Characterization of a 3S PRAME VLD-Specific T Cell Receptor and Its Use in Investigational Medicinal Products for TCR-T Therapy of Patients with Myeloid Malignancies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of VLD-TCR T4.8-1-29 and Generation of VLD-TCR-T Cells (MDG1011)
2.2. Cell Culture of Target Cell Lines
2.3. Quantitative Real-Time PCR (qPCR)
2.4. Cytokine Release Assay
2.5. Live-Cell Imaging Cytotoxicity Assay
2.6. Flow Cytometry
3. Results
3.1. Preclinical Studies of MDG1011 Effector Cells of Healthy Donors
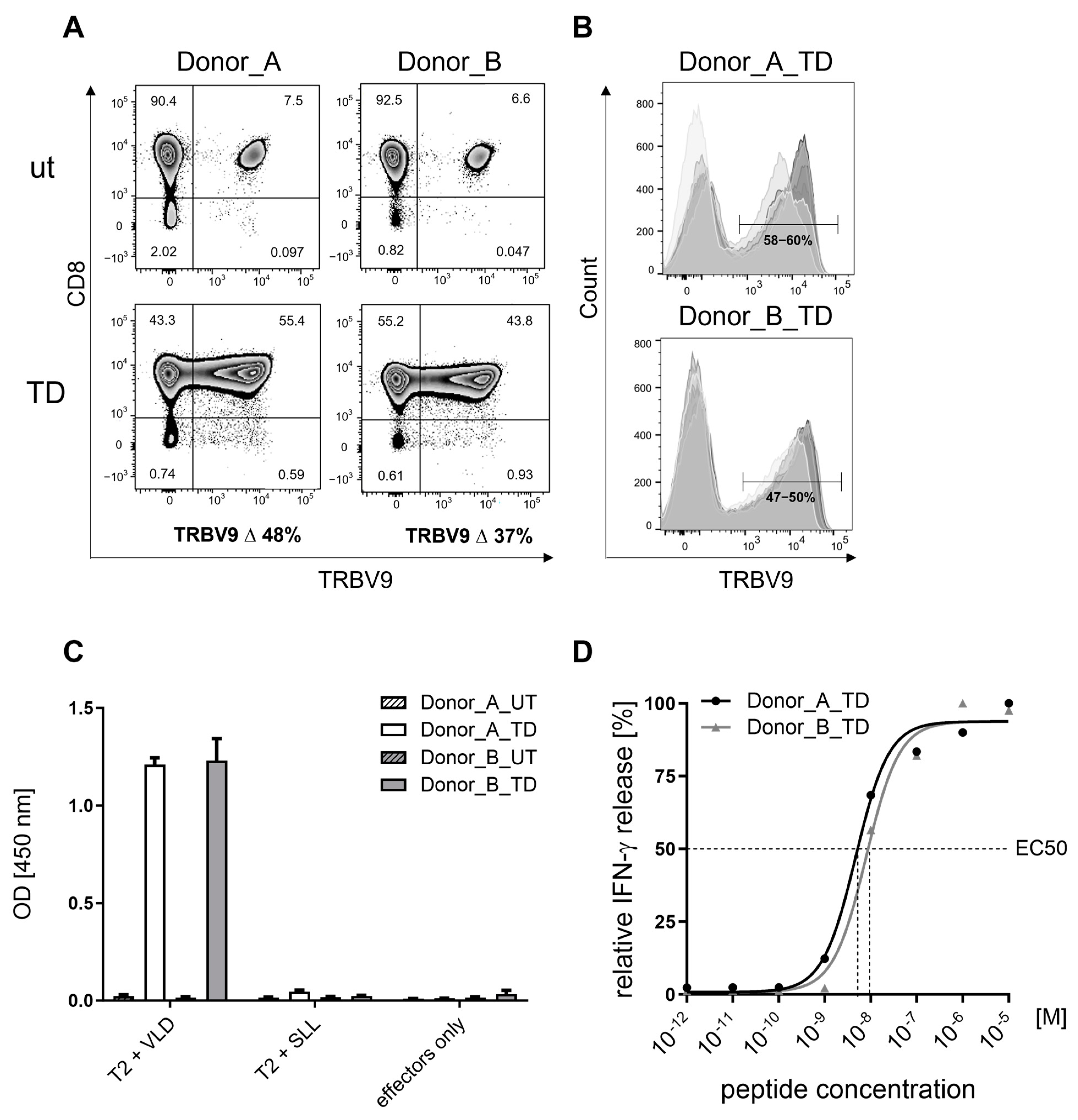
3.1.1. Surface Expression of Recombinant VLD-TCR T4.8-1-29 in CD8-Enriched T Cells
3.1.2. Specificity of MDG1011
3.1.3. Sensitivity of MDG1011
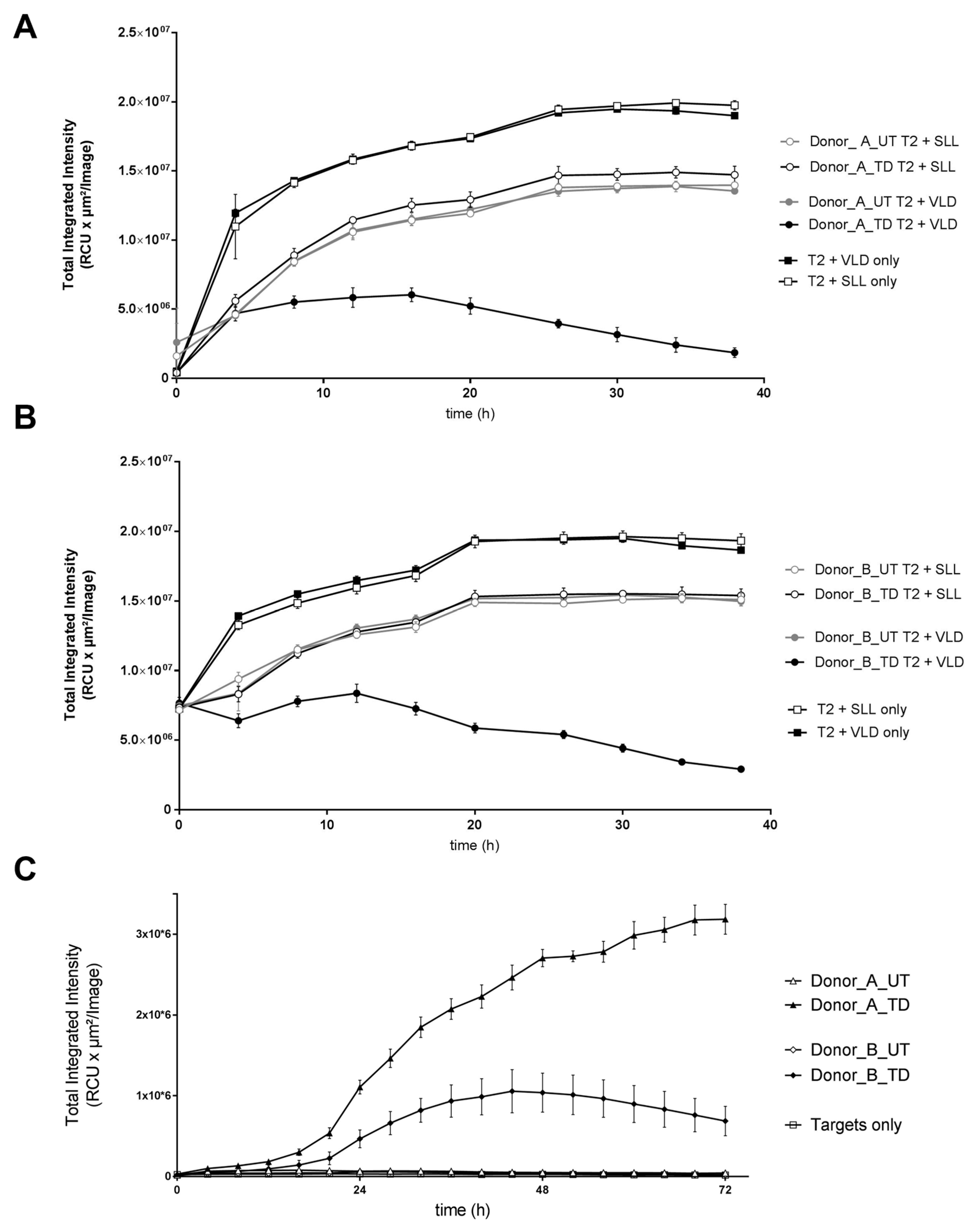
3.1.4. Cytotoxic Activity of MDG1011
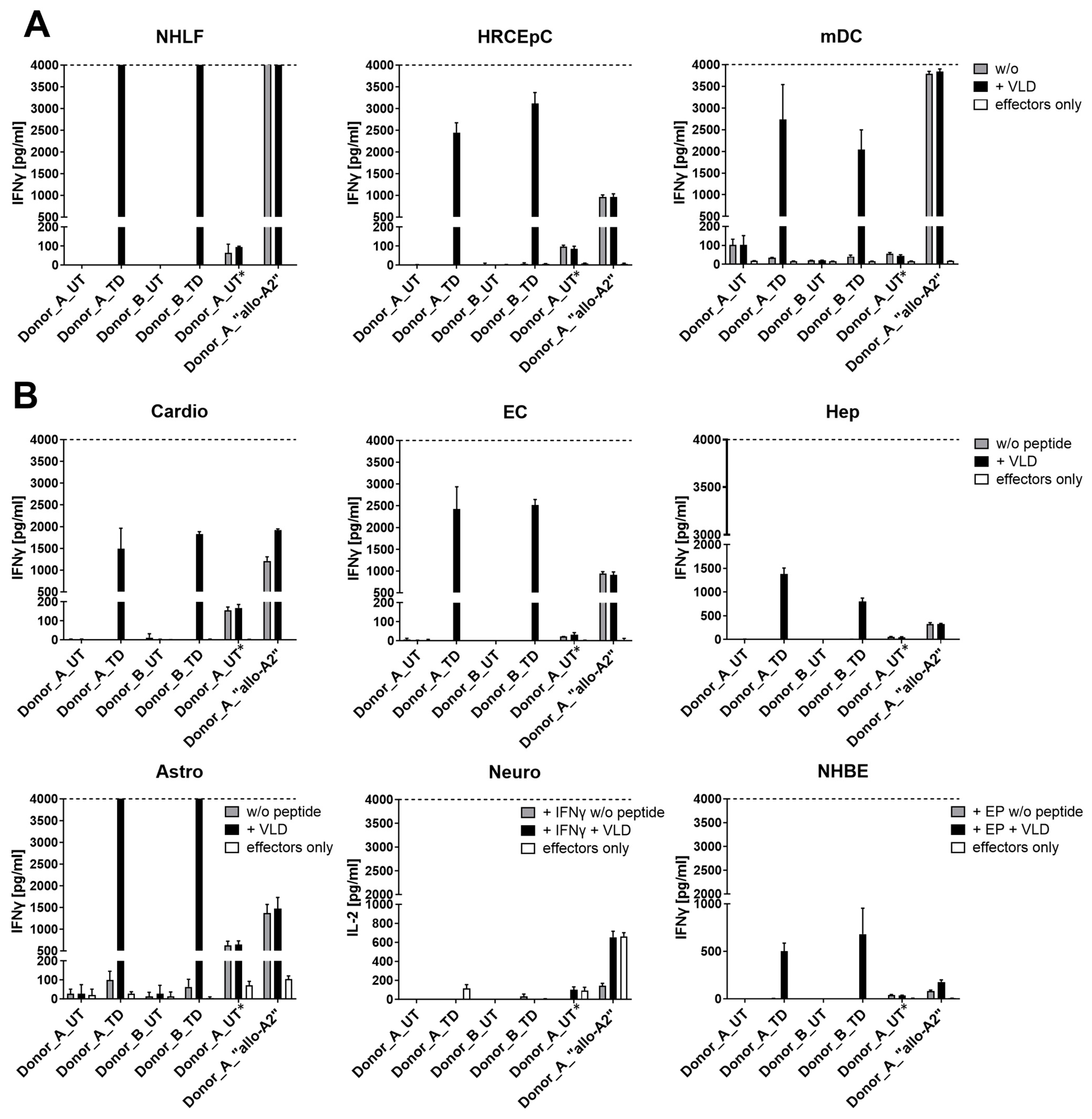
3.1.5. Safety Profile of MDG1011
3.2. Characterization of MDG1011 Investigational Medicinal Products (IMPs)
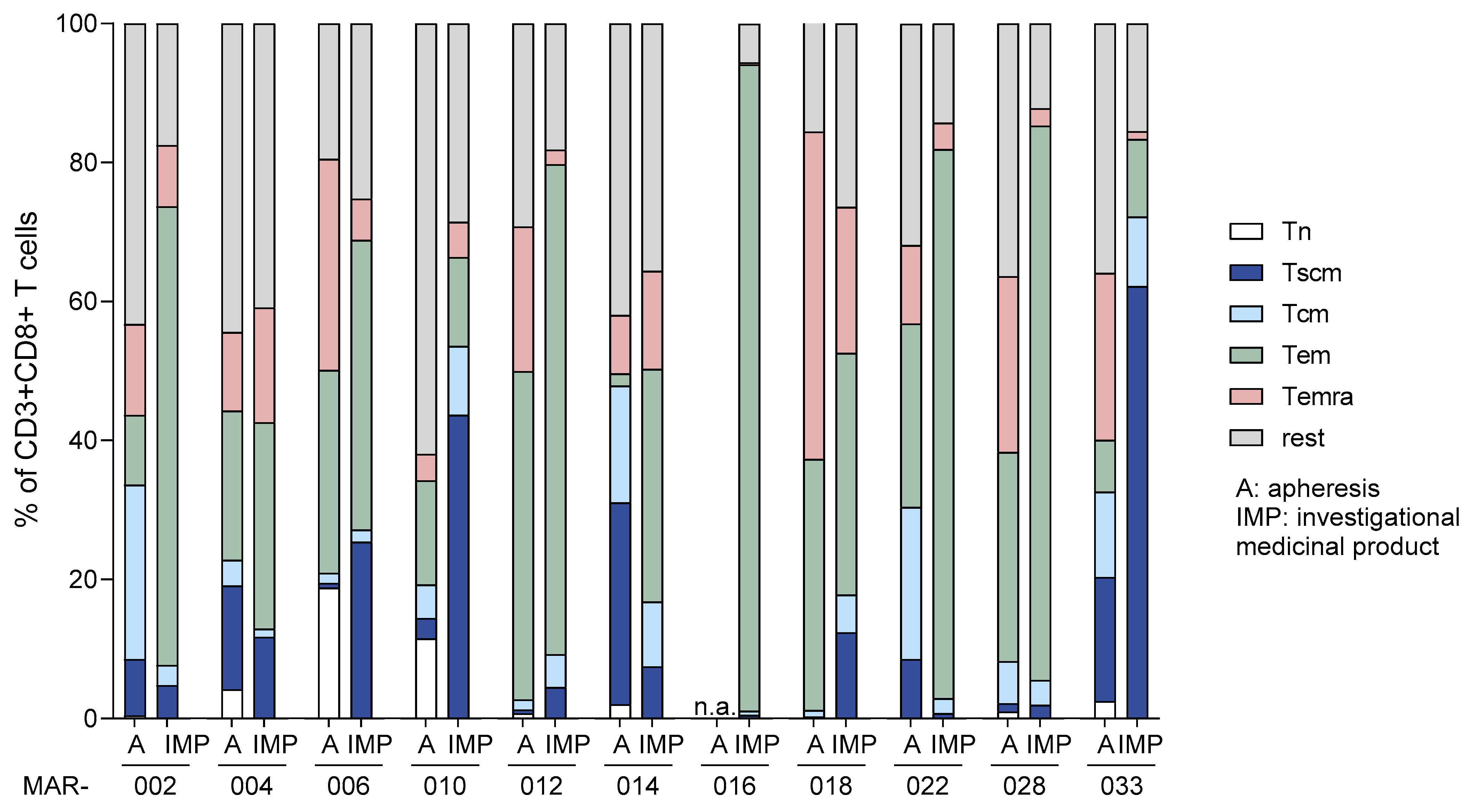
3.2.1. Cellular Composition of Starting Materials and Final MDG1011 IMPs
3.2.2. VLD-TCR Expression by MDG1011 IMPs
3.2.3. Functional Assessment of MDG1011 IMPs
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cappell, K.M.; Kochenderfer, J.N. Long-Term Outcomes Following CAR T Cell Therapy: What We Know so Far. Nat. Rev. Clin. Oncol. 2023, 20, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene Maraleucel for Patients with Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma (CARTITUDE-1): A Phase 1b/2 Open-Label Study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Amorós-Pérez, B.; Rivas-Pardo, B.; Gómez del Moral, M.; Subiza, J.L.; Martínez-Naves, E. State of the Art in CAR-T Cell Therapy for Solid Tumors: Is There a Sweeter Future? Cells 2024, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Müller, F.; Taubmann, J.; Mackensen, A.; Wang, W.; Furie, R.A.; Gold, R.; Haghikia, A.; Merkel, P.A.; Caricchio, R.; et al. Advancements and Challenges in CAR T Cell Therapy in Autoimmune Diseases. Nat. Rev. Rheumatol. 2024, 20, 531–544. [Google Scholar] [CrossRef]
- Biernacki, M.A.; Brault, M.; Bleakley, M. T-Cell Receptor-Based Immunotherapy for Hematologic Malignancies. Cancer J. 2019, 25, 179–190. [Google Scholar] [CrossRef]
- Baulu, E.; Gardet, C.; Chuvin, N.; Depil, S. TCR-Engineered T Cell Therapy in Solid Tumors: State of the Art and Perspectives. Sci. Adv. 2023, 9, eadf3700. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Araujo, D.M.; Abdul Razak, A.R.; Agulnik, M.; Attia, S.; Blay, J.Y.; Carrasco Garcia, I.; Charlson, J.A.; Choy, E.; Demetri, G.D.; et al. Afamitresgene Autoleucel for Advanced Synovial Sarcoma and Myxoid Round Cell Liposarcoma (SPEARHEAD-1): An International, Open-Label, Phase 2 Trial. Lancet 2024, 403, 1460–1471. [Google Scholar] [CrossRef]
- FDA. FDA Approves First Gene Therapy to Treat Adults with Metastatic Synovial Sarcoma. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treat-adults-metastatic-synovial-sarcoma (accessed on 15 October 2024).
- Falkenburg, J.H.F.; Heslop, H.E.; Barrett, A.J. T Cell Therapy in Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2008, 14, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, M.; Ye, J.; Ma, H. Targeting PRAME for Acute Myeloid Leukemia Therapy. Front. Immunol. 2024, 15, 1378277. [Google Scholar] [CrossRef] [PubMed]
- Epping, M.T.; Bernards, R. A Causal Role for the Human Tumor Antigen Preferentially Expressed Antigen of Melanoma in Cancer. Cancer Res. 2006, 66, 10639–10642. [Google Scholar] [CrossRef]
- Lezcano, C.; Jungbluth, A.A.; Nehal, K.S.; Hollmann, T.J.; Busam, K.J. PRAME Expression in Melanocytic Tumors. Am. J. Surg. Pathol. 2018, 42, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.L.; De Pas, T.; Rittmeyer, A.; Valliéres, E.; Kubisa, B.; Levchenko, E.; Wiesemann, S.; Masters, G.A.; Shen, R.; Tjulandin, S.A.; et al. Safety and Immunogenicity of the Prame Cancer Immunotherapeutic in Patients with Resected Non-Small Cell Lung Cancer: A Phase I Dose Escalation Study. J. Thorac. Oncol. 2016, 11, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Roszik, J.; Wang, W.-L.; Livingston, J.A.; Roland, C.L.; Ravi, V.; Yee, C.; Hwu, P.; Futreal, A.; Lazar, A.J.; Patel, S.R.; et al. Overexpressed PRAME Is a Potential Immunotherapy Target in Sarcoma Subtypes. Clin. Sarcoma Res. 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.L.; Van Der Steen, D.M.; Van Loenen, M.M.; Hagedoorn, R.S.; De Boer, R.; Kester, M.D.G.; De Ru, A.H.; Lugthart, G.J.; Van Kooten, C.; Hiemstra, P.S.; et al. PRAME-Specific Allo-HLA-Restricted T Cells with Potent Antitumor Reactivity Useful for Therapeutic T-Cell Receptor Gene Transfer. Clin. Cancer Res. 2011, 17, 5615–5625. [Google Scholar] [CrossRef] [PubMed]
- Sailer, N.; Fetzer, I.; Salvermoser, M.; Braun, M.; Brechtefeld, D.; Krendl, C.; Geiger, C.; Mutze, K.; Noessner, E.; Schendel, D.; et al. T-Cells Expressing a Highly Potent PRAME-Specific T-Cell Receptor in Combination with a Chimeric PD1-41BB Co-Stimulatory Receptor Show a Favorable Preclinical Safety Profile and Strong Anti-Tumor Reactivity. Cancers 2022, 14, 1998. [Google Scholar] [CrossRef]
- Schendel, D.J. Evolution by Innovation as a Driving Force to Improve TCR-T Therapies. Front. Oncol. 2023, 13, 1216829. [Google Scholar] [CrossRef]
- Medigene. Medigene Reports Preliminary Efficacy and Immune Monitoring Data of Phase I of Phase I/II MDG1011 Trial in Blood Cancers. Available online: https://medigene.com/medigene-reports-preliminary-efficacy-and-immune-monitoring-data-of-phase-i-of-phase-i-ii-mdg1011-trial-in-blood-cancers-1/ (accessed on 15 October 2024).
- Wilde, S.; Sommermeyer, D.; Frankenberger, B.; Schiemann, M.; Milosevic, S.; Spranger, S.; Pohla, H.; Uckert, W.; Busch, D.H.; Schendel, D.J. Dendritic Cells Pulsed with RNA Encoding Allogeneic MHC and Antigen Induce T Cells with Superior Anti-Tumor Activity and Higher TCR Functional Avidity. Blood 2009, 114, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Davari, K.; Holland, T.; Prassmayer, L.; Longinotti, G.; Ganley, K.P.; Pechilis, L.J.; Diaconu, I.; Nambiar, P.R.; Magee, M.S.; Schendel, D.J.; et al. Development of a CD8 Co-Receptor Independent T-Cell Receptor Specific for Tumor-Associated Antigen MAGE-A4 for next Generation T-Cell-Based Immunotherapy. J. Immunother. Cancer 2021, 9, e002035. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, M.; Engels, B.; Meyerhuber, P.; Kieback, E.; Sommermeyer, D.; Xue, S.A.; Reuß, S.; Stauss, H.; Uckert, W. Enhanced Functionality of T Cell Receptor-Redirected T Cells Is Defined by the Transgene Cassette. J. Mol. Med. 2008, 86, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Jonuleit, H.; Kühn, U.; Müller, G.; Steinbrink, K.; Paragnik, L.; Schmitt, E.; Knop, J.; Enk, A.H. Pro-Inflammatory Cytokines and Prostaglandins Induce Maturation of Potent Immunostimulatory Dendritic Cells under Fetal Calf Serum-Free Conditions. Eur. J. Immunol. 1997, 27, 3135–3142. [Google Scholar] [CrossRef]
- Haase, K.; Raffegerst, S.; Schendel, D.J.; Frishman, D. Expitope: A Web Server for Epitope Expression. Bioinformatics 2015, 31, 1854–1856. [Google Scholar] [CrossRef][Green Version]
- Sanderson, J.P.; Crowley, D.J.; Wiedermann, G.E.; Quinn, L.L.; Crossland, K.L.; Tunbridge, H.M.; Cornforth, T.V.; Barnes, C.S.; Ahmed, T.; Howe, K.; et al. Preclinical Evaluation of an Affinity-Enhanced MAGE-A4-Specific T-Cell Receptor for Adoptive T-Cell Therapy. Oncoimmunology 2020, 9, e1682381. [Google Scholar] [CrossRef]
- Valitutti, B.S.; Müller, S.; Salio, M.; Lanzavecchia, A. Complexes after Antigenic Stimulation. J. Exp. Med 1997, 185, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, N.; Wargo, J.A.; Yu, Z.; Rao, M.; Frankel, T.L.; Riley, J.P.; Hong, J.J.; Parkhurst, M.R.; Feldman, S.A.; Schrump, D.S.; et al. A TCR Targeting the HLA-A*0201–Restricted Epitope of MAGE-A3 Recognizes Multiple Epitopes of the MAGE-A Antigen Superfamily in Several Types of Cancer. J. Immunol. 2011, 186, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Cameron, B.J. Identification of a Titin-Derived HLA-A1–Presented Peptide for MAGE A3 T Cells. Sci. Transl. Med. 2013, 5, 197ra103. [Google Scholar] [CrossRef]
- Linette, G.P.; Stadtmauer, E.A.; Maus, M.V.; Rapoport, A.P.; Levine, B.L.; Emery, L.; Litzky, L.; Bagg, A.; Carreno, B.M.; Cimino, P.J.; et al. Cardiovascular Toxicity and Titin Cross-Reactivity of Affinity-Enhanced T Cells in Myeloma and Melanoma. Blood 2013, 122, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Zhong, X.S.; Palmer, D.C.; Ji, Y.; Hinrichs, C.S.; Yu, Z.; Wrzesinski, C.; Boni, A.; Cassard, L.; Garvin, L.M.; et al. Wnt Signaling Arrests Effector T Cell Differentiation and Generates CD8 + Memory Stem Cells. Nat. Med. 2009, 15, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, C.; Lu, Y.; Wu, Z.; Guo, Y.; Liu, Y.; Wei, J.; Wang, C.; Yang, Q.; Han, W. Characteristics of Premanufacture CD8+T Cells Determine CAR-T Efficacy in Patients with Diffuse Large B-Cell Lymphoma. Signal Transduct. Target. Ther. 2023, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Cao, K.; Wang, M. A Correlation Between Differentiation Phenotypes of Infused T Cells and Anti-Cancer Immunotherapy. Front. Immunol. 2021, 12, 745109. [Google Scholar] [CrossRef] [PubMed]
- Kishton, R.J.; Sukumar, M.; Restifo, N.P. Metabolic Regulation of T Cell Longevity and Function in Tumor Immunotherapy. Cell Metab. 2017, 26, 94–109. [Google Scholar] [CrossRef]
- Yang, J.; He, J.; Zhang, X.; Li, J.; Wang, Z.; Zhang, Y.; Qiu, L.; Wu, Q.; Sun, Z.; Ye, X.; et al. Next-Day Manufacture of a Novel Anti-CD19 CAR-T Therapy for B-Cell Acute Lymphoblastic Leukemia: First-in-Human Clinical Study. Blood Cancer J. 2022, 12, 104. [Google Scholar] [CrossRef] [PubMed]


| Assessment | Test System | Method | Results |
|---|---|---|---|
| On-target/off-tumor toxicity | PRAME mRNA-positive healthy cells, with or without PRAME VLD | Co-cultures of MDG1011 and healthy test cells: IFN-γ secretion at 24 h Live-cell imaging assay 0–38 h | No recognition of PRAME mRNA-positive healthy cells |
| Off-target/off-tumor toxicity | Human LCL panel (N = 52) with common HLA allotypes: unloaded or loaded with PRAME VLD | Co-cultures of MDG1011 and LCL panel cells: IFN-γ secretion at 24 h | No recognition of unloaded LCL; four PRAME VLD-loaded LCL were recognized that expressed HLA-A*02:07, HLA-A*02:16, and HLA-A*02:17 |
| Mismatched peptide test panel identified using Expitope® 2.0 database: 15 peptides loaded on T2 cells: 1 peptide was expressed as ivtRNA in tumor cell lines | Co-cultures of MDG1011 and mis-matched peptide-pulsed T2 cells or ivtRNA-transfected tumor cell lines: IFN-γ secretion at 24 h | One mismatched peptide cross-recognized on peptide- loaded T2 cells; no recognition of mismatched peptide-encoding ivtRNA in tumor cell lines | |
| PRAME mRNA-negative panel of primary human healthy cell types; unloaded or loaded with VLD peptide | Co-cultures of MDG1011 and healthy test cells: IFN-γ secretion at 24 h Live-cell imaging assay 0–38 h | No cross-recognition of PRAME mRNA-negative test cells of the healthy cell panel |
| IMP | Indication | Age | % CD34+ Tumor Blasts in Apheresis Products 1 |
|---|---|---|---|
| MAR-002 | AML | 58 | 85 |
| MAR-004 | MM | 64 | 0 |
| MAR-006 | AML | 67 | 13 |
| MAR-010 | AML | 58 | 57 |
| MAR-012 | AML | 55 | 1 |
| MAR-014 # | AML | 77 | 82 |
| MAR-016 | AML | 60 | 92 |
| MAR-018 | MM | 60 | 0 |
| MAR-022 # | AML | 65 | 26 |
| MAR-024 * | AML | 77 | 4 |
| MAR-028 | AML | 65 | 7 |
| MAR-030 #* | AML | 69 | 86 |
| MAR-033 | MDS | 80 | 17 |
| Secreted IFN-γ (±SD) 1 | |||
|---|---|---|---|
| IMP | T2_VLD | K562-A2 | Mel624.38 |
| MAR-002 | 1886 (±55) | 2795 (±68) | 988 (±27) |
| MAR-004 | 3443 (±237) | 3420 (±173) | ND |
| MAR-006 | 1738 (±115) | 3287 (±1236) | 971 (±33) |
| MAR-010 | 2200 (±61) | 2461 (±357) | 205 (±12) |
| MAR-012 | 1771 (±83) | 2093 (±557) | 238 (±05) |
| MAR-014 | 772 (±19) | 678 (±92) | 141 (±26) |
| MAR-016 | 1526 (±44) | 931 (±48) | 661 (±83) |
| MAR-018 | 1928 (±44) | 1434 (±80) | 972 (±58) |
| MAR-022 | 455 (±11) | 611 (±49) | 191 (±14) |
| MAR-028 | 1673 (±200) | 2016 (±69) | 589 (±61) |
| MAR-033 | 293 (±19) | 448 (±23) | 51 (±8) |
| % Killing of Target Cells (±SD) 1 | |||
|---|---|---|---|
| IMP | T2_VLD | K562-A2 | Mel624.38 |
| MAR-002 | ND | ND | ND |
| MAR-004 2 | 63 (±2.6) | 66 (±0.3) | ND |
| MAR-006 | 75 (±7.1) | 12 (ND) | 40 (ND) |
| MAR-010 | 66 (±1.0) | 9 (±0.9) | 76 (±6.1) |
| MAR-012 | 45 (±4.8) | 31 (±1.4) | 99 (±0.2) |
| MAR-014 | 96 (±3.3) | 34 (±2.9) | 32 (±20.9) |
| MAR-016 | 64 (±6.2) | 9 (±3.0) | 99 (±0.4) |
| MAR-018 | 85 (±2.0) | 24 (±1.1) | 99 (±0.5) |
| MAR-022 | ND | ND | ND |
| MAR-028 | 99 (±0.9) | 24 (±2.4) | 100 (±0.1) |
| MAR-033 | 95 (±1.0) | 49 (±2.0) | 46 (±9.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bürdek, M.; Prinz, P.U.; Mutze, K.; Tippmer, S.; Geiger, C.; Longinotti, G.; Schendel, D.J. Characterization of a 3S PRAME VLD-Specific T Cell Receptor and Its Use in Investigational Medicinal Products for TCR-T Therapy of Patients with Myeloid Malignancies. Cancers 2025, 17, 242. https://doi.org/10.3390/cancers17020242
Bürdek M, Prinz PU, Mutze K, Tippmer S, Geiger C, Longinotti G, Schendel DJ. Characterization of a 3S PRAME VLD-Specific T Cell Receptor and Its Use in Investigational Medicinal Products for TCR-T Therapy of Patients with Myeloid Malignancies. Cancers. 2025; 17(2):242. https://doi.org/10.3390/cancers17020242
Chicago/Turabian StyleBürdek, Maja, Petra U. Prinz, Kathrin Mutze, Stefanie Tippmer, Christiane Geiger, Giulia Longinotti, and Dolores J. Schendel. 2025. "Characterization of a 3S PRAME VLD-Specific T Cell Receptor and Its Use in Investigational Medicinal Products for TCR-T Therapy of Patients with Myeloid Malignancies" Cancers 17, no. 2: 242. https://doi.org/10.3390/cancers17020242
APA StyleBürdek, M., Prinz, P. U., Mutze, K., Tippmer, S., Geiger, C., Longinotti, G., & Schendel, D. J. (2025). Characterization of a 3S PRAME VLD-Specific T Cell Receptor and Its Use in Investigational Medicinal Products for TCR-T Therapy of Patients with Myeloid Malignancies. Cancers, 17(2), 242. https://doi.org/10.3390/cancers17020242







