Cytokine-Induced Killer Cell Immunotherapy Reduces Recurrence in Patients with Early-Stage Hepatocellular Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design and Treatment Protocol
2.3. Endpoints and Treatment Evaluation
2.4. Statistical Analyses
3. Results
3.1. Patients
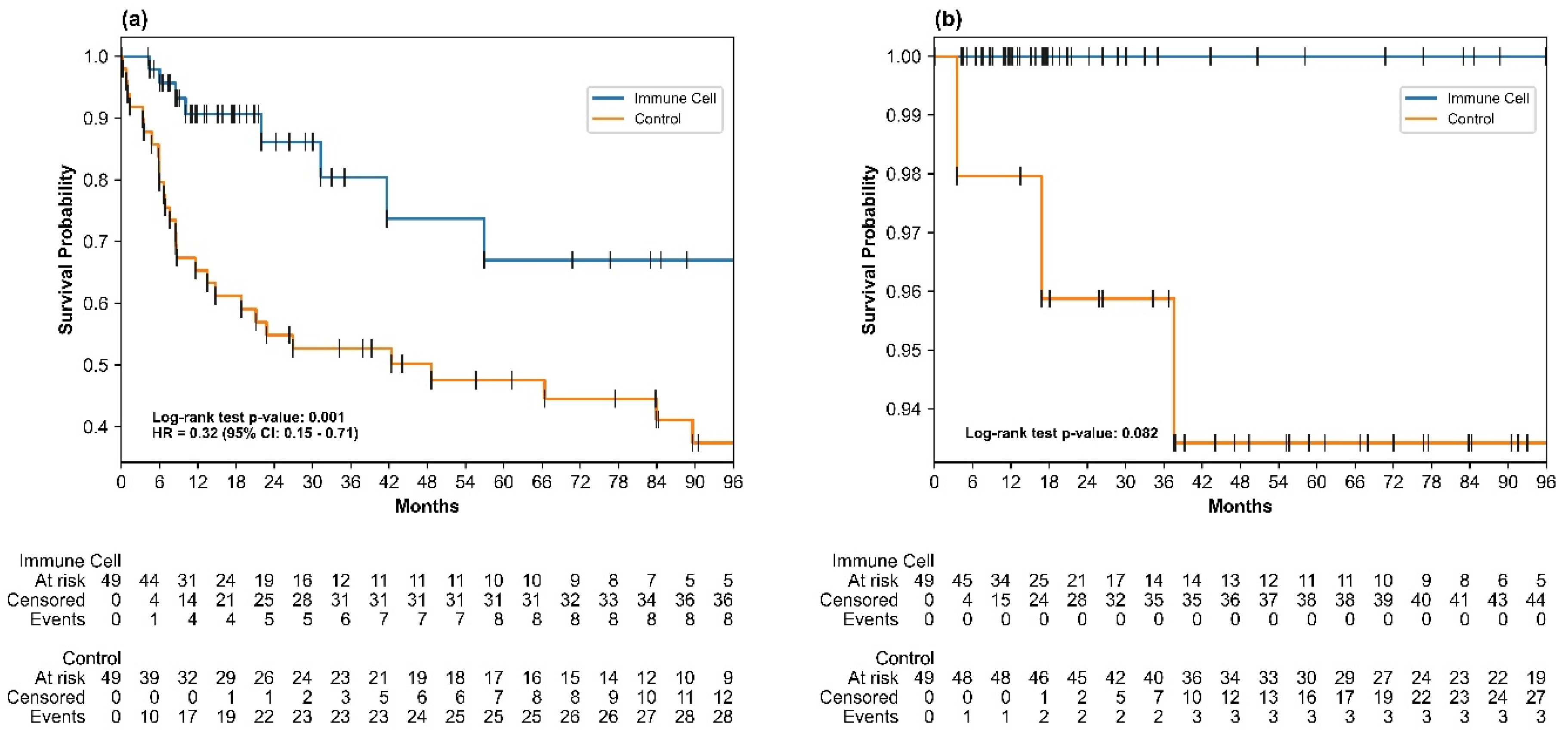
3.2. Recurrence-Free Survival
3.3. Overall Survival
3.4. Safety
3.5. Changes in Tumor Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kim, D.Y. Changing etiology and epidemiology of hepatocellular carcinoma: Asia and worldwide. J. Liver Cancer 2024, 24, 622–670. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M. Surveillance for hepatocellular carcinoma. Best Pract. Research. Clin. Gastroenterol. 2014, 28, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Alberts, C.J.; Clifford, G.M.; Georges, D.; Negro, F.; A Lesi, O.; Hutin, Y.J.-F.; de Martel, C. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: A systematic review. Lancet Gastroenterol. Hepatol. 2022, 7, 724–735. [Google Scholar] [CrossRef]
- Kim, B.H.; Yun, E.H.; Lee, J.-H.; Hong, G.; Park, J.Y.; Shim, J.H.; Kim, E.; Kong, H.-J.; Jung, K.-W.; Lim, Y.-S. Advancing Korean nationwide registry for hepatocellular carcinoma: A systematic sampling approach utilizing the Korea Central Cancer Registry database. J. Liver Cancer 2024, 24, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Chapter One—Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. In Advances in Cancer Research; Sarkar, D., Fisher, P.B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–61. [Google Scholar]
- Ramakrishna, G.; Rastogi, A.; Trehanpati, N.; Sen, B.; Khosla, R.; Sarin, S.K. From Cirrhosis to Hepatocellular Carcinoma: New Molecular Insights on Inflammation and Cellular Senescence. Liver Cancer 2013, 2, 367–383. [Google Scholar] [CrossRef]
- Tabori, N.E.; Sivananthan, G. Treatment Options for Early-Stage Hepatocellular Carcinoma. Semin. Interv. Radiol. 2020, 37, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin. Mol. Hepatol. 2016, 22, 7–17. [Google Scholar] [CrossRef]
- Qin, S.; Chen, M.; O Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.C.; Zhou, J.; Wang, L.; Wen, X.; Heo, J.; et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1835–1847. [Google Scholar] [CrossRef]
- Mo, S.; Dai, W.; Wang, H.; Lan, X.; Ma, C.; Su, Z.; Xiang, W.; Han, L.; Luo, W.; Zhang, L.; et al. Early detection and prognosis prediction for colorectal cancer by circulating tumour DNA methylation haplotypes: A multicentre cohort study. eClinicalMedicine 2022, 55, 101717. [Google Scholar] [CrossRef] [PubMed]
- Yago, A.; Haruta, S.; Ueno, M.; Hamada, Y.; Ogawa, Y.; Ohkura, Y.; Urabe, M.; Udagawa, H. Adequate period of surveillance in each stage for curatively resected gastric cancer: Analyzing the time and rates of recurrence. Gastric Cancer 2021, 24, 752–761. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, C. Advances in the early diagnosis of hepatocellular carcinoma. Genes Dis. 2020, 7, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.-Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.-H.; Tak, W.Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Ding, Y.; Qin, Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1133308. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, L.C.; Schmeel, F.C.; Coch, C.; Schmidt-Wolf, I.G.H. Cytokine-induced killer (CIK) cells in cancer immunotherapy: Report of the international registry on CIK cells (IRCC). J. Cancer Res. Clin. Oncol. 2014, 141, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, F.; Yazdanpanah, N.; Rezaei, N. Cytokine-induced killer cells mediated pathways in the treatment of colorectal cancer. Cell Commun. Signal. 2022, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Cappuzzello, E.; Sommaggio, R.; Zanovello, P.; Rosato, A. Cytokines for the induction of antitumor effectors: The paradigm of Cytokine-Induced Killer (CIK) cells. Cytokine Growth Factor Rev. 2017, 36, 99–105. [Google Scholar] [CrossRef]
- Yu, R.; Yang, B.; Chi, X.; Cai, L.; Liu, C.; Yang, L.; Wang, X.; He, P.; Lu, X. Efficacy of cytokine-induced killer cell infusion as an adjuvant immunotherapy for hepatocellular carcinoma: A systematic review and meta-analysis. Drug Des. Dev. Ther. 2017, 11, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J.; Kim, Y.; Shuang, Z.-Y.; Zhang, Y.-J.; Lao, X.-M.; Li, Y.-Q.; Chen, M.-S.; Pawlik, T.M.; Xia, J.-C.; et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. OncoImmunology 2015, 5, e1083671. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lim, Y.-S.; Yeon, J.; Song, T.-J.; Yu, S.; Kim, K.; Kim, Y.; Yoon, J.-H. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: An extended 5-year follow-up. J. Hepatol. 2018, 68, S37–S38. [Google Scholar] [CrossRef]
- Yang, C.-K.; Huang, C.-H.; Hu, C.-H.; Fang, J.-H.; Chen, T.-C.; Lin, Y.-C.; Lin, C.-Y. Immunophenotype and antitumor activity of cytokine-induced killer cells from patients with hepatocellular carcinoma. PLoS ONE 2023, 18, e0280023. [Google Scholar] [CrossRef]
- Korean Liver Cancer Association; National Cancer Center Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 583–705. [Google Scholar] [CrossRef]
- Song, B.G.; Lee, J.-H.; Lee, H.Y.; Kim, S.W.; Chang, Y.; Bin Lee, Y.; Cho, E.J.; Yu, S.J.; Sinn, D.H.; Kim, Y.J.; et al. Adjuvant cytokine-induced killer cell immunotherapy for hepatocellular carcinoma: A propensity score-matched analysis of real-world data. BMC Cancer 2019, 19, 523. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, Y.S.; Yeon, J.E.; Song, T.J.; Yu, S.J.; Gwak, G.Y.; Kim, K.M.; Kim, Y.J.; Lee, J.W.; Yoon, J.H.; et al. Adjuvant Immunotherapy With Autologous Cytokine-Induced Killer Cells for Hepatocellular Carcinoma. Gastroenterology 2015, 148, 1383–1391.e6. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Lee, Q.; Yu, X.; Yu, W. The value of PIVKA-Ⅱ versus AFP for the diagnosis and detection of postoperative changes in hepatocellular carcinoma. J. Interv. Med. 2021, 4, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.I.; Kim, H.S.; Kim, W.J.; Shin, W.G.; Kim, D.J.; Kim, K.H.; Jang, M.K.; Lee, J.H.; Kim, J.S.; Kim, H.Y.; et al. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Wahed, A. Chapter 13—Tumor markers, in Clinical Chemistry. In Immunology and Laboratory Quality Control, 2nd ed.; Dasgupta, A., Wahed, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 269–293. [Google Scholar]
- Patil, M.; Sheth, K.A.; Adarsh, C.K. Elevated Alpha Fetoprotein, No Hepatocellular Carcinoma. J. Clin. Exp. Hepatol. 2013, 3, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.I.; Kim, S.S.; Choi, B.Y.; Lee, S.H.; Kim, S.J.; Park, H.W.; Kim, H.S.; Shin, W.G.; Kim, K.H.; Lee, J.H.; et al. Clinical significance of elevated serum alpha-fetoprotein (AFP) level in acute viral hepatitis A (AHA). Hepatogastroenterology 2013, 60, 1592–1596. [Google Scholar] [PubMed]
- Nanashima, A.; Abo, T.; Taura, N.; Shibata, H.; Ichikawa, T.; Takagi, K.; Arai, J.; Oyama, S.; Nagayasu, T. NX-PVKA levels before and after hepatectomy of hepatocellular carcinoma as predictors of patient survival: A preliminary evaluation of an improved assay for PIVKA-II. Anticancer Res. 2013, 33, 2689–2697. [Google Scholar]
- Nanashima, A.; Sumida, Y.; Tobinaga, S.; Shibata, K.; Shindo, H.; Obatake, M.; Shibasaki, S.; Ide, N.; Nagayasu, T. Postoperative changes in protein-induced vitamin K absence or antagonist II levels after hepatectomy in patients with hepatocellular carcinoma: Relationship to prognosis. HPB 2006, 8, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Andrade, C. Mean Difference, Standardized Mean Difference (SMD), and Their Use in Meta-Analysis: As Simple as It Gets. J. Clin. Psychiatry 2020, 81, 11349. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-D.; Li, K.-S.; Sun, H.-C. Adjuvant therapies after curative treatments for hepatocellular carcinoma: Current status and prospects. Genes Dis. 2020, 7, 359–369. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, Y.; Zhu, H. Immunotherapy or targeted therapy as the first-line strategies for unresectable hepatocellular carcinoma: A network meta-analysis and cost-effectiveness analysis. Front. Immunol. 2023, 13, 1103055. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Introna, M.; Correnti, F. Innovative Clinical Perspectives for CIK Cells in Cancer Patients. Int. J. Mol. Sci. 2018, 19, 358. [Google Scholar] [CrossRef]
- Zhang, Y.; Schmidt-Wolf, I.G.H. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J. Cell. Physiol. 2020, 235, 9291–9303. [Google Scholar] [CrossRef]
- Tang, S.; Ning, Q.; Yang, L.; Mo, Z.; Tang, S. Mechanisms of immune escape in the cancer immune cycle. Int. Immunopharmacol. 2020, 86, 106700. [Google Scholar] [CrossRef] [PubMed]
- Korangy, F.; Höchst, B.; Manns, M.P.; Greten, T.F. Immune Responses in Hepatocellular Carcinoma. Dig. Dis. 2010, 28, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Jin, H.; Li, M.; Xu, J.; Xu, D.; Hu, J.; He, H.; Li, W.; Cui, J. In vitro analysis of the proliferative capacity and cytotoxic effects of ex vivo induced natural killer cells, cytokine-induced killer cells, and gamma-delta T cells. BMC Immunol. 2015, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.; Wang, Y.; Zhang, W.; Cao, Y. Phenotypic characterization and anticancer capacity of CD8+ cytokine-induced killer cells after antigen-induced expansion. PLoS ONE 2017, 12, e0175704. [Google Scholar] [CrossRef] [PubMed]
- Ruli, T.M.; Pollack, E.D.; Lodh, A.; Evers, C.D.; Price, C.A.; Shoreibah, M. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma and Their Hepatic-Related Side Effects: A Review. Cancers 2024, 16, 2042. [Google Scholar] [CrossRef]
- Weng, D.-S.; Zhou, J.; Zhou, Q.-M.; Zhao, M.; Wang, Q.-J.; Huang, L.-X.; Li, Y.-Q.; Chen, S.-P.; Wu, P.-H.; Xia, J.-C. Minimally Invasive Treatment Combined With Cytokine-induced Killer Cells Therapy Lower the Short-term Recurrence Rates of Hepatocellular Carcinomas. J. Immunother. 2008, 31, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Qiang, L.; Jian, W.; Ti, Z.; Da-Lu, K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig. Liver Dis. 2009, 41, 36–41. [Google Scholar] [CrossRef]
- Michel, M.-L.; Deng, Q.; Mancini-Bourgine, M. Therapeutic vaccines and immune-based therapies for the treatment of chronic hepatitis B: Perspectives and challenges. J. Hepatol. 2011, 54, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, C.M.; McGrath, N.A.; Fu, J.; Xie, C. Immunotherapy of hepatocellular carcinoma with infection of hepatitis B or C virus. Hepatoma Res. 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Baker, J.; Beilhack, A.; Zeiser, R.; Olson, J.A.; Sega, E.I.; Karimi, M.; Negrin, R.S. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood 2008, 112, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Alvarnas, J.C.; Linn, Y.-C.; Hope, E.G.; Negrin, R.S. Expansion of cytotoxic CD3+ CD56+ cells from peripheral blood progenitor cells of patients undergoing autologous hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2001, 7, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Sekine, T.; Makuuchi, M.; Yamasaki, S.; Kosuge, T.; Yamamoto, J.; Shimada, K.; Sakamoto, M.; Hirohashi, S.; Ohashi, Y.; et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: A randomised trial. Lancet 2000, 356, 802–807. [Google Scholar] [CrossRef]
- Toubert, C.; Guiu, B.; Al Taweel, B.; Assenat, E.; Panaro, F.; Souche, F.R.; Ursic-Bedoya, J.; Navarro, F.; Herrero, A. Prolonged Survival after Recurrence in HCC Resected Patients Using Repeated Curative Therapies: Never Give Up! Cancers 2022, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Nevola, R.; Ruocco, R.; Criscuolo, L.; Villani, A.; Alfano, M.; Beccia, D.; Imbriani, S.; Claar, E.; Cozzolino, D.; Sasso, F.C.; et al. Predictors of early and late hepatocellular carcinoma recurrence. World J. Gastroenterol. 2023, 29, 1243–1260. [Google Scholar] [CrossRef]
- Park, H.; Park, J.Y. Clinical Significance of AFP and PIVKA-II Responses for Monitoring Treatment Outcomes and Predicting Prognosis in Patients with Hepatocellular Carcinoma. BioMed Res. Int. 2013, 2013, 310427. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Ma, C.; Heinrich, B.; Brown, Z.J.; Sandhu, M.; Zhang, Q.; Fu, Q.; Agdashian, D.; Rosato, U.; Korangy, F.; et al. Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma. J. Hepatol. 2018, 70, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Nam, J.Y.; Chang, Y.; Cho, H.; Kang, S.H.; Cho, Y.Y.; Cho, E.; Lee, J.-H.; Yu, S.J.; Kim, Y.J.; et al. Synergistic effect of cytokine-induced killer cell with valproate inhibits growth of hepatocellular carcinoma cell in a mouse model. Cancer Biol. Ther. 2016, 18, 67–75. [Google Scholar] [CrossRef]
- Luo, Y.-Z.; Zhu, H. Immunotherapy for advanced or recurrent hepatocellular carcinoma. World J. Gastrointest. Oncol. 2023, 15, 405–424. [Google Scholar] [CrossRef]
- Nishida, N. Metabolic disease as a risk of hepatocellular carcinoma. Clin. Mol. Hepatol. 2021, 27, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef]
- Srivastava, D.K.; George, E.O.; Lu, Z.; Rai, S.N. Impact of unequal censoring and insufficient follow-up on comparing survival outcomes: Applications to clinical studies. Stat. Methods Med. Res. 2021, 30, 2057–2074. [Google Scholar] [CrossRef]
- Beca, J.M.; Chan, K.K.W.; Naimark, D.M.J.; Pechlivanoglou, P. Impact of limited sample size and follow-up on single event survival extrapolation for health technology assessment: A simulation study. BMC Med. Res. Methodol. 2021, 21, 282. [Google Scholar] [CrossRef] [PubMed]



| Immune Cell Group (n = 49) | Control (Before Matching) (n = 2091) | p Value (Before Matching) | Control (After Matching) (n = 49) | p Value (After Matching) | SMD (After Matching) | |
|---|---|---|---|---|---|---|
| Age, years | 59.0 (53.0–66.0) | 60.0 (53.0–67.0) | 0.405 | 60.0 (48.0–65.0) | 0.876 | 0.081 |
| AST, IU/L | 33.0 (26.0–42.0) | 31.0 (24.0–46.5) | 0.508 | 30.0 (23.0–38.0) | 0.192 | 0.082 |
| ALT, IU/L | 31.0 (25.0–40.0) | 29.0 (20.0–45.0) | 0.393 | 29.0 (21.0–39.0) | 0.296 | 0.042 |
| T.bil, mg/dL | 0.8 (0.6–0.9) | 0.8 (0.6–1.1) | 0.420 | 0.8 (0.6–1.0) | 0.841 | 0.081 |
| Albumin, g/dL | 4.5 (4.3–4.8) | 4.4 (4.0–4.6) | 0.003 | 4.4 (4.1–4.7) | 0.136 | 0.213 |
| PT INR | 1.0 (0.97–1.04) | 1.0 (0.9–1.1) | 0.622 | 1.0 (0.9–1.1) | 0.284 | 0.034 |
| PLT, ×103/mm3 | 191.0 (152.0–237.0) | 170.0 (129.0–214.0) | 0.068 | 202.0 (163.0–236.0) | 0.359 | 0.161 |
| AFP, ng/mL | 8.5 (2.8–100.3) | 7.4 (3.2–45.6) | 0.825 | 8.9 (2.9–48.9) | 0.840 | 0.046 |
| PIVKA-II, mAU/mL | 79 (29.0–491.0) | 37.0 (24.0–138.0) | 0.021 | 80.0 (26.0–314.0) | 0.696 | 0.285 |
| Sex | 0.589 | 0.814 | 0.095 | |||
| Male | 36 (73.5) | 1626 (77.8) | 38 (77.6) | |||
| Female | 13 (26.5) | 465 (22.2) | 11 (22.4) | |||
| Hypertension | 0.508 | 0.836 | 0.084 | |||
| Yes | 29 (59.2) | 975 (46.6) | 18 (36.7) | |||
| No | 20 (40.8) | 1116 (53.4) | 31 (63.3) | |||
| Diabetes | 0.097 | 1.000 | 0.048 | |||
| Yes | 11 (22.4) | 730 (34.9) | 12 (24.5) | |||
| No | 38 (77.6) | 1361 (65.1) | 37 (75.5) | |||
| Dyslipidemia | 0.451 | 1.000 | 0.000 | |||
| Yes | 6 (12.2) | 364 (17.4) | 6 (12.2) | |||
| No | 43 (87.8) | 1727 (82.6) | 43 (87.8) | |||
| Fatty Liver | <0.001 | 0.809 | 0.098 | |||
| Yes | 12 (24.5) | 167 (8.0) | 10 (20.4) | |||
| No | 37 (75.5) | 1924 (92.0) | 39 (79.6) | |||
| Liver Cirrhosis | 0.263 | 0.839 | 0.082 | |||
| Yes | 28 (57.1) | 1004 (48.0) | 26 (53.1) | |||
| No | 21 (42.9) | 1087 (52.0) | 23 (46.9) | |||
| HCC Cause | 0.205 | 0.819 | 0.132 | |||
| HBV HCV | 40 (81.6) | 1461 (69.9) | 40 (81.6) | |||
| 2 (4.1) | 129 (6.2) | 1 (2.0) | ||||
| Others | 7 (14.3) | 501 (24.0) | 8 (16.3) | |||
| Treatment | 0.820 | 1.000 | 0.057 | |||
| Resection | 41 (83.7) | 1701 (81.3) | 42 (85.7) | |||
| RFA | 8 (16.3) | 390 (18.7) | 7 (14.3) |
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age > 59 vs. ≤59 years | 0.84 (0.43–1.62) | 0.600 | ||
| AST > 30 vs. ≤30 IU/L | 1.32 (0.68–2.57) | 0.406 | ||
| ALT > 30 vs. ≤30 IU/L | 1.23 (0.64–2.36) | 0.545 | ||
| T.bil > 0.80 vs. ≤0.80 mg/dL | 0.98 (0.50–1.91) | 0.951 | ||
| Albumin > 4.40 vs. ≤4.40 g/dL | 1.33 (0.69–2.58) | 0.390 | ||
| PT INR > 1.01 vs. ≤1.01 | 1.03 (0.53–1.97) | 0.940 | ||
| PLT > 198 vs. ≤198 × 103/mm3 | 1.01 (0.52–1.95) | 0.976 | ||
| AFP > 8.69 vs. ≤8.69 ng/mL | 1.46 (0.75–2.85) | 0.270 | ||
| PIVKA-II > 79.50 vs. ≤79.50 mAU/mL | 2.35 (1.19–4.65) | 0.014 | 2.10 (1.05–4.21) | 0.036 |
| Male Sex | 2.58 (1.00–6.65) | 0.050 | 1.94 (0.74–5.09) | 0.178 |
| Hypertension | 1.29 (0.67–2.49) | 0.447 | ||
| Diabetes | 1.51 (0.74–3.06) | 0.258 | ||
| Dyslipidemia | 0.90 (0.35–2.33) | 0.829 | ||
| Fatty Liver | 0.88 (0.40–1.93) | 0.740 | ||
| Liver Cirrhosis | 1.35 (0.68–2.67) | 0.384 | ||
| HCC cause | 0.314 | |||
| HBV | 1 (Reference) | |||
| HCV | 1.14 (0.15–8.34) | |||
| Others | 1.90 (0.87–4.19) | |||
| Resection vs. RFA | 2.06 (0.72–5.85) | 0.177 | ||
| Immune cell vs. Control | 0.32 (0.15–0.71) | 0.005 | 0.32 (0.15–0.71) | 0.005 |
| (a) Stratification by Post-Treatment AFP Levels. | ||||||
| Values | Post-Treatment AFP > 10 ng/mL | Post-Treatment AFP ≤ 10 ng/mL | ||||
| Pre-CIK | Post-CIK | p value | Pre-CIK | Post-CIK | p value | |
| AFP (ng/mL) | 15.3 (5.2–28.4) | 1.3 (1.7–3.0) | 0.002 | 2.8 (1.3–5.0) | 2.1 (1.3–2.7) | 0.552 |
| PIVKA-II (mAU/mL) | 26.0 (21.0–34.0) | 26.0 (21.0–29.0) | 0.307 | 31.5 (24.5–57.5) | 29.5 (20.5–33.8) | 0.097 |
| (b) Stratification by Post-Treatment PIVKA-II Levels. | ||||||
| Values | Post-Treatment PIVKA-II > 40 mAU/mL | Post-Treatment PIVKA-II ≤ 40 mAU/mL | ||||
| Pre-CIK | Post-CIK | p value | Pre-CIK | Post-CIK | p value | |
| AFP (ng/mL) | 10.4 (1.3–15.3) | 2.4 (1.7–7.9) | 0.084 | 3.0 (2.0–5.7) | 1.5 (1.3–2.3) | 0.001 |
| PIVKA-II (mAU/mL) | 42.0 (27.0–74.0) | 27.0 (23.0–33.0) | 0.019 | 25.0 (21.0–30.3) | 28.0 (17.8–33.0) | 0.932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Kim, E.M.; Lee, J.S.; Kim, M.N.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Choi, G.H.; Ahn, S.H.; Lee, H.W.; et al. Cytokine-Induced Killer Cell Immunotherapy Reduces Recurrence in Patients with Early-Stage Hepatocellular Carcinoma. Cancers 2025, 17, 566. https://doi.org/10.3390/cancers17040566
Kim DH, Kim EM, Lee JS, Kim MN, Kim BK, Kim SU, Park JY, Choi GH, Ahn SH, Lee HW, et al. Cytokine-Induced Killer Cell Immunotherapy Reduces Recurrence in Patients with Early-Stage Hepatocellular Carcinoma. Cancers. 2025; 17(4):566. https://doi.org/10.3390/cancers17040566
Chicago/Turabian StyleKim, Dong Hyun, Eun Min Kim, Jae Seung Lee, Mi Na Kim, Beom Kyung Kim, Seung Up Kim, Jun Yong Park, Gi Hong Choi, Sang Hoon Ahn, Hye Won Lee, and et al. 2025. "Cytokine-Induced Killer Cell Immunotherapy Reduces Recurrence in Patients with Early-Stage Hepatocellular Carcinoma" Cancers 17, no. 4: 566. https://doi.org/10.3390/cancers17040566
APA StyleKim, D. H., Kim, E. M., Lee, J. S., Kim, M. N., Kim, B. K., Kim, S. U., Park, J. Y., Choi, G. H., Ahn, S. H., Lee, H. W., & Kim, D. Y. (2025). Cytokine-Induced Killer Cell Immunotherapy Reduces Recurrence in Patients with Early-Stage Hepatocellular Carcinoma. Cancers, 17(4), 566. https://doi.org/10.3390/cancers17040566








