Real-World Evidence of Bevacizumab and Panitumumab Drug Resistance and Drug Ineffectiveness from EudraVigilance Database
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Criteria and Selection Process
2.3. Statistics
- ROR = reporting odds ratio;
- a = evaluated ADR for the targeted drug;
- b = other ADRs for the targeted drug;
- c = evaluated ADR for the drug used for comparison;
- d = other ADRs for the drug used for comparison.
- CI = confidence interval;
- SE = standard error.
2.4. Ethics
3. Results
3.1. Comparative Analysis of ADRs Related to PAN and BEV
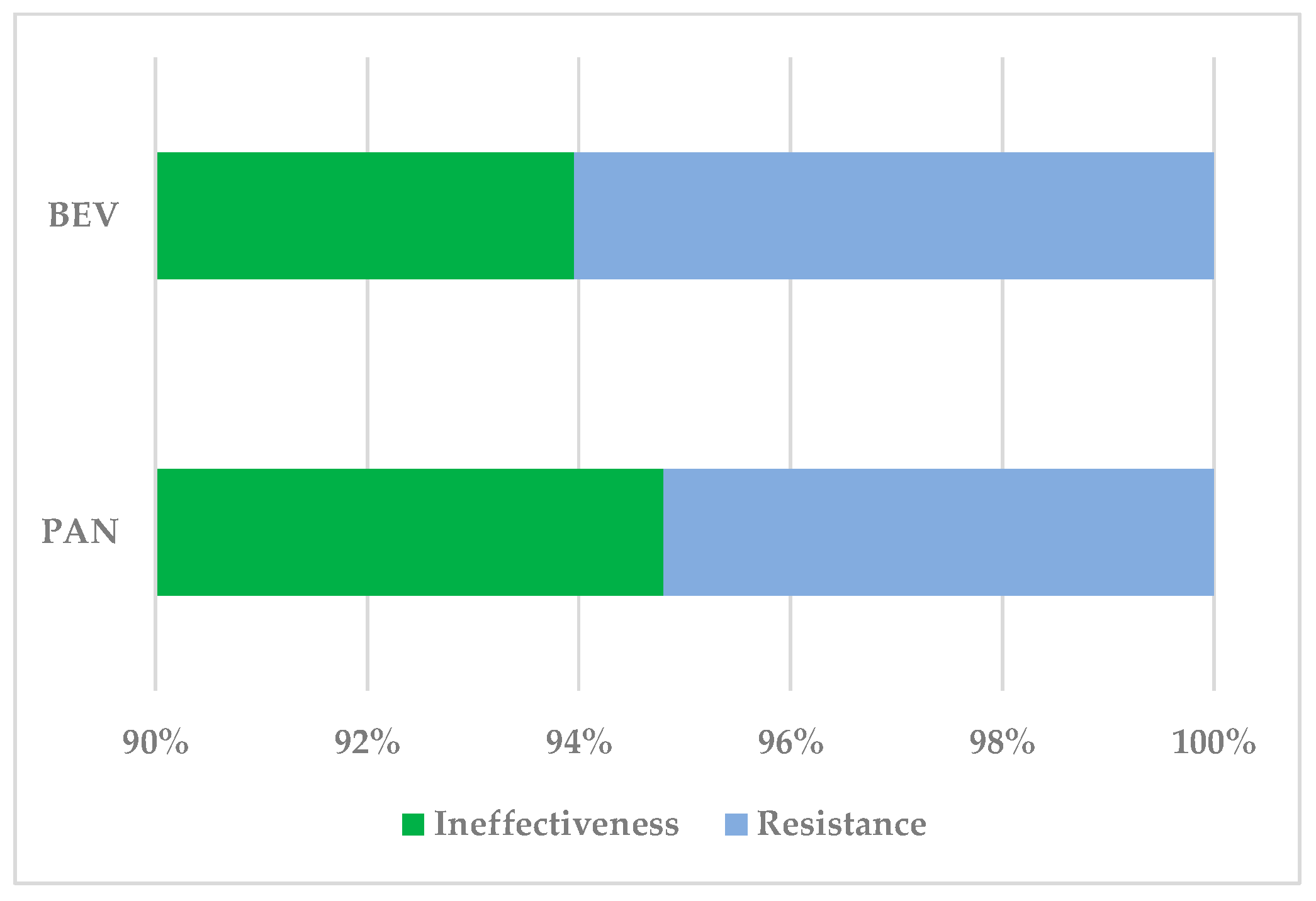
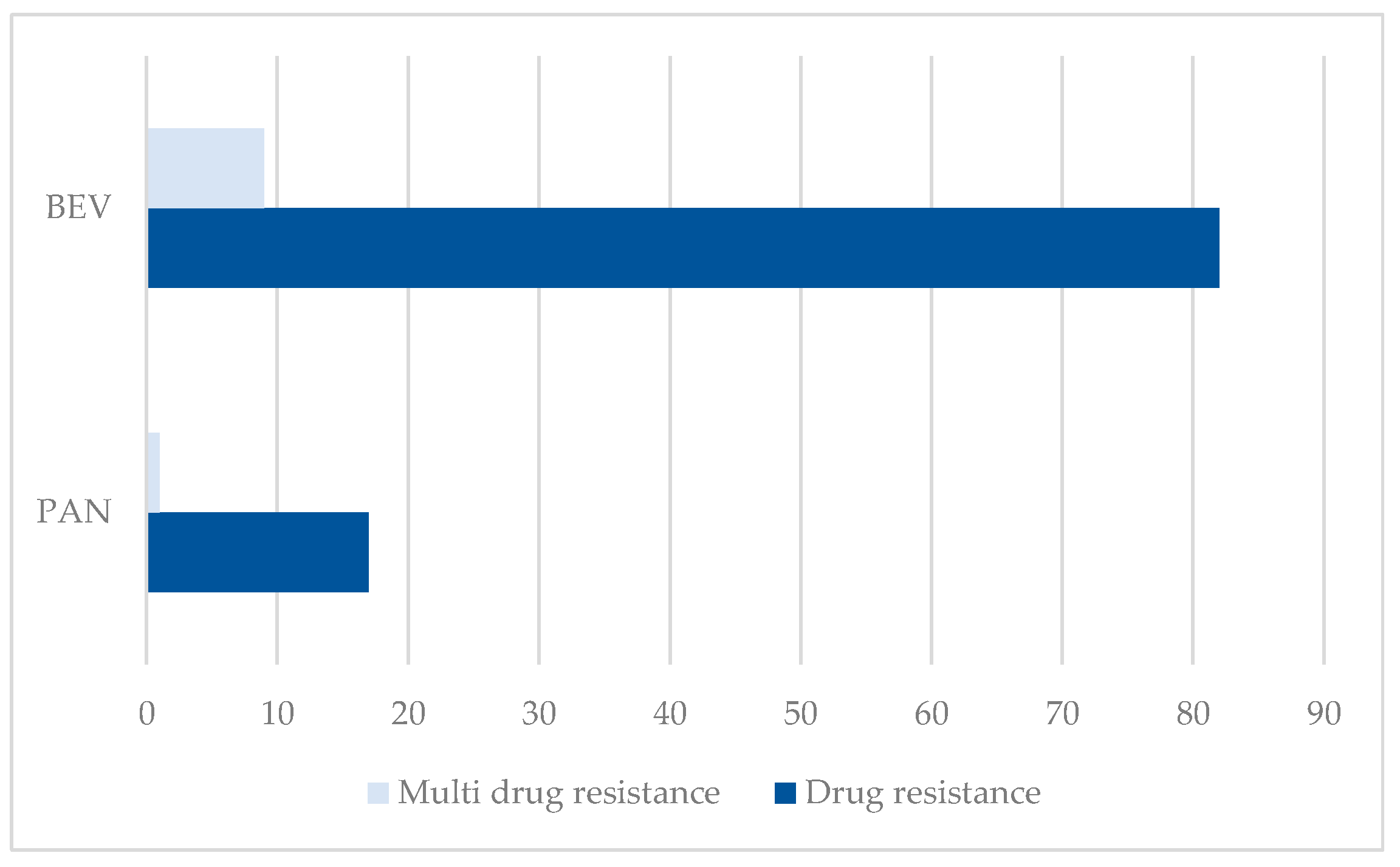
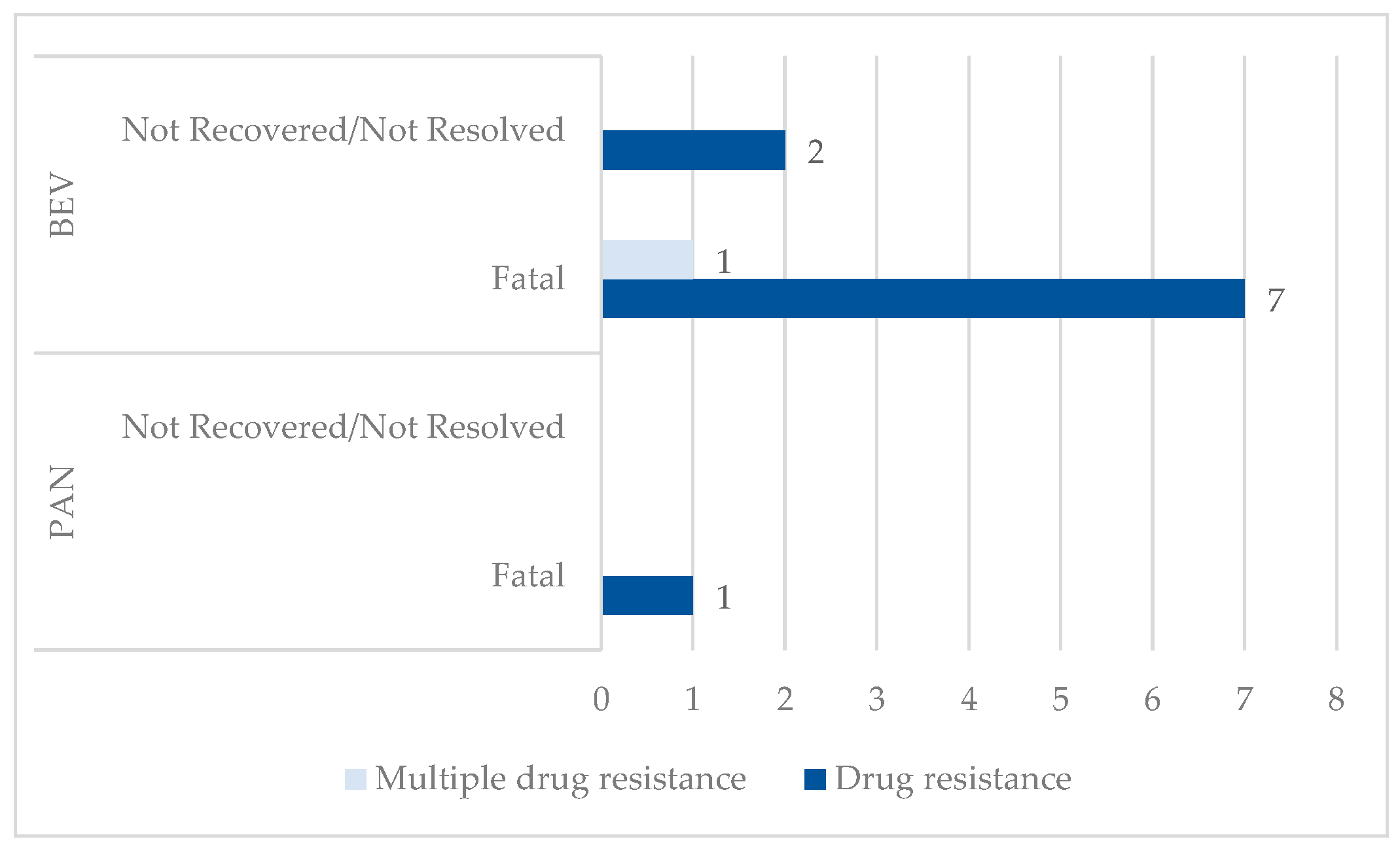
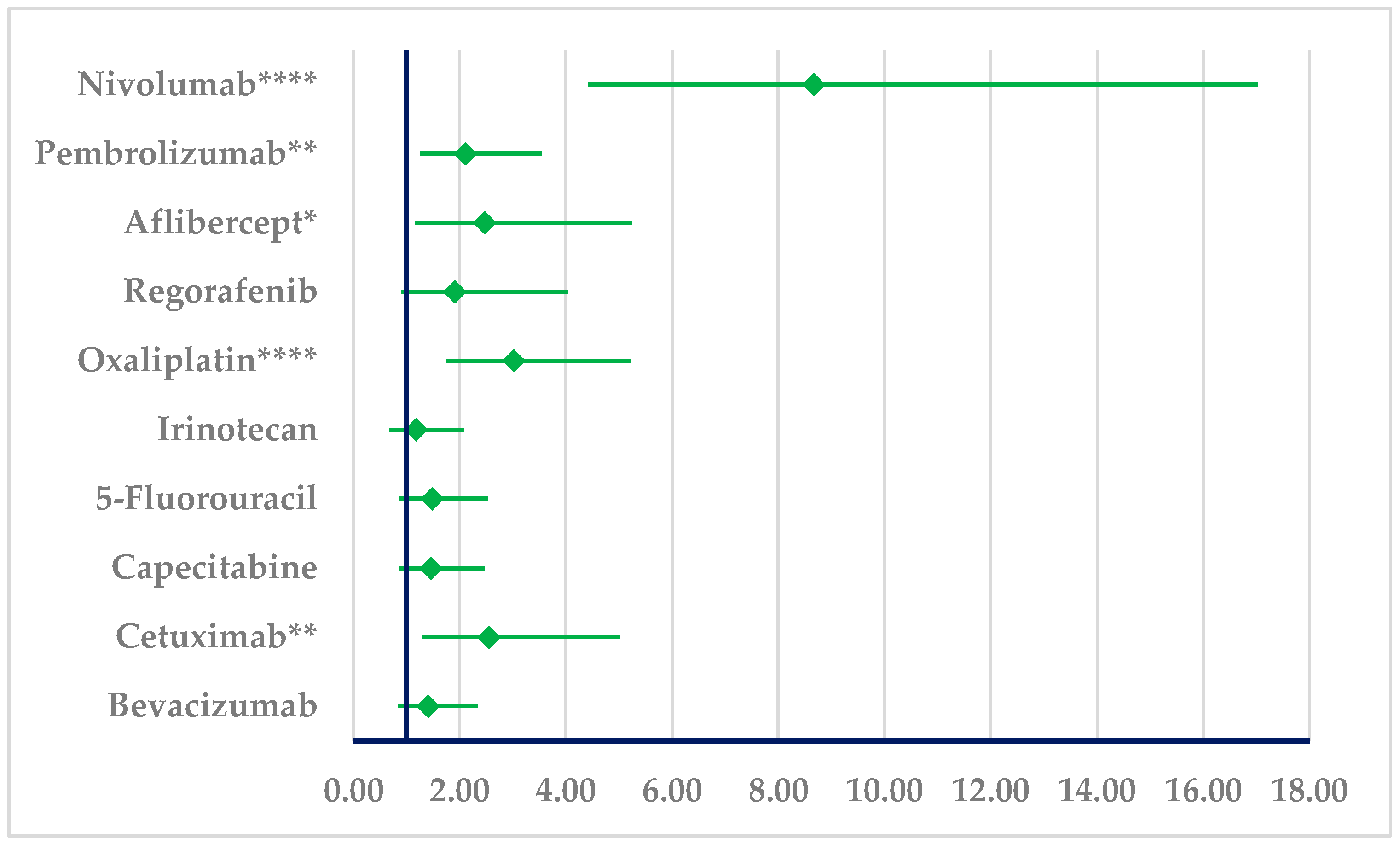
3.1.1. Drug Resistance
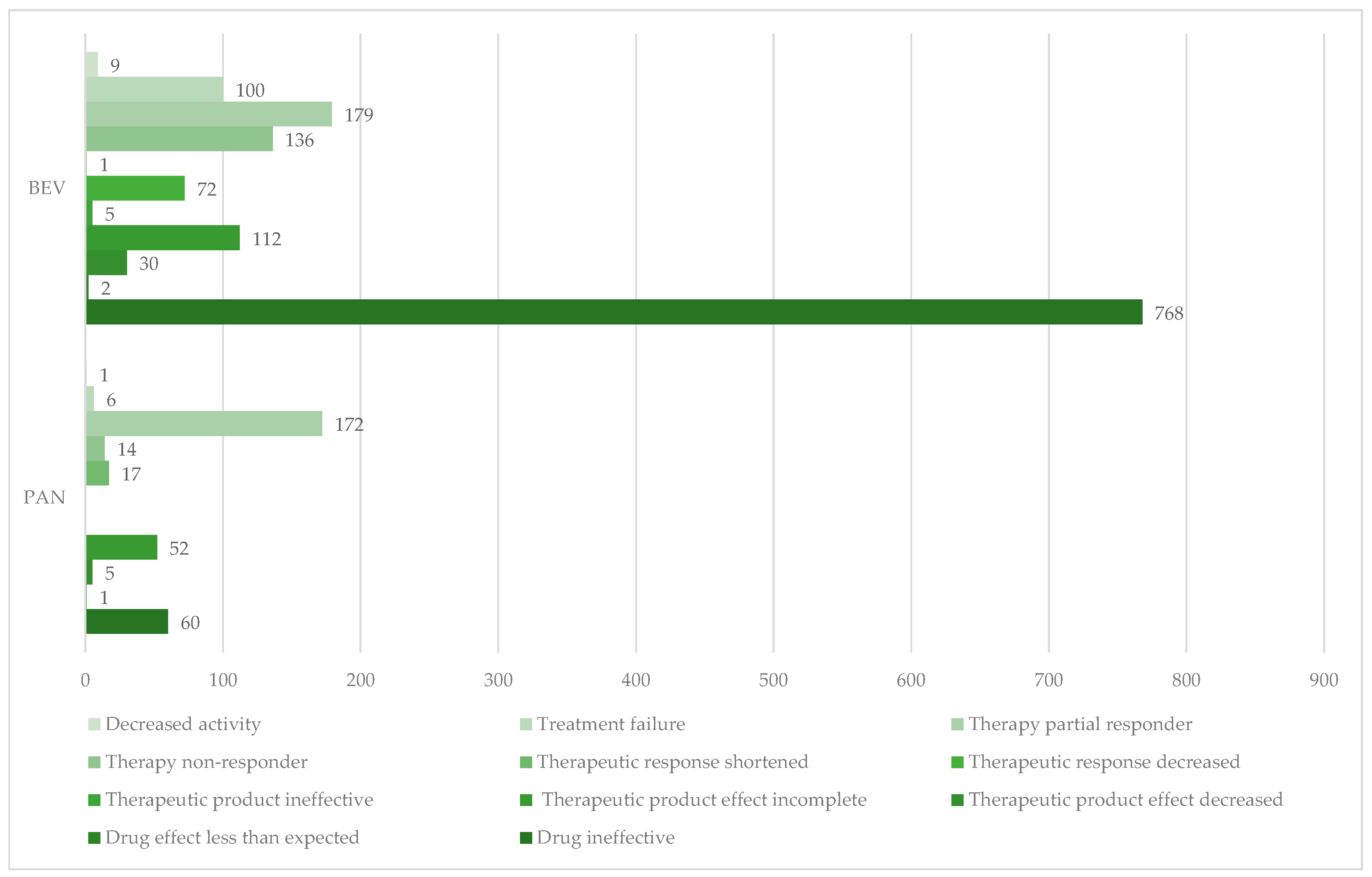
3.1.2. Drug Ineffectiveness
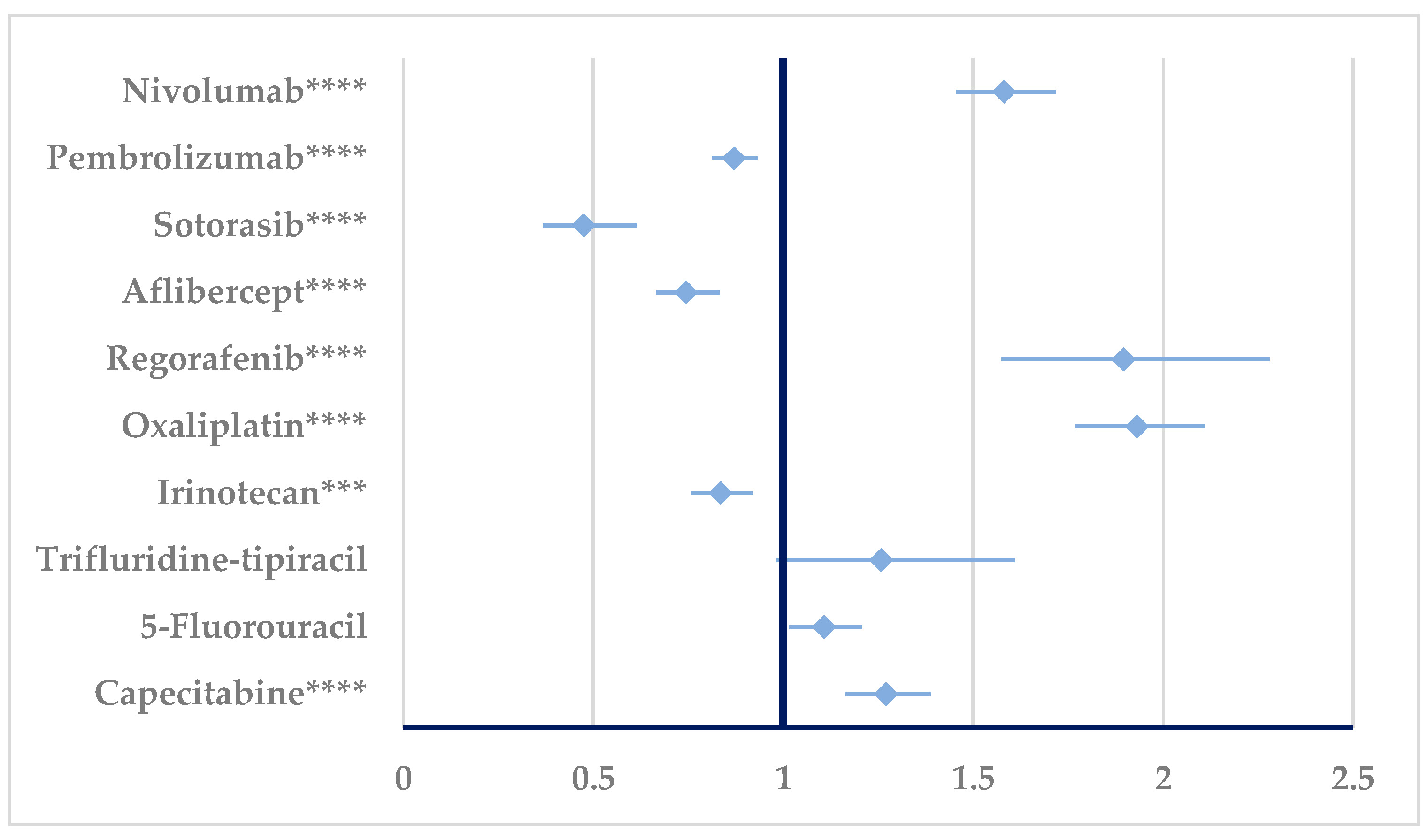
3.2. Disproportionality Analysis
3.2.1. Drug Resistance
3.2.2. Drug Ineffectiveness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weitz, J.; Koch, M.; Debus, J.; Höhler, T.; Galle, P.R.; Büchler, M.W. Colorectal Cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Popovici, D.; Stanisav, C.; Saftescu, S.; Negru, S.; Dragomir, R.; Ciurescu, D.; Diaconescu, R. Exploring the Influence of Age, Gender and Body Mass Index on Colorectal Cancer Location. Medicina 2023, 59, 1399. [Google Scholar] [CrossRef] [PubMed]
- Barkhatov, L.; Aghayan, D.L.; Scuderi, V.; Cipriani, F.; Fretland, Å.A.; Kazaryan, A.M.; Ratti, F.; Armstrong, T.; Belli, A.; Dagher, I.; et al. Long-Term Oncological Outcomes after Laparoscopic Parenchyma-Sparing Redo Liver Resections for Patients with Metastatic Colorectal Cancer: A European Multi-Center Study. Surg. Endosc. 2022, 36, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.E.; Giancotti, F.G.; Rustgi, A.K. Metastatic Colorectal Cancer: Mechanisms and Emerging Therapeutics. Trends Pharmacol. Sci. 2023, 44, 222–236. [Google Scholar] [CrossRef]
- Chis, A.A.; Arseniu, A.M.; Morgovan, C.; Dobrea, C.M.; Frum, A.; Juncan, A.M.; Butuca, A.; Ghibu, S.; Gligor, F.G.; Rus, L.L. Biopolymeric Prodrug Systems as Potential Antineoplastic Therapy. Pharmaceutics 2022, 14, 1773. [Google Scholar] [CrossRef]
- Grozav, A.; Miclaus, V.; Vostinaru, O.; Ghibu, S.; Berce, C.; Rotar, I.; Mogosan, C.; Therrien, B.; Loghin, F.; Popa, D.-S. Acute Toxicity Evaluation of a Thiazolo Arene Ruthenium (II) Complex in Rats. Regul. Toxicol. Pharmacol. 2016, 80, 233–240. [Google Scholar] [CrossRef]
- Morgovan, C.; Dobrea, C.M.; Butuca, A.; Arseniu, A.M.; Frum, A.; Rus, L.L.; Chis, A.A.; Juncan, A.M.; Gligor, F.G.; Georgescu, C.; et al. Safety Profile of the Trastuzumab-Based ADCs: Analysis of Real-World Data Registered in EudraVigilance. Biomedicines 2024, 12, 953. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.M.; Rus, L.-L.; Frum, A.; Morgovan, C.; Butuca, A.; Totan, M.; Juncan, A.M.; Gligor, F.G.; Arseniu, A.M. Dendrimers as Non-Viral Vectors in Gene-Directed Enzyme Prodrug Therapy. Molecules 2021, 26, 5976. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Stintzing, S.; Modest, D.P.; Rossius, L.; Lerch, M.M.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; et al. FOLFIRI plus Cetuximab versus FOLFIRI plus Bevacizumab for Metastatic Colorectal Cancer (FIRE-3): A Post-Hoc Analysis of Tumour Dynamics in the Final RAS Wild-Type Subgroup of This Randomised Open-Label Phase 3 Trial. Lancet Oncol. 2016, 17, 1426–1434. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, S.Y.; Lee, W.S.; Yoo, J.S.; Sun, J.-M.; Lee, J.; Park, S.H.; Park, J.O.; Ahn, M.-J.; Lim, H.Y.; et al. Phase I Trial and Pharmacokinetic Study of Tanibirumab, a Fully Human Monoclonal Antibody to Vascular Endothelial Growth Factor Receptor 2, in Patients with Refractory Solid Tumors. Investig. New Drugs 2017, 35, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Physical Traits of Cancer. Science 2020, 370, eaaz0868. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, L.M.; Brunold, M.; Liwschitz, M.; Goede, V.; Loges, S.; Wroblewski, M.; Quaas, A.; Alakus, H.; Stippel, D.; Bruns, C.J.; et al. A Combination of Low-Dose Bevacizumab and Imatinib Enhances Vascular Normalisation without Inducing Extracellular Matrix Deposition. Br. J. Cancer 2017, 116, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Kedrin, D.; Incio, J.; Liu, H.; Ho, W.W.; Nia, H.T.; Edrich, C.M.; Jung, K.; Daubriac, J.; Chen, I.; et al. Anti-VEGF Therapy Induces ECM Remodeling and Mechanical Barriers to Therapy in Colorectal Cancer Liver Metastases. Sci. Transl. Med. 2016, 8, 360ra135. [Google Scholar] [CrossRef]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a Therapeutic Target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing Tumor Microenvironment to Treat Cancer: Bench to Bedside to Biomarkers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 2205–2218. [Google Scholar] [CrossRef]
- Popovici, D.; Stanisav, C.; Sima, L.V.; Negru, A.; Murg, S.I.; Carabineanu, A. Influence of Biomarkers on Mortality among Patients with Hepatic Metastasis of Colorectal Cancer Treated with FOLFOX/CAPOX and FOLFIRI/CAPIRI, Including Anti-EGFR and Anti-VEGF Therapies. Medicina 2024, 60, 1003. [Google Scholar] [CrossRef]
- Feier, F.C.V.I.; Olariu, O.A.; Olariu, O.S. The Influence of Several Biological Parameters on the Management of Patients with Colon Cancer Undergoing Elective Surgery. Timis. Med. J. 2022, 2022. [Google Scholar] [CrossRef]
- Tâlvan, C.-D.; Tâlvan, E.-T.; Mohor, C.I.; Budișan, L.; Grecu, V.; Mihalache, M.; Zănoagă, O.; Chira, S.; Berindan-Neagoe, I.; Cristea, V.; et al. Exploring MiRNA Profiles in Colon Cancer: A Focus on MiR101-3p, MiR106a-5p, and MiR326. Cancers 2024, 16, 2285. [Google Scholar] [CrossRef]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular Channel Formation by Human Melanoma Cells In Vivo and In Vitro: Vasculogenic Mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Feier, C.V.I.; Paunescu, I.A.; Faur, A.M.; Cozma, G.V.; Blidari, A.R.; Muntean, C. Sexual Functioning and Impact on Quality of Life in Patients with Early-Onset Colorectal Cancer: A Systematic Review. Diseases 2024, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, S.; Wang, B.; Wu, Y.; Chen, Z.; Lv, M.; Lin, Y.; Yang, J. Potential Biomarkers for Anti-EGFR Therapy in Metastatic Colorectal Cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 11645–11655. [Google Scholar] [CrossRef] [PubMed]
- Kast, J.; Dutta, S.; Upreti, V.V. Panitumumab: A Review of Clinical Pharmacokinetic and Pharmacology Properties After Over a Decade of Experience in Patients with Solid Tumors. Adv. Ther. 2021, 38, 3712–3723. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T.; Sorensen, A.G.; di Tomaso, E.; Zhang, W.-T.; Duda, D.G.; Cohen, K.S.; Kozak, K.R.; Cahill, D.P.; Chen, P.-J.; Zhu, M.; et al. AZD2171, a Pan-VEGF Receptor Tyrosine Kinase Inhibitor, Normalizes Tumor Vasculature and Alleviates Edema in Glioblastoma Patients. Cancer Cell 2007, 11, 83–95. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, J.; Liu, W.; Zhou, Y.; Plocinik, R.M.; Li, H.; Hu, Q.; Ghosh, G.; Adams, J.A.; Rosenfeld, M.G.; et al. The Akt-SRPK-SR Axis Constitutes a Major Pathway in Transducing EGF Signaling to Regulate Alternative Splicing in the Nucleus. Mol. Cell 2012, 47, 422–433. [Google Scholar] [CrossRef]
- Hughes, R.; Qian, B.-Z.; Rowan, C.; Muthana, M.; Keklikoglou, I.; Olson, O.C.; Tazzyman, S.; Danson, S.; Addison, C.; Clemons, M.; et al. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 2015, 75, 3479–3491. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- LaFargue, C.J.; Amero, P.; Noh, K.; Mangala, L.S.; Wen, Y.; Bayraktar, E.; Umamaheswaran, S.; Stur, E.; Dasari, S.K.; Ivan, C.; et al. Overcoming Adaptive Resistance to Anti-VEGF Therapy by Targeting CD5L. Nat. Commun. 2023, 14, 2407. [Google Scholar] [CrossRef]
- Casanovas, O.; Hicklin, D.J.; Bergers, G.; Hanahan, D. Drug Resistance by Evasion of Antiangiogenic Targeting of VEGF Signaling in Late-Stage Pancreatic Islet Tumors. Cancer Cell 2005, 8, 299–309. [Google Scholar] [CrossRef]
- Lawler, S.E. Digging Deeper for New Targets in Bevacizumab Resistance. Neuro-Oncology 2023, 25, 261–262. [Google Scholar] [CrossRef]
- Lahiguera, Á.; Hyroššová, P.; Figueras, A.; Garzón, D.; Moreno, R.; Soto-Cerrato, V.; McNeish, I.; Serra, V.; Lazaro, C.; Barretina, P.; et al. Tumors Defective in Homologous Recombination Rely on Oxidative Metabolism: Relevance to Treatments with PARP Inhibitors. EMBO Mol. Med. 2020, 12, e11217. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L.; Febbraio, M. CD36, a Scavenger Receptor Involved in Immunity, Metabolism, Angiogenesis, and Behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chelariu-Raicu, A.; Umamaheswaran, S.; Nick, A.M.; Stur, E.; Hanjra, P.; Jiang, D.; Jennings, N.B.; Chen, X.; Corvigno, S.; et al. Endothelial P130cas Confers Resistance to Anti-Angiogenesis Therapy. Cell Rep. 2022, 38, 110301. [Google Scholar] [CrossRef] [PubMed]
- Vonica, R.C.; Butuca, A.; Vonica-Tincu, A.L.; Morgovan, C.; Pumnea, M.; Cipaian, R.C.; Curca, R.O.; Batar, F.; Vornicu, V.; Solomon, A.; et al. The Descriptive and Disproportionality Assessment of EudraVigilance Database Reports on Capecitabine Induced Cardiotoxicity. Cancers 2024, 16, 3847. [Google Scholar] [CrossRef] [PubMed]
- Formica, V.; Roselli, M. Targeted Therapy in First Line Treatment of RAS Wild Type Colorectal Cancer. World J. Gastroenterol. 2015, 21, 2871–2874. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; Lerchenmueller, J.; Kahl, C.; Seipelt, G.; et al. Randomized Comparison of FOLFIRI plus Cetuximab versus FOLFIRI plus Bevacizumab as First-Line Treatment of KRAS Wild-Type Metastatic Colorectal Cancer: German AIO Study KRK-0306 (FIRE-3). J. Clin. Oncol. 2024, 31, LBA3506. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, L.; Qiu, H.; Zhang, M.; Sun, L.; Peng, P.; Yu, Q.; Yuan, X. Mechanisms of Resistance to Anti-EGFR Therapy in Colorectal Cancer. Oncotarget 2017, 8, 3980–4000. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Peeters, M.; Siena, S.; Humblet, Y.; Hendlisz, A.; Neyns, B.; Canon, J.-L.; Van Laethem, J.-L.; Maurel, J.; Richardson, G.; et al. Open-Label Phase III Trial of Panitumumab Plus Best Supportive Care Compared with Best Supportive Care Alone in Patients with Chemotherapy-Refractory Metastatic Colorectal Cancer. J. Clin. Oncol. 2007, 25, 1658–1664. [Google Scholar] [CrossRef]
- Van Emburgh, B.O.; Sartore-Bianchi, A.; Di Nicolantonio, F.; Siena, S.; Bardelli, A. Acquired Resistance to EGFR-Targeted Therapies in Colorectal Cancer. Mol. Oncol. 2014, 8, 1084–1094. [Google Scholar] [CrossRef]
- Yonesaka, K.; Zejnullahu, K.; Okamoto, I.; Satoh, T.; Cappuzzo, F.; Souglakos, J.; Ercan, D.; Rogers, A.; Roncalli, M.; Takeda, M.; et al. Activation of ERBB2 Signaling Causes Resistance to the EGFR-Directed Therapeutic Antibody Cetuximab. Sci. Transl. Med. 2011, 3, 99ra86. [Google Scholar] [CrossRef]
- Troiani, T.; Martinelli, E.; Napolitano, S.; Vitagliano, D.; Ciuffreda, L.P.; Costantino, S.; Morgillo, F.; Capasso, A.; Sforza, V.; Nappi, A.; et al. Increased TGF-α as a Mechanism of Acquired Resistance to the Anti-EGFR Inhibitor Cetuximab through EGFR–MET Interaction and Activation of MET Signaling in Colon Cancer Cells. Clin. Cancer Res. 2013, 19, 6751–6765. [Google Scholar] [CrossRef] [PubMed]
- Cutsem, E.; Van Köhne, C.-H.; Hitre, E.; Zaluski, J.; Chien, C.-R.C.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.L.; Iida, M.; Kruser, T.J.; Nechrebecki, M.M.; Dunn, E.F.; Armstrong, E.A.; Huang, S.; Harari, P.M. Epidermal Growth Factor Receptor Cooperates with Src Family Kinases in Acquired Resistance to Cetuximab. Cancer Biol. Ther. 2009, 8, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Nakamura, Y.; Yamanaka, T.; Kuboki, Y.; Yamaguchi, D.; Yuki, S.; Yoshino, T.; Komatsu, Y.; Sakamoto, N.; Okamoto, W.; et al. Prognostic and Predictive Value of HER2 Amplification in Patients with Metastatic Colorectal Cancer. Clin. Color. Cancer 2018, 17, 198–205. [Google Scholar] [CrossRef]
- Loboda, A.; Nebozhyn, M.V.; Watters, J.W.; Buser, C.A.; Shaw, P.M.; Huang, P.S.; Van’t Veer, L.; Tollenaar, R.A.E.M.; Jackson, D.B.; Agrawal, D.; et al. EMT Is the Dominant Program in Human Colon Cancer. BMC Med. Genom. 2011, 4, 9. [Google Scholar] [CrossRef]
- Nelson, H.; Ramsey, P.S.; McKean, D.J.; Dozois, R.R.; Donohue, J.H. Anti-Tumor × Anti-Lymphocyte Heteroconjugates Augment Colon Tumor Cell Lysisin Vitro and Prevent Tumor Growthin Vivo. Dis. Colon Rectum 1991, 34, 140–147. [Google Scholar] [CrossRef]
- Bibeau, F.; Lopez-Crapez, E.; Di Fiore, F.; Thezenas, S.; Ychou, M.; Blanchard, F.; Lamy, A.; Penault-Llorca, F.; Frébourg, T.; Michel, P.; et al. Impact of FcγRIIa-FcγRIIIa Polymorphisms and KRAS Mutations on the Clinical Outcome of Patients with Metastatic Colorectal Cancer Treated with Cetuximab Plus Irinotecan. J. Clin. Oncol. 2009, 27, 1122–1129. [Google Scholar] [CrossRef]
- Arisi, M.F.; Dotan, E.; Fernandez, S.V. Circulating Tumor DNA in Precision Oncology and Its Applications in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 4441. [Google Scholar] [CrossRef]
- Pàez-Ribes, M.; Allen, E.; Hudock, J.; Takeda, T.; Okuyama, H.; Viñals, F.; Inoue, M.; Bergers, G.; Hanahan, D.; Casanovas, O. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell 2009, 15, 220–231. [Google Scholar] [CrossRef]
- Ebos, J.M.L.; Lee, C.R.; Cruz-Munoz, W.; Bjarnason, G.A.; Christensen, J.G.; Kerbel, R.S. Accelerated Metastasis after Short-Term Treatment with a Potent Inhibitor of Tumor Angiogenesis. Cancer Cell 2009, 15, 232–239. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO Consensus Conference Recommendations on Ovarian Cancer: Pathology and Molecular Biology, Early and Advanced Stages, Borderline Tumours and Recurrent Disease. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, S.; Hanna, D.L.; Cao, S.; Zhang, W.; Yang, D.; Ning, Y.; Sunakawa, Y.; Okazaki, S.; Berger, M.D.; Miyamato, Y.; et al. Prognostic Impact of IL6 Genetic Variants in Patients with Metastatic Colorectal Cancer Treated with Bevacizumab-Based Chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 3218–3226. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.; Van Cutsem, E.; McDermott, R.S.; Hill, A.G.; et al. Nivolumab (NIVO) + Low-Dose Ipilimumab (IPI) in Previously Treated Patients (Pts) with Microsatellite Instability-High/Mismatch Repair-Deficient (MSI-H/DMMR) Metastatic Colorectal Cancer (MCRC): Long-Term Follow-Up. J. Clin. Oncol. 2019, 37, 635. [Google Scholar] [CrossRef]
- Boige, V.; Malka, D.; Bourredjem, A.; Dromain, C.; Baey, C.; Jacques, N.; Pignon, J.-P.; Vimond, N.; Bouvet-Forteau, N.; De Baere, T.; et al. Efficacy, Safety, and Biomarkers of Single-Agent Bevacizumab Therapy in Patients with Advanced Hepatocellular Carcinoma. Oncologist 2012, 17, 1063–1072. [Google Scholar] [CrossRef]
- Nixon, A.B.; Halabi, S.; Shterev, I.; Starr, M.; Brady, J.C.; Dutcher, J.P.; Hopkins, J.O.; Hurwitz, H.; Small, E.J.; Rini, B.I.; et al. Identification of Predictive Biomarkers of Overall Survival (OS) in Patients (Pts) with Advanced Renal Cell Carcinoma (RCC) Treated with Interferon Alpha (I) with or without Bevacizumab (B): Results from CALGB 90206 (Alliance). J. Clin. Oncol. 2013, 31, 4520. [Google Scholar] [CrossRef]
- Weickhardt, A.J.; Williams, D.; Lee, C.; Simes, J.; Murone, C.; Wilson, K.; Cummins, M.; Asadi, K.; Price, T.J.; Mariadason, J.; et al. Vascular Endothelial Growth Factors (VEGF) and VEGF Receptor Expression as Predictive Biomarkers for Benefit with Bevacizumab in Metastatic Colorectal Cancer (MCRC): Analysis of the Phase III MAX Study. J. Clin. Oncol. 2011, 29, 3531. [Google Scholar] [CrossRef]
- Nixon, A.B.; Pang, H.; Starr, M.D.; Friedman, P.N.; Bertagnolli, M.M.; Kindler, H.L.; Goldberg, R.M.; Venook, A.P.; Hurwitz, H.I. Prognostic and Predictive Blood-Based Biomarkers in Patients with Advanced Pancreatic Cancer: Results from CALGB80303 (Alliance). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 6957–6966. [Google Scholar] [CrossRef]
- Miles, D.W.; de Haas, S.L.; Dirix, L.Y.; Romieu, G.; Chan, A.; Pivot, X.; Tomczak, P.; Provencher, L.; Cortés, J.; Delmar, P.R.; et al. Biomarker Results from the AVADO Phase 3 Trial of First-Line Bevacizumab plus Docetaxel for HER2-Negative Metastatic Breast Cancer. Br. J. Cancer 2013, 108, 1052–1060. [Google Scholar] [CrossRef]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O’Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B. Bevacizumab in Combination with Oxaliplatin, Fluorouracil, and Leucovorin (FOLFOX4) for Previously Treated Metastatic Colorectal Cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007, 25, 1539–1544. [Google Scholar] [CrossRef]
- Kabbinavar, F.; Hurwitz, H.I.; Fehrenbacher, L.; Meropol, N.J.; Novotny, W.F.; Lieberman, G.; Griffing, S.; Bergsland, E. Phase II, Randomized Trial Comparing Bevacizumab plus Fluorouracil (FU)/Leucovorin (LV) with FU/LV Alone in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 60–65. [Google Scholar] [CrossRef]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; et al. Continuation of Bevacizumab after First Progression in Metastatic Colorectal Cancer (ML18147): A Randomised Phase 3 Trial. Lancet. Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Ryoo, B.-Y.; Hsu, C.-H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.; Spahn, J.; Liu, B.; Abdullah, H.; et al. LBA39—Randomised Efficacy and Safety Results for Atezolizumab (Atezo) + Bevacizumab (Bev) in Patients (Pts) with Previously Untreated, Unresectable Hepatocellular Carcinoma (HCC). Ann. Oncol. 2019, 30, v875. [Google Scholar] [CrossRef]
- Venkatasubbaiah, M.; Reddy, P.D.; Satyanarayana, S.V. Knowledge, Attitude, and Practices (KAP) of the Pharm.D Interns towards Adverse Drug Reaction (ADR) Reporting and Pharmacovigilance. Pharm. Educ. 2021, 21, 186–193. [Google Scholar] [CrossRef]
- Alomar, M.; Palaian, S.; Al-tabakha, M.M. Pharmacovigilance in Perspective: Drug Withdrawals, Data Mining and Policy Implications [Version 1; Peer Review: 2 Approved]. F1000Research 2019, 8, 2109. [Google Scholar] [CrossRef]
- Peters, T.; Soanes, N.; Abbas, M.; Ahmad, J.; Delumeau, J.-C.; Herrero-Martinez, E.; Paramananda, M.; Piper, J.; Smail-Aoudia, F.; van der Spuij, W.; et al. Effective Pharmacovigilance System Development: EFPIA-IPVG Consensus Recommendations. Drug Saf. 2021, 44, 17–28. [Google Scholar] [CrossRef]
- Stagi, L.; Bocchi, I.; Bernardini, D.; Ciappa, M.; Dellon, S.; Castiglione, G.N.; Romano, S.; Fabrizi, E.; Mattavelli, A.; Grisoni, I.; et al. The Evolution of the Pharmacovigilance Department in the Pharmaceutical Industry: Results of an Italian National Survey. Ther. Adv. Drug Saf. 2024, 15, 20420986241293296. [Google Scholar] [CrossRef]
- Data Source. EudraVigilance—European Database of Suspected Adverse Drug Reaction Reports. Available online: https://www.adrreports.eu/en/index.html (accessed on 25 August 2024).
- Habarugira, J.M.V.; Härmark, L.; Figueras, A. Pharmacovigilance Data as a Trigger to Identify Antimicrobial Resistance and Inappropriate Use of Antibiotics: A Study Using Reports from The Netherlands Pharmacovigilance Centre. Antibiotics 2021, 10, 1512. [Google Scholar] [CrossRef]
- Pop, G.; Farcaș, A.; Butucă, A.; Morgovan, C.; Arseniu, A.M.; Pumnea, M.; Teodoru, M.; Gligor, F.G. Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals 2022, 15, 1536. [Google Scholar] [CrossRef]
- EMA/849944/2016; European Medicines Agengy—Science Medicines Health. European Medicines Agengy: London, UK, 2016.
- MedCalc Software Ltd. Odds Ratio Calculator. Version 23.0.6. Available online: https://www.medcalc.org/calc/odds_ratio.php (accessed on 3 November 2024).
- Postigo, R.; Brosch, S.; Slattery, J.; van Haren, A.; Dogné, J.-M.; Kurz, X.; Candore, G.; Domergue, F.; Arlett, P. EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection. Drug Saf. 2018, 41, 665–675. [Google Scholar] [CrossRef]
- Vintila, B.I.; Arseniu, A.M.; Butuca, A.; Sava, M.; Bîrluțiu, V.; Rus, L.L.; Axente, D.D.; Morgovan, C.; Gligor, F.G. Adverse Drug Reactions Relevant to Drug Resistance and Ineffectiveness Associated with Meropenem, Linezolid, and Colistin: An Analysis Based on Spontaneous Reports from the European Pharmacovigilance Database. Antibiotics 2023, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Sugimoto, M.; Harada, S.; Yorozu, K.; Kurasawa, M.; Yamamoto, K. Continuous Administration of Bevacizumab plus Capecitabine, Even after Acquired Resistance to Bevacizumab, Restored Anti-Angiogenic and Antitumor Effect in a Human Colorectal Cancer Xenograft Model. Oncol. Rep. 2016, 36, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.J.; Macias, R.I.; Monte, M.J.; Herraez, E.; Peleteiro-Vigil, A.; de Blas, B.S.; Sanchon-Sanchez, P.; Temprano, A.G.; Espinosa-Escudero, R.A.; Lozano, E.; et al. Cellular Mechanisms Accounting for the Refractoriness of Colorectal Carcinoma to Pharmacological Treatment. Cancers 2020, 12, 2605. [Google Scholar] [CrossRef] [PubMed]
- Itatani, Y.; Kawada, K.; Yamamoto, T.; Sakai, Y. Resistance to Anti-Angiogenic Therapy in Cancer—Alterations to Anti-VEGF Pathway. Int. J. Mol. Sci. 2018, 19, 1232. [Google Scholar] [CrossRef]
- Ellis, L.M.; Hicklin, D.J. VEGF-Targeted Therapy: Mechanisms of Anti-Tumour Activity. Nat. Rev. Cancer 2008, 8, 579–591. [Google Scholar] [CrossRef]
- Sackstein, P.E.; Chintapally, N.; Wilgucki, M.; Hartley, M.L.; Alqahtani, A.; Weinberg, B.A. Overcoming the Hurdles: Surmounting Acquired Resistance to Anti-EGFR Therapy in Metastatic Colorectal Cancer. Clin. Adv. Hematol. Oncol. 2023, 21, 572–583. [Google Scholar]
- Misale, S.; Bozic, I.; Tong, J.; Peraza-Penton, A.; Lallo, A.; Baldi, F.; Lin, K.H.; Truini, M.; Trusolino, L.; Bertotti, A.; et al. Vertical Suppression of the EGFR Pathway Prevents Onset of Resistance in Colorectal Cancers. Nat. Commun. 2015, 6, 8305. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Heyes, D.J.; Hardman, S.J.O.; Pedersen, M.N.; Woodhouse, J.; De La Mora, E.; Wulff, M.; Weik, M.; Cammarata, M.; Scrutton, N.S.; Schirò, G. Light-Induced Structural Changes in a Full-Length Cyanobacterial Phytochrome Probed by Time-Resolved X-Ray Scattering. Commun. Biol. 2019, 2, 1. [Google Scholar] [CrossRef]
- Han, B.; Zhang, H.; Tian, R.; Liu, H.; Wang, Z.; Wang, Z.; Tian, J.; Cui, Y.; Ren, S.; Zuo, X.; et al. Exosomal EPHA2 Derived from Highly Metastatic Breast Cancer Cells Promotes Angiogenesis by Activating the AMPK Signaling Pathway through Ephrin A1-EPHA2 Forward Signaling. Theranostics 2022, 12, 4127–4146. [Google Scholar] [CrossRef]
- Corbeil, D.; Karbanová, J.; Fargeas, C.A.; Jászai, J. Prominin-1 (CD133): Molecular and Cellular Features Across Species. In Prominin-1 (CD133): New Insights on Stem & Cancer Stem Cell Biology; Corbeil, D., Ed.; Springer: New York, NY, USA, 2013; pp. 3–24. ISBN 978-1-4614-5894-4. [Google Scholar]
- Brocco, D.; Simeone, P.; Buca, D.; Di Marino, P.; De Tursi, M.; Grassadonia, A.; De Lellis, L.; Martino, M.T.; Veschi, S.; Iezzi, M.; et al. Blood Circulating CD133+ Extracellular Vesicles Predict Clinical Outcomes in Patients with Metastatic Colorectal Cancer. Cancers 2022, 14, 1357. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Beninger, P. Pharmacovigilance: An Overview. Clin. Ther. 2018, 40, 1991–2004. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in Cancer Treatment: A Review of 15 Years of Clinical Experience and Future Outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Galon, J.; Pagès, F.; Marincola, F.M.; Angell, H.K.; Thurin, M.; Lugli, A.; Zlobec, I.; Berger, A.; Bifulco, C.; Botti, G.; et al. Cancer Classification Using the Immunoscore: A Worldwide Task Force. J. Transl. Med. 2012, 10, 205. [Google Scholar] [CrossRef]
- Negri, F.; Bottarelli, L.; de’Angelis, G.L.; Gnetti, L. KRAS: A Druggable Target in Colon Cancer Patients. Int. J. Mol. Sci. 2022, 23, 4120. [Google Scholar] [CrossRef]
- Stein, A.; Atanackovic, D.; Bokemeyer, C. Current Standards and New Trends in the Primary Treatment of Colorectal Cancer. Eur. J. Cancer 2011, 47, S312–S314. [Google Scholar] [CrossRef]
- Spoelstra, E.C.; Dekker, H.; Schuurhuis, G.J.; Broxterman, H.J.; Lankelma, J. P-Glycoprotein Drug Efflux Pump Involved in the Mechanisms of Intrinsic Drug Resistance in Various Colon Cancer Cell Lines: Evidence for a Saturation of Active Daunorubicin Transport. Biochem. Pharmacol. 1991, 41, 349–359. [Google Scholar] [CrossRef]
- Fojo, A.T.; Ueda, K.; Slamon, D.J.; Poplack, D.G.; Gottesman, M.M.; Pastan, I. Expression of a Multidrug-Resistance Gene in Human Tumors and Tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 265–269. [Google Scholar] [CrossRef]
- McIntyre, A.; Harris, A.L. Metabolic and Hypoxic Adaptation to Anti-Angiogenic Therapy: A Target for Induced Essentiality. EMBO Mol. Med. 2015, 7, 368–379. [Google Scholar] [CrossRef]
- Tomida, C.; Yamagishi, N.; Aibara, K.; Yano, C.; Uchida, T.; Abe, T.; Ohno, A.; Hirasaka, K.; Nikawa, T.; Teshima-Kondo, S. Chronic Exposure of VEGF Inhibitors Promotes the Malignant Phenotype of Colorectal Cancer Cells. J. Med. Invest. 2015, 62, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Bagley, R.G.; Ren, Y.; Weber, W.; Yao, M.; Kurtzberg, L.; Pinckney, J.; Bangari, D.; Nguyen, C.; Brondyk, W.; Kaplan, J.; et al. Placental Growth Factor Upregulation Is a Host Response to Antiangiogenic Therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Berger, A.; Camus, M.; Sanchez-Cabo, F.; Costes, A.; Molidor, R.; Mlecnik, B.; Kirilovsky, A.; Nilsson, M.; Damotte, D.; et al. Effector Memory T Cells, Early Metastasis, and Survival in Colorectal Cancer. N. Engl. J. Med. 2005, 353, 2654–2666. [Google Scholar] [CrossRef]
- Hirai, H.; Sootome, H.; Nakatsuru, Y.; Miyama, K.; Taguchi, S.; Tsujioka, K.; Ueno, Y.; Hatch, H.; Majumder, P.K.; Pan, B.-S.; et al. MK-2206, an Allosteric Akt Inhibitor, Enhances Antitumor Efficacy by Standard Chemotherapeutic Agents or Molecular Targeted Drugs In Vitro and In Vivo. Mol. Cancer Ther. 2010, 9, 1956–1967. [Google Scholar] [CrossRef]
- Fusaroli, M.; Raschi, E.; Poluzzi, E.; Hauben, M. The Evolving Role of Disproportionality Analysis in Pharmacovigilance. Expert Opin. Drug Saf. 2024, 23, 981–994. [Google Scholar] [CrossRef]
- van Stekelenborg, J.; Ellenius, J.; Maskell, S.; Bergvall, T.; Caster, O.; Dasgupta, N.; Dietrich, J.; Gama, S.; Lewis, D.; Newbould, V.; et al. Recommendations for the Use of Social Media in Pharmacovigilance: Lessons from IMI WEB-RADR. Drug Saf. 2019, 42, 1393–1407. [Google Scholar] [CrossRef]
- Morgovan, C.; Dobrea, C.M.; Chis, A.A.; Juncan, A.M.; Arseniu, A.M.; Rus, L.L.; Gligor, F.G.; Ardelean, S.A.; Stoicescu, L.; Ghibu, S.; et al. A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database. Pharmaceuticals 2023, 16, 455. [Google Scholar] [CrossRef]
- Fusaroli, M.; Salvo, F.; Begaud, B.; AlShammari, T.M.; Bate, A.; Battini, V.; Brueckner, A.; Candore, G.; Carnovale, C.; Crisafulli, S.; et al. The REporting of A Disproportionality Analysis for DrUg Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Explanation and Elaboration. Drug Saf. 2024, 47, 585–599. [Google Scholar] [CrossRef]




| Medical Condition | Code | PT |
|---|---|---|
| Resistance | 10059866 | Drug resistance |
| 10048723 | Multiple drug resistance | |
| Ineffectiveness | 10013709 | Drug ineffective |
| 10083365 | Drug effect less than expected | |
| 10082201 | Therapeutic product effect decreased | |
| 10082200 | Therapeutic product effect incomplete | |
| 10060769 | Therapeutic product ineffective | |
| 10043414 | Therapeutic response decreased | |
| 10078575 | Therapeutic response shortened | |
| 10051082 | Therapy non-responder | |
| 10078115 | Therapy partial responder | |
| 10066901 | Treatment failure | |
| 10011953 | Decreased activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vonica, R.C.; Morgovan, C.; Butuca, A.; Pumnea, M.; Cipaian, R.C.; Frum, A.; Dobrea, C.M.; Vonica-Tincu, A.L.; Pacnejer, A.-M.; Batar, F.; et al. Real-World Evidence of Bevacizumab and Panitumumab Drug Resistance and Drug Ineffectiveness from EudraVigilance Database. Cancers 2025, 17, 663. https://doi.org/10.3390/cancers17040663
Vonica RC, Morgovan C, Butuca A, Pumnea M, Cipaian RC, Frum A, Dobrea CM, Vonica-Tincu AL, Pacnejer A-M, Batar F, et al. Real-World Evidence of Bevacizumab and Panitumumab Drug Resistance and Drug Ineffectiveness from EudraVigilance Database. Cancers. 2025; 17(4):663. https://doi.org/10.3390/cancers17040663
Chicago/Turabian StyleVonica, Razvan Constantin, Claudiu Morgovan, Anca Butuca, Manuela Pumnea, Remus Calin Cipaian, Adina Frum, Carmen Maximiliana Dobrea, Andreea Loredana Vonica-Tincu, Aliteia-Maria Pacnejer, Florina Batar, and et al. 2025. "Real-World Evidence of Bevacizumab and Panitumumab Drug Resistance and Drug Ineffectiveness from EudraVigilance Database" Cancers 17, no. 4: 663. https://doi.org/10.3390/cancers17040663
APA StyleVonica, R. C., Morgovan, C., Butuca, A., Pumnea, M., Cipaian, R. C., Frum, A., Dobrea, C. M., Vonica-Tincu, A. L., Pacnejer, A.-M., Batar, F., Vornicu, V., Ghibu, S., & Gligor, F. G. (2025). Real-World Evidence of Bevacizumab and Panitumumab Drug Resistance and Drug Ineffectiveness from EudraVigilance Database. Cancers, 17(4), 663. https://doi.org/10.3390/cancers17040663






