Is Extraordinary Response and Long-Term Remission of Metastatic Castration-Resistant Prostate Cancer (mCRPC) After [¹⁷⁷Lu]Lu-PSMA Radioligand Therapy Due to an Immunomodulatory Effect (Radiovaccination)? A Dual Center Experience on Super-Responders
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
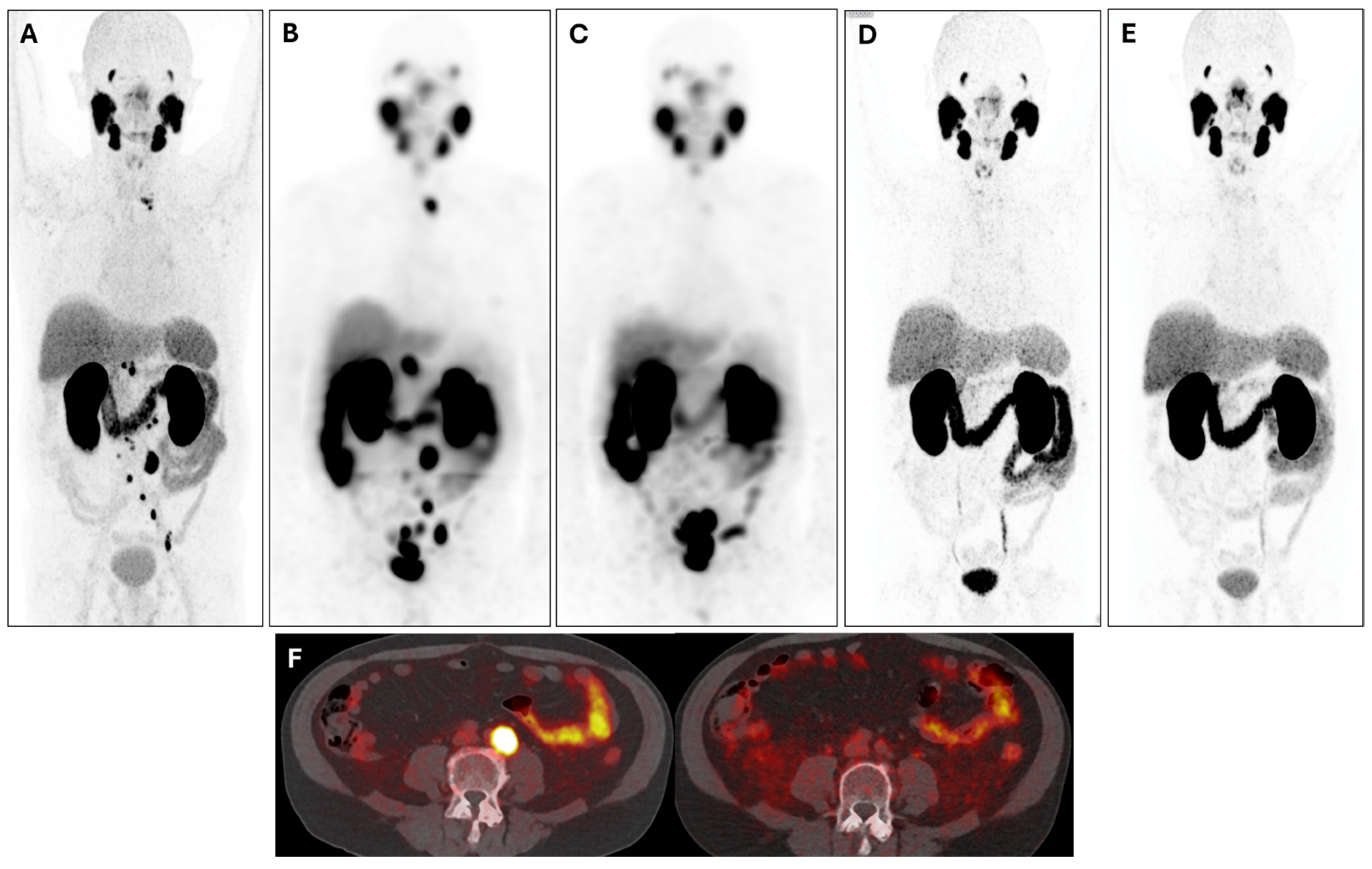
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Kulkarni, H.R.; Singh, A.; Baum, R.P. Complete regression of lung metastases in a patient with metastatic castration-resistant prostate cancer using 177Lu-PSMA Radioligand Therapy. Clin. Nucl. Med. 2020, 45, e48–e50. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Telli, T.; Lapa, C.; Desaulniers, M.; Hekimsoy, T.; Weber, W.A.; Pfob, C.; Hadaschik, B.; Bögemann, M.; Schäfers, M.; et al. Safety and efficacy of extended therapy with [177Lu]Lu-PSMA: A German Multicenter Study. J. Nucl. Med. 2024, 65, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Voter, A.; Shesadri, S.; Spitz, A.; Marshall, C.H.; Rowe, S.P.; Markowski, M.C.; Pomper, M.G.; Civelek, A.C.; Carducci, M.A.; et al. Initial experience with [177Lu]Lu-PSMA-617 after regulatory approval for metastatic castration-resistant prostate cancer: Efficacy, safety, and outcome prediction. J. Nucl. Med. 2024, 65, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, M.; Heslop, L.; Govender, T.; Korowlay, N.; Singh, A.; Choudhary, P.; Sathekge, M. The outcome and safety of Re-challenge Lutetium-177 PSMA (177Lu-PSMA) Therapy with low-dose Docetaxel as a Radiosensitizer-a promising combination in metastatic castrate-resistant prostate cancer (mCRPC): A case report. Nucl. Med. Mol. Imaging 2021, 55, 136–140. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Shiota, M.; Yokomizo, A.; Adachi, T.; Koga, H.; Yamaguchi, A.; Imada, K.; Takeuchi, A.; Kiyoshima, K.; Inokuchi, J.; Tatsugami, K.; et al. The oncological outcomes and risk stratification in docetaxel chemotherapy for castration-resistant prostate cancer. Jpn. J. Clin. Oncol. 2014, 44, 860–867. [Google Scholar] [CrossRef]
- Rouyer, M.; Oudard, S.; Joly, F.; Fizazi, K.; Tubach, F.; Jove, J.; Lacueille, C.; Lamarque, S.; Guiard, E.; Balestra, A.; et al. Overall and progression-free survival with cabazitaxel in metastatic castration-resistant prostate cancer in routine clinical practice: The FUJI cohort. Br. J. Cancer 2019, 121, 1001–1008. [Google Scholar] [CrossRef]
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef]
- Kluge, A.; Baum, R.P.; Bitterlich, N.; Harshad, R.K.; Schorr-Neufing, U.; van Echteld, C.J.A. Immune response to molecular radiotherapy with 177Lu-DOTATOC: Predictive value of blood cell counts for therapy outcome. Cancer Biother. Radiopharm. 2024, 39, 541–550. [Google Scholar] [CrossRef]
- Beauregard, J.M.; Cadieux, P.; Buteau, F.A.; Beaulieu, A.; GuÃrin, B.; Turcotte, A. Development of Theranostic Response Criteria In Solid Tumors (THERCIST) and tumor burden quantification methods for 68Ga-PET/CT and 177Lu-QSPECT/CT. J. Nucl. Med. 2019, 60, 626. [Google Scholar]
- Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Seifert, R.; Kessel, K.; Bögemann, M.; Kulkarni, H.R.; Zhang, J.; Gerke, C.; Fimmers, R.; et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial). Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Di Nicolantonio, F.; Mercer, S.J.; Knight, L.A.; Gabriel, F.G.; Whitehouse, P.A.; Sharma, S.; Fernando, A.; Glaysher, S.; Di Palma, S.; Johnson, P.; et al. Cancer cell adaptation to chemotherapy. BMC Cancer 2005, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Jehle, D.V.K.; Nguyen, N.; Garza, M.A.; Kim, D.K.; Paul, K.K.; Bilby, N.J.; Bogache, W.K.; Chevli, K.K. PSA levels and mortality in prostate cancer patients. Clin. Genitourin. Cancer 2024, 22, 102162. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Huang, C.Y.; Lu, Y.C.; Huang, K.H.; Chow, P.M.; Chang, Y.K.; Hung, F.-C.; Chen, C.-H.; Jaw, F.-S.; Hong, J.-H. Association between low prostate-specific antigen levels and greater disease progression in high-grade locally-advanced prostate cancer. J. Formos. Med. Assoc. 2021, 120, 483–491. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Kim, D.; Kim, H.J.; Oh, K.T.; Kim, S.J.; Choi, Y.D.; Giesel, F.L.; Kopka, K.; Hoepping, A.; et al. Combination of [18F]FDG and [18F]PSMA-1007 PET/CT predicts tumour aggressiveness at staging and biochemical failure postoperatively in patients with prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1763–1772. [Google Scholar] [CrossRef]
- Halabi, S.; Kelly, W.K.; Ma, H.; Zhou, H.; Solomon, N.C.; Fizazi, K.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Hussain, M.; et al. Meta-Analysis evaluating the impact of site of metastasis on Overall Survival in men with castration-resistant prostate cancer. J. Clin. Oncol. 2016, 34, 1652–1659. [Google Scholar] [CrossRef]
- Parker, D.; Zambelli, J.; Lara, M.K.; Wolf, T.H.; McDonald, A.; Lee, E.; Abou-Elkacem, L.; Gordon, E.J.; Baum, R.P. Case report: Long-term complete response to PSMA-targeted radioligand therapy and abiraterone in a metastatic prostate cancer patient. Front. Oncol. 2023, 13, 1192792. [Google Scholar] [CrossRef]
- Maharaj, M.; Heslop, L.; Govender, T.; Korowlay, N. Exceptional response of rare plasmacytoid variant prostate cancer post 177Lu-PSMA Therapy seen on 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2023, 48, e69–e70. [Google Scholar] [CrossRef]
- Dutta, S.; Ganguly, A.; Chatterjee, K.; Spada, S.; Mukherjee, S. Targets of immune escape mechanisms in cancer: Basis for development and evolution of cancer immune checkpoint inhibitors. Biology 2023, 12, 218. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The evasion mechanisms of cancer immunity and drug intervention in the tumor microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Zhao, L.; Pang, Y.; Zhou, Y.; Zhao, L.; Pang, Y.; Zhou, Y.; Chen, J.; Fu, H.; Guo, W.; Xu, W.; et al. Antitumor efficacy and potential mechanism of FAP-targeted radioligand therapy combined with immune checkpoint blockade. Signal Transduct. Target. Ther. 2024, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shao, C. Radiotherapy-mediated immunomodulation and anti-tumor Abscopal Effect combining immune checkpoint blockade. Cancers 2020, 12, 2762. [Google Scholar] [CrossRef] [PubMed]
- Zagardo, V.; Harikar, M.; Ferini, G. Is an immune-oriented use of radiation therapy possible? An increasingly open question under the spotlight of immunotherapy. Oncologie 2024, 26, 487–491. [Google Scholar] [CrossRef]
- InformedHealth.org. In Brief: The Innate and Adaptive Immune Systems. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279396/ (accessed on 10 November 2024).
- Neves, B.M.; Almeida, C.R. Signaling pathways governing activation of innate immune cells. In Tissue-Specific Cell Signaling; Silva, J.V., Freitas, M.J., Fardilha, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 93–131. [Google Scholar]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Shiga, T.; Tamaki, N. Clinical perspectives of theranostics. Molecules 2021, 26, 2232. [Google Scholar] [CrossRef]
- Constanzo, J.; Bouden, Y.; Godry, L.; Kotzki, P.O.; Deshayes, E.; Pouget, J.P. Immunomodulatory effects of targeted radionuclide therapy. Int. Rev. Cell Mol. Biol. 2023, 378, 105–136. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Lee, D.; Cheng, Y.; Baumhover, N.J.; Marks, B.M.; Sagastume, E.A.; Ballas, Z.K.; Johnson, F.L.; Morris, Z.S.; et al. Targeted Alpha-Particle Radiotherapy and immune checkpoint inhibitors induces cooperative inhibition on tumor growth of malignant melanoma. Cancers 2021, 13, 3676. [Google Scholar] [CrossRef]
- Gorin, J.B.; Ménager, J.; Gouard, S.; Maurel, C.; Guilloux, Y.; Faivre-Chauvet, A.; Morgenstern, A.; Bruchertseifer, F.; Chérel, M.; Davodeau, F.; et al. Antitumor immunity induced after α irradiation. Neoplasia 2014, 16, 319–328. [Google Scholar] [CrossRef]
- Yang, M.; Liu, H.; Lou, J.; Zhang, J.; Zuo, C.; Zhu, M.; Zhang, X.; Yin, Y.; Zhang, Y.; Qin, S.; et al. Alpha-Emitter Radium-223 induces STING-dependent pyroptosis to trigger robust antitumor immunity. Small 2024, 20, e2307448. [Google Scholar] [CrossRef]
- Pouget, J.P.; Constanzo, J. Revisiting the radiobiology of targeted alpha therapy. Front. Med. 2021, 8, 692436. [Google Scholar] [CrossRef]
- Patel, R.B.; Hernandez, R.; Carlson, P.; Grudzinski, J.; Bates, A.M.; Jagodinsky, J.C.; Erbe, A.; Marsh, I.R.; Arthur, I.; Aluicio-Sarduy, E.; et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci. Transl. Med. 2021, 13, eabb3631. [Google Scholar] [CrossRef] [PubMed]
- Jagodinsky, J.C.; Jin, W.J.; Bates, A.M.; Hernandez, R.; Grudzinski, J.J.; Marsh, I.R.; Chakravarty, I.; Arthur, I.S.; Zangl, L.M.; Brown, R.J.; et al. Temporal analysis of type 1 interferon activation in tumor cells following external beam radiotherapy or targeted radionuclide therapy. Theranostics 2021, 11, 6120–6137. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.P.; Sheehan-Klenk, J.; Grudzinski, J.J.; Adam, D.P.; Nguyen, T.P.T.; Ferreira, C.A.; Bates, A.M.; Jin, W.J.; Kwon, O.; Olson, A.P.; et al. Effects of clinically relevant radionuclides on the activation of a type I interferon response by radiopharmaceuticals in syngeneic murine tumor models. bioRxiv 2024. [Google Scholar] [CrossRef]
- Shea, A.G.; Idrissou, M.B.; Torres, A.I.; Chen, T.; Hernandez, R.; Morris, Z.S.; Sodji, Q.H. Immunological effects of radiopharmaceutical therapy. Front. Nucl. Med. 2024, 4, 1331364. [Google Scholar] [CrossRef]
- Ghodadra, A.; Bhatt, S.; Camacho, J.C.; Kim, H.S. Abscopal effects and Yttrium-90 radioembolization. Cardiovasc. Intervent Radiol. 2016, 39, 1076–1080. [Google Scholar] [CrossRef]
- Xuan, L.; Bai, C.; Ju, Z.; Luo, J.; Guan, H.; Zhou, P.K.; Huang, R. Radiation-targeted immunotherapy: A new perspective in cancer radiotherapy. Cytokine Growth Factor. Rev. 2024, 75, 1–11. [Google Scholar] [CrossRef]
- Haifen, L.; Wen, M.; Qi, C.; Zhen, Y.; Yunlu, D. Radiotherapy-activated tumor immune microenvironment: Realizing radiotherapy-immunity combination therapy strategies. Nano Today 2023, 53, 102042. [Google Scholar] [CrossRef]
- Frey, B.; Rubner, Y.; Wunderlich, R.; Weiss, E.M.; Pockley, A.G.; Fietkau, R.; Gaipl, U.S. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation—Implications for cancer therapies. Curr. Med. Chem. 2012, 19, 1751–1764. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Keep, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Bezu, L.; Wu Chuang, A.; Humeau, J.; Kroemer, G.; Keep, O. Quantification of eIF2alpha phosphorylation during immunogenic cell death. Methods Enzymol. 2019, 629, 53–69. [Google Scholar] [CrossRef]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Chiriva-Internati, M.; Grizzi, F.; Pinkston, J.; Morrow, K.J.; D’Cunha, N.; Frezza, E.E.; Muzzio, P.C.; Kast, W.M.; Cobos, E. Gamma-radiation upregulates MHC class I/II and ICAM-I molecules in multiple myeloma cell lines and primary tumors. In Vitro Cell Dev. Biol. Anim. 2006, 42, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Probst, H.C.; Vuong, V.; Landshammer, A.; Muth, S.; Yagita, H.; Schwendener, R.; Pruschy, M.; Knuth, A.; van den Broek, M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J. Immunol. 2012, 189, 558–566. [Google Scholar] [CrossRef]
- Kramer, C.S.; Zhang, J.; Baum, R.P. Extraordinary therapeutic effect of PSMA radioligand therapy in treatment-refractory progressive metastatic prostate cancer with the transketolase inhibitor benfo-oxythiamine as a radiosensitizer—A case report. Front. Med. 2024, 11, 1462234. [Google Scholar] [CrossRef]
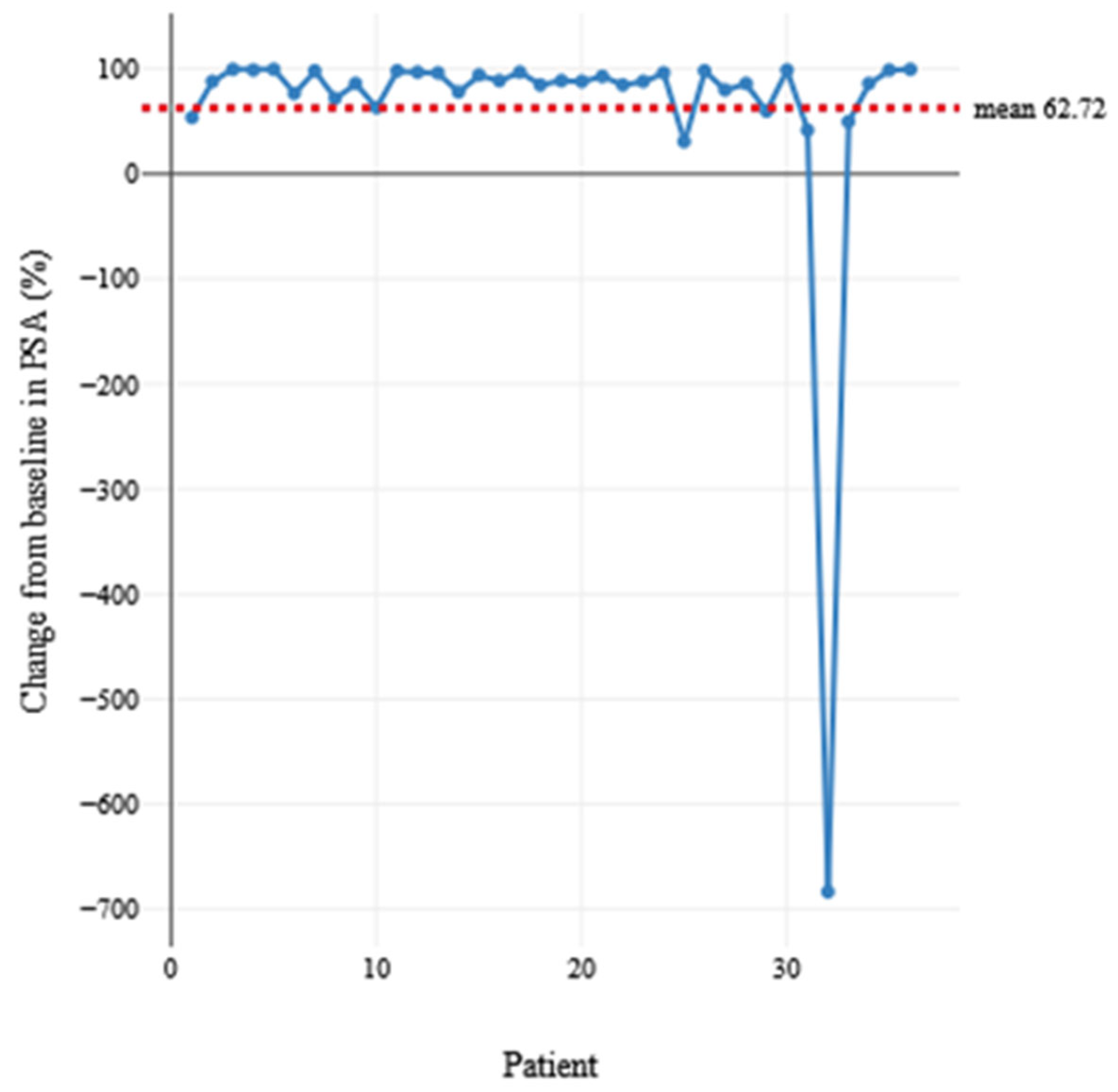
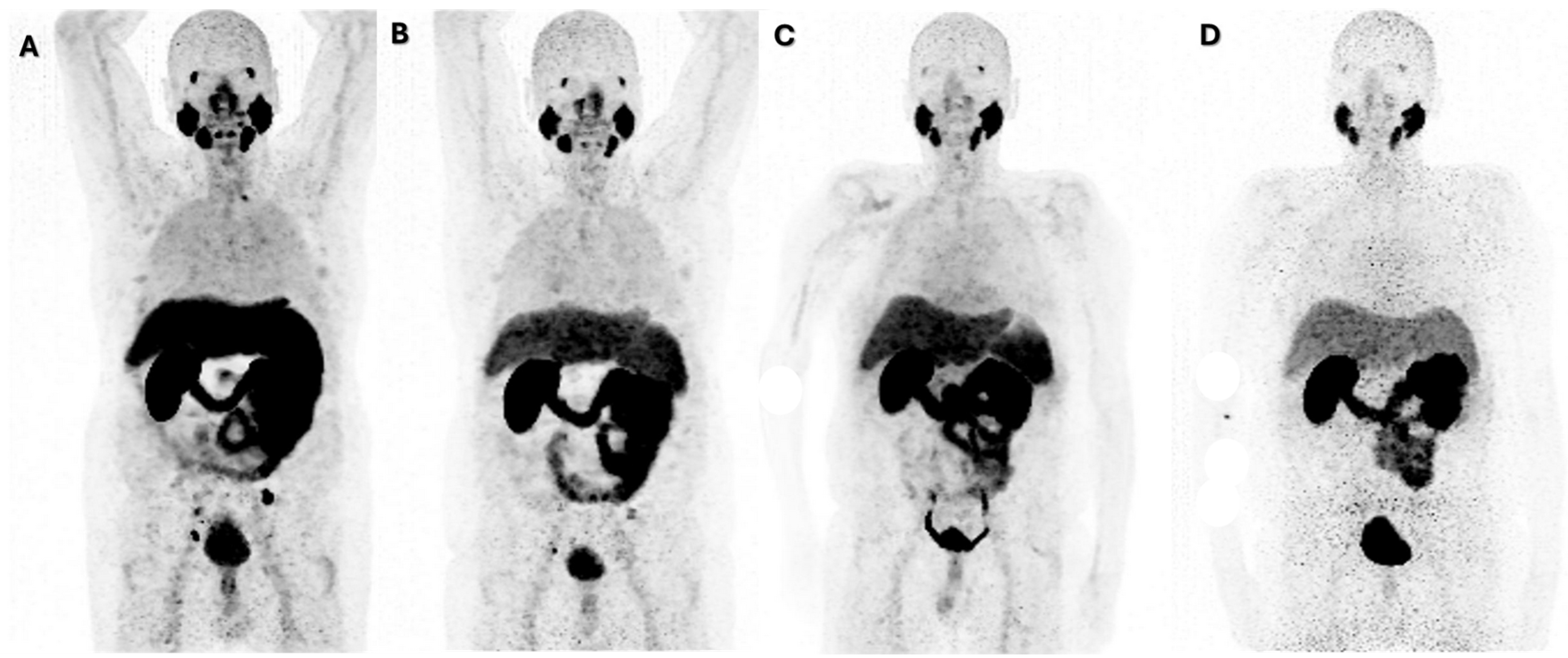
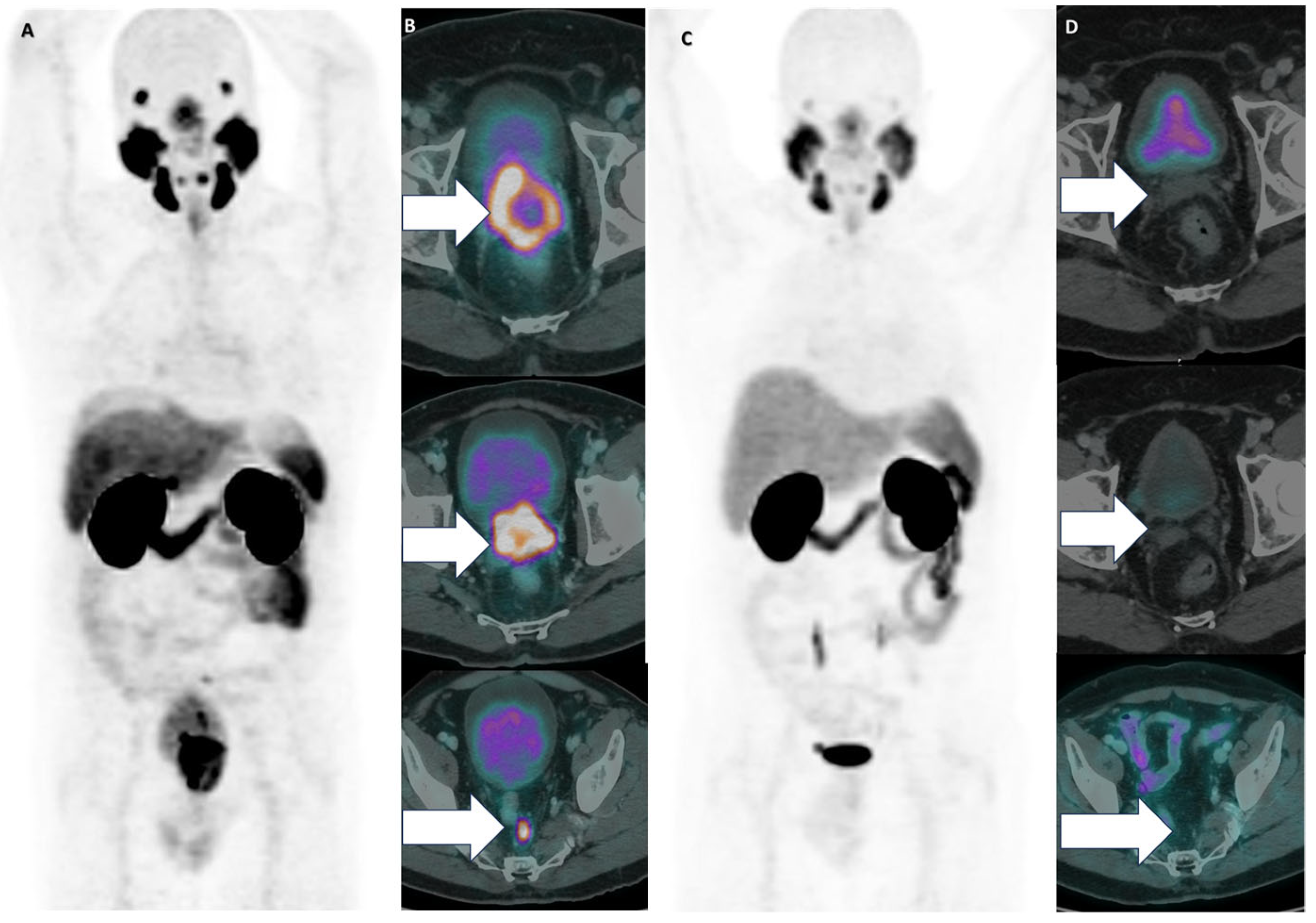
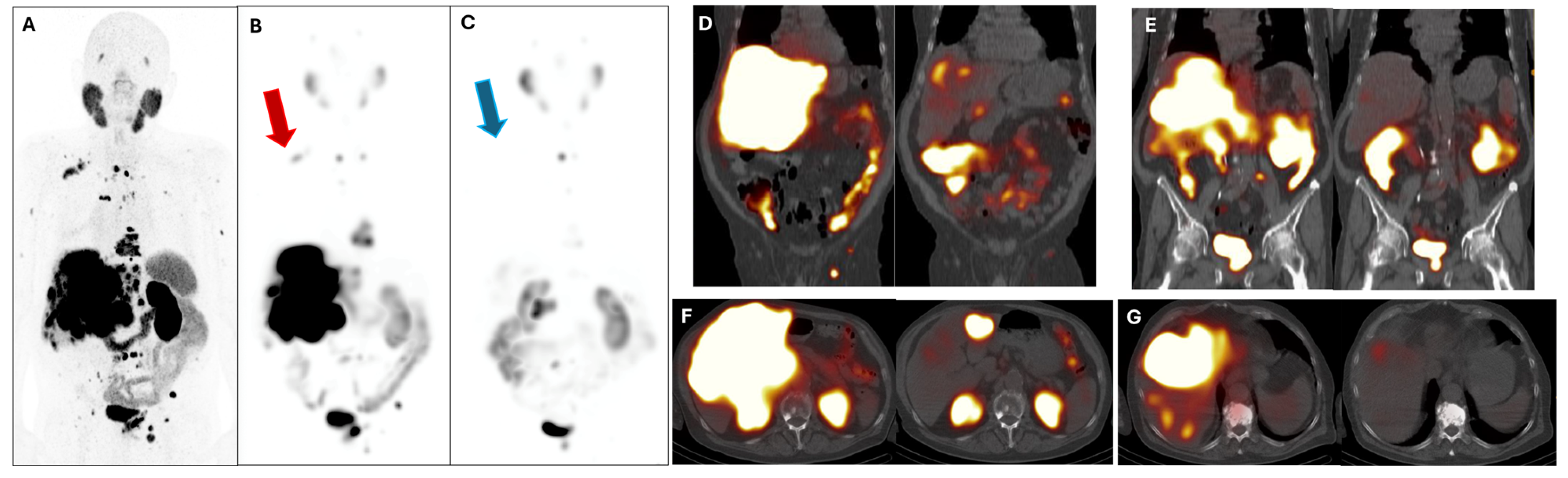
| Age at the Time of Presentation for PRLT | No. of Cycles to Response | No. of Months of Maintained Response | Baseline PSA (ng/mL) | PSA (ng/mL) at Response | ||
|---|---|---|---|---|---|---|
| Mean | 71.78 | 2.81 | 17.44 | 175.15 | 31.51 | |
| Std. Deviation | 8.529 | 1.238 | 21.836 | 329.263 | 72.958 | |
| Percentiles | 25 | 65.25 | 2.00 | 4.00 | 9.44 | 0.33 |
| 50 | 71.00 | 3.00 | 11.00 | 56.95 | 2.51 | |
| 75 | 79.50 | 3.00 | 17.00 | 139.75 | 16.63 | |
| Paired Differences | T | df | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. Deviation | Std. Error Mean | 95% Confidence Interval of the Difference | One-Sided p | Two-Sided p | ||||
| Lower | Upper | ||||||||
| Baseline PSA (ng/mL)—PSA (ng/mL) at response | 143.64 | 274.920 | 45.820 | 50.627 | 236.666 | 3.135 | 35 | 0.002 | 0.003 |
| Variable | Number of Months of Maintained Response | p-Value | ||
|---|---|---|---|---|
| <6 Months | 6–<12 Months | ≥12 Months | ||
| Age at presentation | 70.91 ± 9.62 | 75.70 ± 7.97 | 69.80 ± 7.68 | 0.224 |
| Gleason score | 4.64 ± 3.0 | 4.20 ± 2.89 | 4.13 ± 1.76 | 0.871 |
| Number of cycles to response | 2.82 ± 1.53 | 3.0 ± 0.94 | 2.67 ± 1.23 | 0.813 |
| Baseline PSA | 390.74 ± 506.4 | 113.46 ± 186.9 | 58.18 ± 106.9 | 0.026 |
| PSA at response | 88.91 ± 112.3 | 13.20 ± 27.70 | 1.62 ± 2.75 | 0.004 |
| No. of Cycles (n) | All Patients (n = 36) | Frequency % |
|---|---|---|
| 1 | 4 | 11.1% |
| 2 | 12 | 33.3% |
| 3 | 12 | 33.3% |
| 4 | 5 | 13.9% |
| 5 | 1 | 2.8% |
| 6 | 2 | 5.6% |
| Previous Treatment for mCRPC | All Patients n = 36 | Mean Maintained Response (Months) | Max. Maintained Response (Months) | Mean Decline in PSA (%) | p-Value |
|---|---|---|---|---|---|
| Docetaxel | 7 (19.4%) | 17 | 83 | 78.14 | 0.172 |
| Cabazitaxel | 5 (13.9%) | 3.4 | 5 | 74.40 | |
| Abiraterone | 10 (28%) | 17.2 | 83 | 85.60 | |
| Enzalutamide | 11 (31%) | 6.45 | 14 | 81.18 | |
| EBRT | 15 (41.7%) | 11.45 | 48 | 84.14 (*) | |
| Radium-223 dichloride | 1 (2.7%) | 14 | 14 | 99.0 | |
| Pattern of disease: | |||||
| Bone metastases | 27 (75%) | 15.1 | 99 | 85.05 | 0.721 |
| Lymph node metastases | 27 (75%) | 17.15 | 99 | 84.48 (*) | |
| Liver metastases | 6 (16.67%) | 4.33 | 11 | 66.0 | |
| Peritoneal metastases | 2 (5.6%) | 4.5 | 5 | 69.50 | |
| Gleason Score (GS) | Maintained Response (Months) | ||||
|---|---|---|---|---|---|
| GS | Frequency | Mean | Std. Deviation | Minimum | Maximum |
| 7 | 11 | 25.64 | 22.85 | 6 | 83 |
| 8 | 8 | 9.13 | 6.03 | 2 | 19 |
| 9 | 6 | 8 | 6.03 | 2 | 17 |
| 10 | 5 | 33.2 | 41.07 | 4 | 99 |
| unknown | 4 | 5.25 | 3.59 | 2 | 10 |
| 6 | 2 | 19 | 24.04 | 2 | 36 |
| Known BRCA Status | Patients (n = 12) | Mean Maintained Response (Months) | Max. Maintained Response (Months) | Mean Decline in PSA (%) |
|---|---|---|---|---|
| BRCA1/2 wild-type | 6 (50%) | 6.67 | 13 | 77.0 |
| BRCA 1/2 negative | 1 (8.33%) | 7 | 7 | 94.0 |
| BRCA germline negative and somatic positive | 1 (8.33%) | 36 | 36 | 50.0 |
| BRCA germline negative, Ssomatic negative | 2 (16.67%) | 27 | 48 | 74.5 |
| BRCA 2 positive | 2 (16.67%) | 43 | 83 | 92.18 |
| Prior Therapies | Gleason Score; Specific Pathology and Sequencing | Pattern of Disease | No. of Cycles to Response | Maintained Response (Months) | %PSA Decline |
|---|---|---|---|---|---|
| Bicalutamide, Enantone | 9 BRCA1/2 wild-type | Bone, LNM, Adrenal | 1 | 12 | 99 |
| Goserelin, Abiraterone, Enzalutamide, Zoledronic Acid, Cabazitaxel, EBRT (Thoracic Spine, Prostate + Left Ilium) | 8 | Bone, LNM | 4 | 12 | 89 |
| Bicalutamide, Prostatectomy + EBRT, Lymphadenectomy, Orchiectomy, Abiraterone, Enzalutamide + Trenantone | 8 BRCA 1/2 wild-type | Bone, LNM, Adrenal, Gerota Fascia | 1 | 13 | 77 |
| Prostatectomy + EBRT, Trenantone + Abiraterone, EBRT Ilium, Enzalutamide | 8 | LNM, Bone, Pleura | 2 | 13 | 98 |
| Irreversible Electroporation (IRE) | 7 | Left seminal vesicle, LNM | 2 | 14 | 85 |
| Prostatectomy + Lymphadenectomy, EBRT (Prostate, Rib + T4), Radium-223 dichloride, Trenantone, Enzalutamide, Bicalutamide, Denosumab | 7 | Bone | 3 | 14 | 99 |
| Bicalutamide, Prostate Electroporation with Bleomycin | 7 | Local recurrence, LNM, | 2 | 15 | 100 |
| Da Vinci Prostatectomy, Bicalutamide, Abiraterone | 9 Dedifferentiated Adenocarcinoma VUS CHEK2 | LNM, Bone | 2 | 17 | 97 |
| IRE | 7 | LNM | 3 | 17 | 89 |
| Prostatectomy, Salvage EBRT of Prostate Bed and Pelvic Lymph Pathways, Leuprorelin, Apalutamid, Darolutamid | 8 | LNM | 2 | 19 | −683 |
| Prostatovesiculectomy + LA, Bicalutamide, IMRT prostate bed | 7 Prostatic cribriform adenocarcinoma | LNM | 2 | 32 | 86 |
| Brachytherapy, Bicalutamide, LHRH Analogue, Interstitial HDR-Afterloading Brachytherapy + Boost | 7 | Local recurrence + seminal vesicles + dorsal bladder wall, LNM | 2 | 33 | 72 |
| Prostatectomy, Goserelin | 6 Invasive prostate acinar adenocarcinoma BRCA germline negative, somatic positive, MSS/PDl1 | Prostate bed, locally advanced | 2 | 36 | 50 |
| Brachytherapy, EBRT, Enzalutamide, Leuprorelin | 7 BRCA germline negative, somatic negative | Bone, LNM, prostate | 6 | 48 | 86 |
| Goserelin, Enzalutamide | 10Plasmacytoid variant MSI-H/PDL1 | LNM, Prostate, Locally advanced in bowel and bladder | 4 | 48 | 100 |
| Degarelix, Denosumab, 6x Docetaxel, Leuprorelin, Abiraterone | 7BRCA 2, TMB 10.53 mut/Mb, VUS CHEK2, CDH1 | Bone and bone marrow | 4 | 83 | 96 |
| Bicalutamide, Da Vinci Prostatovesiculectomy + Lymphadenectomy, Buserelin, Leuprorelin, Finasterid | 10 Acinar partial neuroendocrine differentiation, immunostaining positive for synaptophysin, CgA, CD-56 | Local recurrence, LNM, Bone | 3 | 99 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharaj, M.; Perrone, E.; Wirtz, R.M.; Heslop, L.; Govender, T.; Korowlay, N.A.; Ghai, K.; Parkar, T.; Baum, R.P. Is Extraordinary Response and Long-Term Remission of Metastatic Castration-Resistant Prostate Cancer (mCRPC) After [¹⁷⁷Lu]Lu-PSMA Radioligand Therapy Due to an Immunomodulatory Effect (Radiovaccination)? A Dual Center Experience on Super-Responders. Cancers 2025, 17, 476. https://doi.org/10.3390/cancers17030476
Maharaj M, Perrone E, Wirtz RM, Heslop L, Govender T, Korowlay NA, Ghai K, Parkar T, Baum RP. Is Extraordinary Response and Long-Term Remission of Metastatic Castration-Resistant Prostate Cancer (mCRPC) After [¹⁷⁷Lu]Lu-PSMA Radioligand Therapy Due to an Immunomodulatory Effect (Radiovaccination)? A Dual Center Experience on Super-Responders. Cancers. 2025; 17(3):476. https://doi.org/10.3390/cancers17030476
Chicago/Turabian StyleMaharaj, Masha, Elisabetta Perrone, Ralph M. Wirtz, Lucille Heslop, Trisha Govender, Nisaar A. Korowlay, Kriti Ghai, Tanay Parkar, and Richard P. Baum. 2025. "Is Extraordinary Response and Long-Term Remission of Metastatic Castration-Resistant Prostate Cancer (mCRPC) After [¹⁷⁷Lu]Lu-PSMA Radioligand Therapy Due to an Immunomodulatory Effect (Radiovaccination)? A Dual Center Experience on Super-Responders" Cancers 17, no. 3: 476. https://doi.org/10.3390/cancers17030476
APA StyleMaharaj, M., Perrone, E., Wirtz, R. M., Heslop, L., Govender, T., Korowlay, N. A., Ghai, K., Parkar, T., & Baum, R. P. (2025). Is Extraordinary Response and Long-Term Remission of Metastatic Castration-Resistant Prostate Cancer (mCRPC) After [¹⁷⁷Lu]Lu-PSMA Radioligand Therapy Due to an Immunomodulatory Effect (Radiovaccination)? A Dual Center Experience on Super-Responders. Cancers, 17(3), 476. https://doi.org/10.3390/cancers17030476








