Activation of Genes by Nuclear Receptor/Specificity Protein (Sp) Interactions in Cancer
Simple Summary
Abstract
1. Introduction
2. Subgroup III Receptors and Their Interactions with Sp TFs
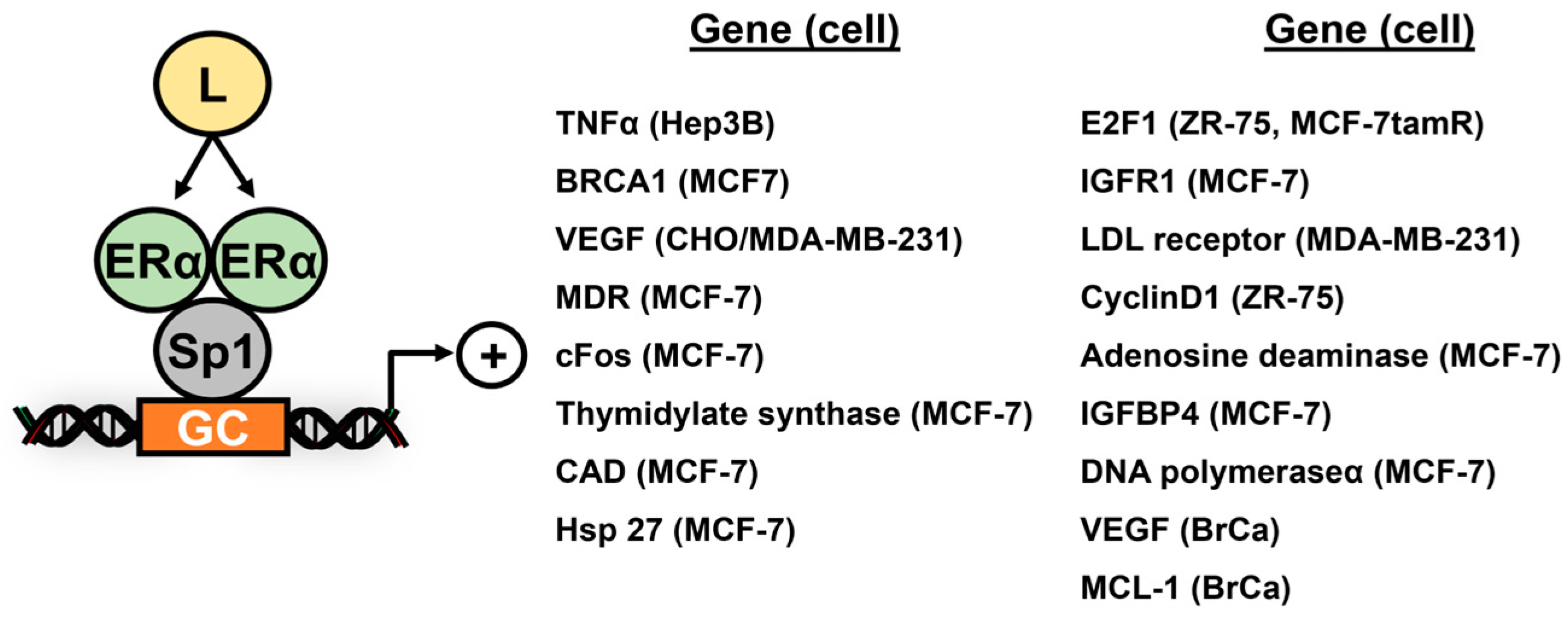
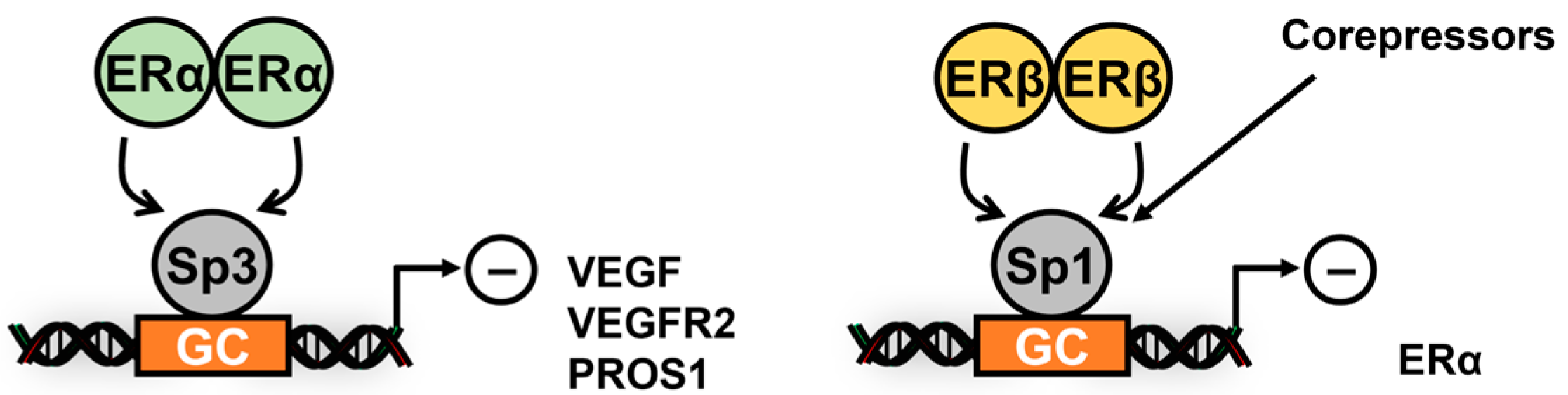
3. Subgroup II Receptor and Their Interactions with Sp TFs
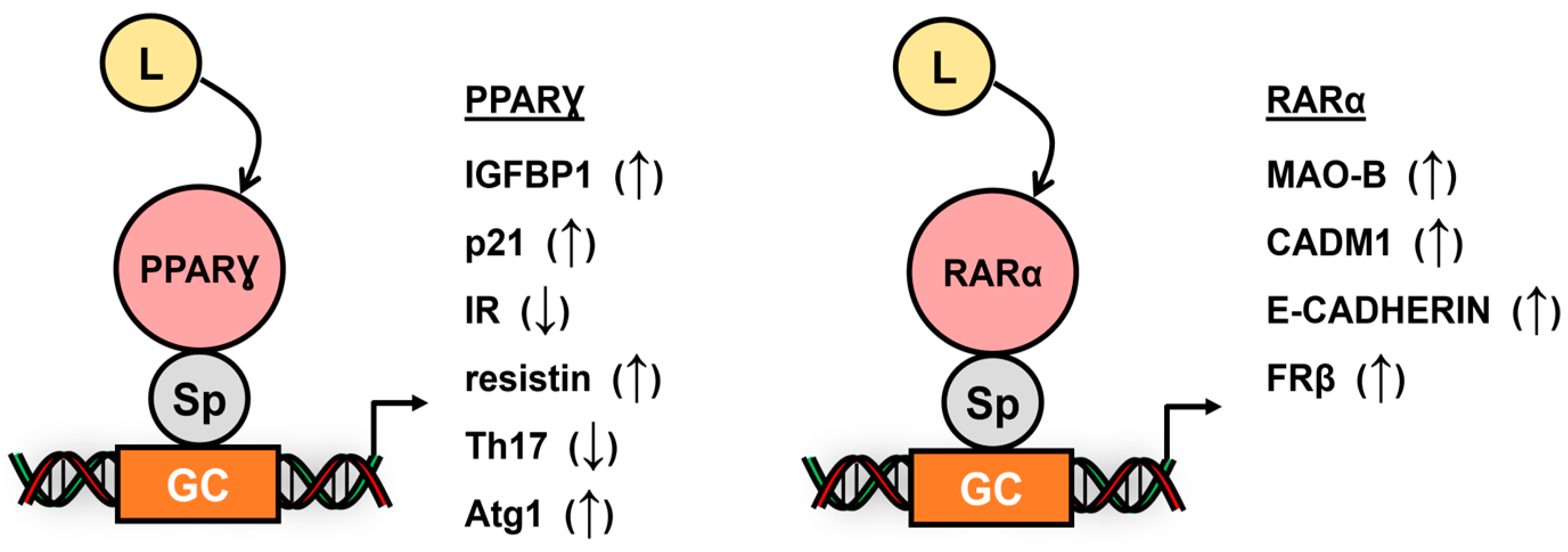
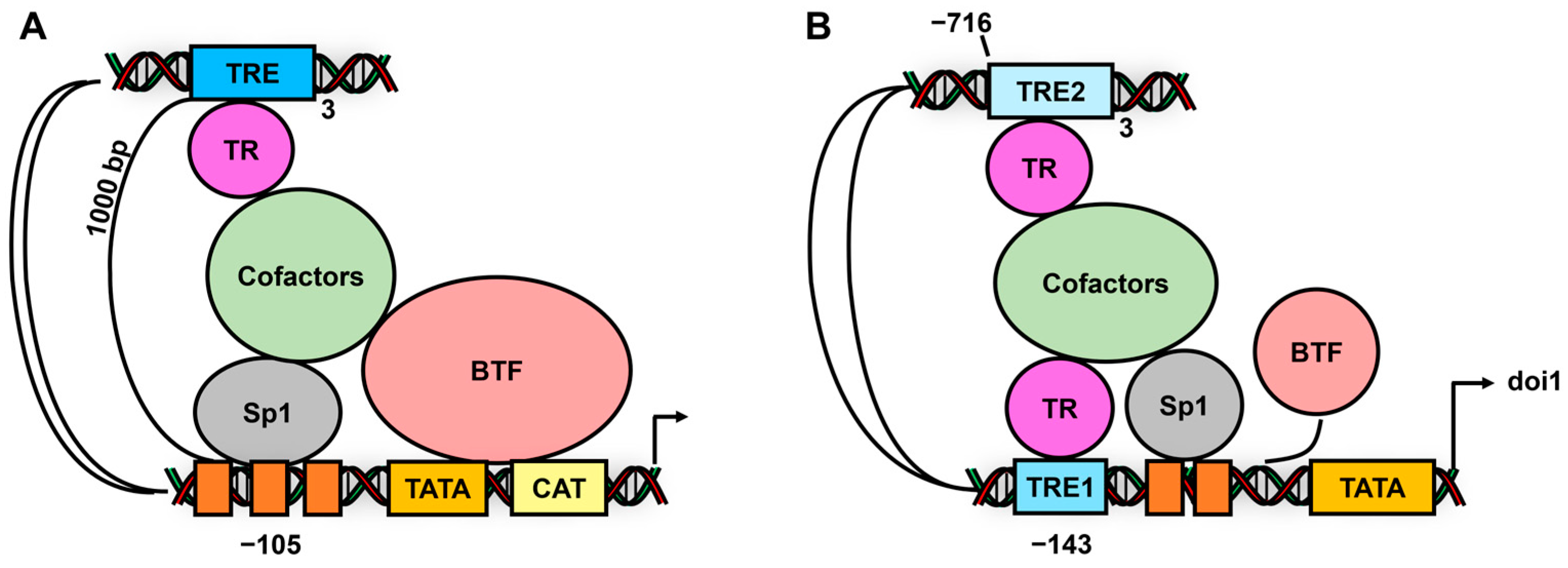
4. Subgroup I Receptors and Their Interactions with Sp TFs
5. Subgroup V, VI and Atypical Receptors Interactions with Sp TFs
6. Subgroup IV Receptors and Their Interactions with Sp1
7. Nuclear Receptor/Sp Variability
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vizcaíno, C.; Mansilla, S.; Portugal, J. Sp1 transcription factor: A long-standing target in cancer chemotherapy. Pharmacol. Ther. 2015, 152, 111–124. [Google Scholar] [CrossRef]
- Kim, C.K.; He, P.; Bialkowska, A.B.; Yang, V.W. SP and KLF Transcription Factors in Digestive Physiology and Diseases. Gastroenterology 2017, 152, 1845–1875. [Google Scholar] [CrossRef]
- Beishline, K.; Azizkhan-Clifford, J. Sp1 and the ‘hallmarks of cancer’. FEBS J. 2015, 282, 224–258. [Google Scholar] [CrossRef]
- Li, L.; Davie, J.R. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann. Anat. 2010, 192, 275–283. [Google Scholar] [CrossRef]
- Dynan, W.S.; Tjian, R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell 1983, 32, 669–680. [Google Scholar] [CrossRef]
- Safe, S. Specificity Proteins (Sp) and Cancer. Int. J. Mol. Sci. 2023, 24, 5164. [Google Scholar] [CrossRef]
- Lou, Z.; O’Reilly, S.; Liang, H.; Maher, V.M.; Sleight, S.D.; McCormick, J.J. Down-regulation of overexpressed sp1 protein in human fibrosarcoma cell lines inhibits tumor formation. Cancer Res. 2005, 65, 1007–1017. [Google Scholar] [CrossRef]
- McCormick, J.J.; Maher, V.M. Malignant transformation of human skin fibroblasts by two alternative pathways. Adv. Exp. Med. Biol. 2011, 720, 191–207. [Google Scholar] [CrossRef]
- Naini, S.; Etheridge, K.T.; Adam, S.J.; Qualman, S.J.; Bentley, R.C.; Counter, C.M.; Linardic, C.M. Defining the cooperative genetic changes that temporally drive alveolar rhabdomyosarcoma. Cancer Res. 2008, 68, 9583–9588. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Baek, H.S.; Ye, D.J.; Shin, S.; Kim, D.; Chun, Y.J. CYP1B1 Enhances Cell Proliferation and Metastasis through Induction of EMT and Activation of Wnt/β-Catenin Signaling via Sp1 Upregulation. PLoS ONE 2016, 11, e0151598. [Google Scholar] [CrossRef]
- Hedrick, E.; Cheng, Y.; Jin, U.H.; Kim, K.; Safe, S. Specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 are non-oncogene addiction genes in cancer cells. Oncotarget 2016, 7, 22245–22256. [Google Scholar] [CrossRef] [PubMed]
- Burris, T.P.; de Vera, I.M.S.; Cote, I.; Flaveny, C.A.; Wanninayake, U.S.; Chatterjee, A.; Walker, J.K.; Steinauer, N.; Zhang, J.; Coons, L.A.; et al. International Union of Basic and Clinical Pharmacology CXIII: Nuclear Receptor Superfamily-Update 2023. Pharmacol. Rev. 2023, 75, 1233–1318. [Google Scholar] [CrossRef]
- Perlmann, T.; Jansson, L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes. Dev. 1995, 9, 769–782. [Google Scholar] [CrossRef]
- Philips, A.; Lesage, S.; Gingras, R.; Maira, M.H.; Gauthier, Y.; Hugo, P.; Drouin, J. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol. Cell. Biol. 1997, 17, 5946–5951. [Google Scholar] [CrossRef]
- Maira, M.; Martens, C.; Philips, A.; Drouin, J. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol. Cell. Biol. 1999, 19, 7549–7557. [Google Scholar] [CrossRef]
- Safe, S. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions. Vitam. Horm. 2001, 62, 231–252. [Google Scholar] [CrossRef]
- Safe, S.; Kim, K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J. Mol. Endocrinol. 2008, 41, 263–275. [Google Scholar] [CrossRef]
- Tu, C.C.; Kumar, V.B.; Day, C.H.; Kuo, W.W.; Yeh, S.P.; Chen, R.J.; Liao, C.R.; Chen, H.Y.; Tsai, F.J.; Wu, W.J.; et al. Estrogen receptor α (ESR1) over-expression mediated apoptosis in Hep3B cells by binding with SP1 proteins. J. Mol. Endocrinol. 2013, 51, 203–212. [Google Scholar] [CrossRef]
- Ngwenya, S.; Safe, S. Cell context-dependent differences in the induction of E2F-1 gene expression by 17 beta-estradiol in MCF-7 and ZR-75 cells. Endocrinology 2003, 144, 1675–1685. [Google Scholar] [CrossRef][Green Version]
- Louie, M.C.; McClellan, A.; Siewit, C.; Kawabata, L. Estrogen receptor regulates E2F1 expression to mediate tamoxifen resistance. Mol. Cancer Res. 2010, 8, 343–352. [Google Scholar] [CrossRef]
- Hockings, J.K.; Degner, S.C.; Morgan, S.S.; Kemp, M.Q.; Romagnolo, D.F. Involvement of a specificity proteins-binding element in regulation of basal and estrogen-induced transcription activity of the BRCA1 gene. Breast Cancer Res. 2008, 10, R29. [Google Scholar] [CrossRef] [PubMed]
- Maor, S.; Mayer, D.; Yarden, R.I.; Lee, A.V.; Sarfstein, R.; Werner, H.; Papa, M.Z. Estrogen receptor regulates insulin-like growth factor-I receptor gene expression in breast tumor cells: Involvement of transcription factor Sp1. J. Endocrinol. 2006, 191, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Brüning, J.C.; Lingohr, P.; Gillette, J.; Hanstein, B.; Avci, H.; Krone, W.; Müller-Wieland, D.; Kotzka, J. Estrogen receptor-alpha and Sp1 interact in the induction of the low density lipoprotein-receptor. J. Steroid Biochem. Mol. Biol. 2003, 86, 113–121. [Google Scholar] [CrossRef]
- Zampieri, L.; Bianchi, P.; Ruff, P.; Arbuthnot, P. Differential modulation by estradiol of P-glycoprotein drug resistance protein expression in cultured MCF7 and T47D breast cancer cells. Anticancer. Res. 2002, 22, 2253–2259. [Google Scholar]
- Castro-Rivera, E.; Samudio, I.; Safe, S. Estrogen regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. J. Biol. Chem. 2001, 276, 30853–30861. [Google Scholar] [CrossRef]
- Dong, L.; Wang, W.; Wang, F.; Stoner, M.; Reed, J.C.; Harigai, M.; Samudio, I.; Kladde, M.P.; Vyhlidal, C.; Safe, S. Mechanisms of transcriptional activation of bcl-2 gene expression by 17beta-estradiol in breast cancer cells. J. Biol. Chem. 1999, 274, 32099–32107. [Google Scholar] [CrossRef]
- Xie, W.; Duan, R.; Safe, S. Estrogen induces adenosine deaminase gene expression in MCF-7 human breast cancer cells: Role of estrogen receptor-Sp1 interactions. Endocrinology 1999, 140, 219–227. [Google Scholar] [CrossRef][Green Version]
- Sun, G.; Porter, W.; Safe, S. Estrogen-induced retinoic acid receptor alpha 1 gene expression: Role of estrogen receptor-Sp1 complex. Mol. Endocrinol. 1998, 12, 882–890. [Google Scholar] [CrossRef][Green Version]
- Duan, R.; Porter, W.; Safe, S. Estrogen-induced c-fos protooncogene expression in MCF-7 human breast cancer cells: Role of estrogen receptor Sp1 complex formation. Endocrinology 1998, 139, 1981–1990. [Google Scholar] [CrossRef][Green Version]
- Qin, C.; Singh, P.; Safe, S. Transcriptional activation of insulin-like growth factor-binding protein-4 by 17beta-estradiol in MCF-7 cells: Role of estrogen receptor-Sp1 complexes. Endocrinology 1999, 140, 2501–2508. [Google Scholar] [CrossRef][Green Version]
- Xie, W.; Duan, R.; Chen, I.; Samudio, I.; Safe, S. Transcriptional activation of thymidylate synthase by 17beta-estradiol in MCF-7 human breast cancer cells. Endocrinology 2000, 141, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Samudio, I.; Vyhlidal, C.; Wang, F.; Stoner, M.; Chen, I.; Kladde, M.; Barhoumi, R.; Burghardt, R.; Safe, S. Transcriptional activation of deoxyribonucleic acid polymerase alpha gene expression in MCF-7 cells by 17 beta-estradiol. Endocrinology 2001, 142, 1000–1008. [Google Scholar] [CrossRef]
- Khan, S.; Abdelrahim, M.; Samudio, I.; Safe, S. Estrogen receptor/Sp1 complexes are required for induction of cad gene expression by 17beta-estradiol in breast cancer cells. Endocrinology 2003, 144, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.; Saville, B.; Hoivik, D.; Safe, S. Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol. Endocrinol. 1997, 11, 1569–1580. [Google Scholar] [CrossRef]
- Stoner, M.; Wormke, M.; Saville, B.; Samudio, I.; Qin, C.; Abdelrahim, M.; Safe, S. Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor alpha and SP proteins. Oncogene 2004, 23, 1052–1063. [Google Scholar] [CrossRef]
- Schacter, J.L.; Henson, E.S.; Gibson, S.B. Estrogen regulation of anti-apoptotic Bcl-2 family member Mcl-1 expression in breast cancer cells. PLoS ONE 2014, 9, e100364. [Google Scholar] [CrossRef]
- Stoner, M.; Wang, F.; Wormke, M.; Nguyen, T.; Samudio, I.; Vyhlidal, C.; Marme, D.; Finkenzeller, G.; Safe, S. Inhibition of vascular endothelial growth factor expression in HEC1A endometrial cancer cells through interactions of estrogen receptor alpha and Sp3 proteins. J. Biol. Chem. 2000, 275, 22769–22779. [Google Scholar] [CrossRef]
- Higgins, K.J.; Liu, S.; Abdelrahim, M.; Vanderlaag, K.; Liu, X.; Porter, W.; Metz, R.; Safe, S. Vascular endothelial growth factor receptor-2 expression is down-regulated by 17beta-estradiol in MCF-7 breast cancer cells by estrogen receptor alpha/Sp proteins. Mol. Endocrinol. 2008, 22, 388–402. [Google Scholar] [CrossRef]
- Suzuki, A.; Sanda, N.; Miyawaki, Y.; Fujimori, Y.; Yamada, T.; Takagi, A.; Murate, T.; Saito, H.; Kojima, T. Down-regulation of PROS1 gene expression by 17beta-estradiol via estrogen receptor alpha (ERalpha)-Sp1 interaction recruiting receptor-interacting protein 140 and the corepressor-HDAC3 complex. J. Biol. Chem. 2010, 285, 13444–13453. [Google Scholar] [CrossRef]
- Bartella, V.; Rizza, P.; Barone, I.; Zito, D.; Giordano, F.; Giordano, C.; Catalano, S.; Mauro, L.; Sisci, D.; Panno, M.L.; et al. Estrogen receptor beta binds Sp1 and recruits a corepressor complex to the estrogen receptor alpha gene promoter. Breast Cancer Res. Treat. 2012, 134, 569–581. [Google Scholar] [CrossRef]
- Jin, W.; Chen, Y.; Di, G.H.; Miron, P.; Hou, Y.F.; Gao, H.; Shao, Z.M. Estrogen receptor (ER) beta or p53 attenuates ERalpha-mediated transcriptional activation on the BRCA2 promoter. J. Biol. Chem. 2008, 283, 29671–29680. [Google Scholar] [CrossRef]
- Mukherjee, T.K.; Reynolds, P.R.; Hoidal, J.R. Differential effect of estrogen receptor alpha and beta agonists on the receptor for advanced glycation end product expression in human microvascular endothelial cells. Biochim. Biophys. Acta 2005, 1745, 300–309. [Google Scholar] [CrossRef]
- Gibson, L.L.; Hahner, L.; Osborne-Lawrence, S.; German, Z.; Wu, K.K.; Chambliss, K.L.; Shaul, P.W. Molecular basis of estrogen-induced cyclooxygenase type 1 upregulation in endothelial cells. Circ. Res. 2005, 96, 518–525. [Google Scholar] [CrossRef]
- Jacobson, D.; Pribnow, D.; Herson, P.S.; Maylie, J.; Adelman, J.P. Determinants contributing to estrogen-regulated expression of SK3. Biochem. Biophys. Res. Commun. 2003, 303, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Saville, B.; Wormke, M.; Wang, F.; Nguyen, T.; Enmark, E.; Kuiper, G.; Gustafsson, J.A.; Safe, S. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J. Biol. Chem. 2000, 275, 5379–5387. [Google Scholar] [CrossRef]
- Kim, K.; Thu, N.; Saville, B.; Safe, S. Domains of estrogen receptor alpha (ERalpha) required for ERalpha/Sp1-mediated activation of GC-rich promoters by estrogens and antiestrogens in breast cancer cells. Mol. Endocrinol. 2003, 17, 804–817. [Google Scholar] [CrossRef]
- Koduri, S.; Goldhar, A.S.; Vonderhaar, B.K. Activation of vascular endothelial growth factor (VEGF) by the ER-alpha variant, ERDelta3. Breast Cancer Res. Treat. 2006, 95, 37–43. [Google Scholar] [CrossRef]
- Kim, K.; Barhoumi, R.; Burghardt, R.; Safe, S. Analysis of estrogen receptor alpha-Sp1 interactions in breast cancer cells by fluorescence resonance energy transfer. Mol. Endocrinol. 2005, 19, 843–854. [Google Scholar] [CrossRef]
- Lee, J.; Safe, S. Coactivation of estrogen receptor alpha (ER alpha)/Sp1 by vitamin D receptor interacting protein 150 (DRIP150). Arch. Biochem. Biophys. 2007, 461, 200–210. [Google Scholar] [CrossRef][Green Version]
- Krishnan, V.; Wang, X.; Safe, S. Estrogen receptor-Sp1 complexes mediate estrogen-induced cathepsin D gene expression in MCF-7 human breast cancer cells. J. Biol. Chem. 1994, 269, 15912–15917. [Google Scholar] [CrossRef]
- Batistuzzo de Medeiros, S.R.; Krey, G.; Hihi, A.K.; Wahli, W. Functional interactions between the estrogen receptor and the transcription activator Sp1 regulate the estrogen-dependent transcriptional activity of the vitellogenin A1 io promoter. J. Biol. Chem. 1997, 272, 18250–18260. [Google Scholar] [CrossRef] [PubMed]
- Vyhlidal, C.; Samudio, I.; Kladde, M.P.; Safe, S. Transcriptional activation of transforming growth factor alpha by estradiol: Requirement for both a GC-rich site and an estrogen response element half-site. J. Mol. Endocrinol. 2000, 24, 329–338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petz, L.N.; Ziegler, Y.S.; Schultz, J.R.; Kim, H.; Kemper, J.K.; Nardulli, A.M. Differential regulation of the human progesterone receptor gene through an estrogen response element half site and Sp1 sites. J. Steroid Biochem. Mol. Biol. 2004, 88, 113–122. [Google Scholar] [CrossRef]
- Stossi, F.; Likhite, V.S.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Estrogen-occupied estrogen receptor represses cyclin G2 gene expression and recruits a repressor complex at the cyclin G2 promoter. J. Biol. Chem. 2006, 281, 16272–16278. [Google Scholar] [CrossRef]
- Shi, J.F.; Yang, N.; Ding, H.J.; Zhang, J.X.; Hu, M.L.; Leng, Y.; Han, X.; Sun, Y.J. ERα directly activated the MDR1 transcription to increase paclitaxel-resistance of ERα-positive breast cancer cells in vitro and in vivo. Int. J. Biochem. Cell Biol. 2014, 53, 35–45. [Google Scholar] [CrossRef]
- Mo, X.M.; Li, L.; Zhu, P.; Dai, Y.J.; Zhao, T.T.; Liao, L.Y.; Chen, G.G.; Liu, Z.M. Up-regulation of Hsp27 by ERα/Sp1 facilitates proliferation and confers resistance to apoptosis in human papillary thyroid cancer cells. Mol. Cell. Endocrinol. 2016, 431, 71–87. [Google Scholar] [CrossRef]
- Wang, F.; Samudio, I.; Safe, S. Transcriptional activation of rat creatine kinase B by 17beta-estradiol in MCF-7 cells involves an estrogen responsive element and GC-rich sites. J. Cell. Biochem. 2001, 84, 156–172. [Google Scholar] [CrossRef]
- Fujita, N.; Kajita, M.; Taysavang, P.; Wade, P.A. Hormonal regulation of metastasis-associated protein 3 transcription in breast cancer cells. Mol. Endocrinol. 2004, 18, 2937–2949. [Google Scholar] [CrossRef]
- Burek, M.; Steinberg, K.; Förster, C.Y. Mechanisms of transcriptional activation of the mouse claudin-5 promoter by estrogen receptor alpha and beta. Mol. Cell. Endocrinol. 2014, 392, 144–151. [Google Scholar] [CrossRef]
- Wu, F.; Khan, S.; Wu, Q.; Barhoumi, R.; Burghardt, R.; Safe, S. Ligand structure-dependent activation of estrogen receptor α/Sp by estrogens and xenoestrogens. J. Steroid Biochem. Mol. Biol. 2008, 110, 104–115. [Google Scholar] [CrossRef]
- Khan, S.; Wu, F.; Liu, S.; Wu, Q.; Safe, S. Role of specificity protein transcription factors in estrogen-induced gene expression in MCF-7 breast cancer cells. J. Mol. Endocrinol. 2007, 39, 289–304. [Google Scholar] [CrossRef]
- Gizard, F.; Robillard, R.; Gross, B.; Barbier, O.; Révillion, F.; Peyrat, J.P.; Torpier, G.; Hum, D.W.; Staels, B. TReP-132 is a novel progesterone receptor coactivator required for the inhibition of breast cancer cell growth and enhancement of differentiation by progesterone. Mol. Cell. Biol. 2006, 26, 7632–7644. [Google Scholar] [CrossRef] [PubMed]
- Faivre, E.J.; Daniel, A.R.; Hillard, C.J.; Lange, C.A. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol. Endocrinol. 2008, 22, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Goldhar, A.S.; Duan, R.; Ginsburg, E.; Vonderhaar, B.K. Progesterone induces expression of the prolactin receptor gene through cooperative action of Sp1 and C/EBP. Mol. Cell. Endocrinol. 2011, 335, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Hardy, D.B.; Mendelson, C.R. Progesterone receptor inhibits proliferation of human breast cancer cells via induction of MAPK phosphatase 1 (MKP-1/DUSP1). J. Biol. Chem. 2011, 286, 43091–43102. [Google Scholar] [CrossRef]
- Dressing, G.E.; Knutson, T.P.; Schiewer, M.J.; Daniel, A.R.; Hagan, C.R.; Diep, C.H.; Knudsen, K.E.; Lange, C.A. Progesterone receptor-cyclin D1 complexes induce cell cycle-dependent transcriptional programs in breast cancer cells. Mol. Endocrinol. 2014, 28, 442–457. [Google Scholar] [CrossRef]
- You, Y.; Tan, W.; Guo, Y.; Luo, M.; Shang, F.F.; Xia, Y.; Luo, S. Progesterone promotes endothelial nitric oxide synthase expression through enhancing nuclear progesterone receptor-SP-1 formation. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H341–H348. [Google Scholar] [CrossRef]
- Yuan, H.; Young, C.Y.; Tian, Y.; Liu, Z.; Zhang, M.; Lou, H. Suppression of the androgen receptor function by quercetin through protein-protein interactions of Sp1, c-Jun, and the androgen receptor in human prostate cancer cells. Mol. Cell. Biochem. 2010, 339, 253–262. [Google Scholar] [CrossRef]
- Chen, S.; Supakar, P.C.; Vellanoweth, R.L.; Song, C.S.; Chatterjee, B.; Roy, A.K. Functional role of a conformationally flexible homopurine/homopyrimidine domain of the androgen receptor gene promoter interacting with Sp1 and a pyrimidine single strand DNA-binding protein. Mol. Endocrinol. 1997, 11, 3–15. [Google Scholar] [CrossRef]
- McDermott, A.; Kim, K.; Kasper, S.; Ho, S.M.; Leung, Y.K. The androgen receptor inhibits transcription of GPER1 by preventing Sp1 and Sp3 from binding to the promoters in prostate cancer cells. Oncotarget 2022, 13, 46–60. [Google Scholar] [CrossRef]
- Diao, X.; Chen, X.; Pi, Y.; Zhang, Y.; Wang, F.; Liu, P.; Gao, Y.; Wang, X.; Yang, S.; Lu, S. Androgen receptor induces EPHA3 expression by interacting with transcription factor SP1. Oncol. Rep. 2018, 40, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
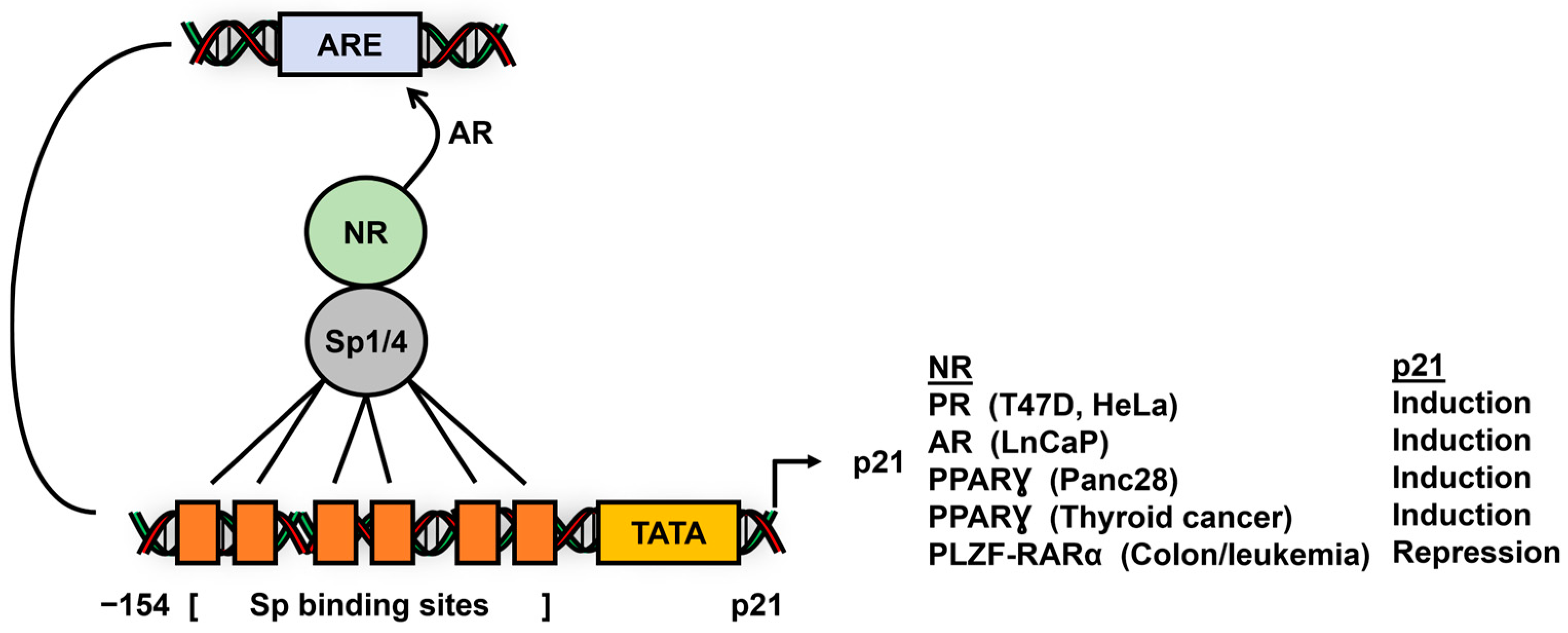
- Lu, S.; Jenster, G.; Epner, D.E. Androgen induction of cyclin-dependent kinase inhibitor p21 gene: Role of androgen receptor and transcription factor Sp1 complex. Mol. Endocrinol. 2000, 14, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Tsao, Y.P.; Wang, C.C.; Chen, S.L. Nuclear receptor interaction protein, a coactivator of androgen receptors (AR), is regulated by AR and Sp1 to feed forward and activate its own gene expression through AR protein stability. Nucleic Acids Res. 2008, 36, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.; Jenkins, S.; Farmer, N.; Anderson, A.C.; Haisenleder, D.J.; Rissman, E.; Wilson, E.M.; Shupnik, M.A. Androgen suppression of GnRH-stimulated rat LHbeta gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Mol. Endocrinol. 2001, 15, 1906–1917. [Google Scholar] [CrossRef][Green Version]
- Ou, X.M.; Chen, K.; Shih, J.C. Glucocorticoid and androgen activation of monoamine oxidase A is regulated differently by R1 and Sp1. J. Biol. Chem. 2006, 281, 21512–21525. [Google Scholar] [CrossRef]
- Chen, K.; Ou, X.M.; Wu, J.B.; Shih, J.C. Transcription factor E2F-associated phosphoprotein (EAPP), RAM2/CDCA7L/JPO2 (R1), and simian virus 40 promoter factor 1 (Sp1) cooperatively regulate glucocorticoid activation of monoamine oxidase B. Mol. Pharmacol. 2011, 79, 308–317. [Google Scholar] [CrossRef]
- Castet, A.; Herledan, A.; Bonnet, S.; Jalaguier, S.; Vanacker, J.M.; Cavaillès, V. Receptor-interacting protein 140 differentially regulates estrogen receptor-related receptor transactivation depending on target genes. Mol. Endocrinol. 2006, 20, 1035–1047. [Google Scholar] [CrossRef]
- El-Mayet, F.S.; Jones, C. Specificity protein 1 (Sp1) and glucocorticoid receptor (GR) stimulate bovine alphaherpesvirus 1 (BoHV-1) replication and cooperatively transactivate the immediate early transcription unit 1 promoter. J. Virol. 2024, 98, e0143623. [Google Scholar] [CrossRef]
- O’Carroll, A.M.; Lolait, S.J.; Howell, G.M. Transcriptional regulation of the rat apelin receptor gene: Promoter cloning and identification of an Sp1 site necessary for promoter activity. J. Mol. Endocrinol. 2006, 36, 221–235. [Google Scholar] [CrossRef][Green Version]
- El-Mayet, F.S.; Santos, V.C.; Wijesekera, N.; Lubbers, S.; Harrison, K.S.; Sadeghi, H.; Jones, C. Glucocorticoid receptor and specificity protein 1 (Sp1) or Sp3, but not the antibiotic Mithramycin A, stimulates human alphaherpesvirus 1 (HSV-1) replication. Antivir. Res. 2024, 225, 105870. [Google Scholar] [CrossRef]
- Nichol, D.; Christian, M.; Steel, J.H.; White, R.; Parker, M.G. RIP140 expression is stimulated by estrogen-related receptor alpha during adipogenesis. J. Biol. Chem. 2006, 281, 32140–32147. [Google Scholar] [CrossRef] [PubMed]
- Meinel, S.; Ruhs, S.; Schumann, K.; Strätz, N.; Trenkmann, K.; Schreier, B.; Grosse, I.; Keilwagen, J.; Gekle, M.; Grossmann, C. Mineralocorticoid receptor interaction with SP1 generates a new response element for pathophysiologically relevant gene expression. Nucleic Acids Res. 2013, 41, 8045–8060. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Yin, P.; Xue, Q.; Yilmaz, B.; Dawson, M.I.; Bulun, S.E. Retinoic acid (RA) regulates 17beta-hydroxysteroid dehydrogenase type 2 expression in endometrium: Interaction of RA receptors with specificity protein (SP) 1/SP3 for estradiol metabolism. J. Clin. Endocrinol. Metab. 2008, 93, 1915–1923. [Google Scholar] [CrossRef]
- Horie, S.; Ishii, H.; Matsumoto, F.; Kusano, M.; Kizaki, K.; Matsuda, J.; Kazama, M. Acceleration of thrombomodulin gene transcription by retinoic acid: Retinoic acid receptors and Sp1 regulate the promoter activity through interactions with two different sequences in the 5′-flanking region of human gene. J. Biol. Chem. 2001, 276, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Thymiakou, E.; Zannis, V.I.; Kardassis, D. Physical and functional interactions between liver X receptor/retinoid X receptor and Sp1 modulate the transcriptional induction of the human ATP binding cassette transporter A1 gene by oxysterols and retinoids. Biochemistry 2007, 46, 11473–11483. [Google Scholar] [CrossRef] [PubMed]
- Shimada, J.; Suzuki, Y.; Kim, S.J.; Wang, P.C.; Matsumura, M.; Kojima, S. Transactivation via RAR/RXR-Sp1 interaction: Characterization of binding between Sp1 and GC box motif. Mol. Endocrinol. 2001, 15, 1677–1692. [Google Scholar] [CrossRef]
- Suzuki, Y.; Shimada, J.; Shudo, K.; Matsumura, M.; Crippa, M.P.; Kojima, S. Physical interaction between retinoic acid receptor and Sp1: Mechanism for induction of urokinase by retinoic acid. Blood 1999, 93, 4264–4276. [Google Scholar] [CrossRef]
- Borel, V.; Marceau, G.; Gallot, D.; Blanchon, L.; Sapin, V. Retinoids regulate human amniotic tissue-type plasminogen activator gene by a two-step mechanism. J. Cell. Mol. Med. 2010, 14, 1793–1805. [Google Scholar] [CrossRef]
- Krey, G.; Mahfoudi, A.; Wahli, W. Functional interactions of peroxisome proliferator-activated receptor, retinoid-X receptor, and Sp1 in the transcriptional regulation of the acyl-coenzyme-A oxidase promoter. Mol. Endocrinol. 1995, 9, 219–231. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Elsner, T.; Ramírez, J.R.; Sanz-Rodriguez, F.; Varela, E.; Bernabéu, C.; Botella, L.M. A cross-talk between hypoxia and TGF-beta orchestrates erythropoietin gene regulation through SP1 and Smads. J. Mol. Biol. 2004, 336, 9–24. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ho, P.C.; Lan, C.Y.; Chang, M.D. Transcriptional regulation of human eosinophil RNase2 by the liver-enriched hepatocyte nuclear factor 4. J. Cell. Biochem. 2009, 106, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Matsuura, N.; Kurokawa, T.; Takahashi, Y.; Miura, T. Co-operation of the transcription factor hepatocyte nuclear factor-4 with Sp1 or Sp3 leads to transcriptional activation of the human haem oxygenase-1 gene promoter in a hepatoma cell line. Biochem. J. 2002, 367, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Kardassis, D.; Tzameli, I.; Hadzopoulou-Cladaras, M.; Talianidis, I.; Zannis, V. Distal apolipoprotein C-III regulatory elements F to J act as a general modular enhancer for proximal promoters that contain hormone response elements. Synergism between hepatic nuclear factor-4 molecules bound to the proximal promoter and distal enhancer sites. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Talianidis, I.; Tambakaki, A.; Toursounova, J.; Zannis, V.I. Complex interactions between SP1 bound to multiple distal regulatory sites and HNF-4 bound to the proximal promoter lead to transcriptional activation of liver-specific human APOCIII gene. Biochemistry 1995, 34, 10298–10309. [Google Scholar] [CrossRef]
- Lavrentiadou, S.N.; Hadzopoulou-Cladaras, M.; Kardassis, D.; Zannis, V.I. Binding specificity and modulation of the human ApoCIII promoter activity by heterodimers of ligand-dependent nuclear receptors. Biochemistry 1999, 38, 964–975. [Google Scholar] [CrossRef]
- Kim, E.; Yang, Z.; Liu, N.C.; Chang, C. Induction of apolipoprotein E expression by TR4 orphan nuclear receptor via 5′ proximal promoter region. Biochem. Biophys. Res. Commun. 2005, 328, 85–90. [Google Scholar] [CrossRef]
- Pipaón, C.; Tsai, S.Y.; Tsai, M.J. COUP-TF upregulates NGFI-A gene expression through an Sp1 binding site. Mol. Cell. Biol. 1999, 19, 2734–2745. [Google Scholar] [CrossRef]
- Rohr, O.; Aunis, D.; Schaeffer, E. COUP-TF and Sp1 interact and cooperate in the transcriptional activation of the human immunodeficiency virus type 1 long terminal repeat in human microglial cells. J. Biol. Chem. 1997, 272, 31149–31155. [Google Scholar] [CrossRef]
- Schwartz, C.; Catez, P.; Rohr, O.; Lecestre, D.; Aunis, D.; Schaeffer, E. Functional interactions between C/EBP, Sp1, and COUP-TF regulate human immunodeficiency virus type 1 gene transcription in human brain cells. J. Virol. 2000, 74, 65–73. [Google Scholar] [CrossRef]
- Sack, M.N.; Disch, D.L.; Rockman, H.A.; Kelly, D.P. A role for Sp and nuclear receptor transcription factors in a cardiac hypertrophic growth program. Proc. Natl. Acad. Sci. USA 1997, 94, 6438–6443. [Google Scholar] [CrossRef]
- Zhang, Y.; Dufau, M.L. Repression of the luteinizing hormone receptor gene promoter by cross talk among EAR3/COUP-TFI, Sp1/Sp3, and TFIIB. Mol. Cell. Biol. 2003, 23, 6958–6972. [Google Scholar] [CrossRef]
- Mehanovic, S.; Mendoza-Villarroel, R.E.; Viger, R.S.; Tremblay, J.J. The Nuclear Receptor COUP-TFII Regulates Amhr2 Gene Transcription via a GC-Rich Promoter Element in Mouse Leydig Cells. J. Endocr. Soc. 2019, 3, 2236–2257. [Google Scholar] [CrossRef] [PubMed]
- Elmi, M.; Matsumoto, Y.; Zeng, Z.J.; Lakshminarasimhan, P.; Yang, W.; Uemura, A.; Nishikawa, S.; Moshiri, A.; Tajima, N.; Agren, H.; et al. TLX activates MASH1 for induction of neuronal lineage commitment of adult hippocampal neuroprogenitors. Mol. Cell. Neurosci. 2010, 45, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wu, J.; Zheng, F.; Hann, S.S.; Chen, Y. Emodin Increases Expression of Insulin-Like Growth Factor Binding Protein 1 through Activation of MEK/ERK/AMPKα and Interaction of PPARγ and Sp1 in Lung Cancer. Cell. Physiol. Biochem. 2017, 41, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, D.; Assante, G.; Moricca, S.; Colantuoni, V.; Lupo, A. Cladosporol A, a new peroxisome proliferator-activated receptor γ (PPARγ) ligand, inhibits colorectal cancer cells proliferation through β-catenin/TCF pathway inactivation. Biochim. Biophys. Acta 2014, 1840, 2361–2372. [Google Scholar] [CrossRef]
- Bonofiglio, D.; Qi, H.; Gabriele, S.; Catalano, S.; Aquila, S.; Belmonte, M.; Andò, S. Peroxisome proliferator-activated receptor gamma inhibits follicular and anaplastic thyroid carcinoma cells growth by upregulating p21Cip1/WAF1 gene in a Sp1-dependent manner. Endocr. Relat. Cancer 2008, 15, 545–557. [Google Scholar] [CrossRef]
- Hong, J.; Samudio, I.; Liu, S.; Abdelrahim, M.; Safe, S. Peroxisome proliferator-activated receptor gamma-dependent activation of p21 in Panc-28 pancreatic cancer cells involves Sp1 and Sp4 proteins. Endocrinology 2004, 145, 5774–5785. [Google Scholar] [CrossRef]
- Costa, V.; Foti, D.; Paonessa, F.; Chiefari, E.; Palaia, L.; Brunetti, G.; Gulletta, E.; Fusco, A.; Brunetti, A. The insulin receptor: A new anticancer target for peroxisome proliferator-activated receptor-gamma (PPARgamma) and thiazolidinedione-PPARgamma agonists. Endocr. Relat. Cancer 2008, 15, 325–335. [Google Scholar] [CrossRef]
- Singh, A.K.; Battu, A.; Mohareer, K.; Hasnain, S.E.; Ehtesham, N.Z. Transcription of human resistin gene involves an interaction of Sp1 with peroxisome proliferator-activating receptor gamma (PPARgamma). PLoS ONE 2010, 5, e9912. [Google Scholar] [CrossRef]
- Miao, Y.; Wu, X.; Xue, X.; Ma, X.; Yang, L.; Zeng, X.; Hu, Y.; Dai, Y.; Wei, Z. Morin, the PPARγ agonist, inhibits Th17 differentiation by limiting fatty acid synthesis in collagen-induced arthritis. Cell Biol. Toxicol. 2023, 39, 1433–1452. [Google Scholar] [CrossRef]
- Roy, D.; Farabaugh, K.T.; Wu, J.; Charrier, A.; Smas, C.; Hatzoglou, M.; Thirumurugan, K.; Buchner, D.A. Coordinated transcriptional control of adipocyte triglyceride lipase (Atgl) by transcription factors Sp1 and peroxisome proliferator-activated receptor γ (PPARγ) during adipocyte differentiation. J. Biol. Chem. 2017, 292, 14827–14835. [Google Scholar] [CrossRef] [PubMed]
- Aguiló, F.; Camarero, N.; Relat, J.; Marrero, P.F.; Haro, D. Transcriptional regulation of the human acetoacetyl-CoA synthetase gene by PPARgamma. Biochem. J. 2010, 427, 255–264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Necela, B.M.; Su, W.; Thompson, E.A. Peroxisome proliferator-activated receptor gamma down-regulates follistatin in intestinal epithelial cells through SP1. J. Biol. Chem. 2008, 283, 29784–29794. [Google Scholar] [CrossRef] [PubMed]
- Sassa, Y.; Hata, Y.; Aiello, L.P.; Taniguchi, Y.; Kohno, K.; Ishibashi, T. Bifunctional properties of peroxisome proliferator-activated receptor gamma1 in KDR gene regulation mediated via interaction with both Sp1 and Sp3. Diabetes 2004, 53, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, A.; Takeuchi, K.; Uruno, A.; Kudo, M.; Sato, K.; Ito, S. Effects of mitogen-activated protein kinase pathway and co-activator CREP-binding protein on peroxisome proliferator-activated receptor-gamma-mediated transcription suppression of angiotensin II type 1 receptor gene. Hypertens. Res. 2003, 26, 623–628. [Google Scholar] [CrossRef][Green Version]
- Sugawara, A.; Uruno, A.; Kudo, M.; Ikeda, Y.; Sato, K.; Taniyama, Y.; Ito, S.; Takeuchi, K. Transcription suppression of thromboxane receptor gene by peroxisome proliferator-activated receptor-gamma via an interaction with Sp1 in vascular smooth muscle cells. J. Biol. Chem. 2002, 277, 9676–9683. [Google Scholar] [CrossRef]
- Meissner, M.; Stein, M.; Urbich, C.; Reisinger, K.; Suske, G.; Staels, B.; Kaufmann, R.; Gille, J. PPARalpha activators inhibit vascular endothelial growth factor receptor-2 expression by repressing Sp1-dependent DNA binding and transactivation. Circ. Res. 2004, 94, 324–332. [Google Scholar] [CrossRef]
- Leuenberger, N.; Pradervand, S.; Wahli, W. Sumoylated PPARalpha mediates sex-specific gene repression and protects the liver from estrogen-induced toxicity in mice. J. Clin. Investig. 2009, 119, 3138–3148. [Google Scholar] [CrossRef]
- Gizard, F.; Amant, C.; Barbier, O.; Bellosta, S.; Robillard, R.; Percevault, F.; Sevestre, H.; Krimpenfort, P.; Corsini, A.; Rochette, J.; et al. PPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J. Clin. Investig. 2005, 115, 3228–3238. [Google Scholar] [CrossRef]
- Husmann, M.; Dragneva, Y.; Romahn, E.; Jehnichen, P. Nuclear receptors modulate the interaction of Sp1 and GC-rich DNA via ternary complex formation. Biochem. J. 2000, 352 Pt. 3, 763–772. [Google Scholar] [CrossRef]
- Wu, J.B.; Chen, K.; Ou, X.M.; Shih, J.C. Retinoic acid activates monoamine oxidase B promoter in human neuronal cells. J. Biol. Chem. 2009, 284, 16723–16735. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Williams-Nate, Y.; Iwai, M.; Tsuboi, Y.; Hagiyama, M.; Ito, A.; Sakurai-Yageta, M.; Murakami, Y. Transcriptional regulation of the CADM1 gene by retinoic acid during the neural differentiation of murine embryonal carcinoma P19 cells. Genes. Cells 2011, 16, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.J.; Jang, K.L. All-trans retinoic acid activates E-cadherin expression via promoter hypomethylation in the human colon carcinoma HCT116 cells. Biochem. Biophys. Res. Commun. 2012, 425, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Qi, H.; Ratnam, M. Modulation of the folate receptor type beta gene by coordinate actions of retinoic acid receptors at activator Sp1/ets and repressor AP-1 sites. Blood 2003, 101, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Yoon, J.H.; Kim, M.Y.; Koh, D.I.; Licht, J.D.; Kim, K.; Hur, M.W. Promyelocytic leukemia zinc finger-retinoic acid receptor α (PLZF-RARα), an oncogenic transcriptional repressor of cyclin-dependent kinase inhibitor 1A (p21WAF/CDKN1A) and tumor protein p53 (TP53) genes. J. Biol. Chem. 2014, 289, 18641–18656. [Google Scholar] [CrossRef]
- Kwon, H.S.; Huang, B.; Ho Jeoung, N.; Wu, P.; Steussy, C.N.; Harris, R.A. Retinoic acids and trichostatin A (TSA), a histone deacetylase inhibitor, induce human pyruvate dehydrogenase kinase 4 (PDK4) gene expression. Biochim. Biophys. Acta 2006, 1759, 141–151. [Google Scholar] [CrossRef]
- Kumar, P.; Garg, R.; Bolden, G.; Pandey, K.N. Interactive roles of Ets-1, Sp1, and acetylated histones in the retinoic acid-dependent activation of guanylyl cyclase/atrial natriuretic peptide receptor-A gene transcription. J. Biol. Chem. 2010, 285, 37521–37530. [Google Scholar] [CrossRef]
- Lv, X.R.; Zheng, B.; Li, S.Y.; Han, A.L.; Wang, C.; Shi, J.H.; Zhang, X.H.; Liu, Y.; Li, Y.H.; Wen, J.K. Synthetic retinoid Am80 up-regulates apelin expression by promoting interaction of RARα with KLF5 and Sp1 in vascular smooth muscle cells. Biochem. J. 2013, 456, 35–46. [Google Scholar] [CrossRef]
- Encarnacao, P.C.; Ramirez, V.P.; Zhang, C.; Aneskievich, B.J. Sp sites contribute to basal and inducible expression of the human TNIP1 (TNFα-inducible protein 3-interacting protein 1) promoter. Biochem. J. 2013, 452, 519–529. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, J.Y.; Hung, W.C. Vitamin D3 receptor/Sp1 complex is required for the induction of p27Kip1 expression by vitamin D3. Oncogene 2004, 23, 4856–4861. [Google Scholar] [CrossRef][Green Version]
- Cheng, H.T.; Chen, J.Y.; Huang, Y.C.; Chang, H.C.; Hung, W.C. Functional role of VDR in the activation of p27Kip1 by the VDR/Sp1 complex. J. Cell. Biochem. 2006, 98, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Fleet, J.C. Phorbol esters enhance 1α,25-dihydroxyvitamin D3-regulated 25-hydroxyvitamin D-24-hydroxylase (CYP24A1) gene expression through ERK-mediated phosphorylation of specific protein 3 (Sp3) in Caco-2 cells. Mol. Cell. Endocrinol. 2012, 361, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.E.; Hetherington, C.J.; Gonzalez, D.A.; Chen, H.M.; Tenen, D.G. Regulation of CD14 expression during monocytic differentiation induced with 1 alpha,25-dihydroxyvitamin D3. J. Immunol. 1994, 153, 3276–3284. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, J.S.; Chung, J.H. In vivo transcription factor recruitment during thyroid hormone receptor-mediated activation. Proc. Natl. Acad. Sci. USA 1999, 96, 10092–10097. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Kim, S.; Harney, J.W.; Larsen, P.R. Further characterization of thyroid hormone response elements in the human type 1 iodothyronine deiodinase gene. Endocrinology 1998, 139, 1156–1163. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.H.; Oh, S.Y.; Cho, D.H.; Kim, S.; Jo, I. Zearalenone-Induced Interaction between PXR and Sp1 Increases Binding of Sp1 to a Promoter Site of the eNOS, Decreasing Its Transcription and NO Production in BAECs. Toxins 2020, 12, 421. [Google Scholar] [CrossRef]
- Schmitz, G.; Langmann, T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim. Biophys. Acta 2005, 1735, 1–19. [Google Scholar] [CrossRef]
- Negoro, H.; Okinami, T.; Kanematsu, A.; Imamura, M.; Tabata, Y.; Ogawa, O. Role of Rev-erbα domains for transactivation of the connexin43 promoter with Sp1. FEBS Lett. 2013, 587, 98–103. [Google Scholar] [CrossRef]
- Liu, Z.; Simpson, E.R. Molecular mechanism for cooperation between Sp1 and steroidogenic factor-1 (SF-1) to regulate bovine CYP11A gene expression. Mol. Cell. Endocrinol. 1999, 153, 183–196. [Google Scholar] [CrossRef]
- Sugawara, T.; Saito, M.; Fujimoto, S. Sp1 and SF-1 interact and cooperate in the regulation of human steroidogenic acute regulatory protein gene expression. Endocrinology 2000, 141, 2895–2903. [Google Scholar] [CrossRef]
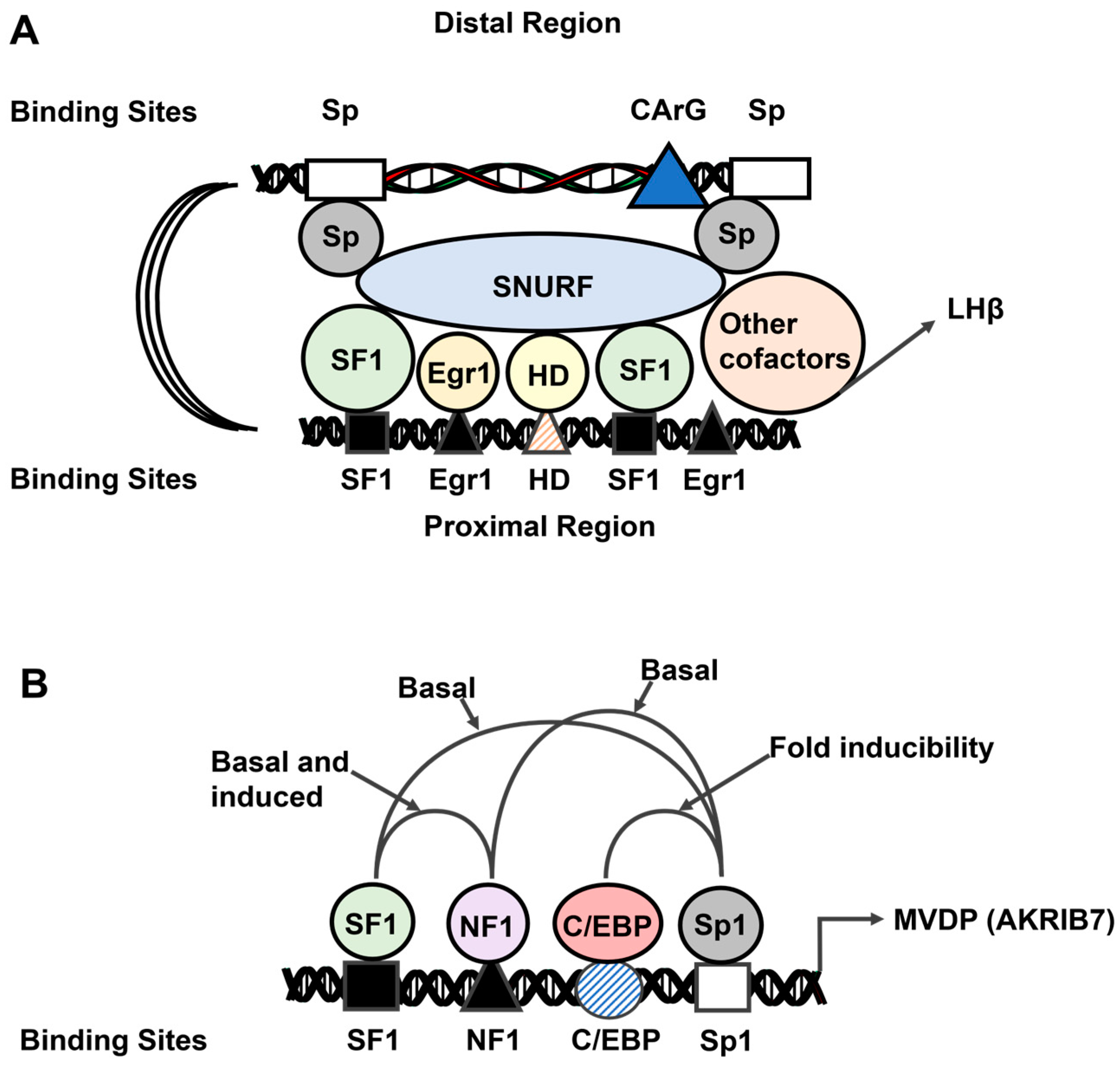
- Kaiser, U.B.; Halvorson, L.M.; Chen, M.T. Sp1, steroidogenic factor 1 (SF-1), and early growth response protein 1 (egr-1) binding sites form a tripartite gonadotropin-releasing hormone response element in the rat luteinizing hormone-beta gene promoter: An integral role for SF-1. Mol. Endocrinol. 2000, 14, 1235–1245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Curtin, D.; Ferris, H.A.; Häkli, M.; Gibson, M.; Jänne, O.A.; Palvimo, J.J.; Shupnik, M.A. Small nuclear RING finger protein stimulates the rat luteinizing hormone-beta promoter by interacting with Sp1 and steroidogenic factor-1 and protects from androgen suppression. Mol. Endocrinol. 2004, 18, 1263–1276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aigueperse, C.; Val, P.; Pacot, C.; Darne, C.; Lalli, E.; Sassone-Corsi, P.; Veyssiere, G.; Jean, C.; Martinez, A. SF-1 (steroidogenic factor-1), C/EBPbeta (CCAAT/enhancer binding protein), and ubiquitous transcription factors NF1 (nuclear factor 1) and Sp1 (selective promoter factor 1) are required for regulation of the mouse aldose reductase-like gene (AKR1B7) expression in adrenocortical cells. Mol. Endocrinol. 2001, 15, 93–111. [Google Scholar] [CrossRef] [PubMed]
- de Santa Barbara, P.; Méjean, C.; Moniot, B.; Malclès, M.H.; Berta, P.; Boizet-Bonhoure, B. Steroidogenic factor-1 contributes to the cyclic-adenosine monophosphate down-regulation of human SRY gene expression. Biol. Reprod. 2001, 64, 775–783. [Google Scholar] [CrossRef][Green Version]
- Chuang, Y.S.; Huang, W.H.; Park, S.W.; Persaud, S.D.; Hung, C.H.; Ho, P.C.; Wei, L.N. Promyelocytic leukemia protein in retinoic acid-induced chromatin remodeling of Oct4 gene promoter. Stem Cells 2011, 29, 660–669. [Google Scholar] [CrossRef]
- Das, A.; Fernandez-Zapico, M.E.; Cao, S.; Yao, J.; Fiorucci, S.; Hebbel, R.P.; Urrutia, R.; Shah, V.H. Disruption of an SP2/KLF6 repression complex by SHP is required for farnesoid X receptor-induced endothelial cell migration. J. Biol. Chem. 2006, 281, 39105–39113. [Google Scholar] [CrossRef]
- Qin, J.; Zhai, J.; Hong, R.; Shan, S.; Kong, Y.; Wen, Y.; Wang, Y.; Liu, J.; Xie, Y. Prospero-related homeobox protein (Prox1) inhibits hepatitis B virus replication through repressing multiple cis regulatory elements. J. Gen. Virol. 2009, 90, 1246–1255. [Google Scholar] [CrossRef]
- Pearen, M.A.; Muscat, G.E. Minireview: Nuclear hormone receptor 4A signaling: Implications for metabolic disease. Mol. Endocrinol. 2010, 24, 1891–1903. [Google Scholar] [CrossRef]
- Maxwell, M.A.; Muscat, G.E. The NR4A subgroup: Immediate early response genes with pleiotropic physiological roles. Nucl. Recept. Signal 2006, 4, e002. [Google Scholar] [CrossRef]
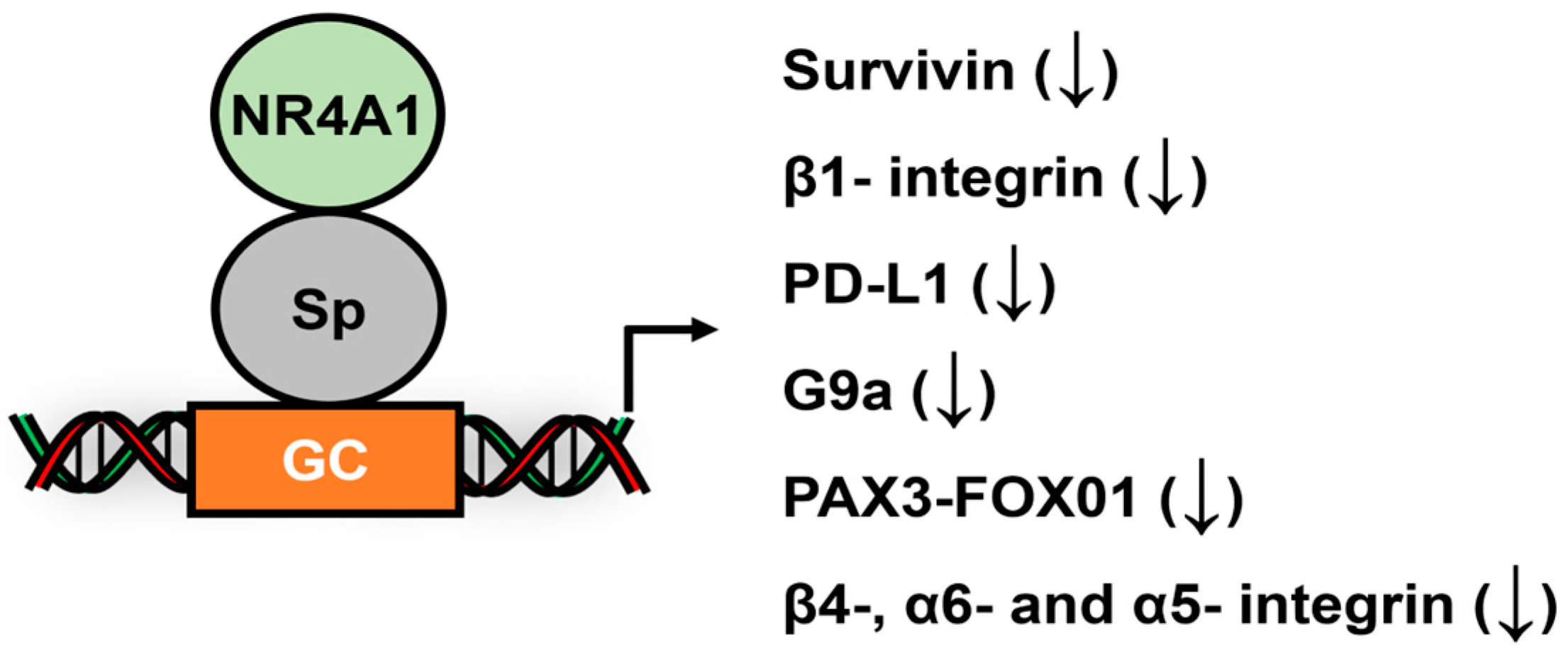
- Safe, S.; Karki, K. The Paradoxical Roles of Orphan Nuclear Receptor 4A (NR4A) in Cancer. Mol. Cancer Res. 2021, 19, 180–191. [Google Scholar] [CrossRef]
- Lee, S.O.; Abdelrahim, M.; Yoon, K.; Chintharlapalli, S.; Papineni, S.; Kim, K.; Wang, H.; Safe, S. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. 2010, 70, 6824–6836. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, E.; Lee, S.O.; Safe, S. The nuclear orphan receptor NR4A1 regulates β1-integrin expression in pancreatic and colon cancer cells and can be targeted by NR4A1 antagonists. Mol. Carcinog. 2017, 56, 2066–2075. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, E.; Li, X.; Safe, S. Penfluridol Represses Integrin Expression in Breast Cancer through Induction of Reactive Oxygen Species and Downregulation of Sp Transcription Factors. Mol. Cancer Ther. 2017, 16, 205–216. [Google Scholar] [CrossRef]
- Lacey, A.; Rodrigues-Hoffman, A.; Safe, S. PAX3-FOXO1A Expression in Rhabdomyosarcoma Is Driven by the Targetable Nuclear Receptor NR4A1. Cancer Res. 2017, 77, 732–741. [Google Scholar] [CrossRef]
- Shrestha, R.; Mohankumar, K.; Jin, U.H.; Martin, G.; Safe, S. The Histone Methyltransferase Gene G9A Is Regulated by Nuclear Receptor 4A1 in Alveolar Rhabdomyosarcoma Cells. Mol. Cancer Ther. 2021, 20, 612–622. [Google Scholar] [CrossRef]
- Karki, K.; Wright, G.A.; Mohankumar, K.; Jin, U.H.; Zhang, X.H.; Safe, S. A Bis-Indole-Derived NR4A1 Antagonist Induces PD-L1 Degradation and Enhances Antitumor Immunity. Cancer Res. 2020, 80, 1011–1023. [Google Scholar] [CrossRef]
- Johnson, S.; Yu, Z.; Li, X.; Zarei, M.; Vaziri-Gohar, A.; Lee, M.; Upadhyay, S.; Du, H.; Zarei, M.; Safe, S. A novel NR4A2-HuR axis promotes pancreatic cancer growth and tumorigenesis that is inhibited by NR4A2 antagonists. Am. J. Cancer Res. 2024, 14, 4337–4352. [Google Scholar] [CrossRef]
- Owen, G.I.; Richer, J.K.; Tung, L.; Takimoto, G.; Horwitz, K.B. Progesterone regulates transcription of the p21(WAF1) cyclin- dependent kinase inhibitor gene through Sp1 and CBP/p300. J. Biol. Chem. 1998, 273, 10696–10701. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Wu, D.; Yu, S.; Wang, Y.; Leung Chan, F. Nuclear receptor HNF4α performs a tumor suppressor function in prostate cancer via its induction of p21-driven cellular senescence. Oncogene 2020, 39, 1572–1589. [Google Scholar] [CrossRef]
- Zhu, A.; Li, Y.; Song, W.; Xu, Y.; Yang, F.; Zhang, W.; Yin, Y.; Guan, X. Antiproliferative Effect of Androgen Receptor Inhibition in Mesenchymal Stem-Like Triple-Negative Breast Cancer. Cell. Physiol. Biochem. 2016, 38, 1003–1014. [Google Scholar] [CrossRef]
- Huang, G.L.; Zhang, W.; Ren, H.Y.; Zhou, P.; Chen, Y.; Chen, Q.X.; Shen, D.Y. Oncogenic retinoic acid receptor α promotes human colorectal cancer growth through simultaneously regulating p21 transcription and GSK3β/β-catenin signaling. Cancer Lett. 2017, 388, 118–129. [Google Scholar] [CrossRef]
- Safe, S.; Abbruzzese, J.; Abdelrahim, M.; Hedrick, E. Specificity Protein Transcription Factors and Cancer: Opportunities for Drug Development. Cancer Prev. Res. 2018, 11, 371–382. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safe, S.; Farkas, E.; Hailemariam, A.E.; Oany, A.R.; Sivaram, G.; Tsui, W.N.T. Activation of Genes by Nuclear Receptor/Specificity Protein (Sp) Interactions in Cancer. Cancers 2025, 17, 284. https://doi.org/10.3390/cancers17020284
Safe S, Farkas E, Hailemariam AE, Oany AR, Sivaram G, Tsui WNT. Activation of Genes by Nuclear Receptor/Specificity Protein (Sp) Interactions in Cancer. Cancers. 2025; 17(2):284. https://doi.org/10.3390/cancers17020284
Chicago/Turabian StyleSafe, Stephen, Evan Farkas, Amanuel E. Hailemariam, Arafat Rahman Oany, Gargi Sivaram, and Wai Ning Tiffany Tsui. 2025. "Activation of Genes by Nuclear Receptor/Specificity Protein (Sp) Interactions in Cancer" Cancers 17, no. 2: 284. https://doi.org/10.3390/cancers17020284
APA StyleSafe, S., Farkas, E., Hailemariam, A. E., Oany, A. R., Sivaram, G., & Tsui, W. N. T. (2025). Activation of Genes by Nuclear Receptor/Specificity Protein (Sp) Interactions in Cancer. Cancers, 17(2), 284. https://doi.org/10.3390/cancers17020284






