. 2021, 48, e772–e806. [Google Scholar] [CrossRef]
- Hectors, S.; Jacobs, I.; Moonen, C.; Strijkers, G.; Nicolay, K. MRI methods for the evaluation of high intensity focused ultrasound tumor treatment: Current status and future needs. Magn. Reson. Med. 2016, 75, 302–317. [Google Scholar] [CrossRef]
- Wimper, Y.; Fütterer, J.; Bomers, J. MR Imaging in Real Time Guiding of Therapies in Prostate Cancer. Life 2022, 12, 302. [Google Scholar] [CrossRef]
- Chow, E.; Harris, K.; Fan, G.; Tsao, M.; Sze, W. Palliative radiotherapy trials for bone metastases: A systematic review. J. Clin. Oncol. 2007, 25, 1423–1436. [Google Scholar] [CrossRef]
- Brown, M.; Farquhar-Smith, P.; Williams, J.; ter Haar, G.; deSouza, N. The use of high-intensity focused ultrasound as a novel treatment for painful conditions-a description and narrative review of the literature. Br. J. Anaesth. 2015, 115, 520–530. [Google Scholar] [CrossRef]
- Napoli, A.; Anzidei, M.; Marincola, B.; Brachetti, G.; Noce, V.; Boni, F.; Bertaccini, L.; Passariello, R.; Catalano, C. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics 2013, 33, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Baal, J.; Chen, W.; Baal, U.; Wagle, S.; Baal, J.; Link, T.; Bucknor, M. Efficacy and safety of magnetic resonance-guided focused ultrasound for the treatment of painful bone metastases: A systematic review and meta-analysis. Skeletal Radiol. 2021, 50, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- McGill, K.; Baal, J.; Bucknor, M. Update on musculoskeletal applications of magnetic resonance-guided focused ultrasound. Skeletal Radiol. 2014, 53, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.; Bratke, G.; Grüll, H. High Intensity Focused Ultrasound for Treatment of Bone Malignancies-20 Years of History. Cancers 2022, 15, 108. [Google Scholar] [CrossRef]
- Hurwitz, M.; Ghanouni, P.; Kanaev, S.; Iozeffi, D.; Gianfelice, D.; Fennessy, F.; Kuten, A.; Meyer, E.; LeBlang, S.; Roberts, A.; et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: Phase III trial results. J. Natl. Cancer Inst. 2014, 106, dju082. [Google Scholar] [CrossRef]
- Huisman, M.; ter Haar, G.; Napoli, A.; Hananel, A.; Ghanouni, P.; Lövey, G.; Nijenhuis, R.; van den Bosch, M.; Rieke, V.; Majumdar, S.; et al. International consensus on use of focused ultrasound for painful bone metastases: Current status and future directions. Int. J. Hyperthermia 2015, 31, 251–259. [Google Scholar] [CrossRef]
- Yuh, B.; Liu, A.; Beatty, R.; Jung, A.; Wong, J.Y.C. Focal therapy using magnetic resonance image-guided focused ultrasound in patients with localized prostate cancer. J. Ther. Ultrasound 2016, 11, 4–8. [Google Scholar] [CrossRef]
- Ghai, S.; Finelli, A.; Corr, K.; Chan, R.; Jokhu, S.; Li, X.; McCluskey, S.; Konukhova, A.; Hlasny, E.; van der Kwast, T.; et al. MRI-guided Focused Ultrasound Ablation for Localized Intermediate-Risk Prostate Cancer: Early Results of a Phase II Trial. Radiology 2021, 298, 695–703. [Google Scholar] [CrossRef]
- Ghai, S.; Finelli, A.; Corr, K.; Lajkosz, K.; McCluskey, S.; Chan, R.; Gertner, M.; van der Kwast, T.; Incze, P.; Zlotta, A.; et al. MRI-guided Focused Ultrasound Focal Therapy for Intermediate-Risk Prostate Cancer: Final Results from a 2-year Phase II Clinical Trial. Radiology 2024, 310, e231473. [Google Scholar] [CrossRef]
- Chin, J.; Billia, M.; Relle, J.; Roethke, M.; Popeneciu, I.; Kuru, T.; Hatiboglu, G.; Mueller-Wolf, M.; Motsch, J.; Romagnoli, C.; et al. Magnetic Resonance Imaging-Guided Transurethral Ultrasound Ablation of Prostate Tissue in Patients with Localized Prostate Cancer: A Prospective Phase 1 Clinical Trial. Eur. Urol. 2016, 70, 447–455. [Google Scholar] [CrossRef]
- Matsutani, A.; Ide, Y.; Miura, S.; Takimoto, M.; Amano, S.; Nakamura, S. Innovative use of magnetic resonance imaging-guided focused ultrasound surgery for non-invasive breast cancer: A report of two cases. Surg. Case Rep. 2020, 6, 294. [Google Scholar] [CrossRef] [PubMed]
- Merckel, L.; Knuttel, F.; Deckers, R.; van Dalen, T.; Schubert, G.; Peters, N.; Weits, T.; van Diest, P.; Mali, W.; Vaessen, P.; et al. First clinical experience with a dedicated MRI-guided high-intensity focused ultrasound system for breast cancer ablation. Eur. Radiol. 2016, 26, 4037–4046. [Google Scholar] [CrossRef] [PubMed]
- Merckel, L.; Bartels, L.; Köhler, M.; van den Bongard, H.; Deckers, R.; Mali, W.; Binkert, C.; Moonen, C.; Gilhuijs, K.; van den Bosch, M. MR-guided high-intensity focused ultrasound ablation of breast cancer with a dedicated breast platform. Cardiovasc. Intervent Radiol. 2013, 36, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, H.; Namba, K.; Thomsen, S.; Akiyama, F.; Bendet, A.; Tanaka, C.; Yasuda, Y.; Nakahara, H. Magnetic resonance-guided focused ultrasound surgery of breast cancer: Reliability and effectiveness. J. Am. Coll. Surg. 2006, 203, 54–63. [Google Scholar] [CrossRef]
- Orsi, F.; Arnone, P.; Chen, W.; Zhang, L. High intensity focused ultrasound ablation: A new therapeutic option for solid tumors. J. Cancer Res. Ther. 2010, 6, 414–420. [Google Scholar]
- Anzidei, M.; Napoli, A.; Sandolo, F.; Marincola, B.; Di Martino, M.; Berloco, P.; Bosco, S.; Bezzi, M.; Catalano, C. Magnetic resonance-guided focused ultrasound ablation in abdominal moving organs: A feasibility study in selected cases of pancreatic and liver cancer. Cardiovasc. Intervent Radiol. 2014, 37, 1611–1617. [Google Scholar] [CrossRef]
- Saeed, M.; Krug, R.; Do, L.; Hetts, S.W.; Wilson, M. Renal ablation using magnetic resonance-guided high intensity focused ultrasound: Magnetic resonance imaging and histopathology assessment. World J. Radiol. 2016, 8, 298–307. [Google Scholar] [CrossRef]
- Thanou, M.; Gedroyc, W. MRI-Guided Focused Ultrasound as a New Method of Drug Delivery. J. Drug Deliv. 2013, 2013, 616197. [Google Scholar] [CrossRef]
- Silvestrini, M.; Ingham, E.; Mahakian, L.; Kheirolomoom, A.; Liu, Y.; Fite, B.; Tam, S.; Tucci, S.; Watson, K.; Wong, A.; et al. Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols. JCI Insight 2017, 2, e90521. [Google Scholar] [CrossRef]
- Joiner, J.; Pylayeva-Gupta, Y.; Dayton, P. Focused Ultrasound for Immunomodulation of the Tumor Microenvironment. J. Immunol. 2020, 205, 2327–2341. [Google Scholar] [CrossRef]
- Lu, P.; Zhu, X.; Xu, Z.; Zhou, Q.; Zhang, J.; Wu, F. Increased infiltration of activated tumor-infiltrating lymphocyte safter high intensity focused ultrasound ablation of human breast cancer. Surgery 2009, 145, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhu, X.; Lu, P.; Zhou, Q.; Zhang, J.; Wu, F. Activation of tumor-infiltrating antigen presenting cells by high intensity focused ultrasound ablation of human breast cancer. Ultrasound Med. Biol. 2009, 35, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Xie, F.; Ran, L.; Xie, X.; Fan, Y.; Wu, F. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med. Biol. 2012, 38, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Del Prete, V.; Crucinio, N.; Serviddio, G.; Vendemiale, G.; Muscatiello, N. Lymphocyte-to-monocyte ratio predicts survival after radiofrequency ablation for colorectal liver metastases. World J. Gastroenterol. 2016, 22, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Antonini, A.; Costa, J.; Śmiłowska, K.; Berg, D.; Corvol, J.C.; Fabbrini, G.; Ferreira, J.; Foltynie, T.; Mir, P. European Academy of Neurology/Movement Disorder Society-European Section Guideline on the Treatment of Parkinson’s Disease: I. Invasive Therapies. Mov. Disord. 2022, 37, 1360–1374. [Google Scholar] [CrossRef]
- Coluccia, D.; Fandino, J.; Schwyzer, L.; O’Gorman, R.; Remonda, L.; Anon, J.; Martin, E.; Werner, B. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J. Ther. Ultrasound 2014, 2, 17. [Google Scholar] [CrossRef]
- McMahon, D.; Poon, C.; Hynynen, K. Evaluating the safety profile of focused ultrasound and microbubble-mediated treatments to increase blood-brain barrier permeability. Expert. Opin. Drug Deliv. 2019, 16, 129–142. [Google Scholar] [CrossRef]
- Beccaria, K.; Canney, M.; Bouchoux, G.; Puget, S.; Grill, J.; Carpentier, A. Blood-brain barrier disruption with low-intensity pulsed ultrasound for the treatment of pediatric brain tumors: A review and perspectives. Neurosurg. Focus. 2020, 48, E10. [Google Scholar] [CrossRef]
- Grasso, G.; Torregrossa, F.; Noto, M.; Bruno, E.; Feraco, P.; Buscemi, F.; Bartolotta, T.; Gagliardo, C. MR-guided focused ultrasound-induced blood-brain barrier opening for brain metastasis: A review. Neurosurg. Focus. 2023, 55, E11. [Google Scholar] [CrossRef]
- Liu, H.; Hsieh, H.; Lu, L.; Kang, C.; Wu, M.; Lin, C. Low-pressure pulsed focused ultrasound with microbubbles promotes an anticancer immunological response. J. Transl. Med. 2012, 10, 221. [Google Scholar] [CrossRef]
- Wu, S.K.; Santos, M.A.; Marcus, S.L.; Hynynen, K. MR-guided Focused Ultrasound Facilitates Sonodynamic Therapy with 5-Aminolevulinic Acid in a Rat Glioma Model. Sci. Rep. 2019, 9, 10465. [Google Scholar] [CrossRef]

| NCT Number | Study Title | Conditions | Status |
|---|---|---|---|
| NCT05291507 | Feasibility Evaluation of the Muse Magnetic Resonance Guided Focused Ultrasound | Breast Cancer | Recruiting |
| NCT05167669 | Pain Relief in Symptomatic Bone Metastases with Adjuvant Hyperthermia MR Guided | Bone Metastases and Pain | Recruiting |
| NCT04791228 | A Pilot Study of Thermodox and MR-HIFU for Treatment of Relapsed Solid Tumors | Solid Tumors | Recruiting |
| NCT04559685 | Study of Sonodynamic Therapy in Participants With Recurrent High-Grade Glioma | High Grade Glioma | Recruiting |
| NCT04307914 | Focused Ultrasound and RadioTHERapy for Noninvasive Palliative Pain Treatment in Patients With Bone Metastases | Cancer Induced Bone Pain | Recruiting |
| NCT04123535 | Focused Ultrasound to Promote Immune Responses for Undifferentiated Pleomorphic Sarcoma | Undifferentiated Pleomorphic Sarcoma | Recruiting |
| NCT03028246 | A Feasibility Safety Study of Benign Centrally-Located Intracranial Tumors in Pediatric and Young Adult Subjects | Benign Centrally-Located Intracranial Tumors | Recruiting |
| NCT02076906 | MR-guided High Intensity Focused Ultrasound (HIFU) on Pediatric Solid Tumors | Relapsed and Refractory Pediatric Solid Tumors | Active |
| 1st Author, Year | Title | Document Type | |
|---|---|---|---|
| Bone Metastases | Leporace, 2024 [2] | Magnetic resonance-guided focused ultrasound versus percutaneous thermal ablation in local control of bone oligometastases: a systematic review and meta-analysis | Meta-Analysis |
| Napoli, 2013 [22] | MR imaging-guided focused ultrasound for treatment of bone metastasis | Review | |
| Baal, 2021 [23] | Efficacy and safety of magnetic resonance-guided focused ultrasound for the treatment of painful bone metastases: a systematic review and meta-analysis | Meta-Analysis | |
| McGill, 2024 [24] | Update on musculoskeletal applications of magnetic resonance-guided focused ultrasound | Review | |
| Yeo, 2022 [25] | High Intensity Focused Ultrasound for Treatment of Bone Malignancies-20 Years of History | Review | |
| Hurwitz, 2014 [26] | Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results | Clinical Trial | |
| Huisman, 2015 [27] | International consensus on use of focused ultrasound for painful bone metastases: Current status and future directions | Review | |
| Prostate Cancer | You, 2016 [28] | Focal therapy using magnetic resonance image-guided focused ultrasound in patients with localized prostate cancer | Research Article |
| Ghai, 2021 [29] | MRI-guided Focused Ultrasound Ablation for Localized Intermediate-Risk Prostate Cancer: Early Results of a Phase II Trial | Clinical Trial | |
| Ghai, 2024 [30] | MRI-guided Focused Ultrasound Focal Therapy for Intermediate-Risk Prostate Cancer: Final Results from a 2-year Phase II Clinical Trial | Clinical Trial | |
| Chin, 2016 [31] | Magnetic Resonance Imaging-Guided Transurethral Ultrasound Ablation of Prostate Tissue in Patients with Localized Prostate Cancer: A Prospective Phase 1 Clinical Trial | Clinical Trial | |
| Breast Cancer | Matsutani, 2020 [32] | Innovative use of magnetic resonance imaging-guided focused ultrasound surgery for non-invasive breast cancer: a report of two cases | Research Article |
| Merckel, 2016 [33] | First clinical experience with a dedicated MRI-guided high-intensity focused ultrasound system for breast cancer ablation | Research Article | |
| Merckel, 2013 [34] | MR-guided high-intensity focused ultrasound ablation of breast cancer with a dedicated breast platform | Review | |
| Furusawa, 2006 [35] | Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness | Clinical Trial | |
| Abdominal Cancers | Orsi, 2010 [36] | High intensity focused ultrasound ablation: a new therapeutic option for solid tumors | Review |
| Anzidei, 2014 [37] | Magnetic resonance-guided focused ultrasound ablation in abdominal moving organs: a feasibility study in selected cases of pancreatic and liver cancer | Research Article | |
| Saeed, 2016 [38] | Renal ablation using magnetic resonance-guided high intensity focused ultrasound: Magnetic resonance imaging and histopathology assessment | Research Article | |
| Targeting Drugs Delivery | Thanou, 2013 [39] | MRI-Guided Focused Ultrasound as a New Method of Drug Delivery | Review Article |
| Immunological Effects | Silvestrini, 2017 [40] | Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols | Research Article |
| Joiner, 2020 [41] | Focused Ultrasound for Immunomodulation of the Tumor Microenvironment | Review | |
| Lu, 2009 [42] | Increased infiltration of activated tumor-infiltrating lymphocyte safter high intensity focused ultrasound ablation of human breast cancer | Randomized Controlled Trial | |
| Xu, 2009 [43] | Activation of tumor-infiltrating antigen presenting cells by high intensity focused ultrasound ablation of human breast cancer | Randomized Controlled Trial | |
| Xia, 2012 [44] | High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes | Research Article | |
| Neuro-Oncology | Coluccia, 2014 [47] | First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound | Case Study |
| McMahon, 2019 [48] | Evaluating the safety profile of focused ultrasound and microbubble-mediated treatments to increase blood-brain barrier permeability | Review | |
| Beccaria, 2020 [49] | Blood-brain barrier disruption with low-intensity pulsed ultrasound for the treatment of pediatric brain tumors: a review and perspectives | Review | |
| Grasso, 2023 [50] | MR-guided focused ultrasound-induced blood-brain barrier opening for brain metastasis: a review | Review |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leporace, M.; Calabria, F.F.; Siciliano, R.; Capalbo, C.; Filippiadis, D.K.; Iezzi, R. The Thermal Ablation with MRgFUS: From Physics to Oncological Applications. Cancers 2025, 17, 36. https://doi.org/10.3390/cancers17010036
Leporace M, Calabria FF, Siciliano R, Capalbo C, Filippiadis DK, Iezzi R. The Thermal Ablation with MRgFUS: From Physics to Oncological Applications. Cancers. 2025; 17(1):36. https://doi.org/10.3390/cancers17010036
Chicago/Turabian StyleLeporace, Mario, Ferdinando F. Calabria, Roberto Siciliano, Carlo Capalbo, Dimitrios K. Filippiadis, and Roberto Iezzi. 2025. "The Thermal Ablation with MRgFUS: From Physics to Oncological Applications" Cancers 17, no. 1: 36. https://doi.org/10.3390/cancers17010036
APA StyleLeporace, M., Calabria, F. F., Siciliano, R., Capalbo, C., Filippiadis, D. K., & Iezzi, R. (2025). The Thermal Ablation with MRgFUS: From Physics to Oncological Applications. Cancers, 17(1), 36. https://doi.org/10.3390/cancers17010036









