Applications of Artificial Intelligence for the Prediction and Diagnosis of Cancer Therapy-Related Cardiac Dysfunction in Oncology Patients
Simple Summary
Abstract
1. Introduction
2. Artificial Intelligence
3. Electrocardiography
3.1. LVSD
3.2. Arrhythmias
3.3. Cardiac Amyloidosis
3.4. Wearables
4. Transthoracic Echocardiography
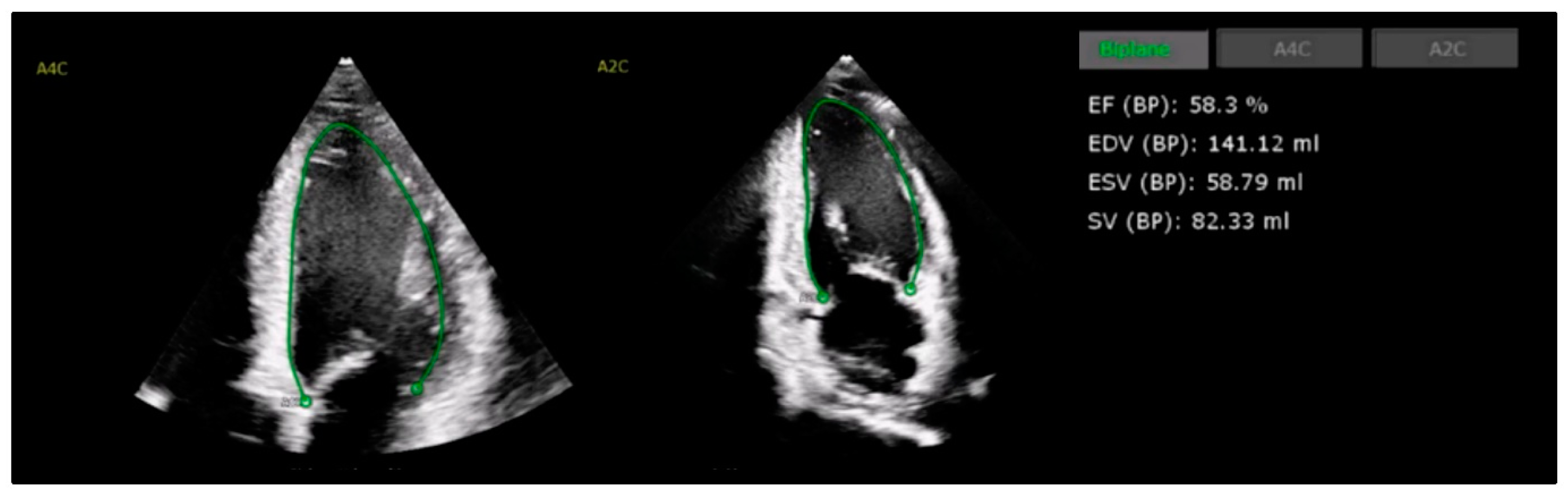
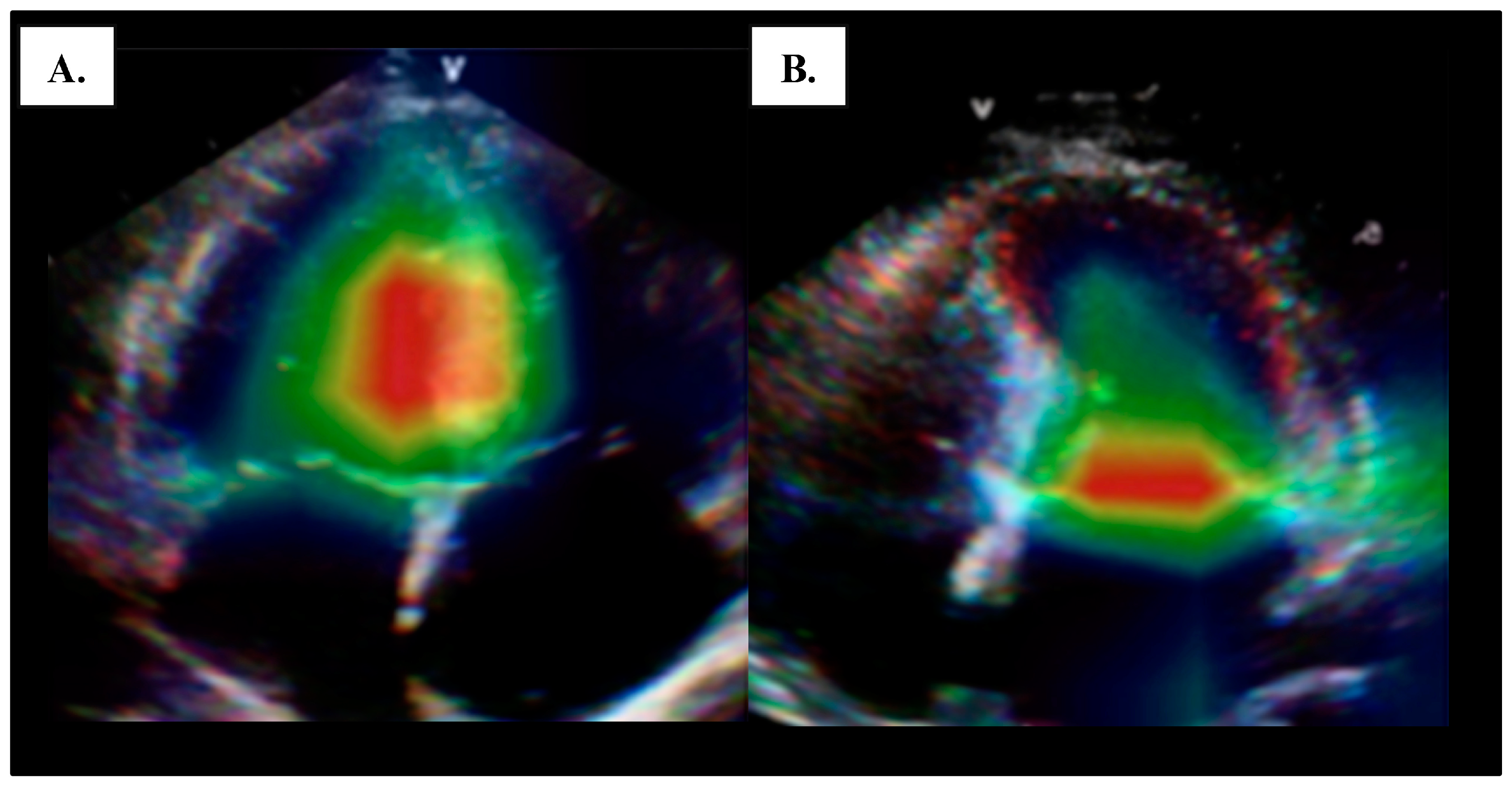
4.1. Left Ventricular Systolic Function
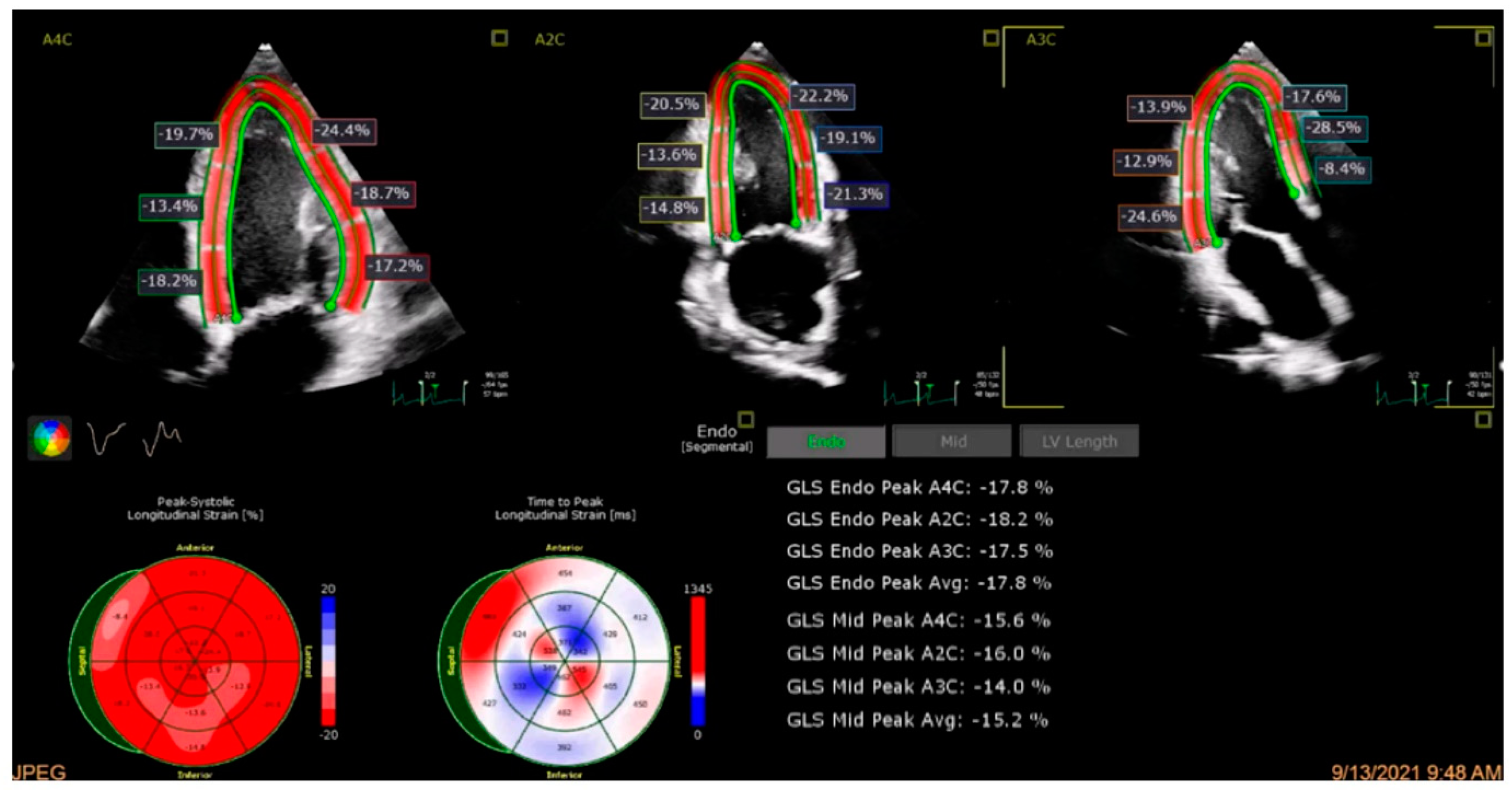
4.2. Global Longitudinal Strain
5. Cardiac Computed Tomography, Cardiac Computed Tomography Angiography, and X-Ray
5.1. Cardiac CT
5.2. CCTA
5.3. X-Ray
6. Cardiac Magnetic Resonance Imaging
6.1. Late Gadolinium Enhancement CMR
6.2. CMR Strain Analysis
6.3. Myocarditis
6.4. Cardiac Amyloidosis
7. Nuclear Imaging
7.1. PET
7.2. SPECT
8. Discussion
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Madan, N.; Lucas, J.; Akhter, N.; Collier, P.; Cheng, F.; Guha, A.; Zhang, L.; Sharma, A.; Hamid, A.; Ndiokho, I.; et al. Artificial intelligence and imaging: Opportunities in cardio-oncology. Am. Heart J. Plus 2022, 15, 100126. [Google Scholar] [CrossRef]
- Awadalla, M.; Hassan, M.Z.O.; Alvi, R.M.; Neilan, T.G. Advanced imaging modalities to detect cardiotoxicity. Curr. Probl. Cancer 2018, 42, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Scalia, I.G.; Gheyath, B.; Tamarappoo, B.K.; Moudgil, R.; Otton, J.; Pereyra, M.; Narayanasamy, H.; Larsen, C.; Herrmann, J.; Arsanjani, R.; et al. Chemotherapy Related Cardiotoxicity Evaluation-A Contemporary Review with a Focus on Cardiac Imaging. J. Clin. Med. 2024, 13, 3714. [Google Scholar] [CrossRef] [PubMed]
- Teske, A.J.; Moudgil, R.; Lopez-Fernandez, T.; Barac, A.; Brown, S.A.; Deswal, A.; Neilan, T.G.; Ganatra, S.; Abdel Qadir, H.; Menon, V.; et al. Global Cardio Oncology Registry (G-COR): Registry Design, Primary Objectives, and Future Perspectives of a Multicenter Global Initiative. Circ. Cardiovasc. Qual. Outcomes 2023, 16, e009905. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Mauro, C.; Capone, V.; Cocchia, R.; Cademartiri, F.; Riccardi, F.; Arcopinto, M.; Alshahid, M.; Anwar, K.; Carafa, M.; Carbone, A.; et al. Exploring the Cardiotoxicity Spectrum of Anti-Cancer Treatments: Definition, Classification, and Diagnostic Pathways. J. Clin. Med. 2023, 12, 1612. [Google Scholar] [CrossRef] [PubMed]
- Celutkiene, J.; Pudil, R.; Lopez-Fernandez, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; von Haehling, S.; et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: A position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 1504–1524. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Lyon, A.R.; Citro, R.; Schneider, B.; Morel, O.; Ghadri, J.R.; Templin, C.; Omerovic, E. Pathophysiology of Takotsubo Syndrome: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 902–921. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-Induced Cardiomyopathy: Clinical Relevance and Response to Pharmacologic Therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef]
- Echefu, G.; Shah, R.; Sanchez, Z.; Rickards, J.; Brown, S.A. Artificial intelligence: Applications in cardio-oncology and potential impact on racial disparities. Am. Heart J. Plus 2024, 48, 100479. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, L.A.; Ganatra, S.; Lopez-Mattei, J.; Yang, E.H.; Zaha, V.G.; Wong, T.C.; Ayoub, C.; DeCara, J.M.; Dent, S.; Deswal, A.; et al. Advances in Multimodality Imaging in Cardio-Oncology: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 1560–1578. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Oikonomou, E.K.; Nadkarni, G.N.; Morley, J.R.; Wiens, J.; Butte, A.J.; Topol, E.J. Transforming Cardiovascular Care with Artificial Intelligence: From Discovery to Practice: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2024, 84, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Mintz, Y.; Brodie, R. Introduction to artificial intelligence in medicine. Minim. Invasive Ther. Allied Technol. 2019, 28, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.A.; Abbas, M.T.; Kanaan, C.N.; Awad, K.A.; Ali, N.B.; Scalia, I.G.; Farina, J.M.; Pereyra, M.; Mahmoud, A.K.; Steidley, D.E.; et al. How Artificial Intelligence Can Enhance the Diagnosis of Cardiac Amyloidosis: A Review of Recent Advances and Challenges. J. Cardiovasc. Dev. Dis. 2024, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Argentiero, A.; Muscogiuri, G.; Rabbat, M.G.; Martini, C.; Soldato, N.; Basile, P.; Baggiano, A.; Mushtaq, S.; Fusini, L.; Mancini, M.E. The applications of artificial intelligence in cardiovascular magnetic resonance—A comprehensive review. J. Clin. Med. 2022, 11, 2866. [Google Scholar] [CrossRef]
- Fotaki, A.; Puyol-Anton, E.; Chiribiri, A.; Botnar, R.; Pushparajah, K.; Prieto, C. Artificial Intelligence in Cardiac MRI: Is Clinical Adoption Forthcoming? Front. Cardiovasc. Med. 2021, 8, 818765. [Google Scholar] [CrossRef] [PubMed]
- Sidey-Gibbons, J.A.M.; Sidey-Gibbons, C.J. Machine learning in medicine: A practical introduction. BMC Med. Res. Methodol. 2019, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A. Artificial Intelligence and Machine Learning in Cardiovascular Health Care. Ann. Thorac. Surg. 2020, 109, 1323–1329. [Google Scholar] [CrossRef]
- Chan, H.P.; Samala, R.K.; Hadjiiski, L.M.; Zhou, C. Deep Learning in Medical Image Analysis. Adv. Exp. Med. Biol. 2020, 1213, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.S.; Noseworthy, P.A.; Akbilgic, O.; Herrmann, J.; Ruddy, K.J.; Hamid, A.; Maddula, R.; Singh, A.; Davis, R.; Gunturkun, F.; et al. Artificial intelligence opportunities in cardio-oncology: Overview with spotlight on electrocardiography. Am. Heart J. Plus 2022, 15, 100129. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Harmon, D.M.; Dugan, J.; Manka, L.; Lopez-Jimenez, F.; Lerman, A.; Siontis, K.C.; Noseworthy, P.A.; Yao, X.; Klavetter, E.W.; et al. Prospective evaluation of smartwatch-enabled detection of left ventricular dysfunction. Nat. Med. 2022, 28, 2497–2503. [Google Scholar] [CrossRef]
- Kwon, J.M.; Lee, S.Y.; Jeon, K.H.; Lee, Y.; Kim, K.H.; Park, J.; Oh, B.H.; Lee, M.M. Deep Learning-Based Algorithm for Detecting Aortic Stenosis Using Electrocardiography. J. Am. Heart Assoc. 2020, 9, e014717. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Galloway, C.D.; Valys, A.V.; Shreibati, J.B.; Treiman, D.L.; Petterson, F.L.; Gundotra, V.P.; Albert, D.E.; Attia, Z.I.; Carter, R.E.; Asirvatham, S.J.; et al. Development and Validation of a Deep-Learning Model to Screen for Hyperkalemia from the Electrocardiogram. JAMA Cardiol. 2019, 4, 428–436. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Attia, Z.I.; Behnken, E.M.; Giblon, R.E.; Bews, K.A.; Liu, S.; Gosse, T.A.; Linn, Z.D.; Deng, Y.; Yin, J.; et al. Artificial intelligence-guided screening for atrial fibrillation using electrocardiogram during sinus rhythm: A prospective non-randomised interventional trial. Lancet 2022, 400, 1206–1212. [Google Scholar] [CrossRef]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef]
- Yagi, R.; Goto, S.; Himeno, Y.; Katsumata, Y.; Hashimoto, M.; MacRae, C.A.; Deo, R.C. Artificial intelligence-enabled prediction of chemotherapy-induced cardiotoxicity from baseline electrocardiograms. Nat. Commun. 2024, 15, 2536. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Sangha, V.; Dhingra, L.S.; Aminorroaya, A.; Coppi, A.; Krumholz, H.M.; Baldassarre, L.A.; Khera, R. Artificial Intelligence-Enhanced Risk Stratification of Cancer Therapeutics-Related Cardiac Dysfunction Using Electrocardiographic Images. MedRxiv 2024. [Google Scholar] [CrossRef]
- Lee, E.; Ito, S.; Miranda, W.R.; Lopez-Jimenez, F.; Kane, G.C.; Asirvatham, S.J.; Noseworthy, P.A.; Friedman, P.A.; Carter, R.E.; Borlaug, B.A.; et al. Artificial intelligence-enabled ECG for left ventricular diastolic function and filling pressure. NPJ Digit. Med. 2024, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.E.J.; Greason, G.; Mangold, K.E.; Wildiers, H.; Willems, R.; Janssens, S.; Noseworthy, P.; Lopez-Jimenez, F.; Voigt, J.U.; Friedman, P.; et al. Artificial intelligence electrocardiogram as a novel screening tool to detect a newly abnormal left ventricular ejection fraction after anthracycline-based cancer therapy. Eur. J. Prev. Cardiol. 2024, 31, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Bungo, B.; Chaudhury, P.; Arustamyan, M.; Rikhi, R.; Hussain, M.; Collier, P.; Kanj, M.; Khorana, A.A.; Mentias, A.; Moudgil, R. Better prediction of stroke in atrial fibrillation with incorporation of cancer in CHA(2)DS(2)VASC score: CCHA(2)DS(2)VASC score. Int. J. Cardiol. Heart Vasc. 2022, 41, 101072. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Misbah, R.; Donnellan, E.; Alkharabsheh, S.; Hou, Y.; Cheng, F.; Crookshanks, M.; Watson, C.J.; Toth, A.J.; Houghtaling, P.; et al. Impact of timing of atrial fibrillation, CHA(2)DS(2)-VASc score and cancer therapeutics on mortality in oncology patients. Open Heart 2020, 7, e001412. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Vincelette, N.D.; Acharya, U.; Abraham, I. Risk of Atrial Fibrillation and Bleeding Diathesis Associated with Ibrutinib Treatment: A Systematic Review and Pooled Analysis of Four Randomized Controlled Trials. Clin. Lymphoma Myeloma Leuk. 2017, 17, 31–37.e13. [Google Scholar] [CrossRef] [PubMed]
- Caron, F.; Leong, D.P.; Hillis, C.; Fraser, G.; Siegal, D. Current understanding of bleeding with ibrutinib use: A systematic review and meta-analysis. Blood Adv. 2017, 1, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, G.; Attia, Z.I.; Achenbach, S.J.; Rabe, K.G.; Call, T.G.; Ding, W.; Leis, J.F.; Muchtar, E.; Kenderian, S.S.; Wang, Y.; et al. Artificial Intelligence Electrocardiography to Predict Atrial Fibrillation in Patients with Chronic Lymphocytic Leukemia. JACC CardioOncol. 2024, 6, 251–263. [Google Scholar] [CrossRef]
- Safdar, M.F.; Nowak, R.M.; Palka, P. Pre-Processing techniques and artificial intelligence algorithms for electrocardiogram (ECG) signals analysis: A comprehensive review. Comput. Biol. Med. 2024, 170, 107908. [Google Scholar] [CrossRef] [PubMed]
- Fiorina, L.; Maupain, C.; Gardella, C.; Manenti, V.; Salerno, F.; Socie, P.; Li, J.; Henry, C.; Plesse, A.; Narayanan, K.; et al. Evaluation of an Ambulatory ECG Analysis Platform Using Deep Neural Networks in Routine Clinical Practice. J. Am. Heart Assoc. 2022, 11, e026196. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Selles, M.; Marina-Breysse, M. Current and Future Use of Artificial Intelligence in Electrocardiography. J. Cardiovasc. Dev. Dis. 2023, 10, 175. [Google Scholar] [CrossRef]
- Khan, L.A.; Shaikh, F.H.; Khan, M.S.; Zafar, B.; Farooqi, M.; Bold, B.; Aslam, H.M.; Essam, N.; Noor, I.; Siddique, A.; et al. Artificial intelligence-enhanced electrocardiogram for the diagnosis of cardiac amyloidosis: A systemic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102860. [Google Scholar] [CrossRef]
- Grogan, M.; Lopez-Jimenez, F.; Cohen-Shelly, M.; Dispenzieri, A.; Attia, Z.I.; Abou Ezzedine, O.F.; Lin, G.; Kapa, S.; Borgeson, D.D.; Friedman, P.A.; et al. Artificial Intelligence-Enhanced Electrocardiogram for the Early Detection of Cardiac Amyloidosis. Mayo Clin. Proc. 2021, 96, 2768–2778. [Google Scholar] [CrossRef]
- Harmon, D.M.; Mangold, K.; Suarez, A.B.; Scott, C.G.; Murphree, D.H.; Malik, A.; Attia, Z.I.; Lopez-Jimenez, F.; Friedman, P.A.; Dispenzieri, A.; et al. Postdevelopment Performance and Validation of the Artificial Intelligence-Enhanced Electrocardiogram for Detection of Cardiac Amyloidosis. JACC Adv. 2023, 2, 100612. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Gorrie, N.; Raftopulos, N.; Olsen, N.; Bart, N. AI for the Detection of Cardiac Amyloidosis: Insights from a Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2024, 83, 400. [Google Scholar] [CrossRef]
- Pereyra Pietri, M.; Farina, J.M.; Mahmoud, A.K.; Scalia, I.G.; Galasso, F.; Killian, M.E.; Suppah, M.; Kenyon, C.R.; Koepke, L.M.; Padang, R.; et al. The prognostic value of artificial intelligence to predict cardiac amyloidosis in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. Digit. Health 2024, 5, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Khunte, A.; Sangha, V.; Oikonomou, E.K.; Dhingra, L.S.; Aminorroaya, A.; Mortazavi, B.J.; Coppi, A.; Brandt, C.A.; Krumholz, H.M.; Khera, R. Detection of left ventricular systolic dysfunction from single-lead electrocardiography adapted for portable and wearable devices. NPJ Digit. Med. 2023, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- Moudgil, R.; Hassan, S.; Palaskas, N.; Lopez-Mattei, J.; Banchs, J.; Yusuf, S.W. Evolution of echocardiography in subclinical detection of cancer therapy-related cardiac dysfunction. Echocardiography 2018, 35, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Hahn, R.T. Valvular Heart Disease Associated with Radiation Therapy: A Contemporary Review. Struct. Heart 2023, 7, 100104. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.H. Chemotherapy-Induced Valvular Heart Disease. JACC Cardiovasc. Imaging 2016, 9, 240–242. [Google Scholar] [CrossRef]
- Mori, S.; Bertamino, M.; Guerisoli, L.; Stratoti, S.; Canale, C.; Spallarossa, P.; Porto, I.; Ameri, P. Pericardial effusion in oncological patients: Current knowledge and principles of management. Cardiooncology 2024, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Chahine, J.; Shekhar, S.; Mahalwar, G.; Imazio, M.; Collier, P.; Klein, A. Pericardial Involvement in Cancer. Am. J. Cardiol. 2021, 145, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Barry, T.; Farina, J.M.; Chao, C.J.; Ayoub, C.; Jeong, J.; Patel, B.N.; Banerjee, I.; Arsanjani, R. The Role of Artificial Intelligence in Echocardiography. J. Imaging 2023, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chao, C.J.; Jeong, J.J.; Farina, J.M.; Seri, A.R.; Barry, T.; Newman, H.; Campany, M.; Abdou, M.; O’Shea, M.; et al. Developing an Echocardiography-Based, Automatic Deep Learning Framework for the Differentiation of Increased Left Ventricular Wall Thickness Etiologies. J. Imaging 2023, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Ghosh, A.K.; Ky, B.; Marwick, T.; Stout, M.; Harkness, A.; Steeds, R.; Robinson, S.; Oxborough, D.; Adlam, D.; et al. BSE and BCOS Guideline for Transthoracic Echocardiographic Assessment of Adult Cancer Patients Receiving Anthracyclines and/or Trastuzumab. JACC CardioOncol. 2021, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Grant, A.D.; Negishi, T.; Plana, J.C.; Popovic, Z.B.; Marwick, T.H. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J. Am. Coll. Cardiol. 2013, 61, 77–84. [Google Scholar] [CrossRef]
- Liu, J.; Banchs, J.; Mousavi, N.; Plana, J.C.; Scherrer-Crosbie, M.; Thavendiranathan, P.; Barac, A. Contemporary Role of Echocardiography for Clinical Decision Making in Patients During and After Cancer Therapy. JACC Cardiovasc. Imaging 2018, 11, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.R.; Mulvagh, S.L.; Abdelmoneim, S.S.; Becher, H.; Belcik, J.T.; Bierig, M.; Choy, J.; Gaibazzi, N.; Gillam, L.D.; Janardhanan, R.; et al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J. Am. Soc. Echocardiogr. 2018, 31, 241–274. [Google Scholar] [CrossRef]
- He, B.; Kwan, A.C.; Cho, J.H.; Yuan, N.; Pollick, C.; Shiota, T.; Ebinger, J.; Bello, N.A.; Wei, J.; Josan, K.; et al. Blinded, randomized trial of sonographer versus AI cardiac function assessment. Nature 2023, 616, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Kagiyama, N.; Abe, Y.; Kusunose, K.; Kato, N.; Kaneko, T.; Murata, A.; Ota, M.; Shibayama, K.; Izumo, M.; Watanabe, H. Multicenter validation study for automated left ventricular ejection fraction assessment using a handheld ultrasound with artificial intelligence. Sci. Rep. 2024, 14, 15359. [Google Scholar] [CrossRef] [PubMed]
- Dadon, Z.; Steinmetz, Y.; Levi, N.; Orlev, A.; Belman, D.; Butnaru, A.; Carasso, S.; Glikson, M.; Alpert, E.A.; Gottlieb, S. Artificial Intelligence-Powered Left Ventricular Ejection Fraction Analysis Using the LVivoEF Tool for COVID-19 Patients. J. Clin. Med. 2023, 12, 7571. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S.L.; Dionysopoulos, D.; Mentesidou, V.; Loga, K.; Michalopoulou, S.; Koukoutzeli, C.; Efthimiadis, K.; Kantartzi, V.; Timotheadou, E.; Styliadis, I.; et al. Artificial intelligence-assisted evaluation of cardiac function by oncology staff in chemotherapy patients. Eur. Heart J. Digit. Health 2024, 5, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, B.; Li, Y.W.; Hothi, S.S. Clinical service evaluation of the feasibility and reproducibility of novel artificial intelligence based-echocardiographic quantification of global longitudinal strain and left ventricular ejection fraction in trastuzumab-treated patients. Front. Cardiovasc. Med. 2023, 10, 1250311. [Google Scholar] [CrossRef]
- Demissei, B.G.; Fan, Y.; Qian, Y.; Cheng, H.G.; Smith, A.M.; Shimamoto, K.; Vedage, N.; Narayan, H.K.; Scherrer-Crosbie, M.; Davatzikos, C.; et al. Left ventricular segmental strain and the prediction of cancer therapy-related cardiac dysfunction. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, K.M.; Mitsis, A.; Grigoriadis, G.; Karanasiou, G.; Lakkas, L.; Mauri, D.; Toli, M.A.; Alexandraki, A.; Keramida, K.; Cardinale, D.; et al. Risk Stratification for Cardiotoxicity in Breast Cancer Patients: Predicting Early Decline of LVEF After Treatment. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Patel, J.; Rikhi, R.; Hussain, M.; Ayoub, C.; Klein, A.; Collier, P.; Moudgil, R. Global longitudinal strain is a better metric than left ventricular ejection fraction: Lessons learned from cancer therapeutic-related cardiac dysfunction. Curr. Opin. Cardiol. 2020, 35, 170–177. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Daraban, A.M.; Unlu, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, A.; Iwasaki, Y.; Kobayashi, M.; Takagi, R.; Yamada, S.; Kubo, T.; Satomi, K.; Tanaka, N. Artificial intelligence-derived left ventricular strain in echocardiography in patients treated with chemotherapy. Int. J. Cardiovasc. Imaging 2024, 40, 1903–1910. [Google Scholar] [CrossRef]
- Myhre, P.L.; Hung, C.L.; Frost, M.J.; Jiang, Z.; Ouwerkerk, W.; Teramoto, K.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; et al. External validation of a deep learning algorithm for automated echocardiographic strain measurements. Eur. Heart J. Digit. Health 2024, 5, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, M.; Urzua Fresno, C.M.; Somerset, E.; Shalmon, T.; Amir, E.; Fan, C.S.; Brezden-Masley, C.; Thampinathan, B.; Thevakumaran, Y.; Yared, K.; et al. A Combined Echocardiography Approach for the Diagnosis of Cancer Therapy-Related Cardiac Dysfunction in Women with Early-Stage Breast Cancer. JAMA Cardiol. 2022, 7, 330–340. [Google Scholar] [CrossRef]
- Serrano, J.M.; Mata, R.; Gonzalez, I.; Del Castillo, S.; Muniz, J.; Morales, L.J.; Espinosa, M.J.; Moreno, F.; Jimenez, R.; Cristobal, C.; et al. Early and late onset cardiotoxicity following anthracycline-based chemotherapy in breast cancer patients: Incidence and predictors. Int. J. Cardiol. 2023, 382, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Nadouri, D.; Osler, E.; Johnson, C.; Dent, S.; Dwivedi, G. Diastolic dysfunction can precede systolic dysfunction on MUGA in cancer patients receiving trastuzumab-based therapy. Nucl. Med. Commun. 2019, 40, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, F.; Zhang, P.; Lin, X.; Wang, W.; Pu, H.; Chen, X.; Chen, Y.; Yu, L.; Deng, Y.; et al. Artificial Intelligence-Assisted Left Ventricular Diastolic Function Assessment and Grading: Multiview Versus Single View. J. Am. Soc. Echocardiogr. 2023, 36, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Lin, M.; Zhang, M.; Qin, Y.; Meng, Y.; Wang, J.; Leng, C.; Zhu, W.; Li, J.; You, J.; et al. Automated echocardiographic diastolic function grading: A hybrid multi-task deep learning and machine learning approach. Int. J. Cardiol. 2024, 416, 132504. [Google Scholar] [CrossRef] [PubMed]
- Turk, E.; Yilmaz, M.; Obeidat, K.; Litvin, R.; Uygun, I.; Hammo, H.; Batra, K. The cardiac outcomes of radiation therapy to thoracic malignancies: Nationwide analysis. J. Clin. Oncol. 2023, 41, e20565. [Google Scholar] [CrossRef]
- Omidi, A.; Weiss, E.; Rosu-Bubulac, M.; Thomas, G.; Wilson, J.S. Quantitative Analysis of Radiation Therapy-Induced Cardiac and Aortic Sequelae in Patients with Lung Cancer via Magnetic Resonance Imaging: A Pilot Study. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.T.; Hudson, M.M.; Kremer, L.C.; Landy, D.C.; Miller, T.L.; et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 2013, 128, 1927–1995. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Huang, Y.M.; Bansal, M.; Ashrafi, A.; Fisher, M.; Shameer, K.; Gall, W.; Dudley, J.T. Cognitive Machine-Learning Algorithm for Cardiac Imaging: A Pilot Study for Differentiating Constrictive Pericarditis from Restrictive Cardiomyopathy. Circ. Cardiovasc. Imaging 2016, 9, e004330. [Google Scholar] [CrossRef]
- Chao, C.J.; Jeong, J.; Arsanjani, R.; Kim, K.; Tsai, Y.L.; Yu, W.C.; Farina, J.M.; Mahmoud, A.K.; Ayoub, C.; Grogan, M.; et al. Echocardiography-Based Deep Learning Model to Differentiate Constrictive Pericarditis and Restrictive Cardiomyopathy. JACC Cardiovasc. Imaging 2023, 17, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Siva, N.K.; Singh, Y.; Hathaway, Q.A.; Sengupta, P.P.; Yanamala, N. A novel multi-task machine learning classifier for rare disease patterning using cardiac strain imaging data. Sci. Rep. 2024, 14, 10672. [Google Scholar] [CrossRef]
- Lopez-Mattei, J.; Yang, E.H.; Baldassarre, L.A.; Agha, A.; Blankstein, R.; Choi, A.D.; Chen, M.Y.; Meyersohn, N.; Daly, R.; Slim, A.; et al. Cardiac computed tomographic imaging in cardio-oncology: An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). Endorsed by the International Cardio-Oncology Society (ICOS). J. Cardiovasc. Comput. Tomogr. 2023, 17, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.A.; Rischpler, C. Cardiovascular imaging in cardio-oncology. J. Thorac. Dis. 2018, 10, S4351–S4366. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Franco, F.X.; McDonald, M.; Rivera, C.; Perez-Villa, B.; Collier, P.; Moudgil, R.; Gupta, N.; Sadler, D.B. Use of computed tomography coronary calcium score for prediction of cardiovascular events in cancer patients: A retrospective cohort analysis. Cardiooncology 2024, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Bergom, C.; Bradley, J.A.; Ng, A.K.; Samson, P.; Robinson, C.; Lopez-Mattei, J.; Mitchell, J.D. Past, Present, and Future of Radiation-Induced Cardiotoxicity: Refinements in Targeting, Surveillance, and Risk Stratification. JACC CardioOncol. 2021, 3, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.H.; Marmagkiolis, K.; Balanescu, D.V.; Hakeem, A.; Donisan, T.; Finch, W.; Virmani, R.; Herrman, J.; Cilingiroglu, M.; Grines, C.L.; et al. Radiation-Induced Vascular Disease-A State-of-the-Art Review. Front. Cardiovasc. Med. 2021, 8, 652761. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, Y.; de Bock, G.H.; de Jong, P.A.; Mali, W.P.; Oudkerk, M.; Vliegenthart, R. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: Systematic review and meta-analysis. Circ. Cardiovasc. Imaging 2013, 6, 514–521. [Google Scholar] [CrossRef]
- Hecht, H.S.; Cronin, P.; Blaha, M.J.; Budoff, M.J.; Kazerooni, E.A.; Narula, J.; Yankelevitz, D.; Abbara, S. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J. Cardiovasc. Comput. Tomogr. 2017, 11, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, C.; Scalia, I.G.; Anavekar, N.S.; Arsanjani, R.; Jokerst, C.E.; Chow, B.J.W.; Kritharides, L. Computed Tomography Evaluation of Coronary Atherosclerosis: The Road Travelled, and What Lies Ahead. Diagnostics 2024, 14, 2096. [Google Scholar] [CrossRef]
- Gennari, A.G.; Rossi, A.; De Cecco, C.N.; van Assen, M.; Sartoretti, T.; Giannopoulos, A.A.; Schwyzer, M.; Huellner, M.W.; Messerli, M. Artificial intelligence in coronary artery calcium score: Rationale, different approaches, and outcomes. Int. J. Cardiovasc. Imaging 2024, 40, 951–966. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, S.G.M.; Lessmann, N.; Velthuis, B.K.; Bank, I.E.M.; van den Bongard, D.; Leiner, T.; de Jong, P.A.; Veldhuis, W.B.; Correa, A.; Terry, J.G.; et al. Deep Learning for Automatic Calcium Scoring in CT: Validation Using Multiple Cardiac CT and Chest CT Protocols. Radiology 2020, 295, 66–79. [Google Scholar] [CrossRef]
- Zeleznik, R.; Foldyna, B.; Eslami, P.; Weiss, J.; Alexander, I.; Taron, J.; Parmar, C.; Alvi, R.M.; Banerji, D.; Uno, M.; et al. Deep convolutional neural networks to predict cardiovascular risk from computed tomography. Nat. Commun. 2021, 12, 715. [Google Scholar] [CrossRef] [PubMed]
- Lessmann, N.; van Ginneken, B.; Zreik, M.; de Jong, P.A.; de Vos, B.D.; Viergever, M.A.; Isgum, I. Automatic Calcium Scoring in Low-Dose Chest CT Using Deep Neural Networks with Dilated Convolutions. IEEE Trans. Med. Imaging 2018, 37, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Eng, D.; Chute, C.; Khandwala, N.; Rajpurkar, P.; Long, J.; Shleifer, S.; Khalaf, M.H.; Sandhu, A.T.; Rodriguez, F.; Maron, D.J.; et al. Automated coronary calcium scoring using deep learning with multicenter external validation. NPJ Digit. Med. 2021, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research, T.; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Douglas, P.S.; Hoffmann, U.; Lee, K.L.; Mark, D.B.; Al-Khalidi, H.R.; Anstrom, K.; Dolor, R.J.; Kosinski, A.; Krucoff, M.W.; Mudrick, D.W.; et al. PROspective Multicenter Imaging Study for Evaluation of chest pain: Rationale and design of the PROMISE trial. Am. Heart J. 2014, 167, 796–803.e791. [Google Scholar] [CrossRef]
- Hoffmann, U.; Ferencik, M.; Udelson, J.E.; Picard, M.H.; Truong, Q.A.; Patel, M.R.; Huang, M.; Pencina, M.; Mark, D.B.; Heitner, J.F.; et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients with Stable Chest Pain: Insights from the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017, 135, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, U.; Truong, Q.A.; Fleg, J.L.; Goehler, A.; Gazelle, S.; Wiviott, S.; Lee, H.; Udelson, J.E.; Schoenfeld, D.; Romicat, I.I. Design of the Rule Out Myocardial Ischemia/Infarction Using Computer Assisted Tomography: A multicenter randomized comparative effectiveness trial of cardiac computed tomography versus alternative triage strategies in patients with acute chest pain in the emergency department. Am. Heart J. 2012, 163, 330–338.e331. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Van Assen, M.; Tesche, C.; De Cecco, C.N.; Chiesa, M.; Scafuri, S.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Guaricci, A.I.; et al. Artificial Intelligence in Coronary Computed Tomography Angiography: From Anatomy to Prognosis. BioMed Res. Int. 2020, 2020, 6649410. [Google Scholar] [CrossRef] [PubMed]
- Baessler, B.; Gotz, M.; Antoniades, C.; Heidenreich, J.F.; Leiner, T.; Beer, M. Artificial intelligence in coronary computed tomography angiography: Demands and solutions from a clinical perspective. Front. Cardiovasc. Med. 2023, 10, 1120361. [Google Scholar] [CrossRef]
- Zreik, M.; van Hamersvelt, R.W.; Wolterink, J.M.; Leiner, T.; Viergever, M.A.; Isgum, I. A Recurrent CNN for Automatic Detection and Classification of Coronary Artery Plaque and Stenosis in Coronary CT Angiography. IEEE Trans. Med. Imaging 2019, 38, 1588–1598. [Google Scholar] [CrossRef]
- Coenen, A.; Kim, Y.H.; Kruk, M.; Tesche, C.; De Geer, J.; Kurata, A.; Lubbers, M.L.; Daemen, J.; Itu, L.; Rapaka, S.; et al. Diagnostic Accuracy of a Machine-Learning Approach to Coronary Computed Tomographic Angiography-Based Fractional Flow Reserve: Result from the MACHINE Consortium. Circ. Cardiovasc. Imaging 2018, 11, e007217. [Google Scholar] [CrossRef] [PubMed]
- Tamarappoo, B.; Otaki, Y.; Doris, M.; Arnson, Y.; Gransar, H.; Hayes, S.; Friedman, J.; Thomson, L.; Wang, F.; Rozanski, A.; et al. Improvement in LDL is associated with decrease in non-calcified plaque volume on coronary CTA as measured by automated quantitative software. J. Cardiovasc. Comput. Tomogr. 2018, 12, 385–390. [Google Scholar] [CrossRef]
- Kolossvary, M.; Karady, J.; Szilveszter, B.; Kitslaar, P.; Hoffmann, U.; Merkely, B.; Maurovich-Horvat, P. Radiomic Features Are Superior to Conventional Quantitative Computed Tomographic Metrics to Identify Coronary Plaques with Napkin-Ring Sign. Circ. Cardiovasc. Imaging 2017, 10, e006843. [Google Scholar] [CrossRef] [PubMed]
- Kolossvary, M.; Park, J.; Bang, J.I.; Zhang, J.; Lee, J.M.; Paeng, J.C.; Merkely, B.; Narula, J.; Kubo, T.; Akasaka, T.; et al. Identification of invasive and radionuclide imaging markers of coronary plaque vulnerability using radiomic analysis of coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- van Rosendael, A.R.; Crabtree, T.; Bax, J.J.; Nakanishi, R.; Mushtaq, S.; Pontone, G.; Andreini, D.; Buechel, R.R.; Grani, C.; Feuchtner, G.; et al. Rationale and design of the CONFIRM2 (Quantitative COroNary CT Angiography Evaluation for Evaluation of Clinical Outcomes: An InteRnational, Multicenter Registry) study. J. Cardiovasc. Comput. Tomogr. 2024, 18, 11–17. [Google Scholar] [CrossRef] [PubMed]
- CONFIRM2: AI-Guided QCT May Help Reduce CV Events in Patients with Suspected CAD. Available online: https://www.acc.org/latest-in-cardiology/articles/2024/10/24/19/43/mon-312pm-confirm2-tct-2024#resources-for-article (accessed on 13 December 2024).
- Belzile-Dugas, E.; Eisenberg, M.J. Radiation-Induced Cardiovascular Disease: Review of an Underrecognized Pathology. J. Am. Heart Assoc. 2021, 10, e021686. [Google Scholar] [CrossRef]
- Layoun, M.E.; Yang, E.H.; Herrmann, J.; Iliescu, C.A.; Lopez-Mattei, J.C.; Marmagkiolis, K.; Budoff, M.J.; Ferencik, M. Applications of Cardiac Computed Tomography in the Cardio-Oncology Population. Curr. Treat. Options Oncol. 2019, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Jellis, C.L.; Kotecha, R.; Johnston, D.R.; Griffin, B.P. Radiation-Associated Cardiac Disease: A Practical Approach to Diagnosis and Management. JACC Cardiovasc. Imaging 2018, 11, 1132–1149. [Google Scholar] [CrossRef]
- Farina, J.M.; Pereyra, M.; Mahmoud, A.K.; Scalia, I.G.; Abbas, M.T.; Chao, C.J.; Barry, T.; Ayoub, C.; Banerjee, I.; Arsanjani, R. Artificial Intelligence-Based Prediction of Cardiovascular Diseases from Chest Radiography. J. Imaging 2023, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Ueda, D.; Matsumoto, T.; Ehara, S.; Yamamoto, A.; Walston, S.L.; Ito, A.; Shimono, T.; Shiba, M.; Takeshita, T.; Fukuda, D.; et al. Artificial intelligence-based model to classify cardiac functions from chest radiographs: A multi-institutional, retrospective model development and validation study. Lancet Digit. Health 2023, 5, e525–e533. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Chao, C.J.; Arsanjani, R.; Ayoub, C.; Lester, S.J.; Pereyra, M.; Said, E.F.; Roarke, M.; Tagle-Cornell, C.; Koepke, L.M.; et al. Opportunistic screening for coronary artery calcium deposition using chest radiographs—A multi-objective models with multi-modal data fusion. medRxiv 2024. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Yang, K.; Wen, Y.; Wang, P.; Hu, Y.; Lai, Y.; Wang, Y.; Zhao, K.; Tang, S.; Zhang, A. Screening and diagnosis of cardiovascular disease using artificial intelligence-enabled cardiac magnetic resonance imaging. Nat. Med. 2024, 30, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Harries, I.; Liang, K.; Williams, M.; Berlot, B.; Biglino, G.; Lancellotti, P.; Plana, J.C.; Bucciarelli-Ducci, C. Magnetic resonance imaging to detect cardiovascular effects of cancer therapy: JACC CardioOncology state-of-the-art review. Cardio Oncol. 2020, 2, 270–292. [Google Scholar]
- Kim, R.J.; de Roos, A.; Fleck, E.; Higgins, C.B.; Pohost, G.M.; Prince, M.; Manning, W.J. Guidelines for training in cardiovascular magnetic resonance (CMR). J. Cardiovasc. Magn. Reson. 2007, 9, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.A.; Venkatesh, B.A. Building confidence in AI-interpreted CMR. Cardiovasc. Imaging 2022, 15, 428–430. [Google Scholar] [CrossRef]
- Dey, D.; Slomka, P.J.; Leeson, P.; Comaniciu, D.; Shrestha, S.; Sengupta, P.P.; Marwick, T.H. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 1317–1335. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Chiesa, M.; Trotta, M.; Gatti, M.; Palmisano, V.; Dell’Aversana, S.; Baessato, F.; Cavaliere, A.; Cicala, G.; Loffreno, A. Performance of a deep learning algorithm for the evaluation of CAD-RADS classification with CCTA. Atherosclerosis 2020, 294, 25–32. [Google Scholar] [CrossRef]
- Schuster, A.; Lange, T.; Backhaus, S.J.; Strohmeyer, C.; Boom, P.C.; Matz, J.; Kowallick, J.T.; Lotz, J.; Steinmetz, M.; Kutty, S.; et al. Fully Automated Cardiac Assessment for Diagnostic and Prognostic Stratification Following Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e016612. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, Z.; Zhang, B.; Guo, S.; Bo, K.; Li, S.; Zhang, N.; Wang, H.; Yang, G.; Zhang, H.; et al. Deep learning-based prognostic model using non-enhanced cardiac cine MRI for outcome prediction in patients with heart failure. Eur. Radiol. 2023, 33, 8203–8213. [Google Scholar] [CrossRef]
- Kustner, T.; Munoz, C.; Psenicny, A.; Bustin, A.; Fuin, N.; Qi, H.; Neji, R.; Kunze, K.; Hajhosseiny, R.; Prieto, C.; et al. Deep-learning based super-resolution for 3D isotropic coronary MR angiography in less than a minute. Magn. Reson. Med. 2021, 86, 2837–2852. [Google Scholar] [CrossRef] [PubMed]
- Steeden, J.A.; Quail, M.; Gotschy, A.; Mortensen, K.H.; Hauptmann, A.; Arridge, S.; Jones, R.; Muthurangu, V. Rapid whole-heart CMR with single volume super-resolution. J. Cardiovasc. Magn. Reson. 2020, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fotaki, A.; Ghadimi, S.; Wang, Y.; Doneva, M.; Wetzl, J.; Delfino, J.G.; O'Regan, D.P.; Prieto, C.; Epstein, F.H. Improving the efficiency and accuracy of cardiovascular magnetic resonance with artificial intelligence-review of evidence and proposition of a roadmap to clinical translation. J. Cardiovasc. Magn. Reson. 2024, 26, 101051. [Google Scholar] [CrossRef]
- Afshin, M.; Ben Ayed, I.; Punithakumar, K.; Law, M.; Islam, A.; Goela, A.; Peters, T.; Shuo, L. Regional assessment of cardiac left ventricular myocardial function via MRI statistical features. IEEE Trans. Med. Imaging 2014, 33, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Sinclair, M.; Tarroni, G.; Oktay, O.; Rajchl, M.; Vaillant, G.; Lee, A.M.; Aung, N.; Lukaschuk, E.; Sanghvi, M.M.; et al. Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. J. Cardiovasc. Magn. Reson. 2018, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Penso, M.; Moccia, S.; Scafuri, S.; Muscogiuri, G.; Pontone, G.; Pepi, M.; Caiani, E.G. Automated left and right ventricular chamber segmentation in cardiac magnetic resonance images using dense fully convolutional neural network. Comput. Methods Programs Biomed. 2021, 204, 106059. [Google Scholar] [CrossRef] [PubMed]
- Lanzafame, L.R.M.; Bucolo, G.M.; Muscogiuri, G.; Sironi, S.; Gaeta, M.; Ascenti, G.; Booz, C.; Vogl, T.J.; Blandino, A.; Mazziotti, S.; et al. Artificial Intelligence in Cardiovascular CT and MR Imaging. Life 2023, 13, 507. [Google Scholar] [CrossRef] [PubMed]
- Zabihollahy, F.; Rajchl, M.; White, J.A.; Ukwatta, E. Fully automated segmentation of left ventricular scar from 3D late gadolinium enhancement magnetic resonance imaging using a cascaded multi-planar U-Net (CMPU-Net). Med. Phys. 2020, 47, 1645–1655. [Google Scholar] [CrossRef]
- El-Rewaidy, H.; Neisius, U.; Mancio, J.; Kucukseymen, S.; Rodriguez, J.; Paskavitz, A.; Menze, B.; Nezafat, R. Deep complex convolutional network for fast reconstruction of 3D late gadolinium enhancement cardiac MRI. NMR Biomed. 2020, 33, e4312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Burrage, M.K.; Lukaschuk, E.; Shanmuganathan, M.; Popescu, I.A.; Nikolaidou, C.; Mills, R.; Werys, K.; Hann, E.; Barutcu, A.; et al. Toward Replacing Late Gadolinium Enhancement with Artificial Intelligence Virtual Native Enhancement for Gadolinium-Free Cardiovascular Magnetic Resonance Tissue Characterization in Hypertrophic Cardiomyopathy. Circulation 2021, 144, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, C.; Ghadimi, S.; Auger, D.C.; Croisille, P.; Viallon, M.; Mangion, K.; Berry, C.; Haggerty, C.M.; Jing, L.; et al. StrainNet: Improved Myocardial Strain Analysis of Cine MRI by Deep Learning from DENSE. Radiol. Cardiothorac. Imaging 2023, 5, e220196. [Google Scholar] [CrossRef]
- Masutani, E.M.; Chandrupatla, R.S.; Wang, S.; Zocchi, C.; Hahn, L.D.; Horowitz, M.; Jacobs, K.; Kligerman, S.; Raimondi, F.; Patel, A.; et al. Deep Learning Synthetic Strain: Quantitative Assessment of Regional Myocardial Wall Motion at MRI. Radiol. Cardiothorac. Imaging 2023, 5, e220202. [Google Scholar] [CrossRef]
- Jordan, J.H.; Hundley, W.G. MRI of Cardiotoxicity. Cardiol. Clin. 2019, 37, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Mabudian, L.; Jordan, J.H.; Bottinor, W.; Hundley, W.G. Cardiac MRI assessment of anthracycline-induced cardiotoxicity. Front. Cardiovasc. Med. 2022, 9, 903719. [Google Scholar] [CrossRef]
- Ong, G.; Brezden-Masley, C.; Dhir, V.; Deva, D.P.; Chan, K.K.W.; Chow, C.M.; Thavendiranathan, D.; Haq, R.; Barfett, J.J.; Petrella, T.M.; et al. Myocardial strain imaging by cardiac magnetic resonance for detection of subclinical myocardial dysfunction in breast cancer patients receiving trastuzumab and chemotherapy. Int. J. Cardiol. 2018, 261, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.H.; Cautela, J.; Palaskas, N.; Baik, A.H.; Meijers, W.C.; Allenbach, Y.; Alexandre, J.; Rassaf, T.; Muller, O.J.; Aras, M.; et al. Clinical Strategy for the Diagnosis and Treatment of Immune Checkpoint Inhibitor-Associated Myocarditis: A Narrative Review. JAMA Cardiol. 2021, 6, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, C.; Appari, L.; Pereyra, M.; Farina, J.M.; Chao, C.-J.; Scalia, I.G.; Mahmoud, A.K.; Abbas, M.T.; Baba, N.A.; Jeong, J.; et al. Multimodal Fusion Artificial Intelligence Model to Predict Risk for MACE and Myocarditis in Cancer Patients Receiving Immune Checkpoint Inhibitor Therapy. JACC Adv. 2025, 4, 101435. [Google Scholar] [CrossRef] [PubMed]
- Łajczak, P.M.; Jóźwik, K. Artificial intelligence and myocarditis—A systematic review of current applications. Heart Fail. Rev. 2024, 29, 1217–1234. [Google Scholar] [CrossRef] [PubMed]
- Baessler, B.; Luecke, C.; Lurz, J.; Klingel, K.; Das, A.; Von Roeder, M.; de Waha-Thiele, S.; Besler, C.; Rommel, K.-P.; Maintz, D. Cardiac MRI and texture analysis of myocardial T1 and T2 maps in myocarditis with acute versus chronic symptoms of heart failure. Radiology 2019, 292, 608–617. [Google Scholar] [CrossRef]
- Agibetov, A.; Kammerlander, A.; Duca, F.; Nitsche, C.; Koschutnik, M.; Dona, C.; Dachs, T.M.; Rettl, R.; Stria, A.; Schrutka, L.; et al. Convolutional Neural Networks for Fully Automated Diagnosis of Cardiac Amyloidosis by Cardiac Magnetic Resonance Imaging. J. Pers. Med. 2021, 11, 1268. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, J.; Moghadasi, N.; Korperich, H.; Weise Valdes, E.; Sciacca, V.; Paluszkiewicz, L.; Burchert, W.; Piran, M. A Machine Learning Challenge: Detection of Cardiac Amyloidosis Based on Bi-Atrial and Right Ventricular Strain and Cardiac Function. Diagnostics 2022, 12, 2693. [Google Scholar] [CrossRef] [PubMed]
- Balaji, V.; Song, T.A.; Malekzadeh, M.; Heidari, P.; Dutta, J. Artificial Intelligence for PET and SPECT Image Enhancement. J. Nucl. Med. 2024, 65, 4–12. [Google Scholar] [CrossRef]
- Cannizzaro, M.T.; Inserra, M.C.; Passaniti, G.; Celona, A.; D’Angelo, T.; Romeo, P.; Basile, A. Role of advanced cardiovascular imaging in chemotherapy-induced cardiotoxicity. Heliyon 2023, 9, e15226. [Google Scholar] [CrossRef]
- Juarez-Orozco, L.E.; Knol, R.J.J.; Sanchez-Catasus, C.A.; Martinez-Manzanera, O.; van der Zant, F.M.; Knuuti, J. Machine learning in the integration of simple variables for identifying patients with myocardial ischemia. J. Nucl. Cardiol. 2020, 27, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.H.; Singh, A.; Dey, D.; Slomka, P. Artificial Intelligence and Cardiac PET/Computed Tomography Imaging. PET Clin. 2022, 17, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, B.; Moody, J.B.; Tang, J. Improved myocardial perfusion PET imaging using artificial neural networks. Phys. Med. Biol. 2020, 65, 145010. [Google Scholar] [CrossRef] [PubMed]
- Lassen, M.L.; Commandeur, F.; Kwiecinski, J.; Dey, D.; Cadet, S.; Germano, G.; Berman, D.; Dweck, M.; Newby, D.; Slomka, P. 10-fold reduction of scan time with deep learning reconstruction of Coronary PET images. J. Nucl. Med. 2019, 60, 244. [Google Scholar]
- Ladefoged, C.; Hasbak, P.; Hansen, J.; Kjer, A.; Hejgaard, L.; Andersen, F. Low-dose PET reconstruction using deep learning: Application to cardiac imaged with FDG. J. Nucl. Med. 2019, 60, 573. [Google Scholar]
- Kelly, J.M.; Babich, J.W. PET Tracers for Imaging Cardiac Function in Cardio-oncology. Curr. Cardiol. Rep. 2022, 24, 247–260. [Google Scholar] [CrossRef]
- Seifert, R.; Weber, M.; Kocakavuk, E.; Rischpler, C.; Kersting, D. Artificial Intelligence and Machine Learning in Nuclear Medicine: Future Perspectives. Semin. Nucl. Med. 2021, 51, 170–177. [Google Scholar] [CrossRef]
- Qi, C.; Wang, S.; Yu, H.; Zhang, Y.; Hu, P.; Tan, H.; Shi, Y.; Shi, H. An artificial intelligence-driven image quality assessment system for whole-body [(18)F]FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Pieszko, K.; Shanbhag, A.; Killekar, A.; Miller, R.J.H.; Lemley, M.; Otaki, Y.; Singh, A.; Kwiecinski, J.; Gransar, H.; Van Kriekinge, S.D.; et al. Deep Learning of Coronary Calcium Scores From PET/CT Attenuation Maps Accurately Predicts Adverse Cardiovascular Events. JACC Cardiovasc. Imaging 2023, 16, 675–687. [Google Scholar] [CrossRef]
- Morf, C.; Sartoretti, T.; Gennari, A.G.; Maurer, A.; Skawran, S.; Giannopoulos, A.A.; Sartoretti, E.; Schwyzer, M.; Curioni-Fontecedro, A.; Gebhard, C.; et al. Diagnostic Value of Fully Automated Artificial Intelligence Powered Coronary Artery Calcium Scoring from 18F-FDG PET/CT. Diagnostics 2022, 12, 1876. [Google Scholar] [CrossRef] [PubMed]
- Song, T.A.; Yang, F.; Dutta, J. Noise2Void: Unsupervised denoising of PET images. Phys. Med. Biol. 2021, 66, 214002. [Google Scholar] [CrossRef] [PubMed]
- Arsanjani, R.; Xu, Y.; Dey, D.; Fish, M.; Dorbala, S.; Hayes, S.; Berman, D.; Germano, G.; Slomka, P. Improved accuracy of myocardial perfusion SPECT for the detection of coronary artery disease using a support vector machine algorithm. J. Nucl. Med. 2013, 54, 549–555. [Google Scholar] [CrossRef]
- Nakajima, K.; Kudo, T.; Nakata, T.; Kiso, K.; Kasai, T.; Taniguchi, Y.; Matsuo, S.; Momose, M.; Nakagawa, M.; Sarai, M.; et al. Diagnostic accuracy of an artificial neural network compared with statistical quantitation of myocardial perfusion images: A Japanese multicenter study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2280–2289. [Google Scholar] [CrossRef] [PubMed]
- Arsanjani, R.; Dey, D.; Khachatryan, T.; Shalev, A.; Hayes, S.W.; Fish, M.; Nakanishi, R.; Germano, G.; Berman, D.S.; Slomka, P. Prediction of revascularization after myocardial perfusion SPECT by machine learning in a large population. J. Nucl. Cardiol. 2015, 22, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Shiri, I.; AmirMozafari Sabet, K.; Arabi, H.; Pourkeshavarz, M.; Teimourian, B.; Ay, M.R.; Zaidi, H. Standard SPECT myocardial perfusion estimation from half-time acquisitions using deep convolutional residual neural networks. J. Nucl. Cardiol. 2021, 28, 2761–2779. [Google Scholar] [CrossRef]
- Ramon, A.J.; Yang, Y.; Pretorius, P.H.; Johnson, K.L.; King, M.A.; Wernick, M.N. Improving Diagnostic Accuracy in Low-Dose SPECT Myocardial Perfusion Imaging with Convolutional Denoising Networks. IEEE Trans. Med. Imaging 2020, 39, 2893–2903. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Wernick, M.N.; Pretorius, P.H.; King, M.A. Deep learning with noise-to-noise training for denoising in SPECT myocardial perfusion imaging. Med. Phys. 2021, 48, 156–168. [Google Scholar] [CrossRef]
- Arabi, H.; AkhavanAllaf, A.; Sanaat, A.; Shiri, I.; Zaidi, H. The promise of artificial intelligence and deep learning in PET and SPECT imaging. Phys. Med. 2021, 83, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Betancur, J.; Commandeur, F.; Motlagh, M.; Sharir, T.; Einstein, A.J.; Bokhari, S.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.; Sinusas, A.J.; et al. Deep Learning for Prediction of Obstructive Disease from Fast Myocardial Perfusion SPECT: A Multicenter Study. JACC Cardiovasc. Imaging 2018, 11, 1654–1663. [Google Scholar] [CrossRef]
- Betancur, J.; Otaki, Y.; Motwani, M.; Fish, M.B.; Lemley, M.; Dey, D.; Gransar, H.; Tamarappoo, B.; Germano, G.; Sharir, T.; et al. Prognostic Value of Combined Clinical and Myocardial Perfusion Imaging Data Using Machine Learning. JACC Cardiovasc. Imaging 2018, 11, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, Z.; Huang, S.; Zhang, N.; Wang, Y.; Hong, S.; Chan, J.S.K.; Chen, K.Y.; Xia, Y.; Zhang, Y.; et al. Machine Learning in Cardio-Oncology: New Insights from an Emerging Discipline. Rev. Cardiovasc. Med. 2023, 24, 296. [Google Scholar] [CrossRef]
- Dreyfuss, A.D.; Bravo, P.E.; Koumenis, C.; Ky, B. Precision Cardio-Oncology. J. Nucl. Med. 2019, 60, 443–450. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalia, I.G.; Pathangey, G.; Abdelnabi, M.; Ibrahim, O.H.; Abdelfattah, F.E.; Pietri, M.P.; Ibrahim, R.; Farina, J.M.; Banerjee, I.; Tamarappoo, B.K.; et al. Applications of Artificial Intelligence for the Prediction and Diagnosis of Cancer Therapy-Related Cardiac Dysfunction in Oncology Patients. Cancers 2025, 17, 605. https://doi.org/10.3390/cancers17040605
Scalia IG, Pathangey G, Abdelnabi M, Ibrahim OH, Abdelfattah FE, Pietri MP, Ibrahim R, Farina JM, Banerjee I, Tamarappoo BK, et al. Applications of Artificial Intelligence for the Prediction and Diagnosis of Cancer Therapy-Related Cardiac Dysfunction in Oncology Patients. Cancers. 2025; 17(4):605. https://doi.org/10.3390/cancers17040605
Chicago/Turabian StyleScalia, Isabel G., Girish Pathangey, Mahmoud Abdelnabi, Omar H. Ibrahim, Fatmaelzahraa E. Abdelfattah, Milagros Pereyra Pietri, Ramzi Ibrahim, Juan M. Farina, Imon Banerjee, Balaji K. Tamarappoo, and et al. 2025. "Applications of Artificial Intelligence for the Prediction and Diagnosis of Cancer Therapy-Related Cardiac Dysfunction in Oncology Patients" Cancers 17, no. 4: 605. https://doi.org/10.3390/cancers17040605
APA StyleScalia, I. G., Pathangey, G., Abdelnabi, M., Ibrahim, O. H., Abdelfattah, F. E., Pietri, M. P., Ibrahim, R., Farina, J. M., Banerjee, I., Tamarappoo, B. K., Arsanjani, R., & Ayoub, C. (2025). Applications of Artificial Intelligence for the Prediction and Diagnosis of Cancer Therapy-Related Cardiac Dysfunction in Oncology Patients. Cancers, 17(4), 605. https://doi.org/10.3390/cancers17040605






